Abstract
Daptomycin is an attractive option for treating prosthetic joint infection, but the 6-mg/kg of body weight/day dose was linked to clinical failure and emergence of resistance. Using a methicillin-resistant Staphylococcus aureus (MRSA) knee prosthesis infection in rabbits, we studied the efficacies of high-dose daptomycin (22 mg/kg given intravenously [i.v.] once daily [o.d.]; equivalent to 8 mg/kg/day in humans) or vancomycin (60 mg/kg given intramuscularly [i.m.] twice daily [b.i.d.]), both either alone or with adjunctive rifampin (10 mg/kg i.m. b.i.d.). After partial knee replacement with a silicone implant, 107 MRSA CFU was injected into the knees. Treatment started 7 days postinoculation and lasted 7 days. Positive cultures were screened for the emergence of mutant strains, defined as having 3-fold-increased MICs. Although in vivo mean log10 CFU/g of daptomycin-treated (4.23 ± 1.44; n = 12) or vancomycin-treated (4.63 ± 1.08; n = 12) crushed bone was significantly lower than that of controls (5.93 ± 1.15; n = 9) (P < 0.01), neither treatment sterilized bone (2/12 and 0/12 rabbits with sterile bone, respectively). Daptomycin mutant strains were found in 6/12, 3/12, and 2/9 daptomycin-treated, vancomycin-treated, and control rabbits, respectively; no resistant strains emerged (MIC was always <1 mg/liter). Adjunctive rifampin with daptomycin (1.47 ± 0.04 CFU/g of bone [detection threshold]; 11/11 sterile bones) or vancomycin (1.5 ± 0.12 CFU/g of bone; 6/8 sterile bones) was significantly more effective than monotherapy (P < 0.01) and prevented the emergence of daptomycin mutant strains. In this MRSA joint prosthesis infection model, combining rifampin with daptomycin was highly effective. Daptomycin mutant strains were isolated in vivo even without treatment, but adjunctive rifampin prevented this phenomenon, previously found after monotherapy in humans.
INTRODUCTION
Orthopedic joint replacement is an increasingly common operation worldwide, reflecting the aging of the population (7). Development of infection in prosthetic joints, although uncommon, is a serious complication, responsible for major morbidity and higher costs (6). Perioperative contamination is responsible for most prosthesis infections, which are caused mainly by Staphylococcus aureus or Staphylococcus epidermidis (7). These microorganisms are often resistant to many commonly used antibiotics. Glycopeptide antibiotics remain the first-line therapy against methicillin-resistant Staphylococcus infections, even though their efficacy might not be optimal (18).
Daptomycin is a novel lipopeptide antibiotic with concentration-dependent bactericidal activity and in vitro activity against a broad range of Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA). It has been approved in the United States and Europe for treatment of patients with complicated skin and skin structure infections (1). Also, daptomycin (6 mg/kg of body weight/day given intravenously, once daily [i.v. o.d.]) was recently designated a first-line parenteral therapy of MRSA bone and joint infections, as was vancomycin, in the Infectious Diseases Society of America (IDSA) Guidelines (12).
Emergence of nonsusceptible isolates during therapy associated with treatment failures has nonetheless been found in humans (12). Most of those cases, recently reviewed (19), concerned bone and joint infections and/or prosthesis device infections and were observed in patients previously treated with standard daptomycin therapy (6 mg/kg/day i.v. o.d.). Prior exposure to vancomycin and elevated vancomycin MICs have also been associated with daptomycin MIC increases, suggesting possible cross-resistance (12). The mechanism of this in vivo loss of daptomycin susceptibility, often found in strains with thickened cell walls, is incompletely understood (21). This phenomenon is a growing concern, especially in the United States (22). An optimal definition of daptomycin dose and regimen to avoid this loss of in vivo efficacy is needed.
