Abstract
The current chemotherapy of alveolar echinococcosis (AE) is based on benzimidazoles such as albendazole and has been shown to be parasitostatic rather than parasiticidal, requiring lifelong duration. Thus, new and more efficient treatment options are urgently needed. By employing a recently validated assay based on the release of functional phosphoglucose isomerase (PGI) from dying parasites, the activities of 26 dicationic compounds and of the (+)- and (−)-erythro-enantiomers of mefloquine were investigated. Initial screening of compounds was performed at 40 μM, and those compounds exhibiting considerable antiparasitic activities were also assessed at lower concentrations. Of the dicationic drugs, DB1127 (a diguanidino compound) with activities comparable to nitazoxanide was further studied. The activity of DB1127 was dose dependent and led to severe structural alterations, as visualized by electron microscopy. The (+)- and (−)-erythro-enantiomers of mefloquine showed similar dose-dependent effects, although higher concentrations of these compounds than of DB1127 were required for metacestode damage. In conclusion, of the drugs investigated here, the diguanidino compound DB1127 represents the most promising compound for further study in appropriate in vivo models for Echinococcus multilocularis infection.
INTRODUCTION
Infection with the larval (metacestode) stage of the fox tapeworm Echinococcus multilocularis causes alveolar echinococcosis (AE), a mainly hepatic disease. AE is fatal if not treated appropriately by surgical and/or chemotherapeutical intervention. For chemotherapeutical treatment, the current drugs of choice are the benzimidazole derivatives albendazole and mebendazole. They are applied either alone without surgery or in combination with surgery (9, 53). Benzimidazoles, however, do not act parasiticidally in vivo. Failures in drug treatment and/or the occurrence of side effects has been reported, leading to discontinuation of treatment or to progressive disease. Due to this unsatisfactory situation, novel drug candidates for the treatment of AE need to be identified (17, 18).
A number of compounds have been investigated by using in vitro-cultured parasites and/or by applying in vivo rodent models. Among these compounds, already commercialized anti-infective or anticancer agents such as other benzimidazole derivatives, including flubendazole, itraconazole, and methiazole, as well as artemether, caspofungin, ivermectin, miltefosine, rifampin, trimethoprim-sulfamethoxazole, and amphotericin B (40, 41), isoprinosine and derivatives (26), nitazoxanide and nitazoxanide derivatives (47, 49, 50), nitazoxanide and albendazole in combination (50), alpha-difluoromethyl-ornithine (33), genistein and respective derivatives (36), pyridinyl imidazoles (13), thioureides (35), and anticancer agents such as 2-methoxyestradiol (43), cyclosporine, and doxorubicin (29, 30), have been studied. Mefloquine, developed in 1971, is a synthetic analogue of quinine. Due to its long half-life, mefloquine is commonly used in malaria prophylaxis and treatment of chloroquine-resistant Plasmodium falciparium malaria (27). Recently, mefloquine was shown to be effective against E. multilocularis metacestodes both in vitro and in vivo (25).
Despite these considerable investigations, most of these compounds did not progress further into clinical development. One exception is amphotericin B deoxycholate, which has been applied as salvage treatment but cannot be used for extended time periods (41). Another compound is nitazoxanide, but this drug has not been as effective in humans as previously indicated by in vivo studies in mice (23, 50, 51).
Nevertheless, independent of how actively a drug is being used in laboratory models, it is unlikely that a drug development candidate will ever reach clinical application for the treatment of a neglected disease such as AE, since the costly development of a novel drug for this limited market is not of interest to the pharmaceutical industry. The focus in drug discovery for AE should therefore be on compounds or compound classes that are either in an advanced stage of development or, even more realistically, already marketed. In the case of echinococcosis, these are (i) compounds with relevance in cancer treatment (24, 30, 43) and (ii) compounds with a broad-spectrum anti-infective activity (reviewed in reference 18).
In recent years, major efforts in drug development have concentrated on neglected diseases, including malaria, trypanosomiasis, leishmaniasis, and schistosomiasis (38). Pentamidine and its analogues represent a class of broad-spectrum antimicrobial compounds with activities against a wide range of intracellular and extracellular protozoan parasites (8). Since its discovery, pentamidine has been successfully used to treat African trypanosomiasis, leishmaniasis, and malaria but also pneumocystis pneumonia caused by Pneumocystis jirovecii and Candida albicans infections (8, 55). Diminazene aceturate, a pentamidine derivative, is commonly used for trypanosome chemotherapy in livestock, but this drug is prone to resistance development. More recently, novel analogues, known as arylimidamides, previously referred to as “reversed amidines,” have been proven effective against visceral leishmaniasis (55), and their development, together with that of mono-arylimidamides, has resulted in improved compounds that exhibit a more favorable pharmacokinetic profile, improved bioavailability, and lower toxicity.
The present study reports on the in vitro efficacies of a series of experimental dicationic pentamidine derivatives, as well as of mefloquine and its erythro-enantiomers, against E. multilocularis metacestodes.
MATERIALS AND METHODS
Media and biochemicals.
If not stated otherwise, all culture media were purchased from Gibco-BRL (Zürich, Switzerland) and biochemical reagents were from Sigma (St. Louis, MO). The dicationic compounds were prepared at the Department of Chemistry, Georgia State University, Atlanta, GA, and at the Department of Chemistry and Physics, Augusta State University, Augusta, GA. Mefloquine was kindly supplied by Mepha Pharma AG (Aesch, BL, Switzerland), and mefloquine enantiomers were obtained from the Walter Reed Army Institute of Research, Silver Spring, MD.
