Abstract
Cellular immune responses of both CD4 and CD8 memory/effector T cells were evaluated in healthy young adults who received two doses of live attenuated influenza A (H5N2) vaccine. The vaccine was developed by reassortment of nonpathogenic avian A/Duck/Potsdam/1402-6/68 (H5N2) and cold-adapted A/Leningrad/134/17/57 (H2N2) viruses. T-cell responses were measured by standard methods of intracellular cytokine staining of gamma interferon (IFN-γ)-producing cells and a novel T-cell recognition of antigen-presenting cells by protein capture (TRAP) assay based on the trogocytosis phenomenon, namely, plasma membrane exchange between interacting immune cells. TRAP enables the detection of activated trogocytosis-positive T cells after virus stimulation. We showed that two doses of live attenuated influenza A (H5N2) vaccine promoted both CD4 and CD8 T-memory-cell responses in peripheral blood of healthy young subjects in the clinical study. Significant differences in geometric mean titers (GMTs) of influenza A (H5N2)-specific IFN-γ+ cells were observed at day 42 following the second vaccination, while peak levels of trogocytosis+ T cells were detected earlier, on the 21st day after the second vaccination. The inverse correlation of baseline levels compared to postvaccine fold changes in GMTs of influenza-specific CD4 and CD8 T cells demonstrated that baseline levels of these specific cells could be considered a predictive factor of vaccine immunogenicity.
INTRODUCTION
Highly pathogenic avian influenza A viruses are considered to be of significant pandemic potentiality in the human population. To 2011, during influenza A (H5N1) outbreaks among poultry and wild birds, more than 500 cases of confirmed human infections were reported in 15 countries in Asia, Europe, and Africa (35). Most human cases with confirmed infection by influenza A (H5N1) manifested severe clinical respiratory disease that progressed rapidly to bilateral pneumonia and cardiac and renal complications with nearly 70% of cases proving fatal (32). Since limited but unsustained human-to-human transmission has been documented (31, 33), WHO has recommended that improvements to vaccines against influenza A (H5N1) vaccine should be pursued to aid in pandemic preparedness.
Clinical trials of inactivated influenza A (H5N1) vaccines have shown prominent induction of antibody responses after the second immunization with antigen (13). Live attenuated influenza A (H5N1) vaccines [A LAIV (H5N1)] have been shown to induce both serum and local antibody responses: studied vaccines have included those generated by reverse genetics and comprised of internal genes from cold-adapted A/Ann Arbor/6/60 (H2N2) virus along with hemagglutinin (HA) and neuraminidase (NA) genes derived from highly pathogenic H5N1 influenza viruses (10, 12, 27), and a vaccine constructed from replication-deficient A (H5N1) virus that lacks the NS1 gene (13, 23).
The live attenuated A (H5N2) influenza vaccine has been developed by a traditional reassortant method combining nonpathogenic avian A/Duck/Potsdam/1402-6/68 (H5N2) and well-characterized cold-adapted, attenuated A/Leningrad/134/17/57 (H2N2) viruses (9, 26). The vaccine strain comprises the HA gene of the avian H5N2 virus and all other genes from the cold-adapted, attenuated strain and has been shown to be safe and protective in mice against live H5N1 virus challenge (9). This same vaccine candidate was also shown to be safe and tolerable in human clinical trials and induced significant antibody titers in both serum and nasal secretion (26). Antibodies elicited in humans by this vaccine were also shown to be cross-reactive to H5N1 virus in standard immunological assays for influenza (26).
However, as vaccinated subjects have not been exposed to highly pathogenic H5N1 influenza virus, it remains unknown whether antibody levels from seasonal vaccination of live attenuated influenza vaccines (LAIV) that have been shown to be protective against seasonal influenza viruses will be sufficient to protect against H5N1 viruses. Furthermore, it has been shown that inactivated vaccines are poor inducers of cellular immunity, which has been shown to play a significant role in protection against H5N1 infection (11). These findings together make the development of vaccines that induce cellular immunity specific to influenza a high priority for pandemic preparedness.
In the present study, CD4 and CD8 memory/effector T-cell immunity was evaluated in healthy young adults who received two doses of live attenuated A (H5N2) vaccine. T-cell responses were measured by standard methods, namely, intracellular cytokine staining (ICCS) of gamma interferon (IFN-γ)-producing cells (19) and a novel T-cell recognition of antigen-presenting cells (APCs) by protein capture (TRAP) assay (3, 8) based on the trogocytosis phenomenon, i.e., plasma membrane exchange between interacting immune cells (17). TRAP enables the detection of activated trogocytosis-positive T cells at initial phases of an immune response during the synaptic complex formation between antigen-presenting cells and T lymphocytes. Recently, TRAP was developed for trogocytosis evaluation in vitro in different cell lines (4, 20, 21) and for the measurement of antigen-specific T-cell responses in mice exposed to ovalbumin (20), lymphocytic choriomeningitis virus (3), or herpesvirus (1). In our study, a modified TRAP assay was used for the evaluation of influenza-specific T-cell responses in humans immunized with live attenuated influenza A (H5N2) vaccine.
MATERIALS AND METHODS
Clinical subjects.
Nineteen healthy adults 18 to 22 years of age met eligibility requirements to participate in the study. Criteria for exclusion from the study were acute infectious and noninfectious diseases, chronic diseases in acute conditions or decompensation, allergy to eggs or any vaccine component, immunodeficiency or immunosuppressive therapy, rhinitis, previous receipt of LAIV, and pregnancy or refusal to use reliable contraception. Written informed consent was obtained from each eligible subject.
Vaccination protocols.