Because clinical trials are very difficult to conduct in the field of bone and joint infections, most data concerning the comparative efficacies of antibiotics come from experimental models of osteomyelitis or tissue cage infections (5). Daptomycin at doses equivalent to 6 mg/kg/day in humans appeared to be as effective as vancomycin in a rat model of chronic MRSA osteomyelitis (15), and its efficacy was enhanced by rifampin in a model of acute osteomyelitis in rabbits (11). In experimental MRSA foreign-body infection, vancomycin or daptomycin (equivalent to 6 mg/kg/day) monotherapy was poorly effective (10). However, combining daptomycin and rifampin (9, 10) or using a higher daptomycin dose (equivalent to 10 mg/kg/day) (9) enhanced the in vivo efficacy in animals and prevented the emergence of daptomycin-resistant strains observed with standard-dose monotherapy (9). At present, no data are available on the comparative efficacies of daptomycin and vancomycin against experimental MRSA prosthetic joint infections that closely mimic the clinical situation.
The primary aim of this study was to compare the efficacies of high-dose daptomycin (equivalent to 8 mg/kg/day in humans) or vancomycin, both alone and with adjunctive rifampin, in an experimental MRSA joint prosthesis infection. Because infected bone/abscess and prosthesis seemed to be favorable conditions for the emergence of these strains in humans, we also examined the in vivo spontaneous emergence of less-susceptible mutants, focusing on infections not previously exposed to antibiotics and those in antibiotic-treated rabbits.
(This work was presented in part at the 50th Interscience Conference on Antimicrobial Agents and Chemotherapy, Boston, MA, 12 to 15 September 2010 [abstr. B-698].)
MATERIALS AND METHODS
Test strain.
This study used MRSA strain S271, which had been isolated from a patient with an infected knee prosthesis. Its virulence was maintained by intraperitoneal injection into mice.
In vitro antibiotic susceptibility testing.
Daptomycin, vancomycin, and rifampin MICs were determined with the Etest method (bioMérieux, La Balme-les-Grottes, France), as recommended by the manufacturer. A single inoculum adjusted to a McFarland standard of 0.5 in distilled water was used. Mueller-Hinton agar plates (Bio-Rad, Marnes-La Coquette, France) were inoculated with swabs saturated with a suspension of the test organism and incubated for 18 h at 37°C. The MIC was defined as the value at which the inhibition zone intersected the scale of the Etest strip.
Time-kill curve studies.
The bactericidal activities of daptomycin or vancomycin alone or combined with rifampin were determined. Overnight cultures were diluted in 10 ml of fresh Mueller-Hinton broth supplemented, for daptomycin, with calcium chloride to contain 50 mg of Ca2+/liter, to yield an inoculum of 106 CFU/ml. The antibiotic concentrations used were equivalent to 2× or 4× MIC for daptomycin and vancomycin and 2× MIC for rifampin. After 0, 3, 6, and 24 h of incubation in a shaking water bath at 37°C, serial dilutions of 0.1-ml samples were subcultured on Mueller-Hinton or Mueller-Hinton II agar plates (Becton Dickinson, Rungis, France) supplemented with calcium chloride (50 mg of Ca2+/liter) for daptomycin and incubated at 37°C for 24 h before CFU were counted. Bactericidal effect was defined as a ≥3-log10 decrease in the initial inoculum. Synergy was defined as a decrease of ≥2 log10 CFU/ml between the combination and its most active constituent after 24 h of incubation.
Experimental prosthesis infection.
New Zealand White rabbits, each weighing between 2.5 and 3 kg, were used. They were housed in individual cages with a natural light-dark cycle. The experimental protocol was in keeping with French legislation on animal experimentation and was approved by the Animal Use Committee of Maison Alfort Veterinary School.
This model was previously described in detail (2). Briefly, the rabbit underwent partial right knee replacement with a tibial component. The operation was carried out under general anesthesia induced by intramuscular (i.m.) injection of ketamine (25 mg/kg of body weight) and then by continuous inhalation of 1% isoflurane. A silicone elastomer implant, commonly used in arthroplasty of the first metatarsophalangeal joint (Silastic, great toe implant HP; Swanson Design, Dow-Corning; provided by Wright Medical France, Créteil, France) was implanted as a tibial prosthetic component. The skin overlying the right leg was shaved 24 h before the operation and cleaned with an iodine solution prior to surgery. A longitudinal skin incision was made, and the knee joint was exposed. After dislocation of the tibia, the epiphyseal plates were removed. The metaphysis was exposed, and the cancellous bone of the medullary cavity of the proximal metaphysis was reamed. The stem of the nail-shaped silicone implant (14 mm long) was inserted into the intramedullary canal of the tibia, with the implant head (15 mm in diameter and 5 mm high) replacing the tibia plateaus. Then, the deep fascia and the skin were closed.
