Abstract
Although acyclovir (ACV) has proven to be of value in the therapy of certain herpes simplex virus (HSV) infections, there is a need for more effective therapies, particularly for serious infections in neonates and immunocompromised individuals, where resistance to this drug can be problematic. CMX001 is an orally bioavailable lipid conjugate of cidofovir that is substantially less nephrotoxic than the parent drug and has excellent antiviral activity against all the human herpesviruses. This compound retains full antiviral activity against ACV-resistant laboratory and clinical isolates. The combined efficacy of CMX001 and ACV was evaluated in a new real-time PCR combination assay, which demonstrated that the combination synergistically inhibited the replication of HSV in cell culture. This was also confirmed in murine models of HSV infection, where the combined therapy with these two drugs synergistically reduced mortality. These results suggest that CMX001 may be effective in the treatment of ACV-resistant HSV infections and as an adjunct therapy in individuals with suboptimal responses to ACV.
INTRODUCTION
Effective therapies are available for the management of both genital herpes (23) and herpes labialis (1). Acyclovir (ACV) can inhibit the replication of both herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) (6), and its oral prodrug, valacyclovir, is well tolerated and suitable for long-term suppressive therapy (38). Prophylactic therapy in individuals with frequent or severe symptoms can reduce the frequency of lesions by 80% to 95% (19) and can also reduce significantly, although not eliminate, asymptomatic virus shedding (8, 11) and transmission of the virus (5). Resistance to the drug is observed infrequently in the general population, even after long-term prophylactic therapy (38), and resistance occurs in immunocompetent individuals at a very low frequency (≤0.5%) (17). However, resistance to the drug has been reported to arise in up to 7% of immunocompromised hosts (38). Drug-resistant isolates selected by ACV therapy are also generally cross-resistant to famciclovir, and secondary therapies such as cidofovir (CDV) and foscarnet are associated with significant toxicities. Thus, there is a clear need for improved therapies for herpes infection in immunocompromised hosts (21, 42).
High-dose ACV therapy can reduce significantly mortality associated with neonatal herpes infections, although the potential for neutropenia in the neonate needs to be considered (16). Similarly, ACV therapy has significantly reduced the mortality associated with herpes simplex encephalitis from 70% to 28% (40); however, serious sequelae are often observed, and viral DNA can persist in the cerebrospinal fluid (CSF) even after 3 weeks of high-dose ACV therapy (20). These successes notwithstanding, there is a need for more effective therapies for severe herpes infections to improve the health and long-term outcome of this population (13).
Recently, we reported that the alkoxyalkyl esters of CDV, including CMX001 (hexadecyloxypropyl CDV), exhibited greatly enhanced in vitro efficacy against all the human herpesviruses, including HSV-1 and HSV-2 (41). The esterification of CDV also imparted oral bioavailability to this compound, and oral administration of this molecule was shown to be effective in vivo against experimental infections in mice with human cytomegalovirus (CMV), murine CMV (3, 14), and several orthopoxvirus infections (22, 28, 34). The modified pharmacokinetic and distribution properties of CMX001 also appeared to reduce its accumulation in the kidneys of infected mice and thus avoided the nephrotoxicity that is observed frequently with CDV (4). CMX001 was also recently reported to penetrate the blood-brain barrier in mice and was more potent than ACV in the treatment of HSV infections in animal models of central nervous system (CNS) infections (29). This compound proved to be well tolerated in phase 1 safety studies and is currently being evaluated in phase 2 clinical studies for treatment of BK virus and CMV infections (ClinicalTrials.gov identifiers NCT00793598 and NCT00942305).
CMX001 has the potential to be a highly effective therapy for neonatal herpes infections and severe CNS infections. Therapy with ACV is the current standard of care, and CMX001 will likely be administered to some patients already receiving this drug; thus, it is important to document the safety as well as confirm the efficacy of this combination. Since CMX001 does not require phosphorylation by the HSV thymidine kinase (TK), it should also be effective against ACV-resistant infections. Further, the coadministration of CMX001 and ACV might also be able to minimize the selection for resistant viruses in immunosuppressed individuals. Studies presented here document the efficacy of CMX001 against ACV-resistant strains of HSV-1 and HSV-2. Additional in vitro studies indicated that combinations of CMX001 and ACV synergistically inhibit the replication of both viruses. These data were confirmed in animal models of HSV-1 and HSV-2 infections, where the combination proved to synergistically reduce mortality of infected mice. Results from this series of experiments support the use of CMX001 in ACV-resistant infections and also suggest that the addition of CMX001 to the standard of care should improve the outcome for patients with severe herpesvirus infections.
MATERIALS AND METHODS
Compounds.
CMX001 was provided by Chimerix, Inc. (Durham, NC), and ACV was purchased from Sigma-Aldrich, St. Louis, MO.
Cells and viruses.
