Abstract
Telaprevir is a hepatitis C virus protease inhibitor that is both a substrate and an inhibitor of CYP3A. Amlodipine and atorvastatin are both substrates of CYP3A and are among the drugs most frequently used by patients with hepatitis C. This study was conducted to examine the effect of telaprevir on atorvastatin and amlodipine pharmacokinetics (PK). This was an open-label, single sequence, nonrandomized study involving 21 healthy male and female volunteers. A coformulation of 5 mg amlodipine and 20 mg atorvastatin was administered on day 1. Telaprevir was taken with food as a 750-mg dose every 8 h from day 11 until day 26, and a single dose of the amlodipine-atorvastatin combination was readministered on day 17. Plasma samples were collected for determination of the PK of telaprevir, amlodipine, atorvastatin, ortho-hydroxy atorvastatin, and para-hydroxy atorvastatin. When administration with telaprevir was compared with administration without telaprevir, the least-square mean ratios (90% confidence limits) for amlodipine were 1.27 (1.21, 1.33) for the maximum drug concentration in serum (Cmax) and 2.79 (2.58, 3.01) for the area under the concentration-time curve from 0 h to infinity (AUC0-∞); for atorvastatin, they were 10.6 (8.74, 12.9) for the Cmax and 7.88 (6.84, 9.07) for the AUC0-∞. Telaprevir significantly increased exposure to amlodipine and atorvastatin, consistent with the inhibitory effect of telaprevir on the CYP3A-mediated metabolism of these agents.
INTRODUCTION
Telaprevir is an orally administered inhibitor of the nonstructural 3/4A (NS3/4A) protease of the hepatitis C virus (HCV) (11). In recent phase 3 studies of patients with chronic hepatitis C (CHC), the addition of telaprevir as part of a combination regimen with pegylated interferon and ribavirin significantly increased the rates of sustained virologic response (8, 17, 23). Telaprevir was recently approved in the United States of America for the treatment of genotype 1 CHC in adult patients with compensated liver disease (3).
Amlodipine is a dihydropyridine calcium channel antagonist used to treat high blood pressure and angina or coronary artery disease. Atorvastatin is a hydroxymethyl glutaryl coenzyme A (HMG-CoA) reductase inhibitor used to lower high cholesterol levels and reduce the risk of heart attack and stroke. These two drugs are frequently prescribed for patients with CHC and are commercially available as a coformulation (Caduet; Pfizer) (1). Both amlodipine and atorvastatin are metabolized primarily by CYP3A (1). CYP3A-mediated metabolism converts atorvastatin into two major active metabolites, ortho-hydroxy atorvastatin and para-hydroxy atorvastatin, and three inactive lactone metabolites corresponding to each acid form (12). Both active acid metabolites are known to be equally as potent as the parent drug and account for ∼70% of the total HMG-CoA reductase inhibitory activity of atorvastatin (6). Excreted primarily in the bile, atorvastatin is also a substrate for P-glycoprotein and organic anion transporter protein (OATP1B1/1B3) (1). Amlodipine is extensively metabolized, primarily involving oxidation to the pyridine derivative with subsequent oxidative deamination of the 2-aminoethyoxymethyl side chain or deesterification at the 5-methoxycarbonyl group. None of the metabolites have any significant calcium antagonist activity relative to amlodipine (20).
Telaprevir inhibits CYP3A-mediated metabolism at therapeutic concentrations and may inhibit and/or saturate P-glycoprotein in the gut. Therefore, this study was designed to evaluate the drug-drug interactions between telaprevir and amlodipine and atorvastatin in healthy volunteers.
MATERIALS AND METHODS
Volunteers.
Twenty-one healthy male (n = 15) and female (n = 6) volunteers were enrolled at the Covance Clinical Research Unit, Inc., Daytona Beach, FL. The female volunteers were documented not to have childbearing potential. At screening, the volunteers were judged to be in good health on the basis of medical history, physical examination, and routine laboratory measurement results. Volunteers had ended any short-duration courses of prescription medications, herbal medications, or dietary supplements (e.g., St. John's wort, ginkgo biloba, garlic supplements), vitamins, Seville oranges, grapefruit, or grapefruit juice at least 14 days before the administration of the first dose of the study drug. Prescription medications were not administered during the study. Volunteers had stopped over-the-counter medications no less than 2 days before the first administration of the study drug. Occasional use of acetaminophen or ibuprofen was allowed during the study for the treatment of pain. Volunteers could not consume alcohol from 72 h before the first dose of the study drug through the follow-up visit and were nonsmokers (subjects who stopped smoking at least 6 months before screening were considered nonsmokers).