Human subjects were randomized in a 1:1 ratio to receive A LAIV (H5N2) (Orvax; Microgen, Russia) or placebo. One group of vaccinated subjects (n = 10) received two doses of A LAIV (H5N2) that contained 107 50% egg infective doses (EID50)/0.5 ml of the influenza A/17/Duck/Potsdam/86/92 (H5N2) vaccine strain in each dose 21 days apart. Another group of participants (n = 9) received placebo (sterile physiological saline) at the same time interval. Blood, nasal swabs, and saliva samples were collected from subjects at baseline before the first vaccination (day 0), before the second vaccination (day 21), 3 weeks (day 42), and 6 weeks (day 63) after the second vaccination.
Safety monitoring.
Each subject was observed for 2 h postvaccination for allergic and respiratory reactions and was instructed to complete a diary card on the day of vaccination and each of the 6 subsequent days to report systemic and respiratory reactions (i.e., rhinitis, cough, sore throat, malaise, nausea, chills, fever, headache, myalgia, and arthralgia). No participants manifested severe reactions postvaccination necessitating a physician's visit or leading to discontinuation of participation in the study.
Microneutralization and HAI assays.
Serum samples were pretreated with receptor-destroying enzyme (Denka Seiken, Tokyo, Japan) overnight at 37°C and subsequently heated at 56°C for 45 min. The hemagglutination inhibition (HAI) assay was performed according to standard procedures (36) from initial serum dilutions of 1:5 and using a 1% suspension of human red blood cells. The test antigen for each assay was live A/17/Duck/Potsdam/86/92 (H5N2). Neutralization antibody titers were determined as previously described (25). Neutralizing antibody titers were expressed as the reciprocal of the highest dilution of serum that gave 50% neutralization of 100 50% tissue culture infective doses (TCID50) of virus in Madin-Darby canine kidney cells.
ELISA of influenza virus-specific antibodies.
Influenza virus-specific IgG antibodies in sera and IgA antibodies in nasal swabs and saliva were tested by enzyme-linked immunosorbent assay (ELISA) (25) using whole purified A/17/Duck/Potsdam/86/92 (H5N2) virus at 16 HA units (HAU) per 0.05 ml for absorption. The endpoint ELISA titers were expressed as the highest dilution that gave an optical density (OD) greater than twice the mean OD of six negative controls.
Cytokine flow cytometry assay.
Peripheral blood mononuclear cells (PBMCs) were collected and prepared with standard Histopaque-1077 (Sigma, St. Louis, MO) gradient centrifugation from heparinized whole blood, washed twice in phosphate-buffered saline (PBS), and resuspended in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) (Biolot, St. Petersburg, Russia). For cell cryopreservation, cell aliquots containing 1.0 × 107 cells were mixed with an equal volume of RPMI-10% FBS with 20% dimethyl sulfoxide (DMSO) (Sigma) and were stored in liquid nitrogen until the day of analysis. Thawed cells were incubated with or without A/17/Duck/Potsdam/86/92 (H5N2) influenza virus purified by continuous 30%-to-60% sucrose gradient centrifugation. Cells (2.0 × 106) were resuspended in 0.25 ml of RPMI without FBS. Purified influenza A (H5N2) virus was added to cells at a multiplicity of infection (MOI) of 1.5 and incubated at 37°C in 5% CO2 for 1 h. The same volume of PBS was used as the negative control. RPMI-10% FBS and antibiotics were then added to a final volume of 1 ml. These cells were incubated for another 16 h, with brefeldin A (Sigma) added to a final concentration of 10 μg/ml for the last 4 h of incubation. Cells were then washed with PBS and fixed by the addition of 0.5 ml 4% paraformaldehyde (Vecton, St. Petersburg, Russia) for 5 min. After fixation, cells were washed with ice-cold PBS with 0.1% bovine serum albumin (BSA) and permeabilized by a 10-min incubation in 1 ml of PBS with 0.1% BSA and 0.1% saponin (Sigma) (PBS-S solution) followed by a 20-min incubation with equal volumes of PBS-S with 4% BSA. Cells were next centrifuged for 5 min at 1,500 × g to separate cells from the supernatant. Isolated cells were then stained with phycoerythrin (PE)-Cy5-labeled anti-CD8, PE-labeled anti-CD3, and fluorescein isothiocyanate (FITC)-labeled anti-IFN-γ (BD Biosciences, San Jose, CA). The stained cells were analyzed with a Coulter EPICS Altra flow cytometer (Beckman Coulter, Miami, FL). Percentages of influenza A virus-specific IFN-γ cells were determined for the following cell subsets after subtraction of the negative control or the background: CD4 T cells (gated on the CD3+ CD8− lymphocyte population) and CD8 T cells (gated on the CD3+ CD8+ lymphocyte population). The limit of detection for the assay was 0.001%. The average background levels were 0.025% for CD4 and 0.016% for CD8 T cells.
TRAP assay.