In vitro culture of E. multilocularis metacestodes.
Culture of E. multilocularis (isolate H95) was carried out as previously described with few modifications (45). In short, metacestodes dissected from experimentally infected BALB/c mice were crushed through a metal tea strainer. The metacestodes were incubated in an antibiotic solution (20 μg/ml levoflaxin [Aventis, Meyrin, Switzerland], 20 μg/ml ciprofloxacin [Bayer, Zürich, Switzerland], in phosphate-buffered saline [PBS]) overnight. The next day, 5 × 106 rat hepatoma cells (RH)/ml (kindly provided by Klaus Brehm, Institute for Hygiene and Microbiology, University of Würzburg) was added to 1 ml of metacestodes, and medium (Dulbecco modified Eagle medium [DMEM], 10% fetal calf serum [FCS], 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate) was added to 50 ml. Tetracycline and levoflaxin have been found to be unnecessary at this stage of culture, in contrast to the procedure described by Spiliotis et al. (45). The metacestode-hepatoma cell cocultures were incubated in culture flasks at 37°C, under 5% CO2, with medium and hepatoma cell changes done once a week. Splitting of cultures was carried out when the total metacestode volume exceeded 20 ml. Metacestodes were used for experimental procedures when they reached diameters of 2 to 4 mm.
The rat hepatoma cells were maintained in the same medium, and the cells were grown to total confluence and trypsinized and diluted 1:20 in fresh culture medium once a week.
In vitro drug treatment of E. multilocularis metacestodes.
E. multilocularis metacestodes were collected after 1 to 2 months of culture and were washed three times in PBS (Fluka Chemie, Buchs, Switzerland) in order to remove medium, debris, and broken vesicles. Treatments were carried out as described by Stadelmann et al. (47). In brief, medium without phenol red (DMEM, 100 U/ml penicillin G, 100 μg/ml streptomycin sulfate, 2 mM l-glutamine) was added to the same volume of vesicles and distributed to 24-well plates (Greiner Bio-One, Frickenhausen, Germany; 2 ml per well, ∼25 to 35 vesicles). The drugs were prepared as stocks of 40 mM in dimethyl sulfoxide (DMSO). Predilutions to 100 times the final concentration were prepared in medium and added to the metacestodes as indicated in Results. Preliminary assays were carried out at 40 μM. Drugs showing good efficacy (i.e., marked increase in phosphoglucose isomerase [PGI] activity in the medium supernatant) were further investigated at concentrations of 20 μM. For the dose-response curve of DB1127, final concentrations of 0.01, 0.05, 0.1, 0.25, 0.5, 1, 5, 10, and 20 μM were tested. As a negative control, the corresponding dilution of DMSO was used. As positive controls, nontreated metacestodes were incubated in (i) 20 μM nitazoxanide and (ii) 1% Triton X-100 in PBS (resulting in complete release of PGI activity). Aliquots of medium supernatant (200 μl) were collected and stored at −20°C until further measurement of PGI activity. In parallel, specimens were viewed by light microscopy to assess potential drug-induced morphological damage.
Assessment of parasite toxicity by PGI assay.
Damage of vesicles incubated with selected drugs under axenic conditions was measured indirectly by detecting the release of PGI, as previously described by Stadelmann et al. (47). The assay was performed in 96-well microtiter plates (Greiner Bio-One). Per well, 95 μl of assay buffer (100 mM Tris-HCl [pH 7.6], 0.5 mM NAD [Fluka], 2 mM EDTA [Merck], and 1 U glucose-6-phosphate dehydrogenase) was mixed with 20 μl of each supernatant aliquot (see above). Measurements were performed in triplicate. As an assay inhibition control, the corresponding concentration of each compound was added to a reaction mix that included metacestode fluid. The reaction was started by the addition of fructose-6-phosphate (Fluka) to 1 mM. NAD reduction to NADH was measured by reading the absorbance at 340 nm (A340) every minute from 0 to 30 min on a 96-well plate reader (2300 EnSpire multilabel reader; Perkin-Elmer, Turku, Finland). Enzyme blanks (no substrate) and substrate blanks (no enzyme) were also included. Absorbance values of the enzyme blanks were subtracted from the enzyme reaction values afterwards. The PGI activity of the untreated group was subtracted from the activity of the treated groups as it represents the activity baseline. PGI activity was calculated from the corresponding linear regression parameters (ΔA340/Δt) and is presented as a percentage relative to the values obtained by treatment of vesicles with 1% Triton X-100. Linear regression analysis was performed using Excel (version 2007).
Morphological and ultrastructural investigations of DB1127-treated metacestodes.
To visualize the damage to metacestodes imposed by drug treatment, parasites were fixed and processed for transmission electron microscopy (TEM) at 1, 2, and 5 days after initiation of treatments with 20 μM DB1127 (15, 16). Briefly, metacestodes were washed once in PBS and then placed into 2.5% glutaraldehyde in 100 mM sodium cacodylate buffer (pH 7.2) for 2 h at room temperature, followed by postfixation in 2% OsO4 in 100 mM sodium cacodylate buffer (pH 7.2) for 2 h at room temperature. Then, samples were washed in distilled water and treated with 1% uranyl acetate for 30 min. Subsequently, the specimens were thoroughly washed in distilled water and dehydrated by sequential incubations in increasing concentrations of ethanol. In a subsequent step, specimens were embedded in Epon 812 resin (Fluka). Polymerization of the resin was carried out at 65°C overnight. Sections were cut on a Reichert and Jung ultramicrotome, loaded onto 300-mesh copper grids (Plano GmbH, Marburg, Germany), and stained with uranyl acetate and lead citrate as previously described (16).