The protocol and informed-consent form were approved in accordance with national procedures. All volunteers provided written informed consent before participating in the study. The study was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local laws and regulations.
Study design.
This was a phase 1, open-label, single-center, nonrandomized study of telaprevir in combination with Caduet tablets containing 5 mg of amlodipine and 20 mg of atorvastatin (amlodipine-atorvastatin). Volunteers received the following treatment: a single dose of amlodipine-atorvastatin alone on day 1, followed by a washout period, telaprevir at a dose of 750 mg every 8 h (q8h) on days 11 through 26, with a single dose of amlodipine-atorvastatin on day 17. Outpatient visits occurred at the screening visit (between 3 and 28 days before the first administration of the study drug); on days 3, 4, 6, 8, 12 through 14, and 21 through 27; and at a final safety visit approximately 6 days after the last dose of the study drug. Volunteers were confined to the clinical research unit on days −1 to 2, 10 to 11, and 15 to 20.
Drugs administered.
Telaprevir (375-mg tablets, Patheon, Mississauga, Ontario, Canada) was administered at 750 mg q8h orally in the fed state (30 min after the start of a meal or snack).
Amlodipine-atorvastatin (25-mg fixed dose combination tablets containing 5 mg amlodipine and 20 mg atorvastatin; Pfizer Incorporated, New York, NY) was administered orally as a single dose in accordance with the package insert 30 min after the start of breakfast (1). During the study, compliance was assessed on an ongoing basis by counting returned dosage units and reviewing the volunteer logs. All volunteers were ≥90% compliant with the telaprevir dosing regimen, and all volunteers received their scheduled doses of amlodipine-atorvastatin (administered at the clinic).
Bioanalysis.
Pharmacokinetic evaluations were based on the concentrations of amlodipine, atorvastatin, ortho-hydroxy atorvastatin, para-hydroxy atorvastatin, and telaprevir in plasma.
Plasma amlodipine, atorvastatin, ortho-hydroxy atorvastatin, and para-hydroxy atorvastatin concentrations were determined predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, 120, 168, and 240 h after a single dose of amlodipine-atorvastatin on day 1 and predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, 24, 48, 72, 120, 168, and 240 h after another single dose of amlodipine-atorvastatin on day 17. Neither amlodipine nor atorvastatin is an inhibitor of CYP3A, while telaprevir is a potent inhibitor of CYP3A. Thus, an effect of amlodipine and atorvastatin on telaprevir pharmacokinetics (PK) was not anticipated. Therefore, telaprevir PK were evaluated only on day 17 and compared to those found historically. Plasma telaprevir concentrations were determined at steady state predose and at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, and 8 h after the morning dose of telaprevir on day 17.
Analysis of all plasma samples was performed using validated liquid chromatography (normal phase) followed by tandem mass spectrometry (LC/MS/MS) methods at Covance Bioanalytical Services, LLC (Indianapolis, IN). Analytes and their internal standards (amlodipine-d4 maleic acid, atorvastatin-d5 sodium salt, ortho-hydroxy atorvastatin-phenyl-d5, para-hydroxy atorvastatin-phenyl-d5, and d11-telaprevir) were extracted from human plasma by liquid-liquid extraction. After evaporation under nitrogen, the residue of all analytes was reconstituted and analyzed using LC/MS/MS with selected ion monitoring in the positive-ion mode. Calibration curves were generated using weighted (1/x2) linear least-square (LS) regression. The standard curve range was 0.0500 to 25.0 ng/ml for amlodipine (lower limit of quantitation [LLOQ], 0.0500 ng/ml), 0.250 to 100 ng/ml for atorvastatin (LLOQ, 0.250 ng/ml), 0.250 to 100 ng/ml for para-hydroxy atorvastatin (LLOQ, 0.250 ng/ml), 0.250 to 100 ng/ml for ortho-hydroxy atorvastatin (LLOQ, 0.250 ng/ml), and 2.00 to 1,000 ng/ml for telaprevir (LLOQ, 2.00 ng/ml).
The calibration curves and quality control data all met the prespecified acceptance criteria for each batch of samples assayed.
Pharmacokinetic analyses.
