Abstract
It is widely accepted that the struggle against malaria depends on the development of new strategies to fight infection. The “magic bullet” thought to be necessary to reach eradication should not only provide treatment for all Plasmodium spp. that infect human red blood cells but should also eliminate the replicative and dormant liver forms of the parasite. Moreover, these goals should ideally be achieved by using different mechanisms of action so as to avoid the development of resistance. To that end, two hybrid molecules with covalently linked primaquine and artemisinin moieties were synthesized, and their effectiveness against the liver and blood stages of infection was compared in vitro and in vivo with those of the parent compounds. Both hybrids displayed enhanced in vitro activities, relative to those of the parent compounds, against Plasmodium berghei liver stages. Both compounds were about as potent as artemisinin against cultured Plasmodium falciparum (50% inhibitory concentration [IC50], ∼10 nM). When used to treat a murine P. berghei infection, one of the molecules displayed better efficacy than an equimolar mixture of the parent pharmacophores, leading to improved cure and survival rates. These results reveal a novel approach to the design and evaluation of antimalarials based on the covalent combination of molecules acting on different stages of the parasite life cycle.
INTRODUCTION
The combination of pharmacological agents may enable synergistic interactions, where the efficacy of a single agent is enhanced by the addition of a second compound. Hybrid (or bifunctional ligand) compounds, where two pharmacophores are combined in a single molecule, can be considered an extension of the concept of drug combinations in which the challenges of ensuring compliance with complex regimens or coformulating different compounds into a single tablet can be avoided. Hybrid molecules have been designed for use in various therapeutic areas, such as inflammation (6, 16), allergy (2), and depression (20). Typically, two pharmacophores are conjugated through a linker unit, creating a single chemical entity that is able to modulate multiple targets. This covalent linkage may present advantages over combinations of the two constituent drugs. These include enhanced uptake of a component due to the physicochemical properties of the other component, stronger synergism between the two components due to their proximity, or improvement of individual pharmacokinetic, stability, or side effect profiles (35).
Malaria remains the most important vector-borne disease in the world, threatening ∼40% of the human population and causing hundreds of millions of infections and about 1 million deaths each year (39). Malaria is caused by protozoan Plasmodium parasites that infect their mammalian hosts in two consecutive stages. The hepatic (liver) stage is initiated when sporozoites injected through the bite of an Anopheles mosquito travel to the liver and infect hepatocytes, where a clinically silent asexual multiplication phase takes place, generating thousands of merozoites. The release of merozoites into the bloodstream marks the beginning of the erythrocytic (blood) stage of infection, during which parasites infect red blood cells (RBCs), undergo repeated asexual replication cycles, and give rise to clinical illness (28).
Plasmodium falciparum is responsible for most malaria-associated mortality worldwide, and it is by far the predominant species in Africa. The other Plasmodium species that infect humans are P. vivax, P. ovale, P. malariae, and P. knowlesi. The asexual blood stages of malaria parasites, which are solely responsible for illness, are the targets of most available drugs. Among these, artemisinin (ART) and its derivatives are highly effective, fast-acting antimalarials and are particularly useful against strains of P. falciparum resistant to other agents. They have proven effective against all species capable of causing human malaria and are generally advocated for the treatment of uncomplicated P. falciparum malaria as components of ART-based combination therapies (ACTs) in which the ART component is partnered with a second, longer-acting agent (37).
P. vivax, which is about as prevalent as P. falciparum, and P. ovale uniquely produce chronic liver forms called hypnozoites, which can remain dormant for extended periods before initiating a blood-stage infection (relapse) (25). Primaquine (PQ) is the only approved drug against hepatic stages of malaria parasites, including parasites acutely infecting the liver and hypnozoites. No other available drugs reliably clear hypnozoites. PQ also acts against sexual stages, known as gametocytes; this activity disrupts the transmission of infection to mosquitoes (21). Thus, PQ is provided in combination with an agent that clears blood-stage parasites to achieve a radical cure of infections with P. vivax or P. ovale and thereby prevent relapses due to the development of subsequent blood-stage infections from hypnozoites (3).
In this work, we describe the synthesis of hybrid molecules containing PQ and ART pharmacophoric units. We report on their efficacies against Plasmodium liver and blood stages, both in vitro and in animal models of malaria, and demonstrate their potential as lead compounds for the development of novel antimalarial drugs.
MATERIALS AND METHODS
Chemical synthesis. (i) General description.
Melting points were determined using a Kofler camera Bock monoscope M and are uncorrected. Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker 400 Ultra-Shield spectrometer. Chemical shifts are reported in ppm using either tetramethylsilane or the solvent peak as an internal standard. Data are reported as follows: chemical shift, integration, multiplicity (s, singlet; brs, broad singlet; d, doublet; t, triplet; dd, double doublet; brd, broad doublet; m, multiplet), and coupling constant (J), reported in hertz. High-resolution mass spectra were recorded by electron impact using a Hewlett-Packard HP5988A system (University of Santiago de Compostela, Santiago de Compostela, Spain). Low-resolution mass spectra were recorded using a Micromass Quattro micro API mass spectrometer. Elemental analyses were performed using a CE Instruments EA 1110 automatic analyzer (University of Santiago de Compostela). Column chromatography was performed with a silica gel (230/400 mesh ASTM; Merck), and preparative thin-layer chromatography (TLC) was performed on silica gel GF254 (Merck). Analytical TLC was performed on precoated silica gel F254 (Merck). Artemisinin was purchased from Fraken Company, China, and was 99% pure. Dihydroartemisinin and artelinic acid were synthesized from artemisinin according to reference 5. 10β-Allyldeoxoartemisinin was prepared according to reference 14. All the other reagent-grade chemicals were bought from Sigma-Aldrich (Spain), Merck (Spain), or Alfa Aesar (Spain). Tetrahydrofuran (THF) was dried by distillation from sodium benzophenone.
(ii) Synthesis of hybrid compound 7.