Cytotoxicity assay in HFF and RH.
Human foreskin fibroblasts (HFF) and rat hepatoma cells (RH) were seeded in 96-well microtiter plates (Greiner Bio-One) at a cell density of 10,000 cells/well. The cells were incubated at 37°C, under 5% CO2, in a mixture of DMEM (without phenol red), 10% FCS, 50 U/ml penicillin G, 50 μg/ml streptomycin, and 2 mM l-glutamine for 24 h, when total confluence was reached in a monolayer. DB1127, mefloquine, and (+)- and (−)-enantiomers of mefloquine solved in DMSO were added to final concentrations of 0.05 to 20 μM. For negative controls, the cells were treated with medium and the same amount of DMSO present in the treated groups. For a positive control, the cells were treated with 1% of Triton X-100. After incubation at 37°C, under 5% CO2, for 72 h, the cell vitality was assessed by an alamarBlue assay (2). In short, a 200× solution (2 g/liter) was made with resazurin and added to each well to a final concentration of 1×. Fluorescence at 595 nm was measured in a multilabel plate reader (Victor, Perkin-Elmer, Turku, Finland) at 0 and 4 h after the addition of resazurin to the cells. The values obtained at 0 h were subtracted from those obtained at 4 h. For each incubation condition, six replicates were measured.
RESULTS
In vitro activities of selected dicationic compounds of mefloquine and mefloquine erythro-enantiomers.
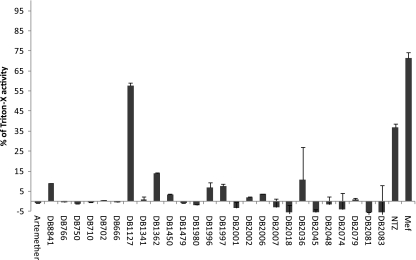
Initial PGI assays were carried out over a period of 5 days at a concentration of 40 μM for each compound. In this assay, the drug-induced damage of the metacestode tissue led to the release of PGI, which is an enzyme normally retained within the vesicle in untreated samples. The enzymatic activity in the medium supernatant was then measured as described previously (25, 47). The treatments over a period of 5 days were performed under axenic conditions, i.e., in the absence of rat hepatoma feeder cells. After culture in the presence of 26 dicationic compounds at a concentration of 40 μM, increased PGI activity was noted with the diarylimidamides DB666, DB702, DB710, DB750, DB766, and DB884, the newly generated monoarylimidamides DB1980, DB1996, DB1997, DB2001, DB2002, DB2006, DB2007, DB2018, DB2036, DB2045, DB2048, DB2074, DB2079, DB2081, and DB2083, the diguanidino analogue DB1127, and the diamidines DB1341, DB1362, DB1450, and DB1479. These dicationic compounds were selected for subsequent testing at 20 μM. In addition, mefloquine and its respective (+)- and (−)-enantiomers also induced elevated levels of PGI release, resulting in clear morphological alterations (loss of turgor, collapse of vesicles), which were visible macroscopically (data not shown).
Figure 1 shows the results of these PGI assays carried out at a 20 μM drug concentration, and the enzymatic activity is given relative to PGI activities obtained from vesicles permeabilized by Triton X-100. As an internal control, treatment with 20 μM nitazoxanide was performed (49). Overall, the most effective drug was the diguanidino derivative DB1127 (Fig. 2).
Fig. 1.
In vitro release of E. multilocularis PGI activities upon metacestode damage induced by dicationic compounds and mefloquine. PGI activity levels for metacestodes treated in vitro for 5 days by a set of different compounds at 20 μM are depicted relative to total vesicle damage induced by exposure to Triton X-100 (1%). Included drug candidates were nitazoxanide (NTZ) and mefloquine (Mef) as positive controls, artemether as a negative control, and dicationic DB compounds.
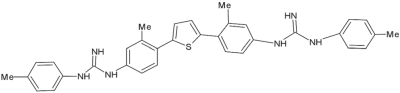
Fig. 2.
Structure of the diguanidino compound DB1127.
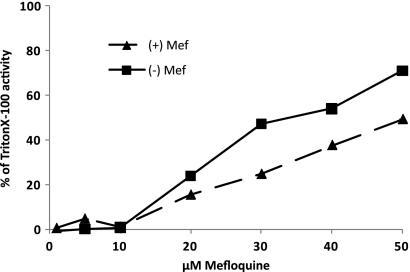
Mefloquine applied at 20 μM for 5 days also exhibited substantial antiparasitic activity, confirming the results of previous in vitro and in vivo studies (25). The corresponding (+)-erythro-enantiomer showed an efficacy similar to that of racemate, while the (−)-erythro-enantiomer appeared to be slightly more effective. A freshly prepared 1:1 mixture of the two enantiomers revealed intermediate activity (results not shown). None of the monoarylimidamides were effective against E. multilocularis metacestodes. Figure 3 demonstrates the dose-dependent activities of the two erythro-enantiomers, with the medium supernatants obtained from the (−)-enantiomer of mefloquine consistently displaying higher PGI levels than the (+)-enantiomer.