All in vitro studies were performed using human foreskin fibroblast (HFF) cells prepared from human foreskin tissue obtained from the University of Alabama at Birmingham tissue procurement facility with approval from its institutional review board (IRB). Strains of HSV-1 used to assess antiviral activity included the wild-type (wt) F strain as well as ACV-resistant isolates DM2.1, PAAr5, SC16-S1, and B-2006. The wt HSV-2 strain G was used to evaluate the activity of the compounds as well as resistant isolates 12247, 13077, 11680, and AG-3. For combined efficacy and animal studies, strains E-377 and MS were used to evaluate efficacy against HSV-1 and HSV-2, respectively. The provenance and phenotypic and genotypic characteristics of each of these viruses were reported recently (26). Mutations in these isolates thought to confer resistance to ACV are in the TK open reading frame.
In vitro susceptibility assays.
Standard plaque reduction assays were performed with monolayers of confluent HFF cells in 6-well plates by methods reported previously (25). Briefly, virus suspensions were used to infect triplicate monolayers of HFF cells in 6-well plates. After adsorption of the virus, the medium was replaced with growth medium containing 6 different concentrations of the compounds. After a 3-day incubation, cell monolayers were stained with crystal violet and plaques were enumerated. Compound concentrations sufficient to reduce plaque numbers by 50% (EC50) were interpolated from the experimental data.
Combination efficacy and cytotoxicity studies.
Combinations of CMX001 and ACV were evaluated using an in vitro antiviral assay to assess combined efficacy using an experimental approach similar to what we described previously (27). Briefly, a checkerboard matrix of drug dilutions was prepared directly in 96-well plates containing monolayers of HFF cells. Four replicate plates were infected with either strain E-377 or MS at a multiplicity of infection (MOI) of 0.01 PFU/cell. At 3 days after infection, total DNA from each well was prepared using the Wizard SV 96 genomic DNA purification system (Promega, Madison, WI). Genome copy number was quantified by real-time PCR using plasmid pMP031 to provide absolute quantification. Amplification primers used were 5′-ACC GCC GAA CTG AGC AGA C-3′ and 5′-TGA GCT TGT AAT ACA CCG TCA GGT-3′, together with the probe 5′-6FAM-CGC GTA CAC CAA CAA GCG CCT G-TAMRA-3′ (where 6FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). These primers detect sequences within the DNA polymerase open reading frame that correspond to coordinates 65824 to 65899 in HSV-1 (GenBank accession number GU734771.1) and 66379 to 66454 in HSV-2 (GenBank accession number Z86099.2). Cells in two replicate plates remained uninfected and were exposed to the same combinations of drug concentrations, and cell viability was determined using a CellTiter-Glo assay (Promega, Madison, WI).
The dynamic range inherent in the real-time PCR data provided information that was superior to that generated in more traditional assays. To take advantage of this new format, the MacSynergy II software that we developed more than 20 years ago (27) was modified to accept the copy number in units of genomic equivalents (ge) and converted it to logarithmic form. The calculations used to evaluate synergy and antagonism were also changed to accommodate the new data but were mathematically equivalent to those used in our established method. The resulting volumes of statistically significant synergy and antagonism reflect the magnitude of these effects and are given in logarithmic form in units of μM2log10ge. For each drug combination study, a concurrent cytotoxicity study was performed with the same cells and matched drug exposure. Cytotoxicity data were evaluated as we described previously to show that the observed effects were not associated with synergistic cytotoxicity (27).
In vivo efficacy against HSV-1 and HSV-2.
Female BALB/c mice, 3 to 4 weeks of age, were obtained from Charles River Laboratories (Raleigh, NC) and utilized in lethal intranasal models of systemic HSV-1 and HSV-2 infections. Mice were housed in microisolator cages and utilized at 15 mice per group. They were obtained, housed, utilized, and euthanized according to policies of USDA and the Association for Assessment and Accreditation for Laboratory Animal Care, International. All animal procedures were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee prior to the initiation of studies. HSV infections were initiated by intranasal inoculation of BALB/c mice with HSV-1 strain E-377 or HSV-2 strain MS with 1 × 103 or 1.1 × 105 PFU/mouse, respectively, which typically results in 90% mortality. Previous studies indicated that the lowest effective dose of ACV was 30 mg/kg of body weight administered orally twice daily, and the lowest effective dose of CMX001 was 5 mg/kg given orally once daily (29). These levels were selected as the highest dosages used, so improved efficacy of the combination could be detected using an experimental design we reported previously (30). All mice were dosed twice daily for 7 days beginning 72 h after viral inoculation using a total volume of 0.2 ml of either the vehicle or drug solution so all animals were subjected to an equal amount of stress. Animals were observed for 21 days for mortality.
Statistical evaluation.
Groups of mice treated with antivirals were compared to vehicle-treated groups for statistical evaluation. Mortality rates were analyzed by Fisher's exact test. The mean-day-of-death data were analyzed by Mann-Whitney U rank sum, which compares the median values nonparametrically. A P value of 0.05 or less was considered significant.
RESULTS
In vitro efficacy against ACV-resistant isolates of HSV-1 and HSV-2.