Immediately after surgery, animals were inoculated with of 5 × 107 MRSA CFU in a final volume of 0.5 ml in phosphate-buffered saline, injected into the knee close to the prosthesis. Each rabbit was given Patch analgesia (Durogesic, Issy-les Moulineaux, France) for 7 days following surgery. Twelve rabbits were randomly assigned to each untreated or treated group. Three rabbits in the untreated group and four rabbits in the vancomycin group died during or within the 24 h following surgery. Their deaths were considered to be secondary to anesthesia or hemorrhage, and these animals were not included in the analysis.
Serum daptomycin levels.
Serum antibiotic levels in uninfected rabbits were determined. Initial doses were selected on the basis of previous experimental studies in rabbits (11), and then we verified that they achieved pharmacokinetic parameters (area under the concentration-time curve [AUC]) equivalent to those obtained in humans given an 8-mg/kg/day dose (8). For that, 3 rabbits received an i.v. injection of daptomycin, and blood was drawn 15 and 30 min and 1, 3, 6, and 8 h thereafter. Peak plasma daptomycin levels in 5 rabbits, treated for 7 days, were also determined 15 min after the last i.v. injection. Daptomycin concentrations were measured by high-performance liquid chromatography with a reverse-phase C18 column and measured by UV detection (λ = 220 nm).
Treatment and its evaluation.
Starting 7 days postinfection, rabbits were treated with daptomycin (22 mg/kg of body weight i.v. o.d.) or vancomycin (60 mg/kg i.m. twice daily [b.i.d.]), alone or combined with rifampin (10 mg/kg i.m. b.i.d.). Vancomycin and rifampin doses were selected based on previous experiments (17) showing that they obtained concentrations close to those recommended for humans (trough vancomycin concentrations of 15 to 20 μg/ml and rifampin dose equivalent to 900-mg daily dose) (12). The daptomycin dose was selected based on the pharmacokinetic study described above. Each regimen was administered for 7 days. Controls were left untreated. Animals were killed by i.v. injection of pentobarbital 3 days after the end of therapy (day 17) to allow for bacterial regrowth after ending treatment while avoiding the persistence of residual antibiotic in the bone. Untreated control rabbits were also killed on day 17.
At the time of death, the right hind leg was dissected, and the tibia and femur were separated from the surrounding soft tissues. A smear of the prosthesis was performed on a blood agar plate. For quantitative bacterial counts, the upper third of the tibia (3 cm long), including compact bone and marrow, was isolated, split with a bone crusher, weighed, cut into small pieces, frozen in liquid nitrogen, and crushed in an autopulverizer (Spex 6700; Freezer/Mill Industries Inc., Metuchen, NJ). The pulverized bone was suspended in 10 ml of sterile saline; serial dilutions were made and plated on Trypticase soy agar. After 24 h of incubation at 37°C, the number of viable microorganisms was determined. The results are expressed as mean log10 CFU/g of bone ± standard deviation (SD) and as the percentage of animals with sterile bone. Bone was considered sterile when the culture showed no growth after incubation for 48 h at 37°C and the number of CFU was recorded as the lowest detectable bacterial count (1.38 to 1.53 CFU/g of bone, depending on sample weight).
In vivo selection of mutants.
Mutant-resistant MRSA was sought in all control and treated rabbits with positive bone cultures at the end of the treatment. Each undiluted bone homogenate (0.1 ml) was plated on Mueller-Hinton II agar supplemented, for daptomycin, with calcium chloride (50 mg of Ca2+/liter) and containing daptomycin (0.5 or 1 μg/ml) or vancomycin (1.5 or 3 μg/ml) to detect potentially emerging resistant mutants after 72 h of incubation at 37°C. When bacterial growth was observed, colonies were counted, identified as S. aureus, and mixed in Trypticase soy broth. The resulting inoculum was adjusted to 0.5 MacFarland and served to determine daptomycin and vancomycin MICs using the Etest method. The number of bacteria grown on antibiotic-containing medium is reported as the number of mutants/g of bone. Mutants were defined as having 3-fold-increased MICs.
Statistical analyses.