Pharmacokinetic analyses were carried out using WinNonlin, version 5.0.1 (Pharsight Corporation, Mountain View, CA). Standard noncompartmental analyses for computation of the area under the concentration-time curve (AUC) were conducted. The maximum observed drug concentration in serum (Cmax) and the time required to achieve the Cmax (tmax) were determined directly from the data obtained. The AUC was computed using the linear trapezoidal rule between increasing concentrations and the log trapezoidal rule between decreasing concentrations. The AUC extrapolated to infinity (AUC0-∞) was computed as the cumulative AUC to the time (tlast) of the last quantifiable concentration (Clast), i.e., AUClast, plus the extrapolated AUC from tlast to infinity. AUCtlast-∞ was estimated by dividing the Clast by the terminal elimination rate constant (λz). The terminal half-life (t1/2) was calculated as ln(2)/λz, and oral clearance (CL/F, where F is oral bioavailability) was calculated by dividing the dose by the AUC0-∞.
For all pharmacokinetic measurements and parameters, appropriate descriptive statistics were calculated, which included the arithmetic mean, the arithmetic standard deviation (SD), and the number of volunteers.
The drug-drug interaction was assessed by the linear mixed-effects modeling method using WinNonlin. The pharmacokinetic parameters (Cmax, AUClast, and AUC0-∞) of amlodipine and atorvastatin following a single dose of amlodipine-atorvastatin coadministered with telaprevir were compared to those measured following a single dose of amlodipine-atorvastatin alone. In the analysis, treatment effect (with or without telaprevir) was considered a fixed effect and subject was a random effect. Geometric LS means for each treatment and 90% confidence interval (CIs) for the geometric LS mean ratio (GLSMR) were reported. The absence of an interaction was to be concluded if the 90% CIs for the GLSMR fell within a range of 0.80 to 1.25 for each pharmacokinetic parameter (2).
Safety assessments.
Adverse events and concomitant medications were monitored throughout the study. Vital signs were assessed predose and at 4, 8, 10, and 24 h postdose; 12-lead electrocardiograms (ECGs) were assessed predose and at 8, 10, and 24 h postdose for amlodipine-atorvastatin when administered alone and when administered in combination with telaprevir. Clinical chemistry and hematology were assessed predose and on days 10, 15, and 20. Urinalysis was performed predose. All safety assessments were repeated at the safety follow-up visit conducted approximately 6 days following the last dose of the study medications.
RESULTS
Demographics and disposition.
Seventy-six percent of our healthy volunteers were Caucasian (n = 16), 19% were black or African American (n = 4), and 5% were Native American or Native Alaskan (n = 1), with a median age of 34 years (range, 21 to 53 years) and a median body mass index of 26.6 (range, 20.7 to 30.1). Twenty-one healthy volunteers were enrolled, and plasma samples from at least 19 volunteers were available following all dosing occasions and analyzed for amlodipine, atorvastatin, ortho-hydroxy atorvastatin, para-hydroxy atorvastatin, and telaprevir. Day 17 plasma samples from 2 volunteers were not available because they discontinued the study after providing blood samples for day 1 amlodipine-atorvastatin PK.
Effect of telaprevir on the PK of amlodipine.
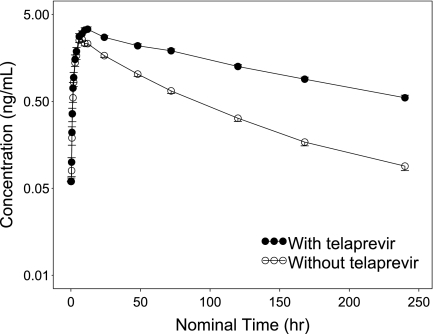
The mean plasma amlodipine concentration-time profile is shown in Fig. 1, and the pharmacokinetic parameter estimates from the noncompartmental analysis of amlodipine concentration data are shown in Table 1. Based on the ratio of the LS means, the mean Cmax and AUC0-∞ of amlodipine were increased 1.27-fold and 2.79-fold, respectively, by the coadministration of telaprevir (Table 1). The mean t1/2 of amlodipine increased from 41.3 h to 95.1 h, and the mean apparent clearance (CL/F) decreased from 38.0 liters/h to 12.3 liters/h.
Fig. 1.
Mean plasma concentration-time profile of amlodipine following oral administration with and without telaprevir. Error bars represent the standard error of the mean.
Table 1.