Previous studies from our laboratory demonstrated that CMX001 exhibited enhanced efficacy against all the human herpesviruses compared to that of CDV (41). It also retained antiviral activity against ganciclovir (GCV)-resistant isolates of CMV, which could also be expected since CDV is a monophosphate analog that does not require an initial phosphorylation by the UL97 viral kinase. This characteristic should also render CMX001 effective against ACV-resistant isolates of both HSV-1 and HSV-2, since most resistant viruses have mutations in the TK open reading frame that reduce the formation of the active metabolite (9, 21). The susceptibility of four ACV-resistant isolates of both HSV-1 and HSV-2 was evaluated for both CMX001 and ACV. Although all resistant isolates exhibited elevated EC50s for ACV, none were resistant to CMX001 (Table 1). These data are consistent with its efficacy against GCV-resistant isolates of CMV and indicate that CMX001 would be useful in the treatment of drug-resistant CMV and HSV infections (41).
Table 1.
Efficacy of CMX001 against ACV-resistant isolates of HSV
| Isolate | EC50 ± SD (μM)a |
|
|---|---|---|
| CMX001 | ACV | |
| HSV-1 | ||
| DM2.1 | 0.008 ± 0.0007 | >100 ± 0b |
| B-2006 | 0.01 ± 0.0007 | >100 ± 0 |
| PAAr5 | 0.01 ± 0 | 14 ± 2.7 |
| SC16-S1 | 0.01 ± 0 | >100 ± 0 |
| F (wt) | 0.015 ± 0.006 | 1.9 ± 0.47 |
| HSV-2 | ||
| AG-3 | 0.023 ± 0.009 | >100 ± 0 |
| 12247 | 0.021 ± 0.012 | >100 ± 0 |
| 11680 | 0.009 ± 0.0007 | 79 ± 10 |
| 11572 | 0.027 ± 0.018 | >100 ± 0 |
| G (wt) | 0.029 ± 0.015 | 2.1 ± 0.99 |
Concentration required to reduce plaque numbers by 50% (EC50), with standard deviation values shown from three independent experiments.
>100 ± 0, all values were >100 μM.
Combined efficacy of CMX001 and ACV.
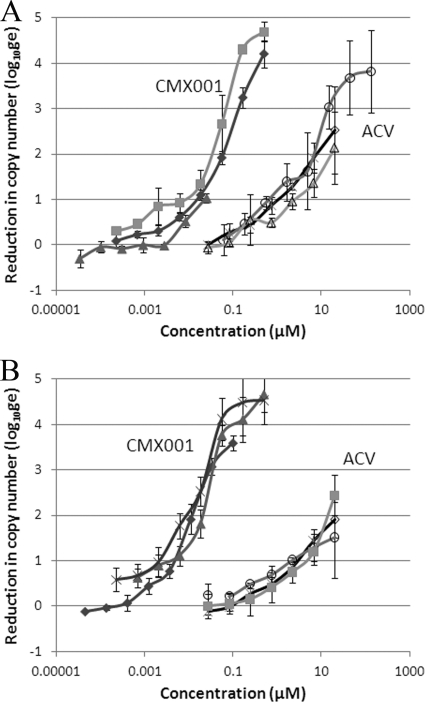
The combined efficacy of CMX001 and ACV against HSV infections was evaluated using methods that we have utilized for the last 20 years (27). However, the application of real-time PCR to quantify genome copy number increased the dynamic range of the assay and facilitated the detection of synergistic interactions over a wider range of drug concentrations. The experimental design yielded complete dose-response curves for both drugs, and CMX001 exhibited more potent antiviral activity than ACV against both HSV-1 and HSV-2 (Fig. 1). At concentrations of 0.5 μM, CMX001 essentially eliminated the replication of viral DNA and reduced levels to below those associated with the inocula and was 100-fold more potent than ACV.
Fig. 1.
Antiviral activity of CMX001 and ACV against HSV. Evaluation of the combined efficacy of CMX001 and ACV includes the activity of the compounds used individually. The efficacy of CMX001 (closed symbols) and ACV (open symbols) is shown as the reduction of the absolute genome copy number (log of genomic equivalents, log10ge) for the E-377 strain of HSV-1 (A) and the MS strain of HSV-2 (B). Results shown for each virus were obtained from three independent studies, with error bars representing the standard deviation values within each experiment.
