Abstract
Rabies is a fatal zoonotic disease of serious public health and economic significance worldwide. The rabies virus glycoprotein (RVG) has been the major target for subunit vaccine development, since it harbors domains responsible for induction of virus-neutralizing antibodies, infectivity, and neurovirulence. The glycoprotein (G) was cloned using the baculovirus expression vector system (BEVS) and expressed in Spodoptera frugiperda (Sf-9) cells. In order to obtain a soluble form of G suitable for experimentation in mice, 18 different combinations of buffers and detergents were evaluated for their ability to solubilize the insect cell membrane-associated G. The combination that involved 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) detergent in lysis buffer 1, formulated with Tris, NaCl, 10% dimethyl sulfoxide (DMSO), and EDTA, gave the highest yield of soluble G, as evidenced by the experimental data. Subsequently, several other parameters, such as the concentration of CHAPS and the duration and temperature of the treatment for the effective solubilization of G, were optimized. The CHAPS detergent, buffered at a concentration of 0.4% to 0.7% (wt/vol) at room temperature (23 to 25°C) for 30 min to 1 h using buffer 1, containing 10% DMSO, resulted in consistently high yields. The G solubilized using CHAPS detergent was found to be immunogenic when tested in mice, as evidenced by high virus-neutralizing antibody titers in sera and 100% protection upon virulent intracerebral challenge with the challenge virus standard (CVS) strain of rabies virus. The results of the mice study indicated that G solubilized with CHAPS detergent retained the immunologically relevant domains in the native conformation, thereby paving the way for producing a cell-free and efficacious subunit vaccine.
INTRODUCTION
Rabies is a zoonotic viral disease that causes acute encephalitis in mammals. The rabies vaccine manufacturing technology has evolved from the usage of crude animal tissue homogenates (nervous tissue vaccine) to the highly purified vaccines produced in defined cell lines. Recently, recombinant viral proteins and DNA have been investigated as vaccine candidates for rabies (2, 7, 26, 27). Glycoprotein (G) is the major surface protein of rabies virus (RV), responsible for the production of neutralizing antibodies (3), and hence, the subunit vaccines that contain G could provide complete protection against RV challenge (4). Moreover, various recombinant protein expression platforms offer the advantage of obtaining scalable protein production without the necessity of handling live RV.
The rabies virus glycoprotein (RVG) has been expressed in various expression systems. The G expressed in Escherichia coli was insoluble and nonimmunogenic and failed to confer protection against rabies (27). Similarly, the RVG expressed in Saccharomyces cerevisiae resulted in an incorrectly folded version which could protect only against an intramuscular challenge and not against an intracerebral (i.c.) challenge (13, 20). The baculovirus expression vector system (BEVS) is one of the most powerful and versatile eukaryotic expression systems available to produce the functionally authentic recombinant proteins. When mammalian proteins are expressed in insect cells, the protein folding and processing are more authentic than in other prokaryotic expression systems, although there are differences in glycosylation (11). The RVG expressed using the BEVS was antigenically conserved with a three-dimensional structure and biological features similar to those of the native protein (18, 22, 23).
The immunogenicity of the BEVS-expressed RVG has been evaluated thus far by immunizing either the intact cells or the crude lysate of insect cells expressing RVG (5, 18). However, vaccine preparations of these kinds, containing an undefined quantity and composition of recombinant proteins, may not be acceptable from the regulatory point of view. Extraction of membrane-expressed G from the host cells without altering its conformation and antigenicity is a challenging task indeed. Several attempts to synthesize recombinant viral membrane G for immunization purposes have failed because of the difficulties in properly isolating them from the cell membrane without affecting their biological and antigenic properties (1).
Cell lysis using detergents is a milder and easier alternative to physical disruption of cell membranes. However, there is no standard protocol available for selecting an appropriate detergent suitable for membrane lysis. The choice of detergent for cell lysis depends on various factors, such as cell type, buffer, pH, salt concentration, temperature, and nature of the proteins (6, 15). In general, nonionic and zwitterionic detergents are milder and less denaturing than ionic detergents and are usually preferred as solubilizing agents whenever preserving the protein structure is critical.
In the present study, the RVG (PV strain) was expressed in insect cells (recombinant RVG [r-RVG]), and the efficiencies of solubilization of three detergents, two nonionic detergents (Nonidet P-40 and Triton X-100) and a zwitterionic detergent (CHAPS), in combination with each of six different buffers were evaluated. The immunogenicity and protective efficacy of the detergent-solubilized r-RVG were assessed in mice. Our findings indicate that baculovirus-derived RVG can be solubilized with a combination of buffers to retain native conformation and that the subunit vaccine provided complete protection against lethal virus challenge by the intracerebral route (18).
MATERIALS AND METHODS
Cells, virus, and animals.
Spodoptera frugiperda (Sf-9) cells (Ingenasa, Spain) were grown using Grace's insect cell medium (Gibco) supplemented with 10% fetal bovine serum (Gibco) at 27°C. Neuro 2a cells (ATCC) were used for rabies serology by a rapid fluorescent-focus inhibition test (RFFIT). The challenge virus standard (CVS; Federal Vaccine Institute, Basel, Switzerland) and CVS 11 (Agence Française de Securité Sanitaire des Aliments, France) strains of RV were used for the mice challenge and the RFFIT, respectively. Swiss albino mice (4 to 6 weeks old; both sexes) procured from the National Institute of Nutrition (NIN), Hyderabad, India, and maintained at a small-animal testing facility (Indian Immunologicals Limited, Hyderabad, India) were used for assessing immunogenicity and protective efficacy.