Fig. 3.
Dose response of PGI release obtained after exposure to mefloquine (+)- and (−)-enantiomers. Drugs were applied at concentrations ranging from 1 to 50 μM for a period of 5 days. Note the consistently higher PGI activity levels in medium supernatants of metacestodes exposed to the (−)-enantiomer.
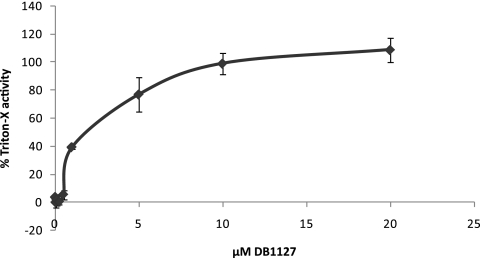
DB1127 was chosen for further studies, and E. multilocularis metacestodes were exposed to different concentrations of the drug ranging from 0 to 20 μM. These investigations revealed that DB1127 displayed considerable activity within a range of 1 to 20 μM, inducing PGI release in a dose-dependent manner (Fig. 4). The drug was largely ineffective at a drug concentration below 1 μM. In addition, treatment with 20 μM DB1127 resulted in a PGI release similar to the level obtained after complete permeabilization of vesicles with 1% Triton X-100.
Fig. 4.
Dose response of PGI release obtained after exposure to DB1127. E. multilocularis metacestodes were incubated in the presence of DB1127 (0 to 20 μM) for a period of 5 days.
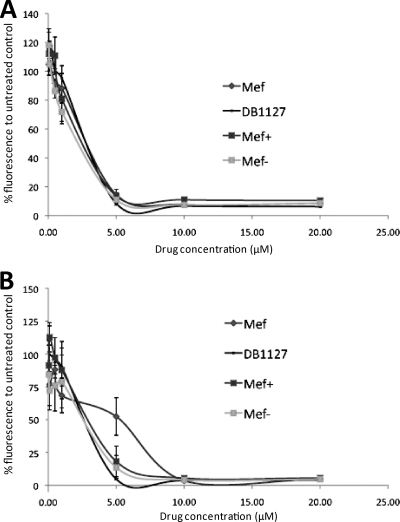
The cytotoxicities of DB1127, mefloquine, and the mefloquine (+)- and (−)-enantiomers were comparatively assessed in HFF and RH by the use of resazurin as an indicator for metabolic activity. Viable cells reduce resazurin to resorufin, a red fluorescent substance (2). As can be seen in Fig. 5, all four compounds exhibited largely similar cytotoxicity values. HFF, which are normal nontransformed cells, were killed by all drugs at 5 μM. No viable RH could be detected after exposure to 5 μM DB1127 or the two mefloquine enantiomers, while mefloquine itself exhibited a slightly less toxic effect (50% viability). However, a concentration of 10 μM mefloquine eliminated all RH.
Fig. 5.
Cytotoxicity of DB1127, mefloquine, and mefloquine (+)- and (−)-enantiomers against human foreskin fibroblasts (A) and rat hepatoma cells (B). Cells were grown to confluence and incubated with different concentrations of the drugs for 72 h. Assessments were made by measuring fluorescence in a resazurin cell viability assay. Values are presented as percentages relative to the values for controls without the compound.
Ultrastructural assessment of DB1127-induced damage in E. multilocularis metacestodes.
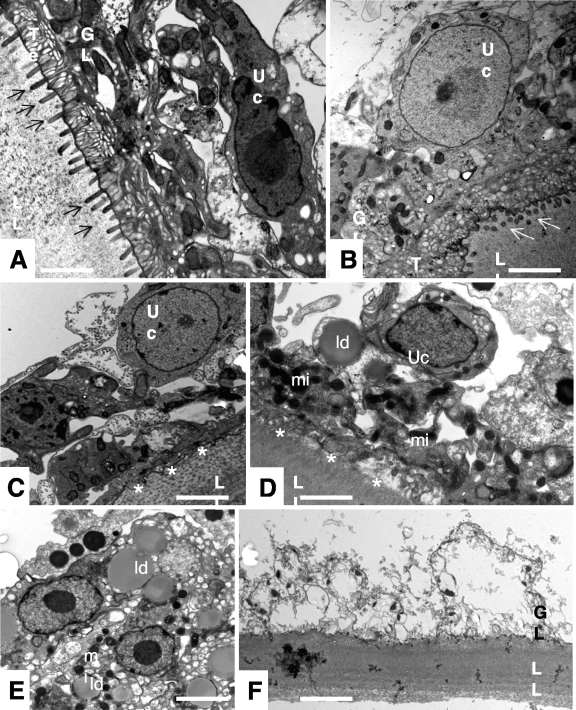
The dramatic increase in PGI release due to DB1127 treatment correlated well with the progressive impairment of metacestode vitality (Fig. 6). Untreated E. multilocularis metacestode vesicles (Fig. 6A and B) obtained from in vitro cultures exhibited typical features, with an acellular laminated layer surrounding the entire parasite. The proximal surface of the laminated layer contains the parasite tissue, with microtriches that protrude from the tegument into the laminated layer. The germinal layer is constituted by a relatively densely packed tissue containing muscle cells, connective tissue, undifferentiated cells, and mostly loaded glycogen storage cells (Fig. 6A and B). TEM micrographs taken 24 h after exposure to DB1127 (Fig. 6C and D) reveal some distinct changes, such as a profound shortening of the microtriches, partial depletion of the glycogen storage cells, and a less dense appearance of the germinal layer tissue (Fig. 6C). In addition, many regions of the germinal layer exhibited large lipid droplets and electron-dense mitochondria, suggesting that these parasites were metabolically impaired in early stages (Fig. 6D). At 48 h of DB1127 treatment, the numbers of lipid droplets found in the germinal layer tissue had increased dramatically, and the tissue was largely filled with vacuolated cells (Fig. 6 E). Five days of drug treatment resulted in complete destruction of the germinal layer, with only residues of the former parasite tissue still detectable (Fig. 6F).