Results are expressed as means ± SD. Bacterial densities in bone were compared between the experimental groups by analysis of variance, followed by Scheffe's test for multiple comparisons. A P value of <0.05 was considered significant.
RESULTS
In vitro studies.
Daptomycin, vancomycin, and rifampin MICs were 0.125, 1.5, and 0.008 μg/ml, respectively.
In vitro killing.
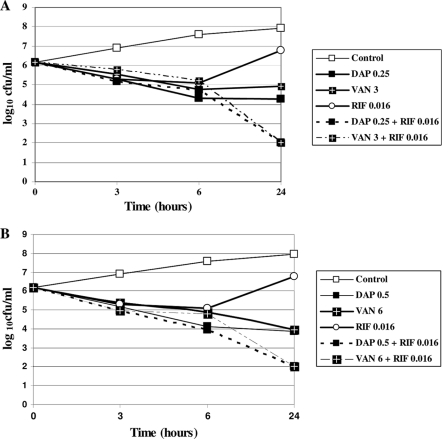
Curves obtained at 2× MIC (Fig. 1A) and 4× MIC (Fig. 1B) show daptomycin and vancomycin bactericidal activities. With rifampin alone, the initial inoculum decreased during the first 6 h, but then regrowth was observed, presumably due to the emergence of resistant mutants. However, adjunctive rifampin with daptomycin or vancomycin increased the killing rates of both combinations by approximately 2 log10 CFU at 24 h.
Fig. 1.
In vitro killing curves for methicillin-resistant Staphylococcus aureus strain S271, using different antibacterial agents and combinations: 2× MIC (A) or 4× MIC, except rifampin (2× MIC) (B). DAP, daptomycin; VAN, vancomycin; RIF, rifampin.
Plasma antibiotic levels in rabbits.
In uninfected animals, following a single injection of daptomycin (22 mg/kg i.v. o.d.), the mean peak plasma concentration (15 min after injection) was 298.67 ± 51 μg/ml and the mean AUC from 0 to 24 h (AUC0–24) was 1,188 ± 311 μg · h/ml. In infected animals, the mean peak plasma daptomycin level was 186.8 ± 31.83 μg/ml.
Therapeutic studies.
All control animals infected with MRSA S271 had positive prosthesis smear cultures and a mean bacterial count of 5.93 ± 1.15 log10 CFU/g of bone (Table 1). Only two of the 12 daptomycin-treated animals had sterile bone; however, their mean bone bacterial density was significantly lower than that of the control animals (P < 0.01). No vancomycin-treated animals had sterile bone, and their mean bacterial count in bone differed significantly from that of controls (P < 0.01). The mean bacterial counts in daptomycin- and vancomycin-treated animals were comparable.
Table 1.
Effects of antibiotic treatment on experimental MRSA prosthetic knee infection in rabbits
| Treatmenta | No. of rabbits with sterile bone/total | Log10 CFU/g of bone (mean ± SD) | No. of rabbits with daptomycin mutant strain/no. infected |
|---|---|---|---|
| None | 0/9 | 5.93 ± 1.15 | 2/9 |
| Daptomycin | 2/12 | 4.23 ± 1.44b | 6/10c |
| Vancomycin | 0/12 | 4.63 ± 1.08b | 3/12 |
| Daptomycin + rifampin | 11/11 | 1.47 ± 0.04d,e | |
| Vancomycin + rifampin | 6/8 | 1.50 ± 0.12d | 0/2d |
For 7 days, rabbits were injected with daptomycin (22 mg/kg i.v. o.d.) or vancomycin (60 mg/kg i.m. b.i.d.) alone or combined with rifampin (10 mg/kg i.m. b.i.d.).
Significantly different from results with untreated controls (P < 0.01).
Other rabbits had sterile bones.
Significantly different from results with monotherapy (P < 0.01).
This value, the limit of detection, was accorded to sterile bone (see Materials and Methods), explaining the absence of a value in the last column.
Adjunctive rifampin with daptomycin or vancomycin was significantly more effective than monotherapy. For the vancomycin-plus-rifampin-treated group, 6/8 animals had sterile bone, while 11/11 of the daptomycin-plus-rifampin-treated animals had sterile bone. For both groups, the mean bacterial counts were significantly lower than those for the untreated controls and the groups treated with either agent alone. The prosthesis smears for animals with sterile bone showed no growth.