A suspension of O,N-dimethylhydroxylamine hydrochloride (90.4 mg; 0.93 mmol) in dichloromethane (1 ml) and triethylamine (129 μl; 0.93 mmol) was added to a solution of artelinic acid, compound 4 (352 mg; 0.84 mmol) in dichloromethane (3 ml), O-(benzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium tetrafluoroborate (TBTU) (270 mg; 0.84 mmol), and triethylamine (118 μl; 0.84 mmol). After stirring for 7 h, the reaction mixture was diluted with dichloromethane (20 ml), and the organic phase was washed with saturated NaHCO3, treated with brine, and dried with anhydrous Na2SO4. Removal of the solvent under reduced pressure gave the hydroxamate compound 5 as a yellow oil (yield, 91%). 1H NMR (CDCl3) δ 7.68 (2H, d, J = 8.0), 7.37 (2H, d, J = 8.0), 5.48 (1H, s), 5.02 to 4.90 (2H, m), 4.58 (1H, d, J = 12.8), 3.58 (3H, s), 3.39 (3H, s), 2.71 (1H, m), 2.40 (1H, m), 2.07 (1H, m), 1.93 to 1.82 (3H, m), 1.66 (1H, m), 1.57 to 1.43 (2H, m), 1.48 (3H, s), 1.37 to 1.23 (3H, m), 0.98 (3H, d, J = 6.4), 0.97 (3H, d, J = 5.6). Lithium aluminum hydride (33.6 mg; 0.89 mmol) was added to a solution of the hydroxamate compound 5 (327 mg; 0.71 mmol) in dry tetrahydrofuran (8 ml) at 0°C. The reaction mixture was stirred for 1 h at 0°C, after which it was quenched using a solution of KHSO4 (191 mg; 1.4 mmol) in water (2 ml), diluted with water (40 ml), and extracted three times with ethyl ether (30 ml). The combined organic extracts were washed with saturated NaHCO3 and brine, dried (with anhydrous NaSO4), and evaporated under reduced pressure to give the aldehyde compound 6 as a colorless oil sufficiently pure to be used in the next step (yield, 78%). 1H NMR (CDCl3) δ 10.02 (1H, s), 7.88 (2H, d, J = 8.0), 7.49 (2H, d, J = 8.0), 5.47 (1H, s), 5.00 (1H, d, J = 13.6), 4.94 (1H, d, J = 3.6), 4.62 (1H, d, J = 13.6), 2.72 (1H, m), 2.39 (1H, m), 2.07 (1H, m), 1.94 to 1.80 (3H, m), 1.65 (1H, m), 1.56 to 1.48 (2H, m), 1.47 (3H, s), 1.39 to 1.25 (3H, m), 0.99 (3H, d, J = 7.2), 0.96 (3H, d, J = 6.0). To a solution of the aldehyde compound 6 (64.0 mg; 0.16 mmol) in methanol (MeOH) (2 ml) and glacial acetic acid (9.15 μl; 0.16 mmol) at 0°C were sequentially added primaquine, compound 1 as a free base (46 mg; 0.176 mmol), and NaBH3CN (11 mg; 0.176 mmol). The reaction mixture was allowed to warm up to room temperature. After the completion of TLC, the reaction mixture was diluted with water (10 ml) and acidified to pH ∼2 to 3 with a 10% (wt/vol) aqueous hydrochloric acid solution. This solution was washed with ethyl ether, neutralized with a 30% (wt/vol) sodium hydroxide solution, and extracted three times with ethyl ether (10 ml). The combined ether extracts were dried over Na2SO4; the solvent was removed under reduced pressure; and the residue was purified by preparative chromatography using ethyl acetate (AcOEt) as an eluent to give the final compound 7 as a yellow syrup (yield, 39%). 1H NMR (CDCl3) δ 8.54 (1H, dd, J = 1.5, 4.2), 7.94 (1H, dd, J = 1.5, 8.2), 7.32 (1H, dd, J = 4.2, 8.2), 7.28 (4H, m), 6.35 (1H, d, J = 2.4), 6.29 (1H, d, J = 2.4), 6.03 (1H, brs), 5.48 (1H, s), 4.92 (1H, d, J = 4.0), 4.90 (1H, d, J = 12.4), 4.52 (1H, d, J = 12.4), 3.91 (3H, s), 3.81 (2H, brs), 3.62 (1H, m), 3.03 (1H, brs), 2.74 to 2.63 (3H, m), 2.40 (1H, m), 2.11 to 2.01 (2H, m), 1.95 to 1.56 (7H, m), 1.55 to 1.20 (5H, m), 1.48 (3H, s), 1.31 (3H, d, J = 6.4), 0.95 (6H, brd); m/z+ 646.38.
(iii) Synthesis of hybrid compound 10.
Sodium periodate (278 mg; 1.3 mmol) and potassium permanganate (31 mg; 0.2 mmol) were added to a stirred solution of 10β-allyldeoxoartemisinin, compound 8 (99 mg; 0.32 mmol) in 20 ml acetone, and 20 ml water at room temperature. On completion of the reaction, the reaction mixture was filtered, and the filtrate was concentrated under reduced pressure, treated with 10 M NaOH until basic, and washed with ethyl ether. The aqueous phase was then acidified to pH 1 with concentrated HCl. The aqueous phase was extracted three times with ethyl ether (20 ml). The combined organic extracts were dried over Na2SO4 and were concentrated to give the carboxylic acid derivative, compound 9, as a yellow oil (yield, 67%). 1H NMR (CDCl3) δ 5.39 (1H, s), 4.88 (1H, m), 2.77 to 2.63 (2H, m), 2.52 (1H, m), 2.35 (1H, m), 2.1 to 1.92 (2H, m), 1.82 (1H, m), 1.77 to 1.66 (2H, m), 1.48 to 1.2 (5H, m), 1.43 (3H, s), 0.99 (3H, d, J = 5.6), 0.90 (3H, d, J = 7.6). To a solution of compound 9 (133 mg; 0.407 mmol) in dimethylformamide (3 ml) stirred at 0°C were added TBTU (142 mg; 0.425 mmol), triethylamine (57 μl; 0.409 mmol), and a solution of primaquine diphosphate salt, compound 1 (183 mg; 0.403 mmol), and triethylamine (115 μl; 0.826 mmol) in dimethylformamide (3 ml). The reaction mixture was allowed to warm up to room temperature, and the reaction was monitored by TLC. After completion, the reaction mixture was diluted with ethyl acetate (25 ml) and was then poured into saturated NaHCO3 (25 ml). The layers were separated, and the aqueous layer was extracted twice with ethyl acetate (25 ml). The combined organic layers were treated with saturated NaHCO3 and brine and were then dried over Na2SO4. The solvent was removed, and the crude product was purified by column chromatography using ethyl acetate–hexane (1:1) to give compound 10 as a yellow solid (yield, 43%), with a melting point (mp) of 127 to 129°C. 1H NMR (CDCl3) δ 8.54 (1H, dd, J = 1.6, 4.0), 7.94 (1H, dd, J = 1.6, 8.0), 7.32 (1H, dd, J = 4.0, 8.0), 7.07 (1H, m), 6.34 (1H, d, J = 2.4), 6.29 (1H, d, J = 2.4), 6.03 (1H, d, J = 8.0), 5.35 (1H, s), 4.75 (1H, m), 3.91 (3H, s), 3.65 (1H, m), 3.44 (1H, m), 3.18 (1H, m), 2.62 to 2.48 (2H, m), 2.37 to 2.23 (2H, m), 2.03 to 1.90 (2H, m), 1.82 to 1.65 (7H, m), 1.30 to 1.10 (5H, m), 1.32 (3H, d, J = 6.4), 1.32 (3H, s), 0.98 (3H, d, J = 5.6), 0.87 (3H, d, J = 7.6). Analysis (C, H, N) calculated for C32H45N3O6 · AcOEt: C, 65.93; H, 8.15; N, 6.41. Found: C, 65.21; H, 7.72; N, 6.73; m/z+ 567.33.