RVG MAbs.
An RVG-specific monoclonal antibody (MAb), M5B4, which binds to the natively folded glycoprotein at antigenic site III, was used to characterize the insect cell-derived r-RVG. The MAb M5B4 was isotyped as IgG2b (17).
Cloning of the RVG gene into a baculovirus transfer vector.
The entire G coding sequence, including the sequences of the leader peptide and the transmembrane domain, was PCR amplified from a plasmid clone (17) using ProofStart DNA polymerase (Invitrogen). The nucleotide sequences of the forward and reverse primers were 5′ACGCTCTAGAATGGTTCCTCAGGCTCTCCT3′ and 5′AGCTGGTACCCAGTCCGGTCTCACCCCC3′, respectively. The recognition sites of the KpnI and XbaI restriction enzymes were added as 5′ overhangs to the forward and reverse primers, respectively, to enable directional cloning. The PCR-amplified DNA fragment was cloned into the multiple cloning site of the baculovirus transfer vector pBacPAK8 (Clontech), downstream of the polyhedrin promoter. The transfer vector pBacPAK8 was modified previously to incorporate a C-terminal His6 tag. The insert was sequence verified using the Bac1 and Bac2 primers (Clontech).
Generation of a recombinant baculovirus containing an RVG expression cassette.
A recombinant baculovirus was generated by cotransfecting Sf-9 cells with 500 ng of transfer vector plasmid DNA, comprising the RVG expression cassette, and 200 ng of FlashBAC baculoviral DNA (Oxford Expression Technologies, United Kingdom) using Lipofectamine transfection reagent (Invitrogen). The cell culture supernatant was collected on day 6 posttransfection, and the recombinant baculovirus clones were plaque purified. The Sf-9 cells were infected with the plaque-purified baculovirus clones, and the cell lysate was subjected to immunoblotting to identify the expression of r-RVG. The immunoblot was probed using anti-His5 MAb-peroxidase conjugate (Qiagen, Germany). The recombinant virus was further amplified in Sf-9 cells, and the virus titer was determined by plaque assay.
Demonstration and characterization of the expressed r-RVG. (i) Immunofluorescence test.
An Sf-9 cell monolayer, grown in a 24-well tissue culture plate, was infected with the recombinant baculovirus clone at a multiplicity of infection (MOI) of 4. On day 3 postinfection, the cells were probed with MAb M5B4 for 45 min at 37°C in a humid chamber. After washing with phosphate-buffered saline (PBS) thrice, the cells were stained with rabbit anti-mouse IgG-fluorescein isothiocyanate (FITC) conjugate (Sigma) and observed under a fluorescence microscope (Olympus). The uninfected Sf-9 cell monolayer used as an internal control was also processed in a similar manner.
(ii) Confocal microscopy.
Sf-9 cells, grown on a coverslip placed inside a 6-well tissue culture plate, were infected with the recombinant baculovirus. On day 3 postinfection, the coverslips were blocked with 0.5% bovine serum albumin (BSA) (wt/vol) for 30 min at room temperature (RT). After washing thrice with PBS, the cells were probed with MAb M5B4 for 60 to 90 min at room temperature. The cells were washed thrice with PBS and stained with goat anti-mouse IgG-Alexa Fluor 488 (Molecular Probes) for 60 to 90 min at room temperature. Subsequently, the cells were washed with PBS and their nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole) (Sigma) for 2 min at room temperature. The coverslips were mounted with Vectashield (Vector Laboratories, United Kingdom) before observation under a confocal microscope (model LSM 510 Meta; Carl Zeiss, Germany) for the specific fluorescence pattern.
(iii) Flow cytometry.
The expression of r-RVG in Sf-9 cells was demonstrated by flow cytometry. Briefly, the recombinant baculovirus-infected Sf-9 cells in suspension were probed with MAb M5B4 for 30 min at 2 to 8°C and then washed using BD stain buffer by centrifugation at 800 × g for 5 min. Subsequently, the cells were stained with goat anti-mouse IgG2b-R-phycoerythrin (RPE) conjugate (AbD Serotec, United Kingdom) for 30 min at 2 to 8°C. The cells were washed as described above and resuspended in staining buffer before analysis in a flow cytometer (BD FACSCanto II). The uninfected Sf-9 cells used as an internal control were also processed in a similar manner.
Production of r-RVG in insect cells.
The Sf-9 cells were grown in a suspension culture in shaker flasks at 27°C and 110 rpm. The cells were infected with the recombinant baculovirus at an MOI of 4 when the cell density reached 1 × 106 cells/ml. The infected cells were observed daily for cytopathic effect (CPE) and harvested when the CPE was almost 100% (∼3 to 4 days). The harvested cells were pelleted by centrifugation at 3,500 × g for 10 min. The cell pellet was washed thrice using PBS and stored frozen at −20°C until further use.
Solubilization of r-RVG using various combinations of salts and detergents.
r-RVG extraction employing buffered detergent solution was done as reported by Astray et al. (1), with some modifications. Eighteen different combinations of buffer-detergent solutions were used: six different buffers in combination with each of three commonly used detergents, CHAPS, NP-40, and Triton X-100, were used to solubilize the r-RVG from Sf-9 cells. All three detergents were sourced from Sigma. The detergents, CHAPS, NP-40, and Triton X-100, were added at final concentrations of 1%, 0.2%, and 0.1%, respectively (1, 19). The cell pellet, stored frozen at −20°C, was thawed at room temperature, and 1 ml of buffer-detergent solution per 2 × 107 cells was added (Table 1). The mixture was then incubated for 30 min by end-to-end constant mixing at room temperature (23 to 25°C). Following the lysis process, the cell lysate was clarified by centrifugation for 5 min at 10,000 × g and the soluble r-RVG was quantified from the supernatant using an immunocapture enzyme-linked immunosorbent assay (IC-ELISA) (17).