Effects of telaprevir on the PK of amlodipine, atorvastatin, and its metabolite ortho-hydroxy atorvastatin
| Substrate and PK analysis day | Interacting drug | No. of subjects with PK data | Median (min, max) tmax (h) | Arithmetic mean (SD) |
GLSMRa (90% CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (ng/ml) | AUC∞ (ng h/ml) | t1/2 (h) | V/F (liters) | CL/F (liters/h) | Cmax | AUC | ||||
| AMLc,g | ||||||||||
| 1 | None | 21 | 8.00 (6.00, 12.0) | 2.75 (0.74) | 142 (37) | 41.3 (8.2) | 2,191 (521) | 38.0 (11.8) | ||
| 17 | Telapreviri | 19 | 12.0 (4.00, 23.8) | 3.55 (0.87) | 425 (83) | 95.1 (23.6) | 1,645 (378) | 12.3 (2.97) | 1.27 (1.21, 1.33) | 2.79 (2.58, 3.01) |
| ATVSd,h | ||||||||||
| 1 | None | 21 | 1.50 (0.75, 6.00) | 3.50 (1.93) | 33.8 (13.6) | 9.44 (2.64) | 8,984 (3,431) | 685 (272) | ||
| 17 | Telapreviri | 19 | 3.00 (1.50, 4.02) | 38.5 (20.0) | 277 (116) | 6.75 (1.55) | 838 (405) | 83.8 (32.7) | 10.60 (8.74, 12.85) | 7.88 (6.84, 9.07) |
| OHATVSf,h | ||||||||||
| 1 | None | 21 | 4.00 (1.50, 10.0) | 2.95 (1.07) | 42.09 (12.76) | 10.16 (1.57) | NAe | NA | ||
| 17 | Telapreviri | 19 | 4.00 (3.00, 12.0) | 1.04 (1.44) | 12.64b (10.50) | 8.53 (2.36) | NA | NA | NA | NA |
Value for substrate with telaprevir/value for substrate without telaprevir.
Four volunteers had an extrapolated component of AUC∞ that was over 25% on day 17; furthermore, the AUC calculation was performed using imputed data by replacing the first value BQL after Clast with half of the LLOQ.
AML, amlodipine.
ATVS, atorvastatin.
NA, not available.
OHATVS, ortho-hydroxy-atorvastatin.
A single 5-mg dose was administered.
A single 20-mg dose of atorvastatin was administered.
A 750-mg dose was administered q8h on days 11 to 26.
Effect of telaprevir on the PK of atorvastatin and metabolites.
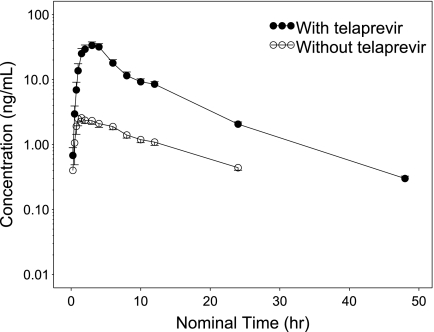
The mean plasma atorvastatin concentration-time profile obtained is shown in Fig. 2, and the pharmacokinetic parameter estimates from the noncompartmental analysis of atorvastatin concentration data are shown in Table 1. Based on the ratio of the LS means, the mean Cmax and the AUC0-∞ were markedly increased 10.6-fold and 7.88-fold, respectively, by the coadministration of telaprevir (Table 1). The mean apparent clearance (CL/F) decreased from 685 liters/h to 83.8 liters/h. The mean (SD) t1/2 decreased from 9.44 (2.64) h to 6.75 (1.55) h with telaprevir coadministration; the difference in t1/2 was not statistically significant.
Fig. 2.
Mean plasma concentration-time profile of atorvastatin following oral administration with and without telaprevir. Error bars represent the standard error of the mean.