Parasites, cells, and mice.
In vitro blood schizonticidal activity assays were performed as reported elsewhere (8). Briefly, synchronized ring-stage P. falciparum strain W2 parasites were cultured with multiple concentrations of test compounds (added from 1,000× stocks in dimethyl sulfoxide [DMSO]) in RPMI 1640 medium with 10% human serum. After 48 h of incubation, when control cultures contained new rings, parasites were fixed with 1% formaldehyde in phosphate-buffered saline (PBS), pH 7.4, for 48 h at room temperature; then they were labeled with YOYO-1 (1 nM; Molecular Probes) in 0.1% Triton X-100 in PBS. Parasitemia was determined from dot plots (forward scatter versus fluorescence) acquired on a FACSort flow cytometer using CellQuest software (Becton Dickinson). Fifty percent inhibitory concentrations (IC50s) for growth inhibition were determined with GraphPad Prism software from plots of the percentage of parasitemia of the control relative to the inhibitor concentration. In each case, the goodness of the curve fit was documented by R2 values of >0.95.
Plasmodium berghei ANKA sporozoites expressing luciferase or green fluorescent protein (GFP) (parasite lines 676m1cl1 and 259cL2, respectively), were obtained from 21- to 28-day infected female Anopheles stephensi mosquitoes (22, 26). Mosquitoes bred at the Insectary of the Instituto de Medicina Molecular, Lisbon, Portugal, were previously infected by feeding on infected mice using standard methods. When required, the salivary glands of the mosquitoes were collected by hand dissection in Dulbecco's modified Eagle medium (DMEM; GIBCO), homogenized with a plastic pestle, and filtered on a cell strainer (70-μm-pore-size nylon; BD Falcon). The free sporozoites were counted in a Neubauer counting chamber using phase-contrast microscopy. For infections by mosquito bite, infected mosquitoes were starved overnight and were then allowed to feed on anesthetized mice for 30 min, at a proportion of 10 mosquitoes per mouse.
Cells of the Huh7 human hepatoma cell line were cultured in RPMI medium (Gibco/Invitrogen) supplemented with 10% fetal calf serum, 1% nonessential amino acids, 1% penicillin-streptomycin, 1% glutamine, and 10 mM HEPES (pH 7) (all from Gibco/Invitrogen) and were maintained at 37°C under 5% CO2. For infection, P. berghei sporozoites obtained from freshly dissected mosquito salivary glands were added directly to the culture medium, and cell culture plates were centrifuged for 5 min at 1,800 × g to promote contact of the parasites with the Huh7 cell monolayer.
C57BL/6 mice were bred and housed in the pathogen-free facility of the Instituto de Medicina Molecular, Lisbon, Portugal. All protocols were approved by the Animal Care Committee of the Instituto de Medicina Molecular.
Luciferase assay for liver-stage infection in vitro.
Huh7 cells at about 50 to 60% confluence were incubated with the test compounds for 1 h and were then infected in 96-well plates at a multiplicity of infection of ∼0.4 with luciferase-expressing P. berghei sporozoites obtained from freshly dissected mosquito salivary glands. The medium was replaced 24 h after infection by new medium containing freshly diluted drugs. At 46 h after infection, cell confluence was assessed by the alamarBlue (Biosource/Invitrogen) assay. Briefly, this assay is based on an oxidation-reduction indicator that both fluoresces and changes color in response to chemical reduction of the culture medium resulting from cell growth. Cells were incubated for 1.5 h with fresh medium containing 5% alamarBlue, and fluorescence was measured (excitation wavelength, 530 nm; emission wavelength, 590 nm) with a Tecan Infinite M200 microplate reader. Following alamarBlue measurement, cells were washed once with PBS and were lysed with 75 μl of cell culture lysis reagent obtained from the Promega luciferase assay system kit. Samples in lysis buffer were either stored at −20°C or processed immediately to measure luminescence intensity. In the latter case, 35 μl of the cell lysate was transferred to white 96-well plates (Nunc) and was mixed with 50 μl of luciferase assay substrate, freshly reconstituted with luciferase assay buffer (Promega luciferase assay system kit). Luminescence was measured over 100 ms with a Tecan Infinite M200 microplate reader within the 3 min following the addition of the luciferase assay substrate.
FACS analysis of in vitro liver-stage infection.
Huh7 cells at about 60% confluence were treated in 24-well plates with the different compounds and were infected 1 h later with GFP-expressing P. berghei sporozoites obtained from freshly dissected mosquito salivary glands at a multiplicity of infection of ∼0.3. Fluorescence-activated cell sorting (FACS) analysis at 4 h and 48 h after infection was performed as previously described (26, 29).