Table 1.
Eighteen different combinations of buffers and detergents used in this studya
| Buffer | Buffer composition | Detergent |
|---|---|---|
| 1 | 50 mM Tris-HCl, 150 mM NaCl, 10% DMSO, 4 mM EDTA | Triton X-100, CHAPS, or NP-40 |
| 2 | 50 mM Tris-HCl, 150 mM NaCl, 10% glycerol, 4 mM EDTA | Triton X-100, CHAPS, or NP-40 |
| 3 | 50 mM Tris-HCl, 150 mM NaCl | Triton X-100, CHAPS, or NP-40 |
| 4 | 25 mM Tris-HCl, 137 mM NaCl, 5 mM KCl, 0.7 mM Na2HPO4 | Triton X-100, CHAPS, or NP-40 |
| 5 | 25 mM Tris-HCl, 25 mM NaCl, 5 mM MgCl2 | Triton X-100, CHAPS, or NP-40 |
| 6 | 150 mM NaCl, 10 mM Tris-HCl, 1 mM EDTA | Triton X-100, CHAPS, or NP-40 |
Six different lysis buffers in combination with one of three detergents (1% CHAPS, 0.2% NP-40, and 0.1% Triton X-100) were analyzed for solubilization of membrane-bound r-RVG from Sf-9 cells. One milliliter of each buffer-detergent solution was added to 2 × 107 Sf-9 cells. The pH of each buffer was 7.4.
Optimization of conditions for solubilization of r-RVG using CHAPS detergent.
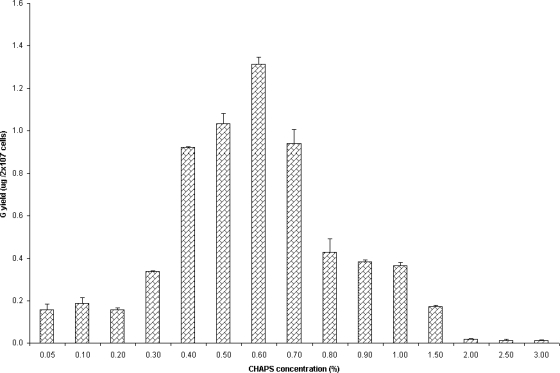
Out of several buffer-detergent combinations evaluated, buffer 1 containing CHAPS detergent had shown the most effective solubilization of r-RVG in Sf-9 cells. Various concentrations of CHAPS detergent, from 0.05% to 3% (wt/vol), were used for solubilization (see Fig. 4) in order to determine the optimal critical micellar concentration (CMC) of CHAPS in buffer 1. Based on the above-described experiment, the concentration of CHAPS detergent that had shown the most effective solubilization of r-RVG was chosen for further experiments to optimize temperature and duration. Subsequently, the solubilization efficiency of buffer 1 with 0.6% CHAPS detergent was evaluated at three different incubation temperature ranges, viz., 2 to 8°C, 23 to 25°C, and 36 to 38°C, and six different incubation periods, viz., 30 min, 1 h, 3 h, 5 h, 8 h, and 24 h (Table 2). In order to determine the effect of dimethyl sulfoxide (DMSO) in solubilizing r-RVG, the Sf-9 cells were treated using buffered detergent solution (buffer 1 containing 0.6% CHAPS) with and without DMSO for 30 min at room temperature (23 to 25°C). The soluble r-RVG content of clarified cell lysate was quantified by IC-ELISA.
Fig. 4.
Determination of the optimal concentration of CHAPS for solubilizing r-RVG from Sf-9 cells. The r-RVG-expressing Sf-9 cells were treated with buffer 1, containing various concentrations of CHAPS (from 0.05 to 3%), for 30 min at RT (23 to 25°C). The G content in the supernatant was quantified by IC-ELISA and the results, expressed in μg, are the means ± SDs of results from three different experiments.
Table 2.
Optimization of temperature and duration for CHAPS treatmenta
| Duration of treatment | G content (μg/2 × 107 cells) at indicated temp |
||
|---|---|---|---|
| 36–38°C | 23–25°C | 2–8°C | |
| 30 min | 0.88 ± 0.02 | 1.56 ± 0.01 | 1.57 ± 0.01 |
| 1 h | 0.50 ± 0.01 | 1.56 ± 0.02 | 1.53 ± 0.02 |
| 3 h | 0.00 | 1.30 ± 0.02 | 1.23 ± 0.01 |
| 5 h | 0.00 | 1.44 ± 0.02 | 1.31 ± 0.01 |
| 8 h | 0.00 | 1.49 ± 0.01 | 1.21 ± 0.02 |
| 24 h | 0.00 | 1.42 ± 0.02 | 1.28 ± 0.03 |
The solubilization of membrane-bound r-RVG from Sf-9 cells by buffer 1 with 0.6% CHAPS was analyzed at different temperatures and for different durations. Three different temperature conditions (2 to 8°C, 23 to 25°C, and 36 to 38°C) and six different incubation periods (30 min, 1 h, 3 h, 5 h, 8 h, and 24 h) were analyzed, and the results are the means ± SDs from three different determinations.
