Abstract
The virological response after an 8-day maraviroc monotherapy has been proposed to be an alternative method to determine whether an CCR5 antagonist should be prescribed to HIV-infected patients. The frequency of patients eligible for a combined antiretroviral therapy which includes maraviroc on the basis of the result of this clinical test is not well-known at the moment. In the same way, clinical and immunovirological factors associated with the virological response after antagonist exposure need to be determined. Ninety consecutive HIV-infected patients were exposed to an 8-day maraviroc monotherapy. The virological response was considered positive if either a reduction of ≥1-log10 HIV RNA copies/ml or an undetectable viral load (<40 HIV RNA copies/ml) was achieved. CXCR4- and CCR5-tropic virus levels were determined by using patients' viral isolates and multiple rounds of infection of indicator cell lines (U87-CXCR4 and U87-CCR5). The frequency of patients with a positive virological response was 72.2% (94.7% and 66.2% for treatment-naïve and pretreated patients, respectively). The positive response rates dramatically decreased in patients with lower CD4+ T-cell counts. The CXCR4-tropic virus level was the only variable independently associated with the virological response after short-term maraviroc exposure. Lower CD4+ T-cell strata were associated with higher CXCR4-tropic virus levels. These results support the suggestion that CCR5 antagonists should be an early treatment option before the expansion of CXCR4-tropic strains.
INTRODUCTION
To gain entry into cells, HIV uses the CCR5 (R5) and/or CXCR4 (X4) coreceptor as well as the CD4 receptor (1). The selective blocking of R5 by the first commercialized coreceptor antagonist, maraviroc (MRV) (4), makes the determination of HIV tropism essential before this drug is prescribed to HIV-infected patients (10). To date, the most widely used coreceptor tropism tests are the recombinant phenotypic Trofile assay (24) and its later version, the enhanced-sensitivity Trofile assay (ESTA) (25).
However, the Trofile assay has some limitations, such as the fact that it requires samples with more than 1,000 HIV RNA copies/ml, the fact that about 20% of the results are nonreportable mainly due to low viral loads, and the reproducibility of this method, which has been described in different studies (12, 20). Other methods, such as genotypic tropism tests (2, 17, 18), have been proposed to be alternatives to the commercialized phenotypic method; however, the main caveats of these tests are the low sensitivity to detect dual/mixed (DM)/X4-tropic viruses (14). Due to these limitations, a short-term exposure to MRV has been proposed to be a method to assay the sensitivity to R5 antagonists (maraviroc clinical test [MCT]) (7). This method has the advantage that the outcome is real-time evidence of drug sensitivity and not a tropism result. However, the frequency of subjects with a virological response after short-term MRV exposure is not well-known at the moment. This frequency has been described in published studies with only smaller sets of patients (7, 9) and is unknown in treatment-naïve patients. On the other hand, it would be interesting to know the factors that are associated with the virological response experienced after a short-term MRV exposure. In this sense, regarding Trofile, CD4 T-cell levels have been independently associated with the DM/X4-tropic result for subjects not eligible for R5 antagonist treatment (16). However, the clinical and immunovirological factors that are associated with the virological response after MCT are unknown.
Thus, the aims of this work were to analyze the frequency of subjects eligible for R5 antagonist treatment on the basis of the MCT result and study the clinical and immunovirological factors associated with the virological response after MCT.
MATERIALS AND METHODS
Patients and intervention.
This study was conducted in the Infectious Diseases Service at Virgen del Rocío University Hospital and the Biomedicine Institute of Seville (IBiS) (Seville, Spain). Ninety consecutive HIV-infected patients who underwent MCT from July 2008 until March 2011 were included in the present study. These patients had a median age of 42 years (age range, 36 to 46 years) and had persistently detectable plasma viral loads (>40 HIV RNA copies/ml), and all of them were asymptomatic at the time of the study. Samples from patients were kindly provided by the HIV BioBank integrated within the Spanish AIDS Research Network (RIS). Patients or legal guardians for those subjects under 18 years old had given written informed consent, and the Ethical Committee of the hospital approved the study. MCT has been previously described (7). Briefly, patients were exposed to an 8-day MRV monotherapy, and the subsequent virological response was analyzed. MCT was considered positive if a significant viral load reduction, defined as a reduction of ≥1 log10 HIV RNA copies/ml, or an undetectable viral load (<40 HIV RNA copies/ml) was achieved on day 8 after addition of MRV. Once the MCT result was obtained, a new highly active antiretroviral therapy (HAART) regimen was started according to the following criteria: (i) previous genotype resistance testing results, (ii) previous antiretroviral exposure, and (iii) response to MCT to determine whether or not to include MRV in the new HAART.