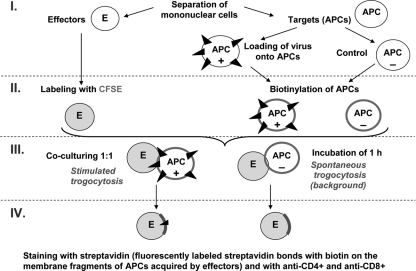
Virus-specific CD8 and CD4 T cells were quantified by a previously described trogocytosis-based method (3, 8) with some modifications (Fig. 1). PBMCs were divided into two groups identified as either “effector” or “target” cells. Target cells were further divided into two groups: control cells and virus-loaded cells. Virus-loaded target cells were plated at a concentration of 2.0 × 106 and were stimulated with 1.5 MOI of purified A/17/Duck/Potsdam/86/92 (H5N2) influenza virus for 1 h in serum-free RPMI at 37°C in 5% CO2. Control target cells were treated with the same conditions except they were exposed to the same volume of PBS only. Then, loaded targets were washed in RPMI-10% FBS and incubated overnight at 37°C in 5% CO2. Virus-loaded target cells were washed twice with PBS and treated with a surface biotinylation reagent (EZ-Link Sulfo-NHS-LC-Biotin, Merck, Darmstadt, Germany) at a final concentration of 1 mg/ml: cells were incubated for 10 min at 25°C and 10 min with an equal volume of FBS at 4°C and then washed three times in RPMI with 10% FBS. Effector cells were plated at a density of 4 × 106 and were labeled with 0.05 μM CFSE (carboxyfluorescein succinimidyl ester; Sigma), incubated for 5 min at room temperature in dark conditions, and washed three times in PBS with 10% FBS. In the next step, effector cells at a density of 1 × 106 were cocultured with either virus-loaded or control target cells at a 1:1 ratio for 1 h at 37°C in 5% CO2. These cocultured cells were next treated with cold PBS-EDTA, washed in PBS, and then stained with ECD (Texas Red)-labeled anti-CD8 (Beckman Coulter), PE-labeled anti-CD4 (BD Biosciences) antibodies, and PE-Cy5-labeled streptavidin (BD Biosciences). Trogocytosis-positive effector cells were identified as CFSE-labeled (CFSE+) cells presenting biotinylated plasma membrane of target cells bound to streptavidin (StAv+ cells). Quantification of CFSE+ StAv+ CD8+ CD4− and CFSE+ StAv+ CD8− CD4+ effector cells was performed with a Coulter EPICS Altra flow cytometer (Beckman Coulter). Percentages of influenza virus-specific trogocytosis-positive cells were determined after subtraction of the corresponding percentage of positive-staining cells from the control sample.
Fig. 1.
Scheme of TRAP assay.
Statistical analysis.
Antibody levels are reported as geometric mean titers (GMTs), and cells staining positive for specific markers are reported as geometric mean percentages (GMPs). Data from microneutralization assay (MNA), HAI, and ELISA were transformed to log2 values, while data from cytokine flow cytometry assays were transformed to log10 values. Mean n-fold changes (FCs) were computed as arithmetical means. Statistical comparisons of various groups were performed by using the Wilcoxon matched-pair test for dependent variables (changes from point to point in one group) and Student's t test for independent samples (comparing vaccine with placebo). Spearman's coefficient (r) was computed for correlations. Attained significance levels with P values of <0.05 were considered to be statistically significant. To evaluate the immunogenicity of the vaccine, the percentage of reliable increases (RIs) was determined. RIs in antibody responses were estimated as ≥4-fold increases of antibody titers. RIs in cell levels were estimated as results exceeding 3 times the standard deviation (SD) of the mean from control cells.
RESULTS
Percentages of influenza A (H5N2)-specific IFN-γ+ and trogocytosis+ T cells.
Memory/effector T-cell responses were evaluated in 19 healthy young human subjects who were inoculated with live attenuated A (H5N2) vaccine. Each subject received two doses of vaccine 21 days apart, and blood samples to evaluate cellular immune responses were collected before vaccine administration (day 0), 21 days after the first vaccination (day 21), 21 days after the second vaccination (day 42), and 42 days after the second vaccination (day 63). Percentages of IFN-γ+ cells among CD4 and CD8 T cells were determined by using standard intracellular cytokine staining (ICCS) methods. In addition to standard methods, a recently developed TRAP assay was used to measure virus-specific CD4 and CD8 T cells by their interactions with antigen-presenting cells via trogocytosis.
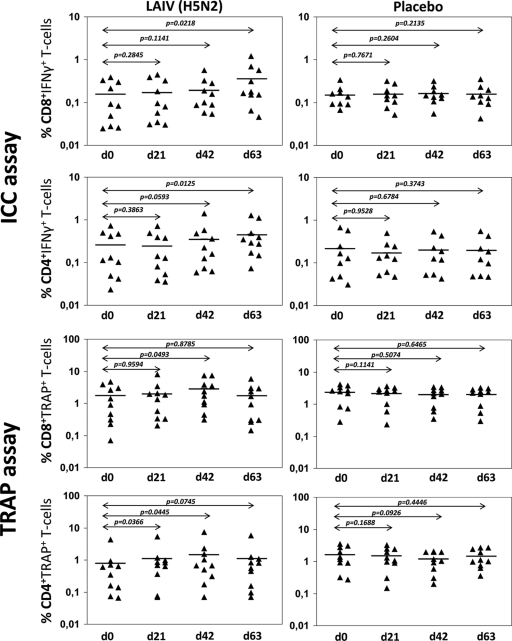
The GMPs of influenza A (H5N2)-specific IFN-γ cells in peripheral blood of vaccinated subjects ranged from 0.025 to 1.206 and from 0.023 to 1.404 in CD4 and CD8 T-cell subsets, respectively (Fig. 2). Significant differences in both CD4 and CD8 IFN-γ-producing T cells were observed only from blood collected at day 63, 42 days after the second vaccination. In contrast, the GMPs of trogocytosis-positive T cells in the TRAP assay were considerably higher than IFN-γ+ cell levels and ranged from 0.070 to 7.761 (for CD8 cells) and from 0.066 to 7.357 (for CD4 cells). Significant differences in influenza A (H5N2)-specific trogocytosis-positive T cells were observed on day 42, 21 days after the second vaccination.
Fig. 2.
Percentages of CD4 and CD8 T cells in TRAP after vaccination with LAIV (H5N2) of young adults. Cells were measured before the first vaccination (d0, baseline), after the first vaccination (d21), at the 21st day after the second vaccination (d42), and at the 42nd day after the second vaccination (d63). The bars denote the GMPs of IFN-γ+ cells; P values were estimated by the Wilcoxon test.
Vaccine-induced changes in percentages of influenza A (H5N2)-specific T cells measured by ICCS and TRAP assays.