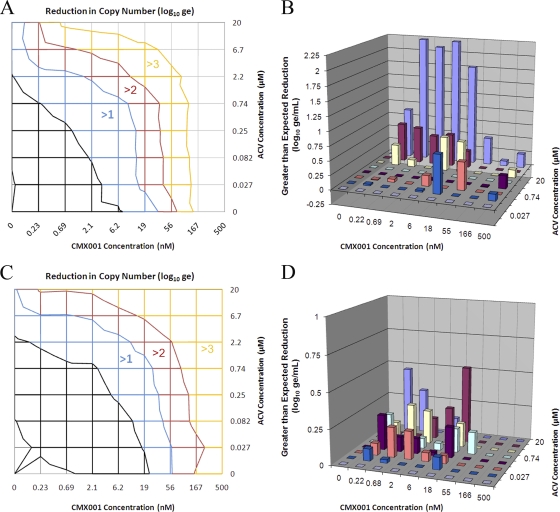
Combinations of ACV and CMX001 were also highly effective in reducing genome copy numbers in cells infected with HSV-1. Reductions in absolute copy numbers in infected cells treated with both drugs individually and in combination are shown in Fig. 2A, with colored regions representing the log10 reduction in genomic equivalents (log10ge). For example, the blue region shows all combinations of concentrations that reduced the viral DNA copy number by at least 1 log10ge. The edge of this region intersects the horizontal axis at 6.2 nM CMX001 (with no ACV), indicating that this concentration was sufficient to reduce the genome copy number by 1 log10ge. Similarly, the intersection of the blue region with the left vertical axes at 2.2 μM ACV indicates that this concentration of ACV as a single agent can also reduce the viral genome copy number by 1 log10ge. The general shift of these lines of equal effects to lower concentrations of ACV reflects its increasing efficacy as concentrations of CMX001 are increased in the combination. To identify concentrations that yield greater-than-expected reductions in genome copy numbers (synergy), the expected effects were calculated from the individual dose-response curves and subtracted from the experimental data. Statistically significant differences were plotted and are shown at concentrations of both drugs where synergy was observed (Fig. 2B). This analysis revealed a rather large range of combinations of concentrations where synergistic interactions were consistently observed. This effect was also reproducible, and the average volume of this interaction from three independent experiments was 23 ± 2.2 μM2log10ge (8.1 ± 2.3 μM2log10ge at 95% confidence). The value given at 95% confidence represents a conservative estimate of the synergy volume using the lower 95% confidence limit of the experimental data rather than the average of the experimental data to calculate synergy (27). Notably, concentrations of ACV and CMX001 that synergistically inhibited viral replication were below the plasma concentrations reported in mice, at 10 μM and 2.3 μM, respectively (10, 12).
Fig. 2.
Combined efficacy of CMX001 and ACV against HSV-1 and HSV-2. Cells infected with E-377 strain HSV-1 were treated with a matrix of combinations of the ACV and CMX001 concentrations indicated. (A) Resulting reductions in the absolute genome copy numbers (log10ge) as determined by real-time PCR are shown at the corresponding concentrations where they were observed, with colors representing the magnitude of the reduction shown. In this topographical representation, colored lines represent equal reductions in genome copy numbers and are shown at concentrations at which they occur. Intersections of these lines with the bottom and left axes represent concentrations of CMX001 and ACV, respectively, that reduce copy numbers to the levels indicated when used individually. (B) Differences between expected reductions in genome copy numbers calculated from the individual dose-response curves and experimentally observed reductions in genome copy numbers (synergy) are represented by columns at each of the combinations of concentrations shown on the two horizontal axes. Synergy values are shown at the 95% confidence level. The same experiment was repeated in cell monolayers infected with the MS strain of HSV-2. Reductions in absolute genome copy numbers (log10ge) are shown at the corresponding concentrations where they occur (C). Differences between expected reductions in genome copy numbers and experimentally determined reductions in genome copy numbers (synergy) are plotted at each of the combinations of concentrations and are shown at the 95% confidence level (D).
Since the combination of ACV and CMX001 would also be useful for the treatment of HSV-2 infections, the study was repeated in cells infected with this virus, and similar results were observed (Fig. 2C and D). Concentrations of ACV required to reduce genome copy number by 1 log10ge were consistently reduced as increasing concentrations of CMX001 were added to the drug combination (Fig. 2C). The synergy plot (Fig. 2D) also revealed a broad range of concentrations of both drugs that synergistically reduced the genome copy number, and the volume of synergy produced by the combination was 16 ± 3.1 μM2log10ge (3.6 ± 1.1 μM2log10ge at 95% confidence), which was similar to that observed with HSV-1. The synergies observed with the in vitro studies against both HSV-1 and HSV-2 were very encouraging and suggested that the combination of ACV and CMX001 should be investigated further in animal models of HSV infection.
All the combination studies included the concurrent evaluation of cytotoxicity to show that the synergistic inhibition of viral replication was a specific effect. For all studies, the observed cytotoxicity was minimal at all combinations of concentrations of both compounds and generally did not reduce cell viability by more than 10%, as determined by CellTiter-Glo assays in parallel cytotoxicity plates (data not presented). Further, the average cell number in the drug matrix was not statistically different from the average cell number in the control wells (P > 0.05).
Synergistic inhibition of mortality in mice infected with HSV-1 and HSV-2.
Recently, we reported the in vivo efficacy of both ACV and CMX001 in mice infected with HSV-1 and HSV-2 (29). To determine if the combination of the two drugs could synergistically reduce mortality in this model, we used an experimental design that used single-drug concentrations that were minimally effective and delayed the onset of therapy for 3 days. In the first study with animals infected with HSV-1, neither ACV nor CMX001 alone at any of the low concentrations tested significantly reduced mortality compared to the mortality of those treated with the vehicle (Table 2). However, animals treated orally with ACV at 30 mg/kg supplemented with each of the three concentrations of CMX001 exhibited significantly reduced mortality (P < 0.01). The same effect was also observed when the dose of ACV was further reduced to 10 mg/kg, and significant reductions in mortality were observed in each of the three groups supplemented with 5, 2.5, or 1.25 mg/kg of CMX001. Similar effects were also apparent for some groups using the mean day of death (MDD) as another indicator of efficacy. No significant increases in the MDD were observed in animals treated with 10 mg/kg of ACV or 1.25 mg/kg of CMX001 alone, yet the combination of these two doses resulted in a significant increase in the MDD. This same effect was also observed with combinations of 3 mg/kg and 1.25 mg/kg of ACV and CMX001, respectively.
