Abstract
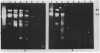
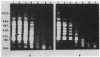
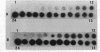
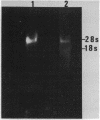
One of the changes accompanying skeletal muscle cell (myoblast) fusion is a dramatic increase in synthesis of muscle specific proteins, one of which is myosin. The underlying mechanism for this burst in synthesis is not yet understood but may occur by two mechanisms: (a) gradual storage of mRNA and translational control as found by others or (b) gene activation and rapid synthesis of mRNA for immediate translation. In this paper we show that the myosin gene changes its organization such that postfusion skeletal muscle cells show an increased susceptibility to DNase I, a recognized probe for gene activation. We also show that this change accompanies an increase in rate of transcription and an increased cell content of myosin heavy chain mRNA. This work shows that transcriptional control is an important mechanism during muscle cell development in addition to the translational control shown by other workers.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affara N. A., Daubas P., Weydert A., Gros F. Changes in gene expression during myogenic differentiation. II. Identification of the proteins encoded by myotube-specific complementary DNA sequences. J Mol Biol. 1980 Jul 15;140(4):459–470. doi: 10.1016/0022-2836(80)90265-x. [DOI] [PubMed] [Google Scholar]
- Affara N. A., Robert B., Jacquet M., Buckingham M. E., Gros F. Changes in gene expression during myogenic differentiation. I. Regulation of messenger RNA sequences expressed during myotube formation. J Mol Biol. 1980 Jul 15;140(4):441–458. doi: 10.1016/0022-2836(80)90264-8. [DOI] [PubMed] [Google Scholar]
- Bellard M., Kuo M. T., Dretzen G., Chambon P. Differential nuclease sensitivity of the ovalbumin and beta-globin chromatin regions in erythrocytes and oviduct cells of laying hen. Nucleic Acids Res. 1980 Jun 25;8(12):2737–2750. doi: 10.1093/nar/8.12.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. Most myosin heavy chain mRNA in L6E9 rat myotubes has a short poly(A) tail. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1853–1857. doi: 10.1073/pnas.76.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmon Y., Czosnek H., Nudel U., Shani M., Yaffe D. DNAase I sensitivity of genes expressed during myogenesis. Nucleic Acids Res. 1982 May 25;10(10):3085–3098. doi: 10.1093/nar/10.10.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Dym H., Turner D. C., Eppenberger H. M., Yaffe D. Creatine kinase isoenzyme transition in actinomycin D-treated differentiating muscle cultures. Exp Cell Res. 1978 Apr;113(1):15–21. doi: 10.1016/0014-4827(78)90082-4. [DOI] [PubMed] [Google Scholar]
- Emerson C. P., Jr, Beckner S. K. Activation of myosin synthesis in fusing and mononucleated myoblasts. J Mol Biol. 1975 Apr 25;93(4):431–447. doi: 10.1016/0022-2836(75)90238-7. [DOI] [PubMed] [Google Scholar]
- Ginder G. D., Wood W. I., Felsenfeld G. Isolation and characterization of recombinant clones containing the chicken adult beta-globin gene. J Biol Chem. 1979 Sep 10;254(17):8099–8102. [PubMed] [Google Scholar]
- Groudine M., Weintraub H. Activation of globin genes during chicken development. Cell. 1981 May;24(2):393–401. doi: 10.1016/0092-8674(81)90329-9. [DOI] [PubMed] [Google Scholar]
- Haigh L. S., Owens B. B., Hellewell O. S., Ingram V. M. DNA methylation in chicken alpha-globin gene expression. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5332–5336. doi: 10.1073/pnas.79.17.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaranis A. S., Heywood S. M. Cytoplasmic utilization of liposome-encapsulated myosin heavy chain messenger ribonucleoprotein particles. During muscle cell differentiation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6898–6902. doi: 10.1073/pnas.78.11.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M., Thibault M. C., Siegel E. Control of gene expression in muscle development. Cell Muscle Motil. 1983;3:157–193. doi: 10.1007/978-1-4615-9296-9_6. [DOI] [PubMed] [Google Scholar]
- Hunter T., Garrels J. I. Characterization of the mRNAs for alpha-, beta- and gamma-actin. Cell. 1977 Nov;12(3):767–781. doi: 10.1016/0092-8674(77)90276-8. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Flavell R. A. A physical map of the DNA regions flanking the rabbit beta-globin gene. Cell. 1977 Oct;12(2):429–439. doi: 10.1016/0092-8674(77)90119-2. [DOI] [PubMed] [Google Scholar]
- Koffer A., Brownson C. Fractionation of nuclear proteins from red and white skeletal muscle, heart and liver of rabbit. Int J Biochem. 1979;10(10):845–857. doi: 10.1016/0020-711x(79)90058-2. [DOI] [PubMed] [Google Scholar]