Fig. 6.
Morphological effects of DB1127 treatment on metacestodes. In vitro-cultivated E. multilocularis metacestodes were either maintained in normal cultivation medium (A, B) or maintained in medium containing 20 μM DB1127 for 24 h (C, D), 48 h (E), or 5 days (F) and fixed and processed for TEM. LL, laminated layer; GL, germinal layer; Te, tegument; Uc, undifferentiated cell. Arrows indicate microtriches. Note the morphological changes of metacestodes upon treatment: shortening of microtriches (indicated by asterisks in panels C and D), occurrence of lipid droplets (indicated by “ld” in panels D and E), electron-dense mitochondria (indicated by “mi” in panels D and E). Bars, 2.2 μm (A and B), 2.4 μm (C and D), 2.5 μm (E), and 2.8 μm (F).
DISCUSSION
In a search for novel treatment options against AE, the in vitro efficacies of selected dicationic compounds and of the (+)- and (−)-enantiomers of mefloquine were investigated. Some of these compounds have proven efficacies against a broad spectrum of parasitic diseases, such as malaria, try-panosomiasis, leishmaniasis, amebiasis, trematode infections, and/or fungal infections (for reviews, see references 24 and 42; see also reference 56).
The literature on treatment of helminth infections with pentamidines or pentamidine derivatives is sparse. Duke (12) reported that pentamidine applied to chimpanzees was not active against experimental infection with Onchocerca volvulus. Another study has reported a lack of activity of pentamidine in the chemotherapy of experimental E. granulosus infection in mice and jirds (20). In contrast, mefloquine has been reported earlier to exhibit filaricidal activity against adults and microfilariae of Brugia patei and Brugia malayi (54), and numerous reports on its activity against Schistosoma adult and larval stages have been published (21, 22, 32). An important characteristic of diamidines is their DNA-binding property. In African trypanosomes, diamidines disrupt the kinetoplast within the single mitochondrion and cause enlargement of the mitochondrion and disruption of the mitochondrial membrane potential (31). Other mechanisms of action include disturbance of polyamine metabolism (9), inhibition of peptidase activity (34), and interference with normal topoisomerase II activity (3). Phenyl-substituted analogues were highly active against Leishmania, Trypanosoma cruzi, Toxoplasma gondii, Neospora caninum, and Besnoitia besnoiti (6, 10, 28, 55).
For E. multilocularis metacestodes, the PGI assay identified the diarylimidamide DB884 and the diamidine DB1362, which triggered a moderate release of E. multilocularis PGI activity when applied at 20 μM, indicating limited parasite drug-induced damage (Fig. 1). None of the tested monoarylimidamides exhibited activity against E. multilocularis metacestodes. However, a third compound, DB1127, was highly active (Fig. 1). DB1127 triggered the release of high levels of PGI activity from metacestodes in a dose-dependent fashion, and the compound was still substantially active at concentrations of 1 μM. This is remarkable, since most other compounds investigated in vitro so far, including albendazole, required concentrations in the range of 10 to 30 μM to exert any antimetacestode effects (for a review, see reference 18). DB1127 belongs to the class of di-N-aryl-diguanidino compounds, some of which have been shown to exhibit antiparasitic activities in vitro (T. brucei rhodesiense and P. falciparum) and in vivo (T. brucei rhodesiense) in the mouse model (14). In addition, diguanidino compounds were demonstrated to be active against Mycobacterium tuberculosis, Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans, and Rhizopus arrhizus (48).
In vitro assays performed with nontransformed HFF monolayers and rat hepatoma cells employing alamarBlue (Fig. 5) revealed that DB1127 treatment at 5 μM caused cytotoxicity, rendering cells largely nonviable. However, similar values were found for mefloquine and its two enantiomers (Fig. 5). This is not surprising, since other studies have demonstrated toxic effects of diamidines in tumor cells, implying that toxicity was mediated by the nuclear or mitochondrial DNA-binding properties and topoisomerase inhibition (3, 37, 46). In any case, toxicity of compounds against cell cultures cannot be automatically translated into the in vivo situation. This has been shown earlier for arylimidamides (6, 55) and also for other drugs, including artemisinins and mefloquine, which exhibited profound cytotoxicity in vitro but not when applied to mice (1, 25, 44). Thus, despite the cytotoxic effects against mammalian cells seen in vitro, DB1127 could still be a valuable drug to be used, and this needs to be addressed in future investigations employing appropriate in vivo models.