In vivo analysis of liver-stage development by luminescence.
Luciferase activity in animals was visualized at 44 h after infection with luciferase-expressing P. berghei sporozoites, as previously described (22). Briefly, d-luciferin (Caliper Life Sciences) dissolved in PBS (100 mg/kg of body weight) was injected subcutaneously in the necks of the animals. Five minutes later, mice were anesthetized by intraperitoneal (i.p.) injection of xylazine (20 mg/kg) and ketamine (120 mg/kg) dissolved in PBS. Bioluminescence measurements were performed in an IVIS Lumina imaging system (Caliper Life Sciences) 10 min after d-luciferin injection.
Assessment of liver parasite loads by qRT-PCR.
Determination of liver parasite loads in vivo was carried out as previously described (27). Briefly, total RNA was isolated from livers by using Qiagen's RNeasy Mini kit according to the manufacturer's instructions. Livers were collected and homogenized in a denaturing solution (4 M guanidine thiocyanate; 25 mM sodium citrate [pH 7], 0.5% sarcosyl, and 0.7% β-mercaptoethanol in diethyl pyrocarbonate-treated water) 40 h after sporozoite injection. Total RNA was extracted using the Qiagen RNeasy Mini kit according to the manufacturer's instructions. RNA for infection measurements was converted into cDNA by using the Roche Transcriptor first-strand cDNA synthesis kit according to the manufacturer's protocol. The quantitative real-time PCRs (qRT-PCRs) used the Applied Biosystems Power SYBR green PCR master mix and were performed according to the manufacturer's instructions on an ABI Prism 7500 Fast system (Applied Biosystems). Amplification reactions were carried out in a total reaction volume of 25 μl, containing 0.8 pmol/ml or 0.16 pmol/ml of the P. berghei ANKA 18S rRNA gene- or housekeeping gene-specific primers, respectively. Relative amounts of P. berghei ANKA mRNA were calculated against the amount of the hypoxanthine guanine phosphoribosyltransferase (HGPRT) housekeeping gene. Primer sequences specific to each gene were as follows: for the P. berghei ANKA 18S rRNA gene, 5′-AAG CAT TAA ATA AAG CGA ATA CAT CCT TAC-3′ and 5′-GGA GAT TGG TTT TGA CGT TTA TGT G-3′; for the mouse HGPRT gene, 5′-TGC TCG AGA TGT GAT GAA GG-3′ and 5′-TCC CCT GTT GAC TGG TCA TT-3′; and for the human HGPRT gene, 5′-TGC TCG AGA TGT GAT GAA GG-3′ and 5′-TCC CCT GTT GAC TGG TCA TT-3′.
Assessment of parasitemia.
Giemsa staining followed by microscopic analysis was performed daily to assess the presence or absence of parasites in the blood, and parasitemia was quantified by flow cytometry as previously described (26).
RESULTS
Design and synthesis of primaquine-artemisinin hybrids.
Hybrid compound 7 was synthesized by starting from artelinic acid (Fig. 1), a derivative of ART previously used in our laboratory to prepare hybrid molecules, including vinyl sulfone inhibitors of falcipain-2 (5). Artelinic acid has been shown to be the most metabolically stable of the derivatives of ART (17). It was converted to the corresponding hydroxamate, compound 5, by reaction with N,O-dimethylhydroxylamine, which was then reduced to the aldehyde compound 6. Reductive amination of compound 6 using PQ in methanol gave the desired hybrid compound 7, with an overall yield of 28%. Hybrid compound 10 was designed to replace the oxygen at the C-10 position with a carbon, a modification expected to produce a compound with greater hydrolytic stability, a longer half-life, and potentially lower toxicity (23). Hybrid compound 10 was synthesized from 10β-allyldeoxoartemisinin, compound 8, in two steps, involving oxidation of the allyl moiety and coupling of the resulting acid with PQ, with an overall yield of 29% (Fig. 1). The structural identification of the hybrid compounds was performed using mass spectrometry and nuclear magnetic resonance spectrometry (correlation spectroscopy [COSY] and heteronuclear correlation spectroscopy [HETCOR] experiments).
Fig. 1.
Syntheses. Shown are the chemical structures of primaquine (compound 1), artemisinin (compound 2), dihydroartemisinin (compound 3), and artelinic acid (compound 4) and synthetic schemes for hybrid compounds 7 and 10.
Inhibition of hepatic P. berghei infection by primaquine-artemisinin hybrids.
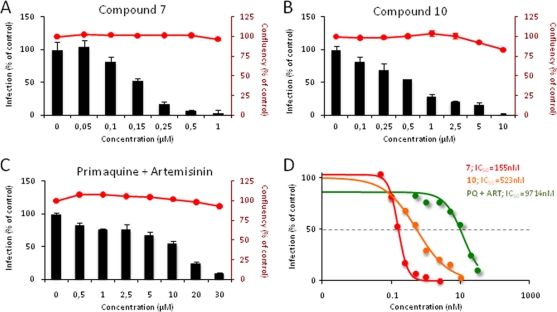
To evaluate the abilities of compounds 7 and compound 10 to inhibit Plasmodium infection of liver cells, we tested them on an established in vitro infection system that employs a human hepatoma cell line (Huh7) and the rodent parasite P. berghei. Huh7 cells were incubated with various amounts of each compound or compound mixture prior to the addition of transgenic, luciferase-expressing P. berghei sporozoites, and total infection loads were quantified 48 h later by luminescence measurements of cell lysates (22). Effects on cell proliferation and survival were assessed by fluorescence measurements following 1.5 h of incubation with alamarBlue. Compounds 7 and 10 had potent parasite-inhibitory effects, displaying IC50 values ∼66 and ∼18 times lower, respectively, than that of a 1:1 PQ-ART mixture (Fig. 2). Treatment with ART had no detectable effect on P. berghei liver-stage infection. Importantly, at the concentrations used in this experiment, none of the hybrid compounds showed any effect on Huh7 cell proliferation, as measured by alamarBlue fluorescence (Fig. 2). Flow cytometry-based analysis of GFP-expressing P. berghei 48 h after the addition of P. berghei to Huh7 cells showed that compounds 7 and 10 and a PQ-ART mixture caused dose-dependent decreases in parasite development, as assessed by the geometrical means of the GFP intensities of infected cells (26) (see Fig. S1 in the supplemental material). Thus, the hybrid molecules acted by preventing intracellular parasite replication, in accordance with our previous data for the parent compound PQ (34). Compound 10 also showed modest inhibition of cell invasion by the parasites, as shown by the infection rate measured 4 h after the addition of parasites to the cells (see Fig. S1A in the supplemental material). Although the IC50s determined by this method were ∼1.5- to 2.5-fold higher than those determined by luminescence, they indicated similar relative efficacies for the three sets of compounds and confirmed that compound 7 displayed a much stronger inhibitory effect than the PQ-ART mixture.