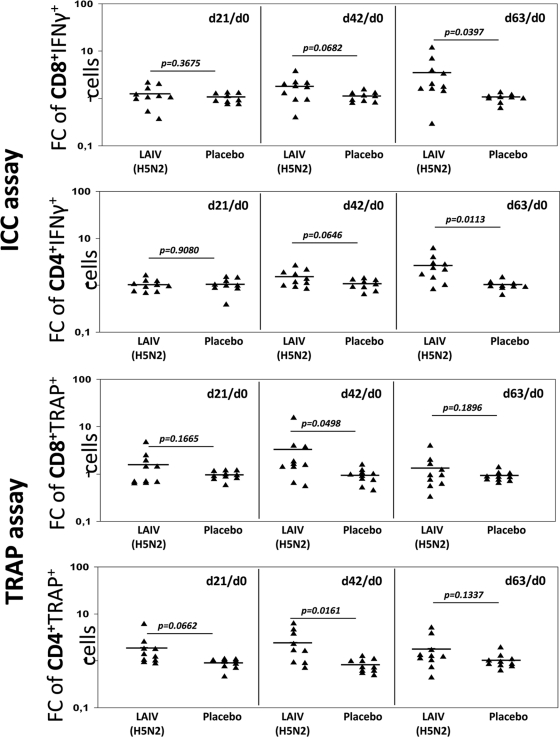
The same trend was observed in n-fold changes in GMPs of IFN-γ+ and trogocytosis+ CD4 and CD8 T cells (Fig. 3). The greatest changes (n-fold) in GMPs of IFN-γ+ cells were observed on day 63, averaging 3.56 in CD8 and 2.60 in CD4 T cells. The maximum n-fold changes in GMPs of influenza-specific T cells measured by the TRAP assay were observed for cells from day 42, 3.29-fold and 2.45-fold in CD4 and CD8 trogocytosis-positive T cells, respectively.
Fig. 3.
Differences in LAIV (H5N2)-induced n-fold changes of CD4 and CD8 T cells versus placebo in young adults. FCs were measured at day 21 after the first vaccination, day 42 (21st day after the second vaccination), and day 63 (42nd day after the second vaccination) and compared to values at day 0 (d21/d0, d42/d0, and d63/d0, respectively). The bars denote the means of n-fold changes in IFN-γ+ cells; P values were estimated by t test for independent variables.
Reliable changes (n-fold) in GMPs of influenza A (H5N2)-specific IFN-γ-producing and trogocytosis-positive T cells were analyzed next. Due to a lack of quantitative standards for cellular immune responses to assess vaccine immunogenicity (7), reliable increases in cell levels were considered as results exceeding 3 times the SD of placebo mean values. The ICCS assay showed gradually increasing percentages of reliable n-fold changes in IFN-γ+ CD8 and CD4 T-cell subsets from day 0 to day 63, whereas the highest percentage of reliable increases in trogocytosis-positive T cells in the TRAP assay was observed earlier for cells collected at day 42.
These data clearly show that healthy young persons who had presumably never been recipients of “avian” influenza vaccines nor had been exposed to H5N1 wild-type viruses were able to respond to live A (H5N2) influenza vaccine, as indicated by significant increases in CD8 and CD4 memory/effector T-cell levels. However, outcomes observed from T-cell response dynamics measured by standard ICCS and by novel TRAP assays did not appear to correlate; no correlation was noted between the CD8 and CD4 T-cell levels estimated by the two assays or in n-fold changes in GMPs of these cells (r ranged from 0.14 to 0.23; P > 0.05).
Relationship between the baseline levels of influenza A-specific cells and changes after vaccination.
It should be noted that influenza A (H5N2)-specific CD8 and CD4 T cells were observed in subjects both before vaccination and in the placebo group. IFN-γ+ T-cell percentages in these subjects ranged from 0.025 to 0.384 in the CD8 subset and from 0.023 to 0.729 in the CD4 subset. Similar percentages were measured by the TRAP assay: 0.070 to 4.796 in CD8 T cells and 0.066 to 3.528 in CD4 T cells. Since a wide range of influenza A (H5N2)-specific T cells was obtained in peripheral blood of nonvaccinated subjects, the relationship between baseline levels of these cells, which reflect cellular immunity to influenza at the time of vaccination, and changes (n-fold) after vaccination, which reflect cellular immune response to the vaccine, were analyzed. Inverse correlations were detected between baseline cell levels (day 0) and changes (n-fold) at all time points (day 21, day 42, and day 63); the Spearman correlation coefficients (r value) ranged from −0.31 to −0.77. However, statistically significant values of r were obtained only at day 42 for IFN-γ+ T cells measured by the ICCS test. As these subjects had never been primed with influenza A (H5N2) virus before vaccination, we hypothesized that T-cell levels after the first vaccination could also be considered a baseline level similar to what is seen with seasonal vaccination when subjects have preexisting cellular immunity to influenza A virus. Estimation of the relationship between cell levels after the first vaccination (day 21) and n-fold changes in GMPs of those cells at subsequent time points (day 42, day 63) yielded significant inverse correlations in IFN-γ+ T cells. These data suggest the influence of baseline influenza-specific CD4 and CD8 T cells on the cellular immune responses after live influenza A (H5N2) vaccine administration.
Antibody immune responses in subjects vaccinated with live influenza A (H5N2) vaccine.
Influenza A (H5N2)-specific antibody levels from peripheral blood were assessed on days 0, 21, 42, and 63 as measured by HAI, MNA, and ELISA. In addition, local IgA antibody levels in nasal swabs and saliva were evaluated by an ELISA (Table 1). No subjects had serum and local antibody titers of more than 1:10 before vaccination. After vaccination, significant increases in influenza A (H5N2)-specific antibody GMTs were observed after the second vaccination on day 42. The greatest percentage of subjects with reliable changes of GMT (≥4-fold increases in antibody levels) detected by HAI was observed at day 63. However, the percentage of subjects with protective antibody titers (≥1:40) was only 20%. Thus, two doses of live influenza A (H5N2) vaccine induced moderate antibody immune responses in serum, nasal swabs, and saliva with a low intensity of antibody formation.