Quantification of r-RVG content by IC-ELISA.
The r-RVG content of clarified cell lysate was quantified by IC-ELISA (17). Briefly, the Maxisorb ELISA plates were coated with MAb M5B4, developed against RVG, and left overnight at 4°C. The unbound sites were blocked using 1% bovine gelatin. The test samples, controls, and in-house reference standard (purified antigen from the PV strain of RV) with known G contents were subjected to serial 2-fold dilutions using PBS-Tween 20 (PBS-T) and incubated for 1 h at 37°C, followed by three washes with PBS-T. The biotinylated MAb M5B4 was added and incubated at 37°C for 1 h. After washing with PBS-T, the plates were incubated with streptavidin-peroxidase conjugate (Sigma) for 1 h at 37°C. After washing thrice with PBS-T, the color was developed using a substrate chromogen mixture (tetramethylbenzidine [Sigma] and hydrogen peroxide [Merck, India]) and incubated for 10 min at room temperature. The reaction was stopped with 1.25 M sulfuric acid, and the optical density (OD) was measured at 450 nm. The r-RVG content was quantified by comparing the OD value of the in-house reference standard with those of the samples.
Determination of immunogenicity of r-RVG in mice.
r-RVG was solubilized using buffer 1 with 0.6% CHAPS for 30 min at 23 to 25°C. Subsequently, the protein was dialyzed against PBS and subjected to immunogenicity testing in mice. Experimental mice were inoculated intraperitoneally (i.p.) with 0.2, 0.3, and 0.4 μg of aluminum hydroxide gel (algel)-adjuvanted solubilized r-RVG. The cell lysate prepared from uninfected Sf-9 cells was used to inoculate control mice. Cell lysate containing an insoluble fraction (50 μg per animal) was also administered to a group of mice. Mice belonging to all the groups received a booster dose of the vaccine of their respective formulation on day 14 postvaccination.
(i) Determination of neutralizing antibody titer by the RFFIT.
The RV-neutralizing antibody titers of experimental mice were determined by the RFFIT (21). Briefly, various dilutions of test and reference sera with standard rabies immunoglobulin (SRIg; NIBSC, United Kingdom) were mixed with the CVS 11 strain of RV (50% fluorescent-focus-forming dose [50 FFD50]) and incubated at 37°C in the presence of 5% CO2 for 90 min. After the incubation period, Neuro 2a cells were added to the mixture and the mixture was incubated again for 20 h at 37°C in the presence of 5% CO2. The cell sheet was then fixed with acetone and stained using an anti-RV nucleocapsid MAb conjugated with FITC (Chemicon). The antibody titers of the sample sera were expressed in IU/ml.
(ii) Determination of protective efficacy of r-RVG by challenge in mice.
The protective efficacy of the solubilized r-RVG was assessed by challenging the vaccinated experimental mice intracerebrally with 30 μl of the mouse brain-passaged CVS strain of RV (30 50% lethal doses [LD50]/mouse) on day 35 postvaccination. Mice were observed for the symptoms of rabies on a daily basis for 14 days. The percentages of protection were calculated for all groups of experimental mice.
RESULTS
Cloning of the RVG gene into a baculovirus transfer vector.
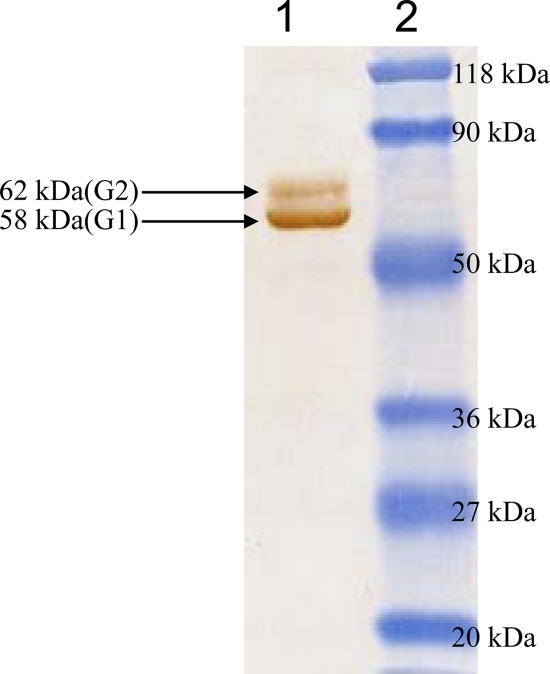
The RVG coding sequence was cloned into a baculovirus transfer vector (modified pBacPAK8). The clone was sequence verified, and the deduced amino acid sequence showed 100% similarity with the published G sequences of the PV strain of RV. A recombinant baculovirus containing the RVG expression cassette was created by cotransfecting the transfer vector construct and FlashBAC baculovirus DNA. The recombinant baculovirus clones were selected by screening the baculovirus-infected Sf-9 cell lysate with an anti-His5 monoclonal antibody in an immunoblot. The positive clones produced doublet protein bands of ∼58 kDa and ∼62 kDa (G1 and G2, respectively) (18, 23) in the immunoblot (Fig. 1).
Fig. 1.
Western blot analysis of detergent-solubilized r-RVG expressed in Sf-9 cells showing reactivity with an anti-His5 MAb conjugated with horseradish peroxidase (HRP). Lane 1, doublet bands corresponding to the insect cell-expressed rabies virus G protein; lane 2, molecular mass markers.