Methods. (i) X4- and R5-tropic virus level determination.
In a subgroup of these consecutive patients (n = 57), the phenotypic tropism test TROCAI (tropism coreceptor assay information) was performed at baseline as previously reported (9). Briefly, TROCAI is based on the production of viral isolates from patients through a coculture and multiple rounds of infection of U87-X4 and U87-R5 indicator cell lines. For the purpose of this study, results were expressed as log viral load (VL) in the U87-X4 and U87-R5 well supernatants (logX4VL and logR5VL, respectively).
(ii) Viral load quantification.
HIV-1 RNA was measured in patients' fresh plasma and in frozen samples of U87-X4 and U87-R5 cell-free supernatants by quantitative PCR (Cobas Ampliprep/Cobas TaqMan HIV-1 test; Roche Molecular Systems, Basel, Switzerland) according to the manufacturer's instructions. The lower detection limit was 40 HIV-1 RNA copies/ml. Plasma samples were tested for anti-hepatitis C virus (anti-HCV) antibodies using an HCV enzyme-linked immunosorbent assay (Siemens Healthcare Diagnosis). A qualitative PCR amplification was performed for plasma HCV RNA amplification (Cobas Amplicor; Roche Diagnostics, Barcelona, Spain), and the lower detection limit was 15 IU/ml.
(iii) CD4 T-cell quantification.
CD4 T-cell counts in fresh whole blood were determined using an Epics XL-MCL flow cytometer (Beckman-Coulter Inc., CA) according to the manufacturer's instructions. Fresh whole blood was stained with directly conjugated monoclonal antibodies, anti-CD3–phycoerythrin (PE) and anti-CD4–fluorescein isothiocyanate (FITC) (BD Bioscience).
(iv) Statistical analysis.
Statistical analyses were performed using the Statistical Package for the Social Sciences software (version 17.0; SPSS, Inc., Chicago, IL). Median and interquartile ranges were used to describe continuous variables and a percentage was used for categorical ones. Pearson's test was used to analyze correlations between variables. Differences between groups were analyzed with the Mann-Whitney U test and a bivariate logistic or linear regression analysis when appropriate. To analyze the independent factors associated with the MCT response or VL changes after MCT, a multivariate logistic or linear regression analysis, respectively, was performed with variables showing P values of <0.1 in the bivariate analysis. Variables showing P values of <0.05 were considered statistically significant.
RESULTS
Frequency of virological response after MCT.
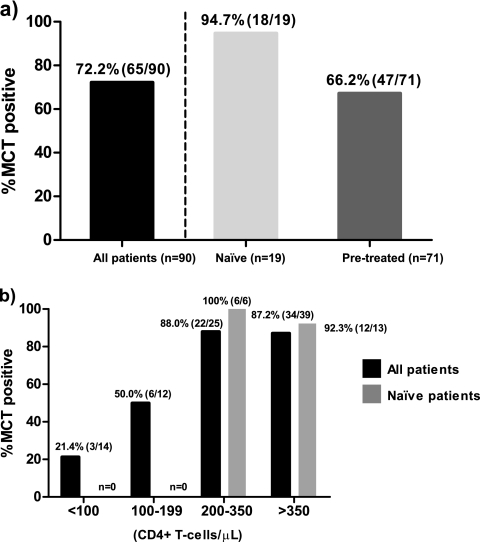
The patients' characteristics at baseline are shown in Table 1. In terms of antiretroviral treatment, 21% of the patients were treatment naïve. After MRV exposure, 72.2% of the all patients experienced a virological response (MCT-positive subjects), which meant that about three-quarters of the patients could benefit from an antiretroviral treatment that includes MRV (Fig. 1a). However, we observed different responses in pretreated and treatment-naïve patients. When we split the populations in these two categories, we observed that almost all treatment-naïve patients were MCT positive (94.7%), whereas only 66.2% of pretreated patients were MCT positive (Fig. 1a). Interestingly, when the virological response was analyzed depending on different baseline CD4+ T-cell strata, higher CD4+ T-cell levels were associated with higher percentages of MCT-positive subjects. There was a dramatic decrease in MCT-positive subjects when CD4+ T-cell levels were below 200 CD4+ T cells/μl (Fig. 1b). Thus, the high percentage of MCT-positive treatment-naïve patients could be associated with higher baseline CD4+ T-cell levels in this group (Fig. 1b).