Table 1.
Antibody immune responses after A LAIV (H5N2) vaccination
| Group | Daya | Serum antibody responses |
Local antibody responses (ELISA IgA) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HAI |
MNA |
ELISA IgG |
Nasal swabs |
Saliva |
||||||||
| GMT | % with ≥4-fold increases in Ab titersb | % with titer ≥1:40 | GMT | % with ≥4-fold increases in Ab titersb | GMT | % with ≥4-fold increases in Ab titersb | GMT | % with ≥4-fold increases in Ab titersb | GMT | % with ≥4-fold increases in Ab titersb | ||
| LAIV (n = 10) | 0 | 5.4 | – | 0 | 5.0 | – | 34.8 | – | 5.7 | – | 4.9 | – |
| 21 | 8.7 | 2 (20%) | 1 (10%) | 6.6 | 2 (20%) | 42.9 | 1 (10%) | 10.6 | 2 (20%) | 7.0 | 0 | |
| 42 | 11.5c,d | 3 (30%) | 2 (20%) | 16.3c,d | 4 (40%) | 113.2c,d | 6 (60%) | 9.9c | 2 (20%) | 9.9d | 3 (30%) | |
| 63 | 14.2c,d | 5 (50%) | 2 (20%) | 16.3c,d | 4 (40%) | 80.0c,d | 6 (60%) | 8.0 | 3 (30%) | 7.0 | 3 (30%) | |
| Placebo (n = 10) | 0 | 4.6 | – | 0 | 5.0 | – | 29.4 | – | 4.7 | – | 6.2 | – |
| 21 | 4.6 | 0 | 0 | 5.0 | 0 | 25.2 | 0 | 5.0 | 0 | 6.7 | 0 | |
| 42 | 4.6 | 0 | 0 | 5.0 | 0 | 27.2 | 0 | 4.0 | 0 | 5.2 | 0 | |
| 63 | 4.6 | 0 | 0 | 5.0 | 0 | 27.2 | 0 | 4.0 | 0 | 5.2 | 0 | |
Days: 0, before vaccination; 21, 21st day after the first vaccination; 42, 21st day after the second vaccination; 63, 42nd day after the second vaccination.
% with ≥4-fold increases in antibody (Ab) titers: % of subjects with reliable ≥4-fold increases in Ab titers after vaccination was not detectable (−) at day 0.
Results were adjusted with a P value of <0.05 compared with the placebo group.
Results were adjusted with a P value of <0.05 compared with baseline Ab levels (at day 0).
Comparability of antibody to cellular immune responses following influenza A (H5N2) vaccine.
The comparability of antibody to cellular immune responses was evaluated following influenza A (H5N2) vaccination. Since immunogenicity of influenza vaccines is traditionally evaluated by the hemagglutination inhibition test, the standard immunogenicity parameter (seroconversion rate: a ≥4-fold increase in HAI antibody titers) was compared to n-fold changes in GMPs of CD4 and CD8 T cells (Table 2).
Table 2.
Comparative results of ICC and TRAP assays
| ID of subject LAIV vaccine recipient | HAI response | Cell-mediated immune responsea |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 21/day 0 |
Day 42/day 0 |
Day 63/day 0 |
|||||||||||
| TRAP assay |
ICC assay |
TRAP assay |
ICC assay |
TRAP assay |
ICC assay |
||||||||
| CD8 | CD4 | IFNγ+ CD8 | IFNγ+ CD4 | CD8 | CD4 | IFNγ+ CD8 | IFNγ+ CD4 | CD8 | CD4 | IFNγ+ CD8 | IFNγ+ CD4 | ||
| 1 | + | − | − | − | − | + | + | − | − | − | − | + | + |
| 3 | + | − | + | + | + | − | − | + | + | − | − | + | + |
| 4 | + | − | + | − | − | − | + | − | − | + | + | + | + |
| 5 | + | + | + | − | − | + | + | + | − | + | + | + | + |
| 6 | + | − | − | − | − | − | − | − | − | − | − | − | − |
| 2 | − | − | − | − | − | − | − | − | − | − | − | + | + |
| 7 | − | − | − | − | − | − | + | + | − | − | − | − | − |
| 8 | − | + | − | − | − | + | − | + | + | + | − | + | + |
| 9 | − | − | − | − | − | − | + | − | − | − | − | − | − |
| 10 | − | − | − | + | − | + | + | − | + | − | − | − | − |
+, Reliable increases; −, absence of such increases. Reliable cell percentage increases were estimated as FC (n-fold change) results exceeding 3 SD of placebo mean value. FCs were evaluated 21 days after the first vaccination (day 21/day 0), 21 days after the second vaccination (day 42/day 0), and 42 days after the second vaccination (day 63/day 0).
HAI titer increases of ≥4-fold were detected in 50% of vaccinated subjects (5 of 10) on day 63. Most subjects (4 of 5) who had increases in HAI titers responded to vaccination by the reliable n-fold changes in GMPs of IFN-γ+ or trogocytosis+ CD4 and CD8 T cells. However, similar cellular responses were detected in subjects without significant increases in HAI titers. Almost every person in the HAI-negative group had reliable n-fold changes in IFN-γ+ and trogocytosis+ CD4 and CD8 T cells, particularly at day 42, following the second vaccination. It should be noted that the cell immune response data in HAI-positive and HAI-negative groups did not coincide. Thus, data on cellular immune responses should be considered as additional information along with the HAI results when vaccine-related immune protection is considered.