Demonstration and characterization of the expressed r-RVG.
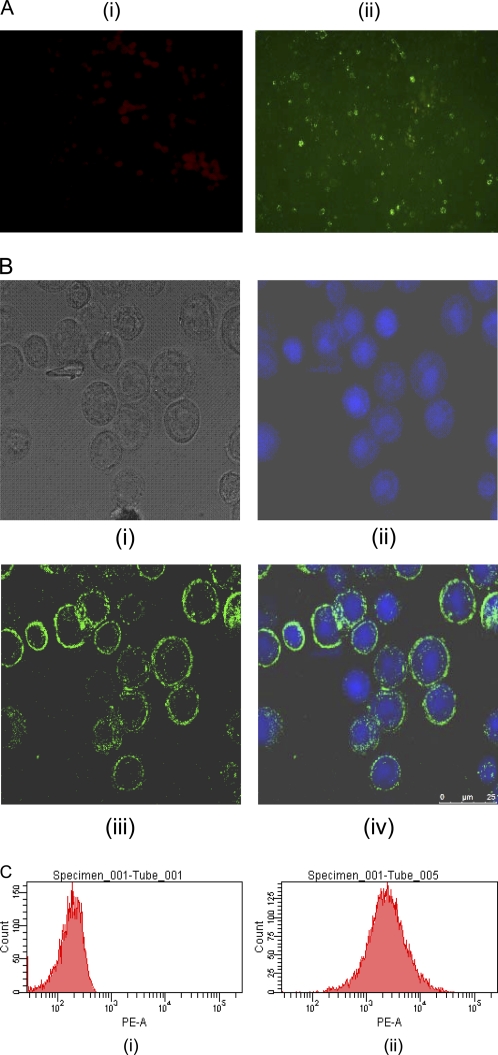
The insect cell-expressed r-RVG was characterized by immunofluorescence tests using an RVG-specific MAb. The recombinant baculovirus-infected Sf-9 cells produced specific membrane fluorescence when stained with MAb M5B4. A similar membrane fluorescence pattern was observed by the confocal microscopy, too. It is understandable that G could get processed and anchored to the cell membrane, owing to the presence of the leader sequence and the transmembrane anchoring domain. Taken together, the experimental data clearly indicated that the native leader sequence of RVG could help target the protein in the endoplasmic reticulum of insect cells (Fig. 2A and B). The results of flow cytometry showed that more than 90% of Sf-9 cells were positive for r-RVG expression on day 3 postinfection, when the cells were infected with an MOI of 4 (Fig. 2C). Therefore, the natively folded conformation of the r-RVG associated with the insect cells could be identified by flow cytometry, immunofluorescence, and confocal microscopy.
Fig. 2.
(A) Immunofluorescence analysis. The r-RVG-expressing and control Sf-9 cells were allowed to react with the MAb specific to RVG and then stained with FITC-conjugated rabbit anti-mouse IgG. (i) Uninfected control Sf-9 cells; (ii) infected, unfixed Sf-9 cells expressing r-RVG. A typical membrane fluorescence pattern was observed using a fluorescence microscope (magnification, ×200). (B) Confocal microscopy analysis. The r-RVG-expressing Sf-9 cells were stained using the MAb specific to the RVG and then with Alexa Fluor 488-conjugated goat anti-mouse IgG. The fluorescence was observed using a confocal microscope (25 μm). (i) Light microscopy image of infected Sf-9 cells; (ii) DAPI-stained cells; (iii) goat anti-mouse IgG-Alexa Fluor 488-stained cells; (iv) composite of DAPI- and Alexa Fluor 488-stained cells. (C) Flow cytometry analysis. The control and infected Sf-9 cells were labeled with the MAb specific to the RVG and then stained using RPE-conjugated anti-IgG2a. The expression of r-RVG was analyzed by flow cytometry. PE-A, phycoerythrin-area.
Recovery of r-RVG from Sf-9 cells using buffered detergent solutions.
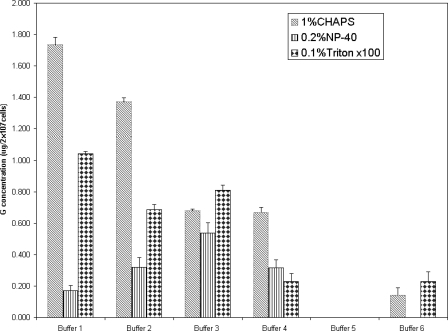
The Sf-9 cells expressing r-RVG were lysed using 18 different combinations of buffered detergent solutions, as indicated in Table 1. The soluble fractions were screened for the presence of r-RVG by IC-ELISA. The experimental data indicated that the detergents NP-40 and Triton X-100 were not as good as CHAPS in solubilizing r-RVG from Sf-9 cells (Fig. 3). Compared to the other five buffer-CHAPS detergent combinations, the solution of buffer 1 with CHAPS had shown considerably higher yields of r-RVG (1.78 μg/2 × 107 cells).
Fig. 3.
Solubilization of r-RVG from Sf-9 cells using various combinations of buffered detergent solutions, as shown in Table 1. The lysis was carried out at RT, and the r-RVG content in the supernatant was quantified by IC-ELISA. The results, expressed in μg, are the means ± SDs of G contents from three independent experiments.