Subpopulation analysis of untreated and treated rabbits.
Subpopulation analysis of MRSA strain S271 before inoculation into rabbits showed no growth on plates containing daptomycin when a high initial inoculum (108 CFU/ml) was used. In contrast, daptomycin mutant strains (MIC of 0.75 μg/ml) emerged in 2/9 untreated rabbits, with 555 and 993 mutant CFU/g bone.
In animals treated with daptomycin or vancomycin alone, daptomycin mutant strains emerged, respectively, in 6/12 (MIC of 0.75 μg/ml [n = 2], MIC of 0.5 μg/ml [n = 2], and MIC of 0.38 μg/ml [n = 2]) or 3/12 (MIC of 0.75 μg/ml [n = 1] and MIC of 0.38 μg/ml [n = 2]) rabbits. Mutant density varied from 24 to 532 or from 23 to 232 CFU/g of bone in animals treated with daptomycin or vancomycin, respectively. The daptomycin MICs of these mutant strains were always below the breakpoint of 1 μg/ml and remained similar after three subcultures in vitro. The vancomycin MICs of these strains were unchanged from those of the initial strain, with values of 1.5 to 2 μg/ml.
Combined therapy with daptomycin or vancomycin and rifampin prevented the emergence of daptomycin mutant strains. Vancomycin and rifampin MICs of MRSA isolated from all control and treated rabbits were unchanged.
DISCUSSION
Daptomycin use has increased in recent years, and concerns have been raised by reports of clinical failures and emergence of nonsusceptible strains during daptomycin therapy (3, 19). Most of those cases were described after standard-dose daptomycin treatment of bone or prosthesis infections, all deep-seated and difficult-to-treat infections that usually require adjunctive surgical intervention (3, 13). This study focused on the in vivo efficacy of high-dose daptomycin alone or combined with rifampin against an experimental MRSA prosthesis joint infection closely resembling human infection and on the in vivo emergence of daptomycin mutants in untreated and treated rabbits. Our results showed that daptomycin monotherapy, at a dose equivalent to 8 mg/kg/day in humans, was as effective as vancomycin. However, despite these high doses, less-susceptible daptomycin mutants were isolated in vivo from more than half of treated animals receiving monotherapy. The daptomycin MICs of these new strains were always below the breakpoint of 1 μg/ml. No cross-resistance with vancomycin was detected.
The emergence of less-susceptible strains was detected by Vaudaux et al. early during experimental staphylococcal foreign-body infection and was thought to reflect suboptimal daptomycin doses, which yielded trough levels in tissue-cage fluid just equivalent to the minimum bactericidal concentration (MBC) of the test strain (20). Similar results were subsequently found with an acute osteomyelitis model (11) and a tissue-cage model (9) treated with daptomycin monotherapy (equivalent to 6 mg/kg/day). In the tissue-cage model, this phenomenon seemed to be prevented by a higher dose (equivalent to 10 mg/kg/day) (9). However, even at that high dose, the authors noted “growth of bacterial haze on agar plates containing daptomycin concentrations higher than the MIC” when high inocula of survival populations from daptomycin experiments were plated (14). This incomplete protective effect of high-dose daptomycin suggests that subtherapeutic doses are not the sole cause of in vivo emergence of daptomycin mutants. Indeed, our findings showed that spontaneous emergence of MRSA with diminished susceptibility to daptomycin could also be detected in 2/9 untreated infected rabbits not exposed to daptomycin. This observation suggests that in vivo factors interacting with the microorganism during the course of the infection could by themselves favor this phenomenon, which was stable after three in vitro subcultures. Notably, emergence of strains with higher vancomycin MICs was not detected in control animals. Daptomycin exposure further enhanced the number of animals in which these less-susceptible subpopulations were detected.
Foreign-body presence, surgical infections with abscess or dead tissues, and too-low doses are factors well known to favor the selective pressure of antibiotics because they contribute to subtherapeutic antibiotic concentrations in the presence of high inocula at the infection focus (13, 19), as encountered in prosthetic joint infections (4, 16). However, to our knowledge, the spontaneous emergence of daptomycin mutant strains after in vivo passage and without previous antibiotic exposure has not been described previously. Because our experimental prosthetic joint model reproduces very closely acute postoperative prosthetic joint infection seen in humans, unlike the subcutaneous tissue-cage model, we think this experimental observation could improve our understanding of daptomycin monotherapy failure seen in humans.
