Abstract
Enterotoxigenic Escherichia coli (ETEC) strains expressing K88 (F4) or F18 fimbriae and heat-labile (LT) and/or heat-stable (ST) toxins are the major cause of diarrhea in young pigs. Effective vaccines inducing antiadhesin (anti-K88 and anti-F18) and antitoxin (anti-LT and anti-ST) immunity would provide broad protection to young pigs against ETEC. In this study, we genetically fused nucleotides coding