Fig. 2.
In vitro inhibition of hepatic Plasmodium berghei infection by compounds 7 and 10. (A to C) Compounds were added to Huh7 hepatoma cells 1 h before infection with luciferase-expressing sporozoites. An amount of DMSO equivalent to that in the highest compound concentration tested was used as a control. Forty-eight hours after the addition of P. berghei sporozoites, cell confluence (red lines on bar plots) was assessed by alamarBlue fluorescence, and the infection rate (bars) was measured by quantifying the luciferase activity by luminescence. The effects of different concentrations of compound 7 (A), compound 10 (B), and a 1:1 mixture of primaquine and artemisinin (C) are shown. Results are expressed as means ± standard deviations. (D) For each compound, the IC50 was calculated by sigmoidal fitting. Red, compound 7; orange, compound 10; green, primaquine plus artemisinin.
We next tested the abilities of the hybrid compounds to inhibit P. berghei liver infection in mice. Mice were infected by intravenous (i.v.) injection of 10,000 luciferase-expressing P. berghei sporozoites, and liver parasite loads were assessed 42 h later following the injection of luciferin (22). We observed significant decreases in parasite loads in the livers of mice treated with a single intraperitoneal (i.p.) injection of 26.5 μmol/kg (equivalent to 12 mg/kg of PQ) of compound 7 or 10, delivered 3 h after infection, although this effect was less marked than that observed in mice similarly treated with PQ (see Fig. S2A in the supplemental material). We then investigated whether administration of the compounds during the liver stage would affect the subsequent appearance of parasitemia. Mice infected with GFP-expressing P. berghei sporozoites and treated 3 h later by i.p. injection of 26.5 μmol/kg of compound 7, compound 10, or PQ were monitored daily for the appearance of parasitemia (see Fig. S2B in the supplemental material) and disease symptoms. Remarkably, while compound 10 had no effect on infection, administration of compound 7 during the liver stage delayed or prevented the appearance of parasites to an extent similar to that of PQ (see Fig. S2B). Importantly, these results are consistent with the relative in vitro efficacies of compounds 7 and 10 against hepatic infection (Fig. 2).
Having established the efficacy of i.p. administration of compound 7 during liver infection, we then assessed efficacy when the compounds were administered orally. We compared the activities of the hybrid molecules with that of an equimolar mixture of PQ and ART. Four 65.9-μmol/kg (equivalent to 30 mg/kg of PQ) doses of the compounds were administered by oral gavage 30 min, 15 h, 23 h, and 39 h after infection of mice with GFP-expressing P. berghei sporozoites. Parasite loads in livers collected 44 h postinfection were then determined by quantitative real-time PCR (Fig. 3A). The results were in agreement with those obtained following i.p. treatment (see Fig. S2A in the supplemental material), with oral administration of compound 7 resulting in near-abrogation of liver infection. Finally, in a parallel experiment, mice were infected with GFP-expressing P. berghei sporozoites and were treated 4 h later with a single 65.9-μmol/kg oral dose of the compounds, followed by daily monitoring of blood for parasitemia. As expected, no parasitemia was detected in mice treated with the PQ-ART mixture (Fig. 3B), presumably due to parasite clearance in the liver by PQ. Four out of 6 mice treated with compound 7 were also blood-stage negative at the end of the experiment. Importantly, parasites were detected in the blood of three mice in this group up to day 5 postinfection but were then cleared, suggesting that the hybrid may remain in the circulation for a considerable time or that an immune response contributed to parasite clearance.
Fig. 3.
In vivo inhibition of Plasmodium berghei liver-stage infection and blood patency by oral administration of compounds 7 and 10. C57BL/6 mice (n = 6) were infected by intravenous injection of 10,000 GFP-expressing P. berghei sporozoites. (A) Mice were treated by oral administration of the different compounds in the amounts and at the times noted in the text. Parasite liver loads (P. berghei ANKA 18S rRNA; relative quantity normalized against that of the host HGPRT gene) were assessed by quantitative real-time PCR 44 h after infection. (B) Mice were treated by a single administration of the compounds in the amounts and at the time noted in the text, and the appearance of parasites in the blood was monitored daily. The percentage of mice with parasitemia under the detection limit (parasite-free mice) is shown.
Overall, these results indicate that the PQ-ART hybrid molecules display strong antiplasmodial effects during the liver stage of infection. In particular, compound 7 displayed strong in vivo efficacy as a liver-stage antimalarial. Since our in vitro results showed that ART alone does not possess significant activity against Plasmodium liver stages, these data suggest that the hybrid molecule constitutes a modification of PQ that potentiates its effect, rendering it more effective at lower molar amounts.
Treatment of Plasmodium parasitemia with primaquine-artemisinin hybrids.
As P. falciparum resistance to chloroquine became increasingly widespread, artemisinin-based therapies became the standard means of treating malaria in nearly all countries where the disease was endemic (18). Thus, we assessed the in vitro activities of compounds 7 and 10 against the erythrocytic stage of P. falciparum and compared these activities with those of PQ and ART. While PQ showed only modest activity in these assays, as expected, compounds 7 and 10 and ART all yielded potent inhibitory effects, with IC50s in the nanomolar range (Table 1).
Table 1.