Mefloquine has been shown recently to exhibit promising in vitro and in vivo activity against trematodes and cestodes, including Schistosoma and E. multilocularis metacestodes (21, 22, 25, 32). How this occurs is not known. The exact mechanism of action of mefloquine on plasmodia has not been elucidated so far, but it has been suggested that a disturbance of hemoglobin metabolism and formation of an insoluble polymer, hemozoin, occurs (11). A hemozoin-related effect is unlikely to be responsible for the activity of mefloquine against E. multilocularis metacestodes, which implies that other targets must be involved. A potential obstacle in a future clinical application of mefloquine might be the neurotoxicity of the drug (52). Mefloquine (+)- and (−)-enantiomers showed similar activities against P. falciparum (5); however, in humans, mice, and rats, the cerebral transport of mefloquine was stereoselective, with concentrations of the (−)-erythro-enantiomer in the plasma and brain being higher than those of the (+)-erythro-enantiomer (4, 7, 39). The stereoselectivity observed in the brain indicates that active cerebral uptake mechanisms could be responsible for transport. As assessed by the PGI assay in the present study, the (−)-erythro-enantiomer of mefloquine also exhibited slightly stronger antimetacestode effects than the (+)-erythro-enantiomer. Possibly, the differential activity of the two enantiomers is also related to differences in the uptake. A 1:1 mixture of the two enantiomers exhibited metacestodicidal effects that mirrored those of the racemate mefloquine. However, further studies on the potential mechanisms of uptake of mefloquine are required to elucidate the nature of the differential effects of the two erythro-enantiomers.
Further investigations by TEM focused on the alterations induced in metacestodes by in vitro treatment with DB1127. When the drug was applied at a concentration of 20 μM, progressive changes occurred in a time-dependent manner, which led to complete loss of viability of DB1127-treated metacestodes within 5 days. The first adverse effects were observed as early as 24 h after treatment, as documented by a loss of microtriches that build up the interface between the living parasite tissue and the acellular laminated layer. Similar findings have been obtained upon treatment of metacestodes with albendazole sulfoxide (19). These findings also include the formation of lipid droplets and the occurrence of electron-dense mitochondria, findings that suggest that the drug interferes with the metabolic activity of the parasites. At 5 days of drug treatment, the germinal layer-associated tissue appeared completely destroyed, with no viable cells present.
In terms of timing, these drug-induced alterations occur at a slightly lower speed, as reported for mefloquine (25), which induced its destructive effects rapidly, within 1 to 2 days, albeit at a slightly higher concentration (24 μM, compared to 20 μM for DB1127 in this study).
However, DB1127 seems to exhibit its in vitro activity at a similar speed as previously observed for nitazoxanide (49) but at a speed much higher than that reported for albendazole (19), artesunate, and dihydroartemisinin (43, 44). This rapid effect of DB1127 against these parasites suggests that this compound could be a useful additive to the currently very limited antimetacestode arsenal based on few benzimidazoles. However, further studies on its in vivo efficacy against experimentally induced echinococcosis are necessary, and bioavailability, pharmacokinetics, in vivo toxicity, and potential side effects need to be carefully evaluated.
In conclusion, the in vitro effects of mefloquine and of dicationic pentamidine derivatives against E. multilocularis metacestodes have been demonstrated. Most notably, DB1127 represents the first diguanidino compound that is reported to exhibit promising in vitro activities against Echinococcus metacestodes. This promising drug candidate should get further attention for treatment of AE and should be tested in in vivo studies with respect to its parasitostatic and parasiticidal effects on E. multilocularis.
Footnotes
Published ahead of print on 18 July 2011.
REFERENCES
- 1. AlKadi H. 2007. Antimalarial drug toxicity: a review. Chemotherapy 53:385–391 [DOI] [PubMed] [Google Scholar]
- 2. Anoopkumar-Dukie S., et al. 2005. Resazurin assay of radiation response in cultured cells. Br. J. Radiol. 78:945–947 [DOI] [PubMed] [Google Scholar]
- 3. Bailly C., et al. 1999. Relationships between topoisomerase II inhibition, sequence-specificity and DNA binding mode of dicationic diphenylfuran derivatives. Anticancer Drug Des. 14:47–60 [PubMed] [Google Scholar]
- 4. Barraud de Lagerie S., et al. 2004. Cerebral uptake of mefloquine enantiomers with and without the P-gp inhibitor elacridar (GF1210918) in mice. Br. J. Pharmacol. 141:1214–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basco L., Gillotin C., Gimenez F., Farinotti R., Le Bras J. 1992. In vitro activity of the enantiomers of mefloquine, halofantrine and enpiroline against Plasmodium falciparum. Br. J. Clin. Pharmacol. 33:517–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Batista D. G., et al. 2010. Arylimidamide DB766, a potential chemotherapeutic candidate for Chagas' disease treatment. Antimicrob. Agents Chemother. 54:2940–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baudry S., et al. 1997. Stereoselective passage of mefloquine through the blood-brain barrier in the rat. J. Pharm. Pharmacol. 49:1086–1090 [DOI] [PubMed] [Google Scholar]