Table 1.
Baseline characteristics of the patients
| Characteristic | Valuea |
|---|---|
| No. (%) male sex | 71 (79) |
| Age (yr) | 42 (36–46) |
| No. (%) of subjects with the | |
| following characteristics: | |
| Sexual transmission | 47 (52) |
| IDUb transmission | 35 (39) |
| No. (%) of subjects naïve for treatment | 19 (21) |
| Time since diagnosis (yr) | 15 (3–19) |
| No. (%) of subjects HCV PCR+c | 31 (34) |
| CD4+ count (cells/ml) | 309 (175–459) |
| Plasma VL (log HIV RNA copies/ml) | 4.5 (3.7–5.0) |
Data are for 90 patients. Values other than number (percent) are expressed as medians (interquartile ranges).
IDU, injecting drug user.
PCR+, PCR positive for hepatitis C virus.
Fig. 1.
Frequency of virological response after MCT. (a) Percentage of subjects with a virological response after a short-term MRV exposure (MCT positive). Black bar, percentage of MCT-positive subjects in the total population. The population was also split into pretreated and treatment-naïve subjects (gray bars). (b) Percentage of MCT-positive subjects depending on different baseline CD4+ T-cell strata. Black bars, total number of subjects; gray bars, treatment-naïve subjects. We did not observe any treatment-naïve subjects in the lower CD4+ T-cell strata (<100 and 100 to 199 cells/μl).
Factors associated with the virological response after MCT.
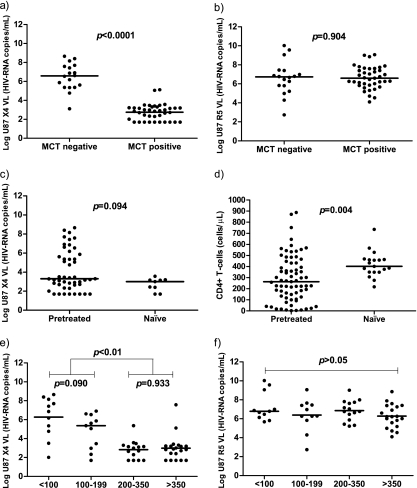
Our aim was to determine which factors were associated with the virological response after MCT. We observed how individuals with no virological response (MCT-negative subjects) differed from the MCT-positive subjects according to several variables; they were mostly pretreated and had been diagnosed for a longer period of time, and the risk of transmission was mainly illicit drug use. According to the results mentioned above, MCT-negative subjects showed almost 3-fold lower CD4+ T-cell levels (Table 2). In fact, when we adjusted for all these variables, the CD4+ T-cell level was the only variable independently associated with a virological response after MCT (Table 2). Interestingly, in a subgroup of these patients (n = 57), we analyzed X4- and R5-tropic virus levels. We expressed the results as the amount of X4/DM-tropic (from here on, X4-tropic) and R5-tropic viruses produced after infection of indicator cell lines that express X4 or R5 by the patients' viral isolates. We observed that the risk of transmission, time since diagnosis, and CD4+ T-cell levels were associated with the virological response after MCT. In addition, the amount of X4-tropic virus was also strongly associated with the virological response after MCT. In this case, however, when all these variables were adjusted, the amount of X4-tropic virus was the only variable independently associated with the virological response after MCT (Table 3). Of note, despite the large differences in X4-tropic virus levels observed, the R5-tropic virus levels were extremely similar between the two groups (Fig. 2a and b). Remarkably, when patients were grouped on the basis of treatment category, the amount of X4-tropic viruses tended to be lower in treatment-naïve patients than pretreated patients (Fig. 2c). This trend was similar regarding R5-tropic virus levels (data not shown). Importantly, we also observed higher CD4+ T-cell levels in treatment-naïve than pretreated patients (Fig. 2d). In addition, when patients were divided according to CD4+ T-cell strata, a dramatic increase in X4-tropic virus levels was observed in patients with CD4+ T-cell levels lower than 200 cells/μl, and again, there were no differences in R5-tropic virus levels on the basis of different CD4+ T-cell levels (Fig. 2e and f).