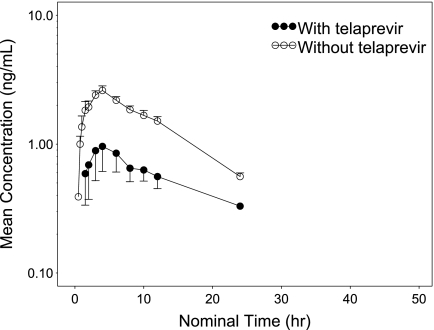
The mean plasma concentration-time profiles of ortho-hydroxy atorvastatin following amlodipine-atorvastatin administration on day 1 and day 17 are shown in Fig. 3. Because a substantial number of sample concentrations for the 240-h sampling interval were close to the LLOQ (both day 1 and day 17), the concentration-versus-time profile of ortho-hydroxy atorvastatin is limited to 24 h postdose. As shown in Table 1, the variability of Cmax and AUC0-∞ on day 17 is quite large. The extrapolated component for AUC0-∞ was over 25% in four volunteers on day 17; furthermore, the AUC calculation was performed using imputed data by replacing the first value below the quantitation limit (BQL) after Clast with half of the LLOQ. Thus, interpretation of these parameters should be done with caution and further interpretation would not be clinically meaningful with limited data. Thus, GLSMRs were not calculated for this analyte.
Fig. 3.
Mean plasma concentration-time profile of ortho-hydroxy-atorvastatin following oral administration with and without telaprevir. Error bars represent the standard error of the mean.
Most of the concentrations of para-hydroxy atorvastatin were below the LLOQ, especially on day 1. Only 2 volunteers showed detectable concentrations of para-hydroxy atorvastatin on day 1. The Cmax of para-hydroxy atorvastatin in these 2 volunteers increased with telaprevir coadministration from 0.30 and 0.54 (mean of 0.42) ng/ml (day 1) to 1.92 and 2.89 (mean of 2.40) ng/ml (day 17).
Steady-state PK of telaprevir after coadministration with amlodipine and atorvastatin.
Selected steady-state pharmacokinetic parameters were calculated for telaprevir on day 17 after coadministration with a single dose of amlodipine-atorvastatin. The mean (SD) Cmax of telaprevir was 3,167 (778) ng/ml, and the mean (SD) AUC0-last of telaprevir was 20,471 (5,317) h · ng/ml. These values are similar to those observed historically (4).
Safety.
There were no serious, life-threatening, or severe adverse events. One volunteer left the study due to an adverse event (herpes zoster) during the telaprevir-alone period. This was considered possibly related to treatment, was mild in severity, and resolved without intervention. With only one exception, all adverse events were considered to be of mild severity. The exception was moderate diarrhea in one volunteer during the telaprevir-amlodipine-atorvastatin combination period.
Thirteen (62%) volunteers reported an adverse event, and 10 (48%) volunteers reported an adverse event that was considered drug related by the investigator. The most frequent adverse events that were considered to be related to the treatment occurred in the nervous system or the gastrointestinal system. The most frequent adverse events (considered either related or unrelated to a study drug by the investigator) included headache (occurred in 5 volunteers [24%] during the amlodipine-atorvastatin-alone period, in 2 volunteers [10%] during the telaprevir-alone period, and in 1 volunteer [5%] during the telaprevir-amlodipine-atorvastatin combination period); dizziness (occurred in 2 volunteers [10%] during the telaprevir-alone period, and in 3 volunteers [15%] during the telaprevir-amlodipine-atorvastatin combination period); diarrhea (occurred in 1 volunteer [5%] during each of the 3 periods); nausea (occurred in 1 volunteer [5%] during the telaprevir-alone period and in 2 volunteers [11%] during the telaprevir-amlodipine-atorvastatin combination period). Rash has been previously reported with telaprevir administration (3). A mild papular rash was reported in a single volunteer (4.8%) on day 24 during the period of coadministration of amlodipine-atorvastatin and telaprevir; the rash resolved without treatment or a change in study drug dosing.
There were no clinically significant changes from the baseline in clinical laboratory values, vital signs, ECG parameters, or physical examination. A creatine kinase level elevation can be associated with increased statin levels (1). However, there were no clinically significant changes in creatine kinase reported as adverse events in any volunteers.
DISCUSSION
Potential drug-drug interactions of telaprevir with amlodipine and atorvastatin were investigated in healthy volunteers by comparing the PK of these drugs with and without the coadministration of telaprevir. A formulation containing a combination of amlodipine and atorvastatin (Caduet) was used in this study for dosing convenience. No significant interaction between amlodipine and atorvastatin was expected.