- 8. Bray P. G., Barrett M. P., Ward S. A., de Koning H. P. 2003. Pentamidine uptake and resistance in pathogenic protozoa: past, present and future. Trends Parasitol. 19:232–239 [DOI] [PubMed] [Google Scholar]
- 9. Brunetti E., Kern P., Vuitton D. A., and Writing Panel for the WHO-IWGE 2010. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 114:1–16 [DOI] [PubMed] [Google Scholar]
- 10. Cortes H. C., Muller N., Boykin D., Stephens C. E., Hemphill A. 2011. In vitro effects of arylimidamides against Besnoitia besnoiti infection in Vero cells. Parasitology 138:583–592 [DOI] [PubMed] [Google Scholar]
- 11. Dorn A., Stoffel R., Matile H., Bubendorf A., Ridley R. G. 1995. Malarial haemozoin/beta-haematin supports haem polymerization in the absence of protein. Nature 374:269–271 [DOI] [PubMed] [Google Scholar]
- 12. Duke B. 1977. The effects of some drugs—pentamidine, stibocaptate, Hoechst 33258, F 151, compound ‘E’ and Nifurtimox—on Onchocerca volvulus in chimpanzees. Tropenmed. Parasitol. 28:447–455 [PubMed] [Google Scholar]
- 13. Gelmedin V., Caballero-Gamiz R., Brehm K. 2008. Characterization and inhibition of a p38-like mitogen-activated protein kinase (MAPK) from Echinococcus multilocularis: antiparasitic activities of p38 MAPK inhibitors. Biochem. Pharmacol. 76:1068–1081 [DOI] [PubMed] [Google Scholar]
- 14. Gonzalez J. L., et al. 2007. Synthesis and antiparasitic evaluation of bis-2,5-[4-guanidinophenyl]thiophenes. Eur. J. Med. Chem. 42:552–557 [DOI] [PubMed] [Google Scholar]
- 15. Hemphill A., Gottstein B. 1995. Immunology and morphology studies on the proliferation of in vitro cultivated Echinococcus multilocularis metacestodes. Parasitol. Res. 81:605–614 [DOI] [PubMed] [Google Scholar]
- 16. Hemphill A., L C. S. 1997. Electron microscopy in parasitology. Springer Verlag, Heidelberg, Germany [Google Scholar]
- 17. Hemphill A., Müller J. 2009. Alveolar and cystic echinococcosis: towards novel chemotherapeutical treatment options. J. Helminthol. 83:99–111 [DOI] [PubMed] [Google Scholar]
- 18. Hemphill A., et al. 2010. Echinococcus metacestodes as laboratory models for the screening of drugs against cestodes and trematodes. Parasitology 137:569–587 [DOI] [PubMed] [Google Scholar]
- 19. Ingold K., et al. 1999. Efficacies of albendazole sulfoxide and albendazole sulfone against in vitro-cultivated Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 43:1052–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kammerer W. S., Perez-Esandi M. V. 1975. Chemotherapy of experimental Echinococcus granulosus infection. Trials in CF1 mice and jirds (Meriones unguiculatus). Am. J. Trop. Med. Hyg. 24:90–95 [DOI] [PubMed] [Google Scholar]
- 21. Keiser J. 2010. In vitro and in vivo trematode models for chemotherapeutic studies. Parasitology 137:589–603 [DOI] [PubMed] [Google Scholar]
- 22. Keiser J., et al. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl. Trop. Dis. 3:e350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kern P., et al. 2008. Critical appraisal of nitazoxanide for the treatment of alveolar echinococcosis. Am. J. Trop. Med. Hyg. 79:119 [Google Scholar]
- 24. Klinkert M., Heussler V. 2006. The use of anticancer drugs in antiparasitic chemotherapy. Mini Rev. Med. Chem. 6:131–143 [DOI] [PubMed] [Google Scholar]
- 25. Küster T., et al. 2011. In vitro and in vivo efficacies of mefloquine-based treatment against alveolar echinococcosis. Antimicrob. Agents Chemother. 55:713–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lawton P., Walchshofer N., Sarciron M. 2001. In vitro effects of isoprinosine and a dipeptide methyl ester on Echinococcus multilocularis protoscoleces. J. Helminthol. 75:251–257 [PubMed] [Google Scholar]
- 27. Leed A., et al. 2002. Solution structures of antimalarial drug-heme complexes. Biochemistry 41:10245–10255 [DOI] [PubMed] [Google Scholar]
- 28. Leepin A., et al. 2008. Host cells participate in the in vitro effects of novel diamidine analogues against tachyzoites of the intracellular apicomplexan parasites Neospora caninum and Toxoplasma gondii. Antimicrob. Agents Chemother. 52:1999–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liance M., et al. 1992. Effects of cyclosporin A on the course of murine alveolar echinococcosis and on specific cellular and humoral immune responses against Echinococcus multilocularis. Int. J. Parasitol. 22:23–28 [DOI] [PubMed] [Google Scholar]
- 30. Liance M., Nemati F., Bories C., Couvreur P. 1993. Experience with doxorubicin-bound polyisohexylcyanoacrylate nanoparticles on murine alveolar echinococcosis of the liver. Int. J. Parasitol. 23:427–429 [DOI] [PubMed] [Google Scholar]
- 31. Macadam R. F., Williamson J. 1972. Drug effects on the fine structure of Trypanosoma rhodesiense: diamidines. Trans. R. Soc. Trop. Med. Hyg. 66:897–904 [DOI] [PubMed] [Google Scholar]