MRSA subpopulations with decreased susceptibility to daptomycin also emerged in 3/12 rabbits given vancomycin alone. Development of daptomycin resistance following vancomycin treatment has been found in patients (19). However, in contrast to what we observed herein, the increased daptomycin MICs were usually linked to increased vancomycin MICs. One explanation could be that the strains were usually isolated from heavily vancomycin-treated patients after treatment failure and had higher daptomycin MICs (>1 mg/liter). Our rabbits were treated for only 7 days with vancomycin, and even though daptomycin MICs were higher than the initial strain's MIC, they remained at <1 μg/ml.
In our study, unlike the case with monotherapy, the daptomycin and rifampin combination sterilized 100% of infected bones and prevented the emergence daptomycin mutant strains. Rifampin is well known for its bactericidal activity against slow-growing biofilm-enclosed bacteria (23), which is in accordance with previous studies of rat foreign-body infection (9) and the experimental osteomyelitis model (11), which showed that daptomycin and rifampin provided the best treatment compared to monotherapy. Our results confirmed the high efficacy of this combination against a prosthetic joint infection model. In contrast to most clinical situations, medical treatment was not combined with surgical debridement. Thus, this experimental model is a stringent model for antibiotics. In this regard, our findings support the recent IDSA recommendations that consider daptomycin or vancomycin as first-line parenteral therapy, combined with rifampin, for the management of MRSA prosthetic joint infection (12).
Our study has several limits. The main limitation is that we used a single MRSA strain, as did most experimental studies. The vancomycin MIC of this clinical strain was 1.5 μg/ml. It was previously shown that elevated MICs (1.5 to 2 μg/ml) enhance the likelihood of vancomycin failure (12). However, it should be noted that in our model, the efficacies of vancomycin and daptomycin were not significantly different. Also, we used a silicone-elastomer implant, and microbiological findings could be modified by the use of other components, e.g., metallic implants, often used in orthopedic surgery. Moreover, treatment was started 7 days after infection to simulate an acute postoperative infection, and antibiotics might be less effective in a more chronic infection. However, because daptomycin and vancomycin were evaluated under the same experimental conditions, our main conclusions concerning their comparative efficacies, alone or combined with rifampin, and the emergence of resistant strains remain valid.
In conclusion, in this experimental MRSA prosthetic joint infection model, high-dose daptomycin was as effective as vancomycin. But even this high dose could not prevent the emergence of daptomycin mutant strains, which were also selected in the absence of antibiotic pressure in untreated control animals. Adjunctive rifampin with daptomycin was highly effective in this experimental model, sterilizing 100% of infected animals and preventing the emergence of these mutants. Therefore, our results support the idea that adjunctive rifampin is crucial for successful daptomycin treatment of prosthetic joint infection.
ACKNOWLEDGMENT
This work was supported in part by a research grant from Novartis Pharma, France.