In vitro blood schizonticidal activity, molecular weight, and ClogP for primaquine, artemisinin, and hybrid compounds 7 and 10
| Compound | IC50 (nM)a | Mol wt | ClogPb |
|---|---|---|---|
| Primaquine | 3,300 ± 55 | 259 | 2.76 |
| Artemisinin | 8.2 ± 0.9 | 282 | 2.90 |
| Compound 7 | 12.5 ± 1.1 | 646 | 5.88 |
| Compound 10 | 9.1 ± 0.6 | 568 | 5.25 |
Determined against the chloroquine-resistant P. falciparum strain W2.
ClogP is an estimate of the value of the logarithm of the partition coefficient between 1-octanol and water, calculated using ALOGPS software (http://www.vcclab.org/lab/alogps/). The logP values measure a compound's lipophilicity (higher values mean higher lipophilicity).
We then compared the in vivo therapeutic efficiencies of the hybrid compounds and ART in the treatment of an established murine blood infection. C57BL/6 mice were infected by i.p. injection of 106 P. berghei-infected RBCs (iRBCs), and their parasitemia was monitored daily until their percentages of iRBCs reached 2 to 3%. At this point, mice were treated daily, for 4 days, by subcutaneous injection of 31.8 μmol/kg (equivalent to 9 mg/kg of ART) of compound 7, compound 10, or ART, an amount corresponding to the reported 90% effective dose (ED90) for ART administered by this route (24), while control mice were treated with equivalent amounts of a solvent. Mice were then monitored daily for parasitemia and disease symptoms (see Fig. S3 in the supplemental material). As expected, the parasitemia of solvent-treated control mice increased steadily until they perished with symptoms of experimental cerebral malaria (19) between days 7 and 8 postinfection. Conversely, the parasitemia of compound-treated mice decreased soon after the initiation of treatment, approaching zero for all mice around day 9 postinfection. However, parasitemia recurred at days 12 to 13 postinfection in mice treated with ART or compound 7 (see Fig. S3A and B in the supplemental material), and all mice in these two groups eventually succumbed on days 15 to 16 and 16 to 17 postinfection, respectively. These outcomes were presumably due to hyperparasitemia, since the mice did not display symptoms of experimental cerebral malaria (see Fig. S3C in the supplemental material). Importantly, mice treated with compound 10 did not experience recurrent parasitemia after initial clearance of infections. Instead, they remained blood-stage negative throughout the duration of the experiment (see Fig. S3A and B) and had a 100% survival rate (see Fig. S3C).
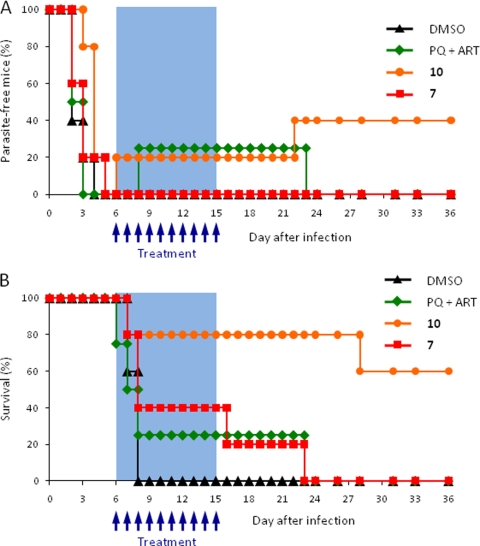
In view of these results, we investigated the efficacy of oral administration of the hybrid molecules in the treatment of a patent blood infection. In this experiment, mice were infected by i.v. injection of 10,000 GFP-expressing P. berghei sporozoites, and treatment was initiated when the level of parasitemia reached 2 to 3%. Both hybrid molecules and an equimolar mixture of PQ and ART were administered daily by oral gavage for 10 days, at 31.8 μmol/kg per dose. We selected a dosage expected to be suboptimal for orally administered artemisinin (24) in order to facilitate comparison of the efficacies of the different treatments. Mice were monitored daily for parasitemia, disease symptoms, and survival (Fig. 4). As expected, all control mice died with symptoms of experimental cerebral malaria between days 7 and 8 after infection. Most noticeably, parasitemia and survival rates were remarkably increased in mice treated with compound 10 over those for the PQ-ART mixture (Fig. 4). These results are in agreement with those obtained after i.p. dosing (see Fig. S3 in the supplemental material) and demonstrate that the hybrid molecules can be administered orally to treat malaria.
Fig. 4.
In vivo treatment of Plasmodium berghei blood infection by oral administration of compounds 7 and 10. C57BL/6 mice (n = 5) were infected by intravenous injection of 105 GFP-expressing sporozoites. Oral administration of the compounds was initiated when parasitemia reached 2 to 3% and was carried out daily for 10 days. (A) Blood parasite patency, monitored daily by flow cytometry. The percentages of mice with parasitemia under the detection limit are shown. (B) Survival plot showing the percentages of live mice throughout the duration of the experiment.
Overall, our results show that both hybrid molecules, and particularly compound 10, can be used to control an ongoing blood-stage infection with greater efficacy than that of the parent compound ART or ART-PQ. Interestingly, although compound 10 had a lower IC50 than compound 7 in vitro, it was more active in vivo, suggesting enhanced pharmacokinetic properties or stability. Indeed, compound 10 was designed to replace the oxygen at the C-10 position with a carbon, a modification expected to produce a compound that was both less toxic and more stable (23).
DISCUSSION
Treatment of malaria relies on agents that eliminate blood-stage infection, notably ART derivatives, and, in the case of P. vivax and P. ovale infections, PQ to eradicate dormant liver-stage parasites (7, 11). Optimal therapy might incorporate agents active against both blood- and liver-stage parasites in the same molecule. To this end, we have synthesized two hybrid molecules, compounds 7 and 10, that covalently combine the PQ and ART pharmacophores. We found that these compounds displayed antimalarial efficacies that in some instances were superior to those of the parent compounds.