Data were then analyzed from another perspective to investigate a possible relationship between humoral immunity measured by HAI and baseline memory T-cell levels. Baseline levels of influenza-specific trogocytosis-positive CD4 T cells measured by TRAP assay in HAI-negative subjects were 2.6 times greater than the ones in volunteers with seroconversions in HAI titers (1.149% and 0.440% of CD4 cells, respectively). Moreover, a significant inverse correlation was observed between baseline levels of trogocytosis-positive CD4 T cells and n-fold increases in anti-hemagglutination antibodies. The Spearman correlation coefficients ranged from −0.48 to −0.55 (P < 0.05) at days 21, 42, and 63. However, there was no significant difference in baseline levels of IFN-γ+ CD4 T cells measured by ICCS between HAI-positive and HAI-negative groups (0.236 and 0.278%, respectively). There was not a detectable difference in baseline levels of CD8 T cells measured by ICCS and TRAP assay. Thus, only baseline levels of trogocytosis-positive influenza-specific CD4 T cells showed a trend of association with the antibody response to the vaccine.
DISCUSSION
The principle of vaccination is based on the ability to stimulate both T and B memory responses that provide immunity against the antigen target. Animal studies have clearly shown a role of antigen-specific T cells in protection from virus infections (28, 30). Evaluation of currently licensed influenza vaccines and those in the development pipeline would help to identify T memory/effector responses that could be harnessed or optimized to improve the vaccine immunogenicity and breadth of protection. In the current study we observed the following attributes associated with vaccination of young adults with live attenuated influenza A (H5N2) vaccine: (i) T memory/effector cellular immune responses; (ii) a correlation between postvaccine induction of IFN-γ+ CD4 and CD8 T cells measured by standard ICCS procedure and trogocytosis-positive CD4 and CD8 T cells identified by novel TRAP assay; and (iii) the comparability of HAI seroconversion rates, the gold standard method to evaluate immunogenicity elicited by influenza vaccines, with postvaccine n-fold changes in T-cell levels.
Data in this study showed that two doses of live influenza A (H5N2) vaccine promoted both CD4 and CD8 memory T-cell responses in peripheral blood of healthy young subjects. It has been shown that about 90% of effector T cells are cleared from the host by apoptosis following elimination of the antigen; remaining T cells acquire resistance to apoptosis and develop into a memory phenotype (34). LAIV strains are generally eliminated from the recipient within the first week after vaccine administration (2, 29). As in the current study, blood samples collected 21 days after vaccination were considered to be memory T cells.
He et al. (15) observed cellular immunity in response to the live attenuated seasonal influenza vaccine FluMist. In their studies, they detected a significant increase in the GMPs of influenza A (H3N2)-specific IFN-γ+ CD4 and CD8 T cells only in children 5 to 9 years old and not in adults. Such data could be explained by the presence of existing immunity to the virus and thus greater baseline levels of virus-specific T cells in adults relative to children in their study. Our results showed that live attenuated influenza A (H5N2) vaccine was able to induce reliable increases in T-cell levels even in adults, due to the possibility that those subjects had not been vaccinated with A LAIV (H5N2) nor exposed to wild-type influenza H5 virus before their participation in the trial and had no preexisting immunity to influenza A (H5N2). We noted an inverse correlation between baseline levels and postvaccine n-fold changes in the GMPs of influenza-specific CD4 and CD8 T cells that compare to the data of He et al. (14) from exposure to seasonal FluMist LAIV. These authors have hypothesized that baseline levels of virus-specific T cells could be a predictive factor for the immunological outcome of vaccination.
We were able to show levels of influenza A (H5N2)-specific CD4 and CD8 T cells in peripheral blood of human clinical trial subjects prior to vaccination as well as in subjects who received only placebo vaccination. Recently, similar findings were observed by other investigators who had detected influenza A (H5N1)-specific T cells in subjects who had never been exposed to avian influenza viruses (18, 24). These authors showed that most of the identified influenza A (H5N1)-reactive T cells were specific to conserved viral proteins such as the influenza matrix or nucleoproteins, and a few identified conserved T-cell epitopes from the influenza hemagglutinin. Data supported the observation that seasonal influenza A (H3N2) and A (H1N1) virus infections were able to elicit significant levels of cross-reactive T cells to avian influenza H5 variant (18). Based on these observations, we hypothesize that preexisting influenza A (H5N2)-specific T cells detected in subjects in our trial were most likely from exposure to seasonal influenza viruses or vaccines and likely recognized conservative, cross-reactive cellular epitopes from the virus.
In our study, a novel TRAP assay modified for human T-cell assessment was utilized to determine influenza A (H5N2)-specific trogocytosis-positive CD4 and CD8 T cells. It was suggested that the TRAP assay was comparable to the ICCS assay with added benefits of technical ease and reduced time (results can be obtained within a day) (8, 17, 20, 21). In addition, the TRAP assay permits the detection of reactive CD4 and CD8 T and B cells in a single assay and provides ability to detect early intercellular interactions such as trogocytosis between APCs and activated lymphocytes within the first minute of interaction. Studies from in vitro and in vivo animal models have shown that TRAP assays are comparable to previously published methods such as ICCS and cellular staining with MHC multimers (3, 20). We have shown dynamic changes in influenza-specific T cells measured by TRAP in vaccinated subjects compared with the placebo group. However, our data obtained by the TRAP assay did not correlate with ICCS results; the times of peak cell levels were different (days 42 and 63, respectively), and there was no correlation between individual levels of trogocytosis+ and IFN-γ+ T cells. Discrepancies between ICCS and TRAP assays seem to depend on different techniques for the measurement of activated T cells. The ICCS method evaluates the terminal step of T-cell activation, namely, the production of cytokines, while the TRAP assay determines early events, i.e., trogocytosis, a plasma membrane exchange between effector and target cells, which begins during the very first minutes of cell-cell interaction (16, 17). Moreover, within the CD3+ CD8− T-cell gate, we were able to detect by ICCS CD4 cells producing IFN-γ which are predominantly related to the Th1 subset, whereas by TRAP assay we obviously estimated both Th1 and Th2 cell subsets, as all the CD4 T-cell subsets were considered to be trogocytosis positive (16). This suggestion corresponds to levels of CD4 T cells measured by TRAP that were higher than those estimated by ICCS. A different timeline of CD4 effector/memory Th1/Th2 cell development and maintenance may provide the observed decrease of trogocytosis-positive cells at day 63 (versus day 42), which has not been found by the ICCS assay (22, 28).