Table 2.
Effect of oral treatment with ACV in combination with CMX001 on the mortality of BALB/c mice inoculated with HSV-1 strain E-377a
| Treatment | Mortality |
P value | MDD ± SD | P value | |
|---|---|---|---|---|---|
| No. | % | ||||
| Untreated | 15/15 | 100 | 6.7 ± 0.9 | ||
| Vehicle (0.4% CMC) | 15/15 | 100 | 6.3 ± 1.1 | ||
| ACV | |||||
| 30 mg/kg | 12/15 | 80 | NS | 7.3 ± 1.4 | <0.05 |
| 10 mg/kg | 11/15 | 73 | NS | 6.9 ± 1.4 | NS |
| 3 mg/kg | 15/15 | 100 | NS | 6.4 ± 0.9 | NS |
| CMX001 | |||||
| 5.0 mg/kg | 11/15 | 73 | NS | 7.6 ± 1.1 | <0.01 |
| 2.5 mg/kg | 15/15 | 100 | NS | 6.6 ± 1.1 | NS |
| 1.25 mg/kg | 13/15 | 87 | NS | 7.0 ± 0.8 | NS |
| ACV and CMX | |||||
| ACV, 30; CMX, 5 mg/kg | 8/14 | 57 | <0.01 | 6.4 ± 1.4 | NS |
| ACV, 30; CMX 2.5 mg/kg | 4/15 | 27 | <0.001 | 7.8 ± 0.5 | <0.05 |
| ACV, 30; CMX, 1.25 mg/kg | 6/15 | 40 | 0.001 | 7.0 ± 0.9 | NS |
| ACV, 10; CMX, 5 mg/kg | 6/15 | 40 | 0.001 | 7.5 ± 1.0 | <0.05 |
| ACV, 10; CMX, 2.5 mg/kg | 9/15 | 60 | <0.05 | 6.2 ± 1.4 | NS |
| ACV, 10; CMX, 1.25 mg/kg | 7/15 | 47 | <0.01 | 8.1 ± 0.9 | <0.01 |
| ACV, 3; CMX, 5 mg/kg | 11/15 | 73 | NS | 7.5 ± 1.0 | <0.01 |
| ACV, 3; CMX, 2.5 mg/kg | 12/15 | 80 | NS | 6.8 ± 1.5 | NS |
| ACV, 3; CMX, 1.25 mg/kg | 13/15 | 87 | NS | 7.8 ± 1.6 | 0.01 |
CMX001 was suspended in 0.4% carboxymethylcellulose (CMC) with or without ACV and given orally in 0.2-ml doses. Acyclovir was prepared in sterile water and given orally in 0.2-ml oral doses. Animals were treated twice daily for 7 days beginning 72 h after viral inoculation. MDD, mean day of death; NS, not significant when compared to the vehicle control group. P values are based on comparison of treatment groups with the vehicle control.
A second study was then conducted in mice infected with HSV-2 to determine if enhanced protection could be demonstrated against this virus, and the results are presented in Table 3. As indicated above for HSV-1, none of the treatment groups with ACV or CMX001 administered as single agents exhibited significantly reduced mortality compared to that of the vehicle control group (P > 0.5). However, the coadministration of 30 mg/kg of ACV with either 0.42 or 0.125 mg/kg of CMX001 significantly reduced mortality (P ≤ 0.01). Similarly, combinations of 10 mg/kg of ACV together with 1.25 mg/kg of CMX001 also resulted in decreased mortality (P ≤ 0.01). Improvements in efficacy were also evident in extending the life of infected animals. Oral administration of 3 mg/kg of ACV alone failed to extend the MDD, as did each of the three oral doses of CMX001. Yet this same dose of ACV supplemented with either 1.25 or 0.42 mg/kg of CMX001 increased the MDD (P ≤ 0.01).
Table 3.