There is consensus that efforts to eradicate malaria should be multifaceted and that new and effective antimalarial drugs will constitute essential tools in this struggle (1, 37). PQ is the compound of reference against the liver stage of mammalian infection by Plasmodium, while ART derivatives, such as artesunate and artemether, are the compounds of reference against blood-stage parasites. PQ is usually employed in combination with blood schizonticides, in particular chloroquine (12) or ART derivatives (9, 32, 33), to prevent relapses in P. vivax infections and to reduce malaria transmission following treatment (31, 33). Two studies on chimeric PQ-containing molecules, in which PQ was linked to statin-based inhibitors of plasmepsins, have been reported (10, 30). Although these double drugs displayed improved in vitro blood schizonticidal activities relative to those of the single compounds, no data were provided on their in vitro effects against Plasmodium liver stages or on their in vivo activities against liver and blood stages. Both hybrids described in the present study displayed marked antiplasmodial activity in vitro against liver stages, with IC50s lower than those reported for PQ (22) (Fig. 2). Our in vitro results suggest that the efficacy of PQ is increased by the introduction of the ART pharmacophoric unit into the hybrid molecules. Additionally, both hybrid molecules significantly reduced parasite loads in the livers of mice following intravenous injection of sporozoites, although this effect was lower than that of PQ (Fig. 3A; see also Fig. S2A in the supplemental material). Taken together, our data suggest that the greater in vitro efficacy of the compounds was, to some extent, counterbalanced by a decrease in their bioavailability, metabolic stability, or other factors necessary for in vivo activity. Activity may be limited by the fact that the drug needs to cross several barriers before reaching the intrahepatic parasite, which resides inside a parasitophorous vacuole. Nevertheless, when sporozoites were delivered by mosquito bite and infection was allowed to proceed to the blood stage, compound 7 displayed significant in vivo efficacy in controlling parasitemia (see Fig. S2B in the supplemental material). Further, compound 7 was also efficacious when administered orally following i.v. injection of sporozoites, although in this case its effect was not as potent as that of the PQ-ART mixture (Fig. 3B). We therefore speculate that the reduced bioavailability of compound 7 is compensated for by its strong efficacy against the liver stage, which, combined with its blood schizonticidal activity (see below), renders it a promising compound for the treatment of P. vivax malaria.
Antimalarial therapy increasingly involves the use of drug combinations as a means of improving treatment efficacy and reducing the development of resistance (4, 38). The emphasis on fixed-dose antimalarial drug combinations has led logically to the development of hybrid molecules that covalently join two distinct antimalarial moieties. Most antimalarial hybrid molecules have been designed to act solely on the asexual blood stages of the parasite (reviewed in reference 35). Only two of these designs included ART as one of the units in the chimeric molecule, coupled with either mefloquine (13) or quinine (36). The ART-mefloquine hybrids were more effective at controlling parasitemia in P. berghei-infected mice than artemether (13). The ART-quinine chimera was tested only in vitro, where it showed anti-P. falciparum activity superior to that of ART alone, quinine alone, or a 1:1 mixture of ART and quinine (36). The data obtained with our hybrid compounds indicate that the covalent linkage of the PQ and ART units leads to enhanced efficacy in the treatment of a patent blood-stage infection over that of the parent compounds, particularly in the case of compound 10 (Fig. 4). It is not clear why a similar effect was not observed for compound 7, despite the similarity of the in vitro activities of compounds 7 and 10 (Table 1). Differences in activity might be explained by differences in compound stability. Another possibility is that the differential activities shown by compounds 7 and 10 against blood and liver stages may result from different preferences of the two compounds for RBCs and hepatocytes.
The design of compounds with a predefined multitarget profile presents several important challenges to medicinal chemists, not the least of which is achieving a design that confers “drug-like” properties on the resulting molecule (15). In this work we describe, for the first time, the ability of hybrid PQ-ART molecules to inhibit the liver and blood stages of mammalian Plasmodium infection. Our data constitute a proof of principle that paves the way for the exploitation of a multistage hybrid approach in drug-based strategies for malaria control and eradication.
Supplementary Material
ACKNOWLEDGMENTS
Inês Albuquerque is gratefully acknowledged for help with the assessment of parasite patency in Giemsa-stained blood smears.
M.P. holds a Ciência 2007 position from the Portuguese Ministry of Science and Technology. R.C. acknowledges FCT for Ph.D. grant SFRH/BD/30418/2006, and G.G.C. acknowledges the FP7 of the European Commission for Marie Curie fellowship FP7-PEOPLE-IEF-2008-235864. This work was supported by Portuguese Foundation for Science and Technology grant PTDC/SAUMII/099118/2008 to M.P. P.J.R. is a Distinguished Clinical Scientist of the Doris Duke Charitable Foundation.
Footnotes
Supplemental material for this article may be found at http://aac.asm.org/.
Published ahead of print on 1 August 2011.