A second set of experiments was carried out to find the optimal concentration of CHAPS to be used in buffer 1 for effective solubilization of r-RVG. From the results, it was clear that 0.4% to 0.7% CHAPS detergent could solubilize r-RVG effectively (Fig. 4). The results of another experiment, attempting to optimize the temperature and duration of treatment for effective solubilization of r-RVG using a combination of buffer 1 and 0.6% CHAPS, clearly indicated that 30-min and 1-h durations of treatments yielded, 1.56 μg of r-RVG per 2 × 107 cells and 1.53 μg to 1.57 μg of r-RVG per 2 × 107 cells, respectively, at room temperature (23 to 25°C) and 2 to 8°C. Treatment of samples at 2 to 8°C or room temperature (23 to 25°C) for 30 min to 1 h resulted in better solubilization of r-RVG than treatment at 36 to 38°C, which resulted in only 50% of the yield of each of the other two ranges. In addition, the natively folded conformation of CHAPS-solubilized r-RVG was proven by specific binding of MAb M5B4 in an IC-ELISA. Addition of 10% (vol/vol) DMSO to a buffered detergent solution (buffer 1) resulted in better solubilization, as evidenced by higher yields of r-RVG (mean ± standard deviation) with DMSO (1.07 ± 0.01 μg per 2 × 107 cells) than without DMSO (0.82 ± 0.02 μg per 2 × 107 cells).
Immunogenicity and protective efficacy of r-RVG.
The immunogenicity of r-RVG was determined by mice experiments, which involved vaccinations on day 0 and day 14 and monitoring for seroconversion on days 0, 7, 14, 21, 28, and 35 postvaccination by the RFFIT. The percentage of mice showing a minimum protective RFFIT titer of 0.5 IU/ml (as recommended by the WHO) was calculated for all the mice belonging to different experimental groups (Table 3). The results indicated that higher percentages of mice had protective RFFIT titers in the groups of mice that received 0.2 μg, 0.3 μg, and 0.4 μg of solubilized r-RVG than in the group of mice vaccinated with 50 μg of insoluble cell lysate. The serum antibody titers of mice immunized with a soluble fraction of r-RVG gradually increased until day 35 postvaccination. The percentage of mice showing protective RFFIT titers reached 100% on day 35 postvaccination in all the groups vaccinated with a soluble fraction of r-RVG. However, only 65 to 67% of the mice that received insoluble cell lysate had the protective RFFIT titers. The untreated control group of mice remained seronegative throughout the experiment.
Table 3.
RVNA titers and percentages of protection of mice vaccinated with insect cell-expressed r-RVGa
| Group | Vaccine | % of mice showing an RFFIT titer of >0.5 IU/ml at day postvaccination: |
% protection after challenge | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 7 | 14 | 21 | 28 | 35 | |||
| 1 | 0.2 μg of solubilized r-RVG | 0 | 50 | 84 | 84 | 100 | 100 | 100 |
| 2 | 0.3 μg of solubilized r-RVG | 0 | 34 | 84 | 100 | 100 | 100 | 100 |
| 3 | 0.4 μg of solubilized r-RVG | 0 | 84 | 84 | 100 | 100 | 100 | 100 |
| 4 | 50 μg of cell lysate | 0 | 17 | 67 | 67 | 67 | 65 | 85 |
| 5 | None (control) | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Swiss albino mice in groups 1, 2, and 3 were vaccinated using the indicated solubilized fractions of r-RVG, and group 4 was vaccinated with the crude cell lysate prepared from the infected Sf-9 cell pellet. All the groups were vaccinated twice, on days 0 and 14, and challenged on day 35. Mice were bled on days 0, 7, 14, 21, 28, and 35 postvaccination. The rabies virus-neutralizing antibody (RVNA) titer was determined by the RFFIT, and the results are the percentages of mice showing RFFIT titers higher than the protective titer value (0.5 IU/ml). All the mice in experimental groups were challenged on day 35 postinfection with 30 LD50 of the rabies virus CVS strain by an intracerebral route. The percentages of protection after challenge were calculated.
To examine whether the RFFIT titers induced by solubilized r-RVG can confer protection against live RV challenge, vaccinated mice were inoculated intracerebrally (i.c.) with 30 LD50 of the RV CVS strain on day 35 postvaccination. It was evident from the results that the solubilized r-RVG could protect all vaccinated mice in the group, while insoluble cell lysate protected only 85% of vaccinated mice (Table 3). Moreover, the solubilized r-RVG conferred 100% protection upon i.c. challenge, even at 0.2 μg of protein, and the results of the challenge study correlated with the protective neutralizing antibody response.
DISCUSSION
Rabies remains a major public health problem in developing countries despite the availability of effective vaccines. The prime immune correlate of protection against RV is the induction of neutralizing antibodies, and the only viral antigen capable of eliciting this is G. Subunit vaccines that contain this antigen provide substantial protection against RV challenge (25, 26).
In the present study, r-RVG was expressed in the BEVS, and the quantitative and qualitative studies of expressed r-RVG were performed by fluorescence-activated cell sorting (FACS), immunofluorescence, confocal microscopy, and immunoblotting techniques. The profile obtained by immunoblotting revealed the existence of a doublet r-RVG (G1 and G2) showing distinct electrophoretic mobilities, probably due to different glycosylation levels or truncation of protein during translation (18, 23). Cellular locations and surface expression of r-RVG in Sf-9 cells were determined by immunofluorescence tests. The typical membrane fluorescence of the unfixed Sf-9 cells indicated that the recombinant G was processed normally, transported, and anchored to the plasma membrane (18). Although the r-RVG expressed in the BEVS has different glycosylation patterns from those of the RVG expressed in mammalian cells, the specific binding of a MAb (M5B4) with the r-RVG-expressing Sf-9 cells in flow cytometry and confocal microscopy confirms the natively folded conformation of r-RVG (17, 18).