A clinical drug-drug interaction study of telaprevir and midazolam showed that telaprevir increased the AUC of oral midazolam almost 9-fold (4), indicating that telaprevir is a potent inhibitor of CYP3A4. Amlodipine is a dihydropyridine calcium antagonist drug and has been reported as a substrate and mild inhibitor of CYP3A from both in vitro liver microsomal incubation and clinical studies (9, 13). Atorvastatin, one of the most commonly prescribed HMG-CoA reductase inhibitors, is also a substrate of CYP3A (6). Adverse events such as rhabdomyolysis and myopathy have been reported with statins, and most of the statin drug interactions are attributed to metabolism catalyzed by cytochrome P450 (22). Thus, clinically significant changes in the PK of amlodipine and atorvastatin, administered as Caduet, were anticipated with the coadministration of telaprevir.
Results from the present study indicate that telaprevir significantly inhibited the metabolism of both amlodipine and atorvastatin. The bioavailability of amlodipine has been reported to be ∼60% (14). It is extensively metabolized by the liver and is very slowly cleared from the body (elimination t1/2, ∼45 h). Its volume of distribution is known to be large (∼21 liters/kg), with a high level of binding to the plasma albumin (∼98%) (15). When amlodipine was coadministered with telaprevir, its AUC0-∞ increased 2.79-fold and its Cmax increased 1.27-fold. The mean (SD) t1/2 increased from 41.3 (8.2) h to 95.1 (23.6) h, and the mean (SD) apparent clearance (CL/F) decreased from 38.0 (11.8) liters/h to 12.3 (2.97) liters/h. The increased t1/2 associated with the clearance decrease signifies the inhibitory effect of telaprevir on the metabolism of amlodipine. An effect of a similar magnitude on amlodipine has been observed in other studies with antiviral agents that are CYP3A inhibitors. For example, combined dosing of indinavir and ritonavir increased the median amlodipine AUC0-24 by 90% (n = 18) (5).
The effect of telaprevir on atorvastatin disposition was more pronounced. Atorvastatin is given in the acid form, and its Cmax is achieved quickly (∼1 to 2 h postdose) (10). Atorvastatin acid undergoes extensive first-pass metabolism in the gut and the liver, and therefore its oral bioavailability is only ∼14%. Its volume of distribution has been reported to be ∼380 liters, with a high degree of plasma protein binding, mainly to albumin (98%) (22).
Upon the coadministration of telaprevir with a single dose of amlodipine-atorvastatin, the atorvastatin AUC0-∞ increased 7.88-fold and the atorvastatin Cmax increased 10.6-fold. These results suggest that the primary effect of telaprevir on atorvastatin is to increase its bioavailability (F) by decreasing its first-pass metabolism by CYP3A and/or increasing its net absorption by inhibiting P-glycoprotein-mediated efflux back to the gut. The effect of telaprevir on the hepatic metabolism of atorvastatin does not appear to be significant in its overall disposition. An effect of a similar magnitude on atorvastatin has been observed in studies using some other CYP3A inhibitors. For example, the AUC and Cmax of atorvastatin were increased about 9-fold when it was coadministered with tipranavir-ritonavir at steady state (16).
With coadministration of telaprevir, the mean (SD) apparent clearance decreased from 685 (272) liters/h to 83.8 (32.7) liters/h and the apparent volume of distribution decreased from 8,984 (3,431) liters to 838 (405) liters. The mean (SD) terminal t1/2 decreased from 9.44 (2.64) h to 6.75 (1.55) h with the coadministration of telaprevir, although this difference was not statistically significant. Inhibition of atorvastatin metabolism would be expected to increase the atorvastatin t1/2. This unexpected result may be caused by the inhibition of transporters involved in the hepatic uptake of atorvastatin, such as organic anion-transporting polypeptide 1B1 (OATP1B1), which could reduce the volume of distribution of atorvastatin to an extent similar to or greater than the observed decrease in systemic clearance. Such a mechanism has been hypothesized for the effect of cyclosporine (an inhibitor of OATP1B1) on rosuvastatin, a statin which is also a substrate of OATP1B1 and whose t1/2 was decreased by half upon the coadministration of cyclosporine (19). Other statins that are substrates of OATP1B1, such as cerivastatin, fluvastatin, and atorvastatin, showed unaltered t1/2s, while severalfold AUC and Cmax increases resulted when the drugs were coadministered with cyclosporine (18). However, at this time, the effect of telaprevir on OATP1B1 is unknown.