We analyzed the relationship between humoral immune responses and levels of trogocytosis-positive CD4 T cells, including Th2 cells, which are known to be responsible for humoral immune responses (22). We noted an inverse correlation between baseline trogocytosis-positive CD4 cells and antibody levels measured by HAI. The influence of CD4 T-cell levels on antibody production has also been shown in the mouse model by adoptive transfer of influenza-specific CD4 T cells followed by measurement of humoral immunity in transfer-recipient animals (6). Since He et al. (14) proposed that baseline levels of T cells could be a predictive marker of postvaccine cellular immune response, we hypothesized that baseline levels of trogocytosis-positive CD4 T cells could be a predictive indicator of both cellular and humoral immunogenicity in response to a vaccine. We consider these findings to be a significant selection factor to be considered for subjects participating in clinical trials.
The immunogenicity raised in response to influenza vaccines has traditionally been evaluated by serologic parameters measured in the hemagglutination inhibition (HAI) assay, including geometric mean titer in pre- and postvaccination specimens, seroconversion rate (typically a ≥4-fold increase in HAI titer), and a postvaccination protection threshold (e.g., an HAI titer of >1:40) (5). However, anti-hemagglutination antibody production may not reflect vaccine efficacy as it does not measure the full scope of the immune response, in particular cellular immunity or memory responses. For prevention from and control of influenza infection, the WHO recommends the development and optimization of assays to determine cellular immunity and the development of appropriate criteria to measure vaccine immunogenicity (7). In our study using a novel influenza A (H5N2) vaccine, we observed a significant discrepancy between the HAI-based immunogenicity results and data obtained from ICCS and TRAP assays used to measure postvaccine memory T-cell immunity. All subjects with negative postvaccine antibody response measured by HAI had significant changes in GMPs of IFN-γ-producing and/or trogocytosis-positive cells. Based on these findings, we highlight the need for measurement of cellular immunity to be able to more clearly evaluate effectiveness of influenza vaccines and to guide us to methods to improve the breadth of immunogenicity that can be achieved in future generations of influenza vaccines.
In summary, this study shows that immunization of healthy young adults with live attenuated A (H5N2) influenza vaccine induced an increase in influenza-specific CD4 and CD8 memory/effector T-cell levels as measured by a standard ICCS method, intracellular IFN-γ staining, and by a novel TRAP assay that detects activated trogocytosis-positive cells. Results obtained by both ICCS and TRAP assays are additive to HAI data in providing additional information about the immunogenicity of influenza vaccines. Baseline levels of influenza-specific CD4 and CD8 T cells target the postvaccine cellular immune response and can be considered as a predictive factor for vaccine effectiveness.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Adamopoulou E., et al. 2007. Human CD4+ T cells displaying viral epitopes elicit a functional virus-specific memory CD8+ T cell response. J. Immunol. 178:5465–5472 [DOI] [PubMed] [Google Scholar]
- 2. Alexandrova G. I., Klimov A. I. 1994. Live influenza vaccine. Nauka, Saint Petersburg, Russia [Google Scholar]
- 3. Beadling C., Slifka M. K. 2006. Quantifying viable virus-specific T cells without a priori knowledge of fine epitope specificity. Nat. Med. 12:1208–1212 [DOI] [PubMed] [Google Scholar]
- 4. Beum P. V., Mack D. A., Pawluczkowycz A. W., Lindorfer M. A., Taylor R. P. 2008. Binding of rituximab, trastuzumab, cetuximab, or mAb T101 to cancer cells promotes trogocytosis mediated by THP-1 cells and monocytes. J. Immunol. 181:8120–8132 [DOI] [PubMed] [Google Scholar]
- 5. Beyer W. E. P., Palache A. M, Luchters G., Nauta J., Osterhaus A. D. M. E. 2004. Seroprotection rate, mean fold increase, seroconversion rate: which parameters adequately expresses seroresponse to influenza vaccination? Virus Res. 103:125–132 [DOI] [PubMed] [Google Scholar]
- 6. Brown D. M., Dilzer A. M., Meents D. L., Swain S. L. 2006. CD4 T-cell mediated protection from lethal influenza: perforin and antibody-mediated mechanism give a one-two punch. J. Immunol. 177:2888–2898 [DOI] [PubMed] [Google Scholar]
- 7. Cassetti M. C., Katz J. M., Wood J. 2006. Report of a consultation on role of immunological assays to evaluate efficacy of influenza vaccines. Initiative for Vaccine Research and Global Influenza Programme, World Health Organization, Geneva, Switzerland, 25 January 2005. Vaccine 24:541–543 [DOI] [PubMed] [Google Scholar]
- 8. Daubeuf S., Puaux A. L., Joly E., Hudrisier D. 2006. A simple trogocytosis-based method to detect, quantify, characterize and purify antigen-specific live lymphocytes by flow cytometry, via their capture of membrane fragments from antigen-presenting cells. Nat. Protoc. 1:2536–2542 [DOI] [PubMed] [Google Scholar]
- 9. Desheva J. A., et al. 2006. Characterization of an influenza A H5N2 reassortant as a candidate for live-attenuated and inactivated vaccines against highly pathogenic H5N1 viruses with pandemic potential. Vaccine 24:6859–6866 [DOI] [PubMed] [Google Scholar]