Since the expressed r-RVG is membrane bound, attempts were made to isolate the membrane protein, which is a tedious, time-consuming, and cumbersome process (14, 16). Effective cell lysis and protein extraction from different cell and tissue types require the correct choice not only of detergents but also of buffers. Hence, we made attempts to prepare combinations of three detergents and six buffers (in total, 18 buffered detergent solutions) for effective solubilization of membrane-bound r-RVG from Sf-9 cells. Our results showed that the detergent CHAPS could yield the largest amount of soluble G, compared to other detergents used in the study. Moreover, the zwitterionic CHAPS detergent is fully dialyzable, which makes the removal process easy before subjecting the r-RVG to in vitro and in vivo tests. The removal of other, nonionic detergents requires tedious methods, such as ion-exchange chromatography and a sucrose density gradient (10, 24).
The amount of detergent needed for optimal protein extraction depends on the type of detergent, CMC, aggregation number, temperature, and nature of the membrane. The solubilization buffer should contain sufficient detergent to provide more than 1 micelle per membrane protein molecule to ensure that individual protein molecules are isolated in separate micelles (9). Further experiments were carried out to find the suitable concentration for the efficient solubilization of r-RVG using the CHAPS-buffer 1 combination. The concentrations of 0.4% to 0.7% CHAPS at room temperature (23 to 25°C) had shown superior solubilization effects when solutions were incubated for 30 min. The optimal concentration is almost closer to the standard CMC of the CHAPS detergent. DMSO is a small amphiphilic molecule which is widely employed in cell biology. The role of DMSO in enhancing the solubilization of r-RVG is not clearly understood. We speculate that the ability of DMSO to induce pores on cell membranes (8), facilitating cell lysis, is one of the reasons for the enhanced solubilization.
The r-RVG expressed in many different expression systems (13, 18, 19, 22, 23, 27) met with variable success when the systems were evaluated for their immunogenicity. Immunization of mice with insect cells expressing RVG induced protective neutralizing antibody titers (18). In raccoons, oral immunization with RVG-containing insect cell lysate induced RV-neutralizing antibody titers that protected most but not all of the animals from lethal challenge (5). A pseudotype baculovirus expressing the G of RV was found to be safe and immunogenic in mice (12). Since the use of cells or cell lysate as a vaccine may not be appropriate, we tried to extract the membrane-bound r-RVG using several combinations of salts and detergents. Most of the RV-neutralizing antibodies bind to conformation-dependent epitopes on the G, and hence, extracting the G without altering its conformation is essential to preserve its immunogenicity. Thus, the immunogenicity and protective efficacy of detergent-solubilized r-RVG were tested in mice. The mice were vaccinated intraperitoneally and challenged intracerebrally with live RV on day 35 postinfection. All the mice vaccinated with solubilized r-RVG had induced the protective RFFIT titer value, whereas only 65 to 67% of the mice that received the insoluble cell lysate had induced the protective titer value. In the challenge experiment, all the mice vaccinated with solubilized r-RVG were protected, even at the lowest dose of protein (0.2 μg) used in the experiment. These results confirm the finding that the native conformation and immunogenicity of r-RVG remains unaltered despite solubilization with CHAPS detergent. There are reports wherein r-RVG-expressing intact cells or crude cell lysate of insect cells was used for immunization (5, 18). Prehaud et al. (18) demonstrated that mice vaccinated with 12 to 120 μg of intact insect cell-expressing RVG (106 to 107 whole insect cells expressing RVG) survived the peripheral challenge, whereas in the present study, even 0.2 μg of the solubilized insect cell-expressed r-RVG showed 100% protection upon i.c. challenge with RV. The protection conferred with the lowest dose (0.2 μg) of solubilized r-RVG was associated with the induction of RV-neutralizing antibodies. This may have been possible because of the use of a solubilized preparation for immunization, which allowed the quantification of the actual G content without the interference of cellular proteins.
In summary, we have optimized a simple and reliable technique which can aid in solubilizing larger amounts of r-RVG without altering its natively folded conformation and immunogenicity. The buffer-detergent combinations helped in formulating a cell-free, soluble-r-RVG-based vaccine. Further studies are planned to determine the immunogenicity of the solubilized r-RVG in dogs, which remain the major reservoir responsible for the spread of rabies to humans and animals in India.