Effect of oral treatment with ACV in combination with CMX001 on the mortality of BALB/c mice inoculated with HSV-2 strain MSa
| Treatment | Mortality |
P value | MDD ± SD | P value | |
|---|---|---|---|---|---|
| No. | % | ||||
| Untreated | 12/15 | 80 | 8.5 ± 0.9 | ||
| Vehicle (0.4% CMC) | 12/15 | 80 | 8.0 ± 1.4 | ||
| ACV | |||||
| 30 mg/kg | 6/15 | 40 | NS | 10.7 ± 2.0 | <0.01 |
| 10 mg/kg | 11/15 | 79 | NS | 11.1 ± 2.4 | <0.01 |
| 3 mg/kg | 15/15 | 100 | NS | 9.0 ± 1.6 | NS |
| CMX001 | |||||
| 1.25 mg/kg | 9/15 | 60 | NS | 9.4 ± 2.9 | NS |
| 0.42 mg/kg | 12/15 | 80 | NS | 9.8 ± 2.6 | NS |
| 0.125 mg/kg | 13/15 | 87 | NS | 9.0 ± 1.0 | NS |
| ACV and CMX001 | |||||
| ACV, 30; CMX, 1.25 mg/kg | 6/15 | 40 | NS | 11.0 ± 4.7 | NS |
| ACV, 30; CMX, 0.42 mg/kg | 4/15 | 27 | <0.01 | 12.8 ± 4.1 | <0.01 |
| ACV, 30; CMX, 0.125 mg/kg | 2/15 | 13 | 0.001 | 10.5 ± 0.7 | <0.05 |
| ACV, 10; CMX, 1.25 mg/kg | 3/15 | 20 | <0.01 | 9.7 ± 0.6 | 0.05 |
| ACV, 10; CMX, 0.42 mg/kg | 8/15 | 53 | NS | 10.4 ± 3.4 | NS |
| ACV, 10; CMX, 0.125 mg/kg | 6/15 | 40 | NS | 9.8 ± 1.7 | <0.05 |
| ACV, 3; CMX, 1.25 mg/kg | 6/15 | 40 | NS | 11.3 ± 2.5 | <0.01 |
| ACV, 3; CMX, 0.42 mg/kg | 8/15 | 53 | NS | 10.3 ± 1.9 | <0.01 |
| ACV, 3; CMX, 0.125 mg/kg | 10/15 | 67 | NS | 9.2 ± 2.2 | NS |
CMX001 was suspended in 0.4% carboxymethylcellulose (CMC) with or without ACV and given orally in 0.2-ml doses. Acyclovir was prepared in sterile water and given orally in 0.2-ml oral doses. Animals were treated twice daily for 7 days beginning 72 h after viral inoculation. MDD, mean day of death; NS, not significant when compared to the vehicle control group. P values are based on comparison of treatment groups with the vehicle control.
The results from the combination studies against HSV-1 and HSV-2 infections in mice were consistent and indicated that the combined oral therapy with ACV and CMX001 could significantly reduce mortality, whereas the compounds at the same doses as single agents were essentially ineffective. The confirmation of synergistic efficacy in animal models of herpes infections further suggests that this combination might be both safe and effective in humans.
DISCUSSION
The purpose of the studies presented here was to evaluate the efficacy of combinations of CMX001 and ACV against HSV infections in vitro and to determine if the combination therapy could demonstrate enhanced protection in animal model infections without exacerbating toxicity. The ultimate goal was to provide data to support the further development of this combination in clinical trials.
The evaluation of drug combinations in cell culture has helped to identify combinations of compounds that synergistically inhibit replication against other viruses, such as HIV and hepatitis C. The data presented here for the combinations of ACV and CMX001 clearly demonstrate the synergistic inhibition of HSV replication. The observed effects were both reproducible as well as robust and supported further studies in animal models of HSV-1 and HSV-2 infection. These studies confirmed that the efficacy of the combination was superior to either drug used individually and indicated that this combination could synergistically reduce mortality in this animal model. This was most apparent in Table 2, where combinations of 10 mg/kg ACV and 1.25 mg/kg CMX001 significantly reduced mortality, whereas three times this dose of ACV used alone and four times this dose of CMX001 used singly were ineffective. But will this effect translate to improved efficacy in humans? Administration of high-dose ACV results in plasma levels of the drug of 10 μM (35), and a single 2-mg/kg dose of CMX001 can achieve 0.62 μM concentrations in plasma (E. R. Lanier, personal communication). Thus, these levels are comparable to those observed in mice and provide exposure to the drugs at levels where significant synergy was observed in vitro (Fig. 2). Equally important, the drug combination did not exhibit synergistic cytotoxicity in cell culture, and the combination appeared to be well tolerated in mice. Additional in vivo studies with higher concentrations of both drugs in combination will be required to further evaluate the potential for combined toxicity. These data taken together suggest that the combination should be both safe and highly effective in humans. It is noteworthy that the clinically achievable levels of ACV and CMX001 in plasma correspond to 100-fold and 10,000-fold reductions of viral DNA, respectively (Fig. 1). The significantly greater control of viral replication afforded by CMX001 might also translate to improved efficacy in the clinic.
Although both HSV-1 and HSV-2 infections respond well to valacyclovir and famciclovir therapy, there is a need for improved therapies. The superior efficacy of CMX001 will be particularly valuable for serious herpesvirus infections. Herpes encephalitis remains a serious disease, and poor outcomes can occur notwithstanding the availability of ACV (15). Severe disseminated neonatal herpesvirus infections also respond well to ACV therapy, but mortality rates remain in excess of 25%, and survivors can also have long-term neurological sequelae (16). Resistance to ACV is also a significant problem and is observed in up to 7% of immunocompromised patients infected with HSV-1 or HSV-2 (36), and additional therapies are needed but not currently available (9). Since about 95% of ACV-resistant clinical isolates exhibit reduced TK activity and DNA polymerase mutations that confer resistance to the drug are uncommon, CMX001 could be predicted to be highly effective against most resistant isolates (2). Existing secondary therapies, such as CDV for resistant infections, are inadequate and suffer from dose-limiting toxicities.