Table 2.
Factors associated with the virological response after MCT in all the study subjectsa
| Characteristic | Value of characteristic by treatment responseb |
Bivariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| MCT positive (n = 65) | MCT negative (n = 25) | P | OR (95% CI)c | P | OR (95% CI) | |
| No. of males/total no. (%) | 50/65 (77) | 21/25 (84) | 0.464 | 0.635 (0.188–2.140) | NAd | |
| Age (yr) | 42 (35–46) | 43 (37–47) | 0.677 | 1.009 (0.967–1.054) | NA | |
| No. treatment naïve/total no. (%) | 18/65 (28) | 1/25 (4) | 0.036 | 9.191 (1.157–73.048) | 0.283 | 3.857 (0.328–45.364) |
| No. infected by sexual transmission/total no. (%) | 40/65 (62) | 7/25 (28) | 0.006 | 4.114 (1.505–11.250) | 0.406 | 1.767 (0.462–6.765) |
| No. infected by IDUe transmission/total no. (%) | 22/65 (34) | 13/25 (52) | 0.117 | 0.472 (0.185–1.206) | NA | |
| Time since diagnosis (yr) | 12 (2–19) | 16 (12–19) | 0.068 | 0.944 (0.888–1.004) | 0.890 | 1.007 (0.911–1.114) |
| No. HCV PCR+f/total no. (%) | 22/65 (34) | 9/25 (36) | 0.847 | 0.910 (0.347–2.387) | NA | |
| CD4+ count (cells/ml) | 355 (226–465) | 149 (21–320) | 0.001 | 1.006 (1.002–1.009) | 0.011 | 1.004 (1.001–1.008) |
| Plasma log VL (HIV RNA copies/ml) | 4.4 (3.7–4.9) | 4.6 (3.5–5.2) | 0.557 | 0.861 (0.521–1.421) | NA | |
Data are for 90 subjects. Bivariate and multivariate analyses were performed using a logistic regression model, and variables showing a P value of <0.1 (boldface) in the bivariate analysis were included in the multivariate analysis. In the multivariate analysis, variables with a P value of <0.05 (boldface) were considered statistically significant.
Values other than number (percent) are expressed as medians (interquartile ranges).
OR, odds ratio; CI, confidence interval.
NA, not applicable.
IDU, injecting drug user.
PCR+, PCR positive for hepatitis C virus.
Table 3.
Factors associated with the virological response after MCTa
| Characteristic | Value of characteristic by treatment responseb |
Bivariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|---|---|
| MCT positive (n = 39) | MCT negative (n = 18) | P | OR (95% CI)c | P | OR (95% CI) | |
| No. male/total no. (%) | 31/39 (80) | 15/18 (83) | 0.733 | 0.775 (0.179–3.348) | NAd | |
| Age (yr) | 42 (36–45) | 41 (27–46) | 0.500 | 1.017 (0.969–1.067) | NA | |
| No. treatment naïve/total no. (%) | 9/39 (23) | 0/18 (0) | NDe | NA | ||
| No. infected by sexual transmission/total no. (%) | 22/39 (56) | 4/18 (22) | 0.021 | 4.529 (1.261–16.271) | 0.221 | 19.832 (0.165–2383.7) |
| No. infected by IDUf transmission/total no. (%) | 14/39 (36) | 9/18 (50) | 0.315 | 0.560 (0.181–1.737) | NA | |
| Time since diagnosis (yr) | 13 (2–19) | 17 (12–18) | 0.081 | 0.929 (0.855–1.009) | 0.428 | 1.142 (0.822–1.587) |
| No. HCV PCR+g/total no. (%) | 11/39 (28) | 6/18 (33) | 0.694 | 0.786 (0.236–2.616) | NA | |
| CD4+ counts (cells/ml) | 349 (223–432) | 105 (14–181) | <0.0001 | 1.010 (1.005–1.015) | 0.920 | 1.001 (0.989–1.012) |
| Plasma log VL (HIV RNA copies/ml) | 4.6 (4.2–5.0) | 4.9 (3.7–5.2) | 0.626 | 0.840 (0.415–1.696) | NA | |
| LogX4VLh (HIV RNA copies/ml) | 2.8 (1.7–3.2) | 6.6 (5.4–7.6) | <0.0001 | 0.115 (0.035–0.374) | 0.007 | 0.096 (0.017–0.535) |
| LogR5VLi (HIV RNA copies/ml) | 6.6 (5.9–7.6) | 6.7 (5.7–7.4) | 0.939 | 1.016 (0.675–1.529) | NA | |
In this analysis, only patients with X4- and R5-tropic virus levels available were included (n = 57). Bivariate and multivariate analyses were performed using a logistic regression model, and variables showing a P value of <0.1 (boldface) in the bivariate analysis were included in the multivariate analysis. In the multivariate analysis, variables with a P value of <0.05 (boldface) were considered statistically significant.