- 10. Fan S., et al. 2009. Immunogenicity and protective efficacy of a live attenuated H5N1 vaccine in nonhuman primates PLoS Pathog. 5:e1000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gambotto A., Barratt-Boyes S. M., de Jong M. D., Neumann G., Kawaoka Y. 2008. Human infection with highly pathogenic H5N1 influenza virus. Lancet 371:1464–1475 [DOI] [PubMed] [Google Scholar]
- 12. Girard M., Palkonyay L., Kieny M. P. 2008. Report of the 4th meeting on the evaluation of pandemic influenza prototype vaccines in clinical trials. Vaccine 26:4975–4977 [DOI] [PubMed] [Google Scholar]
- 13. Hayden F. G., Howard W. A., Palkonyay L., Kieny M. P. 2009. Report of the 5th meeting on the evaluation of pandemic influenza prototype vaccines in clinical trials: World Health Organization, Geneva, Switzerland, 12–13 February 2009. Vaccine 27:4079–4089 [DOI] [PubMed] [Google Scholar]
- 14. He X. S., et al. 2008. Baseline levels of influenza-specific CD4 memory T-cells affect T-cell responses to influenza vaccines. PLoS One 3:2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. He X. S., et al. 2006. Cellular immune responses in children and adults receiving inactivated or live attenuated influenza vaccines. J. Virol. 80:11756–11766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hudrisier D., Aucher A., Puaux A. L., Bordier C., Joly E. 2007. Capture of target cell membrane components via trogocytosis is triggered by a selected set of surface molecules on T or B cells. J. Immunol. 178:3637–3647 [DOI] [PubMed] [Google Scholar]
- 17. Joly E., Hudrisier D. 2003. What is trogocytosis and what is its purpose? Nat. Immunol. 4:815. [DOI] [PubMed] [Google Scholar]
- 18. Lee L. Y., et al. 2008. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J. Clin. Invest. 118:3478–3490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Prussin C., Metcalfe D. D. 1995. Detection of intracytoplasmic cytokine using flow cytometry and directly conjugated anti-cytokine antibodies. J. Immunol. Methods 188:117–128 [DOI] [PubMed] [Google Scholar]
- 20. Puaux A. L., et al. 2006. A very rapid and simple assay based on trogocytosis to detect and measure specific T and B cell reactivity by flow cytometry. Eur. J. Immunol. 36:779–788 [DOI] [PubMed] [Google Scholar]
- 21. Rechavi O., et al. 2007. Intercellular transfer of oncogenic H-Ras at the immunological synapse. PLoS One 2:e1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Romagnani S. 1991. Type 1 T helper and type 2 T helper cells: functions, regulation and role in protection and disease. Int. J. Clin. Lab. Res. 21:152–158 [DOI] [PubMed] [Google Scholar]
- 23. Romanova J., et al. 2009. Preclinical evaluation of a replication-deficient intranasal DeltaNS1 H5N1 influenza vaccine. PLoS One 4:e5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roti M., et al. 2008. Healthy human subjects have CD4+ T cells directed against H5N1 influenza virus. J. Immunol. 180:1758–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rowe T., et al. 1999. Detection of antibody to avian influenza A(H5N1) virus in human serum by using a combination of serologic assays. J. Clin. Microbiol. 37:937–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rudenko L., et al. 2008. Safety and immunogenicity of live attenuated influenza reassortant H5 vaccine (phase I-II clinical trials). Influenza Other Respi. Viruses 2:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Subbarao K., et al. 2003. Evaluation of a genetically modified reassortant H5N1 influenza A virus vaccine candidate generated by plasmid-based reverse genetics. Virology 305:192–200 [DOI] [PubMed] [Google Scholar]
- 28. Swain S. L., et al. 2006. CD4+ T-cell memory: generation and multi-faceted roles for CD4+ T cells in protective immunity to influenza. Immunol. Rev. 211:8–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tamura S., Tanimoto T., Kurata T. 2005. Mechanisms of broad cross-protection provided by influenza virus infection and their application to vaccines. Jpn. J. Infect. Dis. 58:195–207 [PubMed] [Google Scholar]
- 30. Thomas P. G., Keating R., Hulse-Post D. J., Doherty P. C. 2006. Cell-mediated protection in influenza infection. Emerg. Infect. Dis. 12:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ungchusak K., et al. 2005. Probable person-to-person transmission of avian influenza A (H5N1). N. Engl. J. Med. 352:333–340 [DOI] [PubMed] [Google Scholar]
- 32. Uyeki T. M. 2008. Global epidemiology of human infections with highly pathogenic avian influenza A (H5N1) viruses. Respirology 13(Suppl. 1):S2–S9 [DOI] [PubMed] [Google Scholar]
- 33. Wang H., et al. 2008. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet 371:1427–1434 [DOI] [PubMed] [Google Scholar]
- 34. Welsh R. M., Selin L. K., Szomolanyi-Tsuda E. 2004. Immunological memory to viral infections. Annu. Rev. Immunol. 22:711–743 [DOI] [PubMed] [Google Scholar]
- 35. World Health Organization. 2011. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/disease/avian_influenza/country/cases_table_2011_03_16/en/index.html. [Google Scholar]
- 36. World Health Organization. 2005. WHO manual on animal influenza diagnosis and surveillance. WHO/CDS/CSR/NCS/2002.5. World Health Organization, Geneva, Switzerland: http://www.who.int/csr/resources/publications/influenza/whocdscsrncs20025rev.pdf. Accessed 20 September 2008 [Google Scholar]