Combination therapy has proven to be highly effective in the management of HIV infections (39). The improved efficacy afforded by these therapies suppresses virus replication to undetectable levels and minimizes the development of resistance (7). A similar strategy will likely be required to manage herpesvirus infections in immunosuppressed individuals that suffer persistent infections from these viruses, which reactivate frequently in the absence of an effective immune response. The addition of CMX001 to standard ACV regimens may significantly increase the barriers to resistance and should be particularly useful in this target population.
This combination may also prove to be useful in the treatment of primary HSV infections with the goal of rapidly reducing viral load to minimize the number of latently infected ganglia that seed subsequent recurrent infections. In animal models of infection, the genome copy number correlates well with the number of latently infected neurons in mice (31–32). The effect of early intervention with high-dose ACV showed reductions in the levels of viral DNA in ganglia and reduced rates of reactivation in these models (33). Famciclovir and valacyclovir have both been reported to work well in reducing viral load and reactivation, and it could be important to compare the efficacy of CMX001 in additional models of HSV infections (18, 24, 37).
The efficacy of CMX001 against HSV-1 and HSV-2 is impressive, and this drug has the potential to be useful in the treatment of severe HSV infections and those that are resistant to ACV. Data presented here suggest that the addition of CMX001 to standard ACV treatment regimens should be particularly effective and also retain efficacy even against ACV-resistant infections. The further development of CMX001 for the treatment of HSV infections will provide a valuable second-line therapy for resistant infections, and concomitant therapy with ACV and CMX001 may also prove to be more effective than and improve adverse outcomes from severe infections compared to ACV alone.
ACKNOWLEDGMENTS
We thank Emma A. Harden, Deborah J. Collins, and Terri L. Rice for expert technical assistance.
This work was supported by National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) contracts N01-AI-15439 (D.C.Q.), N01-AI-30049 (M.N.P.), and HHSN2722011000010C (M.N.P.).
Footnotes
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Arduino P. G., Porter S. R. 2006. Oral and perioral herpes simplex virus type 1 (HSV-1) infection: review of its management. Oral Dis. 12:254–270 [DOI] [PubMed] [Google Scholar]
- 2. Bacon T. H., Levin M. J., Leary J. J., Sarisky R. T., Sutton D. 2003. Herpes simplex virus resistance to acyclovir and penciclovir after two decades of antiviral therapy. Clin. Microbiol. Rev. 16:114–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bidanset D. J., Beadle J. R., Wan W. B., Hostetler K. Y., Kern E. R. 2004. Oral activity of ether lipid ester prodrugs of cidofovir against experimental human cytomegalovirus infection. J. Infect. Dis. 190:499–503 [DOI] [PubMed] [Google Scholar]
- 4. Ciesla S. L., et al. 2003. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 59:163–171 [DOI] [PubMed] [Google Scholar]
- 5. Corey L., et al. 2004. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. 350:11–20 [DOI] [PubMed] [Google Scholar]
- 6. Elion G. B. 1982. Mechanism of action and selectivity of acyclovir. Am. J. Med. 73:7–13 [DOI] [PubMed] [Google Scholar]
- 7. Este J. A., Cihlar T. 2010. Current status and challenges of antiretroviral research and therapy. Antiviral Res. 85:25–33 [DOI] [PubMed] [Google Scholar]
- 8. Fife K. H., et al. 2006. Effect of valacyclovir on viral shedding in immunocompetent patients with recurrent herpes simplex virus 2 genital herpes: a U.S.-based randomized, double-blind, placebo-controlled clinical trial. Mayo Clin. Proc. 81:1321–1327 [DOI] [PubMed] [Google Scholar]
- 9. Gilbert C., Bestman-Smith J., Boivin G. 2002. Resistance of herpesviruses to antiviral drugs: clinical impacts and molecular mechanisms. Drug Resist. Updat. 5:88–114 [DOI] [PubMed] [Google Scholar]
- 10. Glasgow L. A., Richards J. T., Kern E. R. 1982. Effect of acyclovir treatment on acute and chronic murine cytomegalovirus infection. Am. J. Med. 73:132–137 [DOI] [PubMed] [Google Scholar]
- 11. Gupta R., et al. 2004. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J. Infect. Dis. 190:1374–1381 [DOI] [PubMed] [Google Scholar]
- 12. Hostetler K. Y. 2009. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: current state of the art. Antiviral Res. 82:A84–A98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. James S. H., Kimberlin D. W., Whitley R. J. 2009. Antiviral therapy for herpesvirus central nervous system infections: neonatal herpes simplex virus infection, herpes simplex encephalitis, and congenital cytomegalovirus infection. Antiviral Res. 83:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kern E. R., et al. 2004. Oral treatment of murine cytomegalovirus infections with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:3516–3522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kimberlin D. W. 2007. Management of HSV encephalitis in adults and neonates: diagnosis, prognosis and treatment. Herpes 14:11–16 [PubMed] [Google Scholar]
- 16. Kimberlin D. W., et al. 2001. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 108:230–238 [DOI] [PubMed] [Google Scholar]