Values other than number (percent) are expressed as medians (interquartile ranges).
OR, odds ratio; CI, confidence interval.
NA, not applicable.
ND, not determined; this contrast was not possible due to the low number of patients.
IDU, injecting drug user.
PCR+, PCR positive for hepatitis C virus.
Number of HIV RNA copies/ml in the U87-CXCR4 cell line well supernatant.
Number of HIV RNA copies/ml in the U87-CCR5 cell line well supernatant.
Fig. 2.
X4- and R5-tropic virus levels and CD4+ T-cell levels depending on treatment category. Log viral loads in the U87-X4 and U87-R5 well supernatants were used to express X4- and R5-tropic virus levels. (a) MCT-positive patients (n = 39) presented significantly lower X4-tropic virus levels than MCT-negative patients (n = 18); (b) R5-tropic virus levels were similar in patients with different responses after MCT; (c) treatment-naïve patients (n = 9) presented a trend toward lower X4-tropic virus levels than pretreated patients (n = 48); (d) in addition, treatment-naïve patients (n = 19) showed higher CD4+ T-cell levels than pretreated patients (n = 71); (e) patients with the lower CD4+ T-cell strata (<100 CD4+ T cells/μl [n = 11] and 100 to 199 CD4+ T cells/μl [n = 11]) presented significantly higher X4-tropic virus levels than patients with higher CD4+ T-cell strata (200 to 350 CD4+ T cells/μl [n = 15] and >350 CD4+ T cells/μl [n = 20]); (f) on the contrary, R5-tropic virus levels were independent of the CD4+ T-cell strata. Black bars, median. The Mann-Whitney U test was used.
The MCT result is categorical (positive or negative), but this result can be transformed into a continuous variable that analyzes the viral load changes after 8 days of drug exposure. We verified how, using viral load changes (six subjects with <1,000 HIV RNA copies/ml were excluded from the analysis; n = 51), sexual transmission, CD4+ T-cell counts, and X4-tropic virus levels were associated with the viral load changes experienced after the clinical test. In the same way, the amount of X4-tropic virus was the only variable independently associated with the viral load changes after MCT (Table 4).
Table 4.
Factors associated with viral load changes after MCTa
| Characteristic | Changes in VL after MCT |
|||
|---|---|---|---|---|
| Bivariate analysis |
Multivariate analysis |
|||
| P | B (95% CIb) | P | B (95% CI) | |
| Male sex | 0.698 | −0.118 (−0.727 to 0.491) | NAc | |
| Age | 0.961 | 0.000 (−0.019 to 0.018) | NA | |
| Treatment naïve | 0.297 | 0.302 (−0.273 to 0.877) | NA | |
| Sexual transmission | 0.022 | 0.469 (0.042 to 0.897) | 0.672 | 0.077 (−0.286 to 0.440) |
| IDUd transmission | 0.205 | −0.289 (−0.742 to 0.164) | NA | |
| Time since diagnosis | 0.169 | −0.021 (−0.050 to 0.009) | NA | |
| HCV PCR+e | 0.243 | −0.289 (−0.779 to 0.202) | NA | |
| CD4+ counts (cells/ml) | 0.001 | 0.002 (0.001 to 0.003) | 0.346 | 0.001 (−0.001 to 0.002) |
| Plasma log VL | 0.523 | −0.120 (−0.497 to 0.256) | NA | |
| LogX4VLf (HIV RNA Copies/ml) | <0.0001 | −0.263 (−0.338 to −0.187) | <0.0001 | −0.234 (−0.322 to −0.146) |
| LogR5VLg (HIV RNA Copies/ml) | 0.227 | −0.089 (−0.253 to 0.074) | NA | |
In this analysis, only patients with X4- and R5-tropic virus levels available and with >1,000 HIV RNA copies/ml were included (n = 51). Bivariate and multivariate analyses were performed using a linear regression model, and variables showing a P value of <0.1 in the bivariate analysis were included in the multivariate analysis. In the multivariate analysis, variables with a P value of <0.05 were considered statistically significant.