The mean AUC0-∞ for ortho-hydroxy atorvastatin decreased by approximately 70% after telaprevir coadministration; however, most concentrations of para-hydroxy atorvastatin were below the LLOQ, making noncompartmental analysis for this metabolite not feasible. In the 2 volunteers in whom para-hydroxy atorvastatin was measurable before (day 1) and after (day 17) the coadministration of telaprevir, the concentrations of this metabolite increased about 6-fold. On day 17, but not day 1, several other volunteers had measurable concentrations of para-hydroxy atorvastatin. While these results were not anticipated, it has been reported that para-hydroxy atorvastatin, but not ortho-hydroxy atorvastatin, is also formed by CYP2C8 in addition to CYP3A (7). Therefore, in the presence of greater systemic exposure of atorvastatin during the coadministration of telaprevir, it is plausible that more para-hydroxy atorvastatin is formed via CYP2C8 and its concentration in plasma is increased.
The PK of telaprevir were evaluated after coadministration at steady state with a single dose of amlodipine-atorvastatin. The Cmax and AUClast were similar to the steady-state estimates obtained in other studies (21). This suggests that adequate telaprevir exposure was achieved in this study and a clinically significant effect of amlodipine or atorvastatin on telaprevir is unlikely.
The coadministration of multiple doses of telaprevir with one dose of amlodipine and atorvastatin administered in 2 periods was well tolerated. There were no serious, life-threatening, or severe adverse events, no volunteers discontinued due to an adverse event, and most of the adverse events reported were mild. The frequently reported adverse events included, headache, dizziness, diarrhea, and nausea, all of which have been reported in other clinical trials after the administration of telaprevir alone (8, 17, 23). The low frequency and/or lack of adverse events commonly associated with amlodipine and atorvastatin may be attributed to the single-dose regimen of these drugs used in this study.
In summary, the results of this study suggest that telaprevir significantly increased exposure to amlodipine and atorvastatin (Cmax and AUC0-∞). Atorvastatin coadministration with telaprevir is contraindicated. When amlodipine is coadministered with telaprevir, caution should be used and an amlodipine dose reduction should be considered. Clinical monitoring is recommended. Please check the INCIVEK package insert for full information and/or updates (3).
ACKNOWLEDGMENTS
We acknowledge the contribution of J. H. Frank Farmer, Jr., of the Covance Clinical Research Unit (Daytona Beach, FL), who was contracted to serve as an investigator in this study. Additionally, we acknowledge the assistance of the following Vertex Pharmaceuticals Incorporated employees and stockholders: Mahlet Woldermarian for study coordination and support, Megan Melch and Lakshmi Viswanathan for assistance in the preparation of the manuscript, Kristin Stephan for editorial coordination and support, and Jonathan Kirk for graphic design support.
This study was supported by Vertex Pharmaceuticals Incorporated and Tibotec BVBA.
We were all employed by either Vertex (V.G., J.E.L., K.A., F.S., and R.K.) or Tibotec (R.V.) at the time of this study and own stock in these companies.
J.E.L. is currently employed by the U.S. Food and Drug Administration. Her contribution to the manuscript was based on her prior employment, and the content of the work does not necessarily reflect any position of the U.S. Food and Drug Administration.
Footnotes
Published ahead of print on 8 August 2011.
REFERENCES
- 1. Anonymous. 2011. Caduet package insert. Pfizer Incorporated, New York, NY: http://labeling.pfizer.com/ShowLabeling.aspx?id=531 [Google Scholar]
- 2. Anonymous. 2006. FDA draft guidance on drug interaction studies: study design, data analysis, and implications for dosing and labeling. Food and Drug Administration, Washington, DC [Google Scholar]
- 3. Anonymous. 2011. INCIVEK package insert. Vertex Pharmaceuticals Incorporated, Cambridge, MA: http://pi.vrtx.com/files/uspi_telaprevir.pdf [Google Scholar]