- 17. Kriesel J. D., Spruance S. L., Prichard M., Parker J. N., Kern E. R. 2005. Recurrent antiviral-resistant genital herpes in an immunocompetent patient. J. Infect. Dis. 192:156–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. LeBlanc R. A., Pesnicak L., Godleski M., Straus S. E. 1999. The comparative effects of famciclovir and valacyclovir on herpes simplex virus type 1 infection, latency, and reactivation in mice. J. Infect. Dis. 180:594–599 [DOI] [PubMed] [Google Scholar]
- 19. Leung D. T., Sacks S. L. 2000. Current recommendations for the treatment of genital herpes. Drugs 60:1329–1352 [DOI] [PubMed] [Google Scholar]
- 20. Mejias A., Bustos R., Ardura M. I., Ramirez C., Sanchez P. J. 2009. Persistence of herpes simplex virus DNA in cerebrospinal fluid of neonates with herpes simplex virus encephalitis. J. Perinatol. 29:290–296 [DOI] [PubMed] [Google Scholar]
- 21. Morfin F., Thouvenot D. 2003. Herpes simplex virus resistance to antiviral drugs. J. Clin. Virol. 26:29–37 [DOI] [PubMed] [Google Scholar]
- 22. Parker S., et al. 2008. Efficacy of therapeutic intervention with an oral ether-lipid analogue of cidofovir (CMX001) in a lethal mousepox model. Antiviral Res. 77:39–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Patel R. 2002. Progress in meeting today's demands in genital herpes: an overview of current management. J. Infect. Dis. 186(Suppl. 1):S47–S56 [DOI] [PubMed] [Google Scholar]
- 24. Prichard M. N., et al. 2005. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine 23:5424–5431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prichard M. N., Keith K. A., Quenelle D. C., Kern E. R. 2006. Activity and mechanism of action of N-methanocarbathymidine against herpesvirus and orthopoxvirus infections. Antimicrob. Agents Chemother. 50:1336–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prichard M. N., et al. 2009. Inhibition of herpesvirus replication by 5-substituted 4′-thiopyrimidine nucleosides. Antimicrob. Agents Chemother. 53:5251–5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prichard M. N., Shipman C., Jr 1990. A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 14:181–205 [DOI] [PubMed] [Google Scholar]
- 28. Quenelle D. C., et al. 2004. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 48:404–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Quenelle D. C., et al. 2010. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J. Infect. Dis. 202:1492–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Quenelle D. C., et al. 2007. Synergistic efficacy of the combination of ST-246 with CMX001 against orthopoxviruses. Antimicrob. Agents Chemother. 51:4118–4124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sawtell N. M. 1998. The probability of in vivo reactivation of herpes simplex virus type 1 increases with the number of latently infected neurons in the ganglia. J. Virol. 72:6888–6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sawtell N. M., Poon D. K., Tansky C. S., Thompson R. L. 1998. The latent herpes simplex virus type 1 genome copy number in individual neurons is virus strain specific and correlates with reactivation. J. Virol. 72:5343–5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sawtell N. M., Thompson R. L., Stanberry L. R., Bernstein D. I. 2001. Early intervention with high-dose acyclovir treatment during primary herpes simplex virus infection reduces latency and subsequent reactivation in the nervous system in vivo. J. Infect. Dis. 184:964–971 [DOI] [PubMed] [Google Scholar]
- 34. Smee D. F., et al. 2004. Effects of four antiviral substances on lethal vaccinia virus (IHD strain) respiratory infections in mice. Int. J. Antimicrob. Agents 23:430–437 [DOI] [PubMed] [Google Scholar]
- 35. Smith J. P., et al. 2010. Pharmacokinetics of acyclovir and its metabolites in cerebrospinal fluid and systemic circulation after administration of high-dose valacyclovir in subjects with normal and impaired renal function. Antimicrob. Agents Chemother. 54:1146–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stranska R., et al. 2005. Survey of acyclovir-resistant herpes simplex virus in the Netherlands: prevalence and characterization. J. Clin. Virol. 32:7–18 [DOI] [PubMed] [Google Scholar]
- 37. Thackray A. M., Field H. J. 1996. Differential effects of famciclovir and valacyclovir on the pathogenesis of herpes simplex virus in a murine infection model including reactivation from latency. J. Infect. Dis. 173:291–299 [DOI] [PubMed] [Google Scholar]
- 38. Tyring S. K., Baker D., Snowden W. 2002. Valacyclovir for herpes simplex virus infection: long-term safety and sustained efficacy after 20 years' experience with acyclovir. J. Infect. Dis. 186(Suppl. 1):S40–S46 [DOI] [PubMed] [Google Scholar]
- 39. Volberding P. A., Deeks S. G. 2010. Antiretroviral therapy and management of HIV infection. Lancet 376:49–62 [DOI] [PubMed] [Google Scholar]
- 40. Whitley R. J., et al. 1986. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N. Engl. J. Med. 314:144–149 [DOI] [PubMed] [Google Scholar]
- 41. Williams-Aziz S. L., et al. 2005. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob. Agents Chemother. 49:3724–3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wilson S. S., Fakioglu E., Herold B. C. 2009. Novel approaches in fighting herpes simplex virus infections. Expert Rev. Anti Infect. Ther. 7:559–568 [DOI] [PMC free article] [PubMed] [Google Scholar]