B, regression coefficient; CI, confidence interval.
Not applicable.
IDU, injecting drug user.
PCR+, PCR positive for hepatitis C virus.
Number of HIV RNA copies/ml in the U87-CXCR4 cell line well supernatant.
Number of HIV RNA copies/ml in the U87-CCR5 cell line well supernatant.
DISCUSSION
Results presented here show that about three-quarters of HIV-infected patients are eligible for R5 antagonist therapy on the basis of the virological response observed after a short-term exposure to the drug. Furthermore, the X4-tropic virus level is the only variable independently associated with this response.
MCT has been proposed to be an alternative method to determine whether to prescribe R5 antagonist treatment in HIV-infected patients. MCT does not give a tropism result but gives the sensitivity to the antiretroviral drug (7). MCT has some potential limitations, such as the possibility of the development of mutations for resistance to MRV during the test. Besides, during MCT the pressure of the R5 antagonist could lead to a switch to X4-tropic virus. However, no deleterious effects after administration of rescue therapy on the basis of the MCT result have been observed (8). Studies to address the safety of MCT and development of resistance are in progress. On the other hand, MCT can overcome the limitations of phenotype and genotype tropism tests, which, due to their high and low sensitivities in detecting X4-tropic strains, respectively, can render variable rates of discordant results for the virological response after MRV exposure (6, 9). Besides, this clinical approach is easy and cheap and can be performed on subjects with <1,000 HIV RNA copies/ml. Another advantage is that a nonreportable result cannot be obtained when using MCT. All these characteristics make MCT an attractive model to analyze the factors associated with the response after exposure to an R5 antagonist.
According to this model, a high proportion of HIV-infected patients (72.2%) could be treated with a combined antiretroviral therapy (cART) which includes MRV. These results, together with the different frequencies of response depending on the treatment category (treatment-naïve versus pretreated patients), have important clinical implications. The fact that the positive response frequencies are higher in treatment-naïve subjects and are associated with higher CD4+ T-cell levels strongly suggests that a cART that includes an R5 antagonist may be used as a first-line treatment for HIV infection, because it is well-known that R5-tropic virus predominates early in the infection and X4-tropic virus appears late in the infection in 50% of the patients (11). These results agree with those of clinical trials that show the noninferiority of a cART which includes MRV compared with efavirenz at achieving undetectable levels of virus (<50 HIV RNA copies/ml) in treatment-naïve patients (3, 15, 21).
On the other hand, knowledge of the factors associated with the virological response after short-term MRV exposure will be particularly interesting in order to optimize R5 antagonist treatment and design strategies to overcome factors associated with the absence of a response. In a first attempt, the CD4+ T-cell level was the only variable independently associated with the MCT response. These results agree with those of previous studies that showed that CD4+ T-cell levels were associated with Trofile results (16, 23). In these studies, Trofile did not communicate the amount of X4-tropic viremia; thus, the extent to which the X4-tropic viral load influenced the tropism result was unknown. However, in a subgroup of consecutive patients where X4- and R5-tropic virus levels were available, only the X4-tropic virus levels were associated with a virological response, after being adjusted by CD4+ T-cell levels and type of transmission. This outcome was reproduced when the MCT result was not considered to be a categorical variable but was considered to be a continuous variable expressed as viral load changes after an 8-day MRV exposure. This result may explain the association of high frequencies of response in treatment-naïve patients, who tended to have lower X4-tropic virus levels and higher CD4 T-cell levels than pretreated patients, and, in general, in those patients with high CD4+ T-cell levels. In fact, an inverse correlation was observed between CD4 T-cell and X4-tropic virus levels (r = −0.499; P < 0.0001, Pearson test). These results agree with the classic concept that syncytium-inducing viral strains are associated with the rate of disease progression and lower CD4 T-cell levels (11).