ACKNOWLEDGMENTS
We gratefully thank T. Nagarajan and S. Rajalakshmi for their valuable suggestions, constant support, and critical reading of the manuscript. We are thankful to Rajan Sriraman, P. Baji Babu, M. Madhan Mohan, B. Bala Obulapathi, M. Loganathan, and Ravindra Sisodiya at the R&D center, Indian Immunologicals Limited, Hyderabad, India, for their excellent technical support and assistance.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Astray R. M., Augusto E., Yokomizo A. Y., Pereira A. 2008. Analytical approach for the extraction of recombinant membrane viral glycoprotein from stably transfected Drosophila melanogaster cells. Biotech. J. 3:98–103 [DOI] [PubMed] [Google Scholar]
- 2. Biswas S., Reddy G. S., Srinivasan V. A., Rangarajan P. N. 2001. Preexposure efficacy of a novel combination DNA and inactivated rabies virus vaccine. Hum. Gene Ther. 12:1917–1922 [DOI] [PubMed] [Google Scholar]
- 3. Dietzschold B., Cox J. H., Schneider G. 1978. Structure and function of rabies virus glycoprotein. Dev. Biol. Stand. 40:45–55 [PubMed] [Google Scholar]
- 4. Ertl H. C. 2009. Novel vaccines to human rabies. PLoS Negl. Trop. Dis. 3:e515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fu Z. F., et al. 1993. Oral vaccination of raccoons (Procyon lotor) with baculovirus-expressed rabies virus glycoprotein. Vaccine 11:925–928 [DOI] [PubMed] [Google Scholar]
- 6. Garavito R. M., Ferguson-Miller S. 2001. Detergents as tools in membrane biochemistry J. Biol. Chem. 276:32403–32406 [DOI] [PubMed] [Google Scholar]
- 7. Gupta P. K., et al. 2005. Immunogenic and antigenic properties of recombinant soluble glycoprotein of rabies virus. Vet. Microbiol. 108:207–214 [DOI] [PubMed] [Google Scholar]
- 8. Gurtovenko A. A., Anwar J. 2007. Modulating the structure and properties of cell membranes: the molecular mechanism of action of dimethyl sulfoxide. J. Phys. Chem. B 111:10453–10460 [DOI] [PubMed] [Google Scholar]
- 9. Helenius A., McCaslin D. R., Fries E., Tanford C. 1979. Properties of detergents. Methods Enzymol. 56:734–749 [DOI] [PubMed] [Google Scholar]
- 10. Hjelmeland L. M. 1990. Removal of detergents from membrane proteins. Methods Enzymol. 182:277–282 [DOI] [PubMed] [Google Scholar]
- 11. Hu Y.-C. 2005. Baculovirus as a highly efficient expression vector in insect and mammalian cells. Acta Pharmacol. Sin. 26(4):405–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang H., et al. 2011. Construction and immunogenicity of a recombinant pseudotype baculovirus expressing the glycoprotein of rabies virus in mice. Arch. Virol. 156:753–758 [DOI] [PubMed] [Google Scholar]
- 13. Klepfer S. R., et al. 1993. Characterization of rabies glycoprotein expressed in yeast. Arch. Virol. 128:269–286 [DOI] [PubMed] [Google Scholar]
- 14. Lenstra J. A., Bloemendal H. 1983. Topography of the total protein population from cultured cells upon fractionation by chemical extractions. Eur. J. Biochem. 135:413–423 [DOI] [PubMed] [Google Scholar]
- 15. Lin S. H., Guidotti G. 2009. Purification of membrane proteins. Methods Enzymol. 463:619–629 [DOI] [PubMed] [Google Scholar]
- 16. Morre J., Morre D. 1989. Preparation of mammalian plasma membranes by aqueous two-phase partitioning. Biotechniques 7:946–958 [PubMed] [Google Scholar]
- 17. Nagarajan T., et al. 2006. A simple immune-capture ELISA to estimate rabies viral glycoprotein antigen in vaccine manufacture. Biologicals 34:21–27 [DOI] [PubMed] [Google Scholar]
- 18. Prehaud C., Takehara K., Flamand A., Bishop D. H. 1989. Immunogenic and protective properties of rabies virus glycoprotein expressed by baculovirus vectors. Virology 173:390–399 [DOI] [PubMed] [Google Scholar]
- 19. Rojas-Anaya E., Loza-Rubio E., Olivera-Flores M. T., Gomez-Lim M. 2009. Expression of rabies virus G protein in carrots (Daucus carota). Transgenic Res. 18:911–919 [DOI] [PubMed] [Google Scholar]
- 20. Sakamoto S., et al. 1999. Studies on the structures and antigenic properties of rabies virus glycoprotein analogues produced in yeast cells. Vaccine 17:205–218 [DOI] [PubMed] [Google Scholar]
- 21. Smith J. S., Yager P. A., Baer G. M. 1996. Rapid fluorescent focus inhibition test for determining rabies virus neutralizing antibodies, p. 181–192In Meslin F. X., Kaplan M. M., Koprowski H.(ed.), Laboratory techniques in rabies, 4th ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 22. Tordo N., Kouknetzoff A. 1993. The rabies virus genome: an overview. Onderstepoort J. Vet. Res. 60:263–269 [PubMed] [Google Scholar]
- 23. Tuchiya K., Matsuura Y., Kawai A., Ishihama A., Ueda S. 1992. Characterization of rabies virus glycoprotein expressed by recombinant baculovirus. Virus Res. 25:1–13 [DOI] [PubMed] [Google Scholar]
- 24. Warren G. B., Toon P. A., Birdsall N. J., Lee A. G., Metcalfe J. C. 1974. Reconstitution of a calcium pump using defined membrane components. Proc. Natl. Acad. Sci. U. S. A. 71:622–626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wiktor T. J., et al. 1984. Protection from rabies by a vaccinia virus recombinant containing the rabies virus glycoprotein gene. Proc. Natl. Acad. Sci. U. S. A. 81:7194–7198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiang Z. Q., et al. 1994. Vaccination with a plasmid vector carrying the rabies virus glycoprotein gene induces protective immunity against rabies virus. Virology 199:132–140 [DOI] [PubMed] [Google Scholar]
- 27. Yelverton E., Norton S., Obijeski J. F., Goeddel D. V. 1983. Rabies virus glycoprotein analogs: biosynthesis in Escherichia coli. Science 219:614–620 [DOI] [PubMed] [Google Scholar]