REFERENCES
- 1. Alonso P. L., et al. 2011. A research agenda for malaria eradication: drugs. PLoS Med. 8:e1000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aslanian R., et al. 2003. Identification of a dual histamine H1/H3 receptor ligand based on the H1 antagonist chlorpheniramine. Bioorg. Med. Chem. Lett. 13:1959–1961 [DOI] [PubMed] [Google Scholar]
- 3. Baird J. K., Hoffman S. L. 2004. Primaquine therapy for malaria. Clin. Infect. Dis. 39:1336–1345 [DOI] [PubMed] [Google Scholar]
- 4. Bell A. 2005. Antimalarial drug synergism and antagonism: mechanistic and clinical significance. FEMS Microbiol. Lett. 253:171–184 [DOI] [PubMed] [Google Scholar]
- 5. Capela R., et al. 2009. Artemisinin-dipeptidyl vinyl sulfone hybrid molecules: design, synthesis and preliminary SAR for antiplasmodial activity and falcipain-2 inhibition. Bioorg. Med. Chem. Lett. 19:3229–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cena C., et al. 2003. Antiinflammatory, gastrosparing, and antiplatelet properties of new NO-donor esters of aspirin. J. Med. Chem. 46:747–754 [DOI] [PubMed] [Google Scholar]
- 7. Chattopadhyay R., et al. 2010. Establishment of an in vitro assay for assessing the effects of drugs on the liver stages of Plasmodium vivax malaria. PLoS One 5:e14275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coteron J. M., et al. 2010. Falcipain inhibitors: optimization studies of the 2-pyrimidinecarbonitrile lead series. J. Med. Chem. 53:6129–6152 [DOI] [PubMed] [Google Scholar]
- 9. Dao N. V., et al. 2007. Vivax malaria: preliminary observations following a shorter course of treatment with artesunate plus primaquine. Trans. R. Soc. Trop. Med. Hyg. 101:534–539 [DOI] [PubMed] [Google Scholar]
- 10. Dell'Agli M., et al. 2006. High antiplasmodial activity of novel plasmepsins I and II inhibitors. J. Med. Chem. 49:7440–7449 [DOI] [PubMed] [Google Scholar]
- 11. Dembele L., et al. 2011. Towards an in vitro model of Plasmodium hypnozoites suitable for drug discovery. PLoS One 6:e18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galappaththy G. N., Omari A. A., Tharyan P. 24 January 2007. Primaquine for preventing relapses in people with Plasmodium vivax malaria. Cochrane Database Syst. Rev. 1:CD004389 doi:10.1002/14651858.CD004389.pub2 [DOI] [PubMed] [Google Scholar]
- 13. Grellepois F., Grellier P., Bonnet-Delpon D., Begue J. P. 2005. Design, synthesis and antimalarial activity of trifluoromethylartemisinin-mefloquine dual molecules. Chembiochem 6:648–652 [DOI] [PubMed] [Google Scholar]
- 14. Hindley S., et al. 2002. Mechanism-based design of parasite-targeted artemisinin derivatives: synthesis and antimalarial activity of new diamine containing analogues. J. Med. Chem. 45:1052–1063 [DOI] [PubMed] [Google Scholar]
- 15. Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. 2001. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46:3–26 [DOI] [PubMed] [Google Scholar]
- 16. Lolli M. L., et al. 2001. A new class of ibuprofen derivatives with reduced gastrotoxicity. J. Med. Chem. 44:3463–3468 [DOI] [PubMed] [Google Scholar]
- 17. Navaratnam V., et al. 2000. Pharmacokinetics of artemisinin-type compounds. Clin. Pharmacokinet. 39:255–270 [DOI] [PubMed] [Google Scholar]
- 18. Nosten F., White N. J. 2007. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 77:181–192 [PubMed] [Google Scholar]
- 19. Pamplona A., et al. 2007. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat. Med. 13:703–710 [DOI] [PubMed] [Google Scholar]
- 20. Perez M., et al. 1998. Design and synthesis of new potent, silent 5-HT1A antagonists by covalent coupling of aminopropanol derivatives with selective serotonin reuptake inhibitors. Bioorg. Med. Chem. Lett. 8:3423–3428 [DOI] [PubMed] [Google Scholar]
- 21. Peters W. 1999. The evolution of tafenoquine—antimalarial for a new millennium? J. R. Soc. Med. 92:345–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ploemen I. H., et al. 2009. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS One 4:e7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Posner G. H., et al. 2003. Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high stability and efficacy. J. Med. Chem. 46:1060–1065 [DOI] [PubMed] [Google Scholar]
- 24. Posner G. H., et al. 1999. Orally active, hydrolytically stable, semisynthetic, antimalarial trioxanes in the artemisinin family. J. Med. Chem. 42:300–304 [DOI] [PubMed] [Google Scholar]
- 25. Price R. N., et al. 2007. Vivax malaria: neglected and not benign. Am. J. Trop. Med. Hyg. 77:79–87 [PMC free article] [PubMed] [Google Scholar]
- 26. Prudencio M., Rodrigues C. D., Ataide R., Mota M. M. 2008. Dissecting in vitro host cell infection by Plasmodium sporozoites using flow cytometry. Cell. Microbiol. 10:218–224 [DOI] [PubMed] [Google Scholar]
- 27. Prudencio M., et al. 2008. Kinome-wide RNAi screen implicates at least 5 host hepatocyte kinases in Plasmodium sporozoite infection. PLoS Pathog. 4:e1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Prudencio M., Rodriguez A., Mota M. M. 2006. The silent path to thousands of merozoites: the Plasmodium liver stage. Nat. Rev. Microbiol. 4:849–856 [DOI] [PubMed] [Google Scholar]
- 29. Rodrigues C. D., et al. 2008. Host scavenger receptor SR-BI plays a dual role in the establishment of malaria parasite liver infection. Cell Host Microbe 4:271–282 [DOI] [PubMed] [Google Scholar]
- 30. Romeo S., et al. 2004. Plasmepsin II inhibition and antiplasmodial activity of Primaquine-Statine ‘double-drugs.’ Bioorg. Med. Chem. Lett. 14:2931–2934 [DOI] [PubMed] [Google Scholar]
- 31. Shekalaghe S., et al. 2007. Primaquine clears submicroscopic Plasmodium falciparum gametocytes that persist after treatment with sulphadoxine-pyrimethamine and artesunate. PLoS One 2:e1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shekalaghe S. A., et al. 2010. In Tanzania, hemolysis after a single dose of primaquine coadministered with an artemisinin is not restricted to glucose-6-phosphate dehydrogenase-deficient (G6PD A−) individuals. Antimicrob. Agents Chemother. 54:1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Smithuis F., et al. 2010. Effectiveness of five artemisinin combination regimens with or without primaquine in uncomplicated falciparum malaria: an open-label randomised trial. Lancet Infect. Dis. 10:673–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vale N., Moreira R., Gomes P. 2009. Primaquine revisited six decades after its discovery. Eur. J. Med. Chem. 44:937–953 [DOI] [PubMed] [Google Scholar]
- 35. Walsh J. J., Bell A. 2009. Hybrid drugs for malaria. Curr. Pharm. Des. 15:2970–2985 [DOI] [PubMed] [Google Scholar]
- 36. Walsh J. J., Coughlan D., Heneghan N., Gaynor C., Bell A. 2007. A novel artemisinin-quinine hybrid with potent antimalarial activity. Bioorg. Med. Chem. Lett. 17:3599–3602 [DOI] [PubMed] [Google Scholar]
- 37. Wells T. N., Alonso P. L., Gutteridge W. E. 2009. New medicines to improve control and contribute to the eradication of malaria. Nat. Rev. Drug Discov. 8:879–891 [DOI] [PubMed] [Google Scholar]
- 38. White N. 1999. Antimalarial drug resistance and combination chemotherapy. Philos. Trans. R. Soc. Lond. B Biol. Sci. 354:739–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. World Health Organization 2010. World malaria report 2010. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/world_malaria_report_2010/en/index.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.