- 4. Garg V., Chandorkar G., Smith F., Alves K., van Heeswijk R. 2011. The effect of telaprevir on the pharmacokinetics of midazolam and digoxin. 6th International Workshop on Clinical Pharmacology of Hepatitis Therapy, 22–23 June 2011, Boston, MA http://regist2.virology-education.com/2011/6HEPPK/docs/08_Garg.pdf [DOI] [PubMed] [Google Scholar]
- 5. Glesby M. J., et al. 2005. Pharmacokinetic interactions between indinavir plus ritonavir and calcium channel blockers. Clin. Pharmacol. Ther. 78:143–153 [DOI] [PubMed] [Google Scholar]
- 6. Hermann M., et al. 2006. Exposure of atorvastatin is unchanged but lactone and acid metabolites are increased several-fold in volunteers with atorvastatin-induced myopathy. Clin. Pharmacol. Ther. 79:532–539 [DOI] [PubMed] [Google Scholar]
- 7. Jacobsen W., et al. 2000. Lactonization is the critical first step in the disposition of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitor atorvastatin. Drug Metab. Dispos. 28:1369–1378 [PubMed] [Google Scholar]
- 8. Jacobson I. M., et al. 2011. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364:2405–2416 [DOI] [PubMed] [Google Scholar]
- 9. Josefsson M., Zackrisson A. L., Ahlner J. 1996. Effect of grapefruit juice on the pharmacokinetics of amlodipine in healthy volunteers. Eur. J. Clin. Pharmacol. 51:189–193 [DOI] [PubMed] [Google Scholar]
- 10. Lennernäs H. 2003. Clinical pharmacokinetics of atorvastatin. Clin. Pharmacokinet. 42:1141–1160 [DOI] [PubMed] [Google Scholar]
- 11. Lin C., Kwong A. D., Perni R. B. 2006. Discovery and development of VX-950, a novel, covalent, and reversible inhibitor of hepatitis C virus NS3.4A serine protease. Infect. Disord. Drug Targets 6:3–16 [DOI] [PubMed] [Google Scholar]
- 12. Lins R. L., et al. 2003. Pharmacokinetics of atorvastatin and its metabolites after single and multiple dosing in hypercholesterolaemic haemodialysis patients. Nephrol. Dial. Transplant. 18:967–976 [DOI] [PubMed] [Google Scholar]
- 13. McGregor D. O., Bailey R. R., Robson R. A. 1997. Amlodipine has a minor effect on cyclosporine metabolism. Clin. Nephrol. 48:336. [PubMed] [Google Scholar]
- 14. Meredith P. A., Elliott H. L. 1992. Clinical pharmacokinetics of amlodipine. Clin. Pharmacokinet. 22:22–31 [DOI] [PubMed] [Google Scholar]
- 15. Murdoch D., Heel R. C. 1991. Amlodipine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cardiovascular disease. Drugs 41:478–505 [DOI] [PubMed] [Google Scholar]
- 16. Pham P. A., et al. 2009. Differential effects of tipranavir plus ritonavir on atorvastatin or rosuvastatin pharmacokinetics in healthy volunteers. Antimicrob. Agents Chemother. 53:4385–4392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sherman K. E., et al. 2010. Telaprevir in combination with peginterferon alfa-2a and ribavirin for 24 or 48 weeks in treatment-naïve genotype 1 HCV volunteers who achieved an extended rapid viral response: final results of phase 3 ILLUMINATE study. 61st Annu. Meet. AASLD, Boston, Ma, 30 October-3 November 2010 http://www.natap.org/2010/AASLD/AASLD_21.htm [Google Scholar]
- 18. Shitara Y. 2011. Clinical importance of OATP1B1 and OATP1B3 in drug-drug interactions. Drug Metab. Pharmacokinet. 26:220–227 [DOI] [PubMed] [Google Scholar]
- 19. Simonson S. G., et al. 2004. Rosuvastatin pharmacokinetics in heart transplant recipient administered and antirejection regimen including cyclosporine. Clin. Pharmacol. Ther. 76:167–177 [DOI] [PubMed] [Google Scholar]
- 20. Stopher D. A., Beresford A. P., Macrae P. V., Humphrey M. J. 1988. The metabolism and pharmacokinetics of amlodipine in humans and animals. J. Cardiovasc. Pharmacol. 12(Suppl. 7):S55–S59 [DOI] [PubMed] [Google Scholar]
- 21. van Heeswijk R., et al. 2008. The pharmacokinetic interaction between tenofovir disoproxil fumarate and the investigational HCV protease inhibitor telaprevir, abstr. A-966. 48th ICAAC, Washington, DC, 25–28 October 2008 [Google Scholar]
- 22. Williams D. 2002. Pharmacokinetic-pharmacodynamic drug interactions with HMG-CoA reductase inhibitors. Clin. Pharmacokinet. 41:343–370 [DOI] [PubMed] [Google Scholar]
- 23. Zeuzem S., et al. 2011. Telaprevir for retreatment of HCV infection. N. Engl. J. Med. 364:2417–2428 [DOI] [PubMed] [Google Scholar]