Footnotes
Published ahead of print on 8 August 2011.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1. Arbeit R. D., Maki D., Tally F. P., Campanero E., Eisenstein B. I., and the Daptomycin 98-01 and 99-01 Investigators 2004. The safety and efficacy of daptomycin for the treatment of complicated skin and skin-structure infections. Clin. Infect. Dis. 38:1673–1681 [DOI] [PubMed] [Google Scholar]
- 2. Belmatoug N., et al. 1996. A new model of experimental prosthetic joint infection due to methicillin-resistant Staphylococcus aureus: a microbiologic, histopathologic and magnetic resonance characterization. J. Infect. Dis. 174:414–417 [DOI] [PubMed] [Google Scholar]
- 3. Boucher H. W., Sakoulas G. 2007. Perspectives on daptomycin resistance, with emphasis on resistance in Staphylococcus aureus. Clin. Infect. Dis. 45:601–608 [DOI] [PubMed] [Google Scholar]
- 4. Crémieux A. C., et al. 1996. Efficacy of sparfloxacin and autoradiographic diffusion pattern of [14C]sparfloxacin in experimental Staphylococcus aureus joint prosthesis infection. Antimicrob. Agents Chemother. 40:2111–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crémieux A. C., Carbon C. 1997. Experimental model of bone and prosthetic joint infections. Clin. Infect. Dis. 25:1295–1302 [DOI] [PubMed] [Google Scholar]
- 6. Darouiche R. O. 2004. Treatment of infections associated with surgical implants. N. Engl. J. Med. 350:1422–1429 [DOI] [PubMed] [Google Scholar]
- 7. Del Pozo J. L., Patel R. 2009. Infection associated with prosthetic joints. N. Engl. J. Med. 361:787–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dvorchik B. H., Brazier D., DeBruin M. F., Arbeit R. D. 2003. Daptomycin pharmacokinetics and safety following administration of escalating doses once daily to healthy subjects. Antimicrob. Agents Chemother. 47:1318–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garrigós C., et al. 2010. Efficacy of usual and high doses of daptomycin in combination with rifampin versus alternative therapies in experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 54:5251–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. John A. K., et al. 2009. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: importance of combination with rifampin. Antimicrob. Agents Chemother. 53:2719–2724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lefebvre M., et al. 2010. Efficacy of daptomycin combined with rifampin for the treatment of experimental MRSA acute osteomyelitis. Int. J. Antimicrob. Agents 36:542–544 [DOI] [PubMed] [Google Scholar]
- 12. Liu C., et al. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin. Infect. Dis. 52:285–292 [DOI] [PubMed] [Google Scholar]
- 13. Moise P. A., North D., Steenbergen J. N., Sakoulas G. 2009. Susceptibility relationship between vancomycin and daptomycin in Staphylococcus aureus: facts and assumptions. Lancet Infect. Dis. 9:617–624 [DOI] [PubMed] [Google Scholar]
- 14. Murillo O., et al. 2009. Efficacy of high doses of daptomycin versus alternative therapies against experimental foreign-body infection by methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:4252–4257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rouse M. S., et al. 2006. Daptomycin treatment of Staphylococcus aureus experimental chronic osteomyelitis. J. Antimicrob. Chemother. 57:301–305 [DOI] [PubMed] [Google Scholar]
- 16. Saleh Mghir A., et al. 1998. Efficacy of teicoplanin and autoradiographic diffusion of pattern [14C]teicoplanin in experimental Staphylococcus aureus joint prosthesis infection. Antimicrob. Agents Chemother. 42:2830–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saleh-Mghir A., et al. 2002. Combination of synercid and rifampin is highly synergistic in experimental Staphylococcus aureus joint prosthesis infection. Antimicrob. Agents Chemother. 46:1122–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steckelberg J. M., Osmon D. R. 2000. Prosthetic joint infections, p. 173–209 In Waldvogel F. A., Bisno A. L. (ed.), Infections associated with indwelling medical devices, 3rd ed ASM Press, Washington, DC [Google Scholar]
- 19. van Hal S. J., Paterson D. L., Gosbell I. B. 2011. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naïve patient—a review of the literature. Eur. J. Clin. Microbiol. Infect. Dis. 30:603–610 [DOI] [PubMed] [Google Scholar]
- 20. Vaudaux P., et al. 2003. Comparative efficacy of daptomycin and vancomycin in the therapy of foreign body infection due to Staphylococcus aureus. J. Antimicrob. Chemother. 52:89–95 [DOI] [PubMed] [Google Scholar]
- 21. Yang S. J., et al. 2010. Cell wall thickening is not a universal accompaniment of the daptomycin nonsusceptibility phenotype in Staphylococcus aureus: evidence for multiple resistance mechanisms. Antimicrob. Agents Chemother. 8:3079–3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang S. J., et al. 2010. Daptomycin-oxacillin combinations in treatment of experimental endocarditis caused by daptomycin-nonsusceptible strains of methicillin-resistant Staphylococcus aureus with evolving oxacillin susceptibility (the “seesaw effect”). Antimicrob. Agents Chemother. 54:3161–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zimmerli W., Trampuz A., Ochsner P. E. 2004. Prosthetic-joint infections. N. Engl. J. Med. 351:1645–1654 [DOI] [PubMed] [Google Scholar]