- Kuo M. T., Mandel J. L., Chambon P. DNA methylation: correlation with DNase I sensitivity of chicken ovalbumin and conalbumin chromatin. Nucleic Acids Res. 1979 Dec 20;7(8):2105–2113. doi: 10.1093/nar/7.8.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Wood W. I., Dolan M., Engel J. D., Felsenfeld G. A 200 base pair region at the 5' end of the chicken adult beta-globin gene is accessible to nuclease digestion. Cell. 1981 Nov;27(1 Pt 2):45–55. doi: 10.1016/0092-8674(81)90359-7. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford R. M., Wydro R. M., Nguyen H. T., Nadal-Ginard B. Cytoplasmic processing of myosin heavy chain messenger RNA: evidence provided by using a recombinant DNA plasmid. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5749–5753. doi: 10.1073/pnas.77.10.5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Sobel A., Changeux J. P., Gros F. Synthesis of acetylcholine receptor during differentiation of cultured embryonic muscle cells. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4028–4032. doi: 10.1073/pnas.72.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Many T., Cedar H. Active gene sequences are undermethylated. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4246–4250. doi: 10.1073/pnas.78.7.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen H. T., Gubits R. M., Wydro R. M., Nadal-Ginard B. Sarcomeric myosin heavy chain is coded by a highly conserved multigene family. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5230–5234. doi: 10.1073/pnas.79.17.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas R. H., Wright C. A., Cockerill P. N., Wyke J. A., Goodwin G. H. The nuclease sensitivity of active genes. Nucleic Acids Res. 1983 Feb 11;11(3):753–772. doi: 10.1093/nar/11.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Shani M., Zevin-Sonkin D., Saxel O., Carmon Y., Katcoff D., Nudel U., Yaffe D. The correlation between the synthesis of skeletal muscle actin, myosin heavy chain, and myosin light chain and the accumulation of corresponding mRNA sequences during myogenesis. Dev Biol. 1981 Sep;86(2):483–492. doi: 10.1016/0012-1606(81)90206-2. [DOI] [PubMed] [Google Scholar]
- Shen C. K., Maniatis T. Tissue-specific DNA methylation in a cluster of rabbit beta-like globin genes. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6634–6638. doi: 10.1073/pnas.77.11.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stalder J., Larsen A., Engel J. D., Dolan M., Groudine M., Weintraub H. Tissue-specific DNA cleavages in the globin chromatin domain introduced by DNAase I. Cell. 1980 Jun;20(2):451–460. doi: 10.1016/0092-8674(80)90631-5. [DOI] [PubMed] [Google Scholar]
- Tolstoshev P., Berg R. A., Rennard S. I., Bradley K. H., Trapnell B. C., Crystal R. G. Procollagen production and procollagen messenger RNA levels and activity in human lung fibroblasts during periods of rapid and stationary growth. J Biol Chem. 1981 Mar 25;256(6):3135–3140. [PubMed] [Google Scholar]
- Umeda P. K., Sinha A. M., Jakovcic S., Merten S., Hsu H. J., Subramanian K. N., Zak R., Rabinowitz M. Molecular cloning of two fast myosin heavy chain cDNAs from chicken embryo skeletal muscle. Proc Natl Acad Sci U S A. 1981 May;78(5):2843–2847. doi: 10.1073/pnas.78.5.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardimon L., Neumann R., Kuhlmann I., Sutter D., Doerfler W. DNA methylation and viral gene expression in adenovirus-transformed and -infected cells. Nucleic Acids Res. 1980 Jun 11;8(11):2461–2473. doi: 10.1093/nar/8.11.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasylyk B., Chambon P. Transcription by eukaryotic RNA polymerases A and B of chromatin assembled in vitro. Eur J Biochem. 1979 Aug 1;98(2):317–327. doi: 10.1111/j.1432-1033.1979.tb13191.x. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Sell S. M. Myosin from fetal hearts contains the skeletal muscle embryonic light chain. Nature. 1980 Aug 14;286(5774):731–733. doi: 10.1038/286731a0. [DOI] [PubMed] [Google Scholar]
- Widmer H. J., Jaggi R. B., Weber R., Ryffel G. U. Enrichment and characterization of the DNA coding for vitellogenin in Xenopus laevis. Eur J Biochem. 1979 Aug 15;99(1):23–29. doi: 10.1111/j.1432-1033.1979.tb13226.x. [DOI] [PubMed] [Google Scholar]
- Wu C., Wong Y. C., Elgin S. C. The chromatin structure of specific genes: II. Disruption of chromatin structure during gene activity. Cell. 1979 Apr;16(4):807–814. doi: 10.1016/0092-8674(79)90096-5. [DOI] [PubMed] [Google Scholar]
- Wydro R. M., Nguyen H. T., Gubits R. M., Nadal-Ginard B. Characterization of sarcomeric myosin heavy chain genes. J Biol Chem. 1983 Jan 10;258(1):670–678. [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen D. R., Boyd M. C.D., Fitschen W., von Holt C. DNA-Dependent RNA polymerase in maturing avian erythrocytes. FEBS Lett. 1973 Mar 1;30(2):195–198. doi: 10.1016/0014-5793(73)80650-7. [DOI] [PubMed] [Google Scholar]