- 32. Manneck T., Haggenmüller Y., Keiser J. 2010. Morphological effects and tegumental alterations induced by mefloquine on schistosomula and adult flukes of Schistosoma mansoni. Parasitology 137:85–98 [DOI] [PubMed] [Google Scholar]
- 33. Miyaji S., et al. 1993. Failure of treatment with alpha-difluoromethylornithine against secondary multilocular echinococcosis in mice. Parasitol. Res. 79:75–76 [DOI] [PubMed] [Google Scholar]
- 34. Morty R. E., et al. 1998. A trypanosome oligopeptidase as a target for the trypanocidal agents pentamidine, diminazene and suramin. FEBS Lett. 433:251–256 [DOI] [PubMed] [Google Scholar]
- 35. Müller J., et al. 2009. Thioureides of 2-(phenoxymethyl)benzoic acid 4-R substituted: a novel class of anti-parasitic compounds. Parasitol. Int. 58:128–135 [DOI] [PubMed] [Google Scholar]
- 36. Naguleswaran A., et al. 2006. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob. Agents Chemother. 50:3770–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Neidle S. 1997. Recent developments in triple-helix regulation of gene expression. Anticancer Drug Des. 12:433–442 [PubMed] [Google Scholar]
- 38. Nwaka S., et al. 2009. Advancing drug innovation for neglected diseases-criteria for lead progression. PLoS Negl. Trop. Dis. 3:e440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pham Y., Nosten F., Farinotti R., White N., Gimenez F. 1999. Cerebral uptake of mefloquine enantiomers in fatal cerebral malaria. Int. J. Clin. Pharmacol. Ther. 37:58–61 [PubMed] [Google Scholar]
- 40. Reuter S., Manfras B., Merkle M., Härter G., Kern P. 2006. In vitro activities of itraconazole, methiazole, and nitazoxanide versus Echinococcus multilocularis larvae. Antimicrob. Agents Chemother. 50:2966–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Reuter S., Merkle M., Brehm K., Kern P., Manfras B. 2003. Effect of amphotericin B on larval growth of Echinococcus multilocularis. Antimicrob. Agents Chemother. 47:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Soeiro M. N., et al. 2009. Experimental chemotherapy for Chagas disease: 15 years of research contributions from in vivo and in vitro studies. Mem. Inst. Oswaldo Cruz 104(Suppl. 1):301–310 [DOI] [PubMed] [Google Scholar]
- 43. Spicher M., et al. 2008. In vitro and in vivo effects of 2-methoxyestradiol, either alone or combined with albendazole, against Echinococcus metacestodes. Exp. Parasitol. 119:475–482 [DOI] [PubMed] [Google Scholar]
- 44. Spicher M., et al. 2008. In vitro and in vivo treatments of echinococcus protoscoleces and metacestodes with artemisinin and artemisinin derivatives. Antimicrob. Agents Chemother. 52:3447–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Spiliotis M., et al. 2008. Transient transfection of Echinococcus multilocularis primary cells and complete in vitro regeneration of metacestode vesicles. Int. J. Parasitol. 38:1025–1039 [DOI] [PubMed] [Google Scholar]
- 46. Spychała J. 2008. The usefulness of cyclic diamidines with different core-substituents as antitumor agents. Bioorg. Chem. 36:183–189 [DOI] [PubMed] [Google Scholar]
- 47. Stadelmann B., Scholl S., Müller J., Hemphill A. 2010. Application of an in vitro drug screening assay based on the release of phosphoglucose isomerase to determine the structure-activity relationship of thiazolides against Echinococcus multilocularis metacestodes. J. Antimicrob. Chemother. 65:512–519 [DOI] [PubMed] [Google Scholar]
- 48. Stephens C. E., et al. 2001. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. J. Med. Chem. 44:1741–1748 [DOI] [PubMed] [Google Scholar]
- 49. Stettler M., et al. 2003. In vitro parasiticidal effect of nitazoxanide against Echinococcus multilocularis metacestodes. Antimicrob. Agents Chemother. 47:467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stettler M., et al. 2004. Secondary and primary murine alveolar echinococcosis: combined albendazole/nitazoxanide chemotherapy exhibits profound anti-parasitic activity. Int. J. Parasitol. 34:615–624 [DOI] [PubMed] [Google Scholar]
- 51. Tappe D., Müller A., Frosch M., Stich A. 2009. Limitations of amphotericin B and nitazoxanide in the treatment of alveolar echinococcosis. Ann. Trop. Med. Parasitol. 103:177–181 [DOI] [PubMed] [Google Scholar]
- 52. Toovey S. 2009. Mefloquine neurotoxicity: a literature review. Travel Med. Infect. Dis. 7:2–6 [DOI] [PubMed] [Google Scholar]
- 53. Vuitton D. 2009. Benzimidazoles for the treatment of cystic and alveolar echinococcosis: what is the consensus? Expert Rev. Anti Infect. Ther. 7:145–149 [DOI] [PubMed] [Google Scholar]
- 54. Walter R., Wittich R., Kuhlow F. 1987. Filaricidal effect of mefloquine on adults and microfilariae of Brugia patei and Brugia malayi. Trop. Med. Parasitol. 38:55–56 [PubMed] [Google Scholar]
- 55. Wang M. Z., et al. 2010. Novel arylimidamides for treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 54:2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wenzler T., et al. 2009. New treatment option for second-stage African sleeping sickness: in vitro and in vivo efficacy of aza analogs of DB289. Antimicrob. Agents Chemother. 53:4185–4192 [DOI] [PMC free article] [PubMed] [Google Scholar]