One limitation of our results concerns what we have called the X4-tropic virus level, which is not actually the X4-tropic viral load present in the patient's peripheral blood but the viral load in the supernatant that is a result of multiple rounds of infection of the U87-X4 cell line with the patient virus isolate. We believe that other methods, such as ultradeep sequencing, should be informative in this respect (22), as no quantitative information can be obtained from Trofile. However, the results shown in this work suggest that the X4-tropic viral load in the supernatant would be a good surrogate marker for X4-tropic viremia. It is important to note that no association was found between R5-tropic virus levels and the virological response after MCT. This indicates that the R5-tropic component of the patient's total viral load is not involved in the virological response to R5 antagonists. This means that an MCT-positive patient will have very low X4-tropic virus levels, and then the changes in viremia during MCT will resemble the changes in R5-tropic viremia, which is the predominant one in this type of patient. Because an MCT-negative patient has variable X4-tropic virus levels, the level of R5-tropic viremia decreases during MCT and will probably be accompanied by increases in X4-tropic viremia after the R5 antagonist exposure, which will lead to no changes in the final viral load after the test. Thus, factors associated with the high levels of X4-tropic viruses at baseline could be associated with the absence of a virological response to R5 antagonists. Findings from previous work suggest the importance of the X4 density in this process (13). In fact, the X4 density on the CD4+ T-cell surface has been associated with the emergence of X4-tropic strains during the course of HIV infection (5). These hypotheses also support the belief that the changes in the rates of proliferation of the T-cell subsets which differentially express R5 and X4 favor X4-tropic virus expansion at lower CD4+ T-cell levels (19).
In summary, our results demonstrate for the first time that X4-tropic virus levels are independently associated with the absence of a response to R5 antagonist therapy. The association of high X4-tropic and low CD4+ T-cell levels agrees with the option to use early R5 antagonist treatment. Further studies are needed to analyze which factors are associated with the expansion of X4-tropic strains in order to optimize treatment with R5 antagonists and to look for immunotherapeutic strategies to avoid the coreceptor switch during the course of HIV infection.
ACKNOWLEDGMENTS
This study has been supported by the Redes Temáticas de Investigación en SIDA (ISCIII RETIC RD06/0006/0021, RD06/0006/0035, and RD06/0006/1004), by the Fondo de Investigación Sanitaria grants PI07/0976 and PS09/01595, and by the Fondos Europeos para el Desarrollo Regional (FEDER). S.F.-M. and E.R.-M. have grants from the Fondo de Investigaciones Sanitarias (CD10/00382 and CP08/00172, respectively).
We particularly acknowledge the patients in this study for their participation. We thank Francisca Cano and Magdalena Rodriguez for their support.
Footnotes
Published ahead of print on 1 August 2011.
REFERENCES
- 1. Berger E. A., et al. 1998. A new classification for HIV-1. Nature 391:240. [DOI] [PubMed] [Google Scholar]
- 2. Chueca N., et al. 2009. Improvement in the determination of HIV-1 tropism using the V3 gene sequence and a combination of bioinformatic tools. J. Med. Virol. 81:763–767 [DOI] [PubMed] [Google Scholar]
- 3. Cooper D. A., et al. 2010. Maraviroc versus efavirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naive subjects with CCR5-tropic HIV-1 infection. J. Infect. Dis. 201:803–813 [DOI] [PubMed] [Google Scholar]
- 4. Dorr P., et al. 2005. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 49:4721–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fiser A. L., et al. 21 September 2010, posting date High CD4+ T-cell surface CXCR4 density as a risk factor for R5 to X4 switch in the course of HIV-1 infection. J. Acquir. Immune Defic. Syndr. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 6. Genebat M., et al. 2011. Discordance rates between Trofile test and short-term virological response to maraviroc. Antiviral Res. 89:182–185 [DOI] [PubMed] [Google Scholar]
- 7. Genebat M., et al. 2009. Correlation between the Trofile(R) test and virological response to a short-term maraviroc exposure in HIV-infected patients. J. Antimicrob. Chemother. 64:845–849 [DOI] [PubMed] [Google Scholar]
- 8. Genebat M., et al. 2010. Long-term immunovirological effect and tolerability of a maraviroc-containing regimen in routine clinical practice. Curr. HIV Res. 8:482–486 [DOI] [PubMed] [Google Scholar]
- 9. Gonzalez-Serna A., et al. 2010. TROCAI (tropism coreceptor assay information): a new phenotypic tropism test and its correlation with Trofile enhanced sensitivity and genotypic approaches. J. Clin. Microbiol. 48:4453–4458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hammer S. M., et al. 2008. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society—USA Panel. JAMA 300:555–570 [DOI] [PubMed] [Google Scholar]
- 11. Koot M., et al. 1993. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 118:681–688 [DOI] [PubMed] [Google Scholar]
- 12. Landovitz R. J., et al. 2008. Phase II study of vicriviroc versus efavirenz (both with zidovudine/lamivudine) in treatment-naive subjects with HIV-1 infection. J. Infect. Dis. 198:1113–1122 [DOI] [PubMed] [Google Scholar]
- 13. Lin Y. L., et al. 2005. CXCR4 overexpression during the course of HIV-1 infection correlates with the emergence of X4 strains. J. Acquir. Immune Defic. Syndr. 39:530–536 [PubMed] [Google Scholar]
- 14. Low A. J., et al. 2007. Current V3 genotyping algorithms are inadequate for predicting X4 co-receptor usage in clinical isolates. AIDS 21:F17–F24 [DOI] [PubMed] [Google Scholar]
- 15. Mills A., et al. 2010. Once-daily maraviroc (MVC) + atazanavir/ritonavir (ATV/r) versus emtricitabine/tenofovir (TDF/FTC) + ATV/r in treatment naïve patients: a week 24 planned interim analysis, abstr. THLBB203. Abstr. XVIII Int. AIDS Conf. [Google Scholar]
- 16. Moreno S., et al. 2009. Prevalence of CCR5-tropic HIV-1 among treatment-experienced individuals in Spain. HIV Clin. Trials 10:394–402 [DOI] [PubMed] [Google Scholar]
- 17. Poveda E., et al. 2009. Design and validation of new genotypic tools for easy and reliable estimation of HIV tropism before using CCR5 antagonists. J. Antimicrob. Chemother. 63:1006–1010 [DOI] [PubMed] [Google Scholar]
- 18. Raymond S., et al. 2008. Correlation between genotypic predictions based on V3 sequences and phenotypic determination of HIV-1 tropism. AIDS 22:F11–F16 [DOI] [PubMed] [Google Scholar]
- 19. Ribeiro R. M., Hazenberg M. D., Perelson A. S., Davenport M. P. 2006. Naive and memory cell turnover as drivers of CCR5-to-CXCR4 tropism switch in human immunodeficiency virus type 1: implications for therapy. J. Virol. 80:802–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schurmann D., et al. 2007. Antiviral activity, pharmacokinetics and safety of vicriviroc, an oral CCR5 antagonist, during 14-day monotherapy in HIV-infected adults. AIDS 21:1293–1299 [DOI] [PubMed] [Google Scholar]
- 21. Sierra-Madero J., et al. 2010. Efficacy and safety of maraviroc versus efavirenz, both with zidovudine/lamivudine: 96-week results from the MERIT study. HIV Clin. Trials 11:125–132 [DOI] [PubMed] [Google Scholar]
- 22. Swenson L. C., et al. 2011. Deep sequencing to infer HIV-1 co-receptor usage: application to three clinical trials of maraviroc in treatment-experienced patients. J. Infect. Dis. 203:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Waters L., et al. 2008. The impact of HIV tropism on decreases in CD4 cell count, clinical progression, and subsequent response to a first antiretroviral therapy regimen. Clin. Infect. Dis. 46:1617–1623 [DOI] [PubMed] [Google Scholar]
- 24. Whitcomb J. M., et al. 2007. Development and characterization of a novel single-cycle recombinant-virus assay to determine human immunodeficiency virus type 1 coreceptor tropism. Antimicrob. Agents Chemother. 51:566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wilkin T. J., et al. 2011. Reanalysis of coreceptor tropism in HIV-1-infected adults using a phenotypic assay with enhanced sensitivity. Clin. Infect. Dis. 52:925–928 [DOI] [PMC free article] [PubMed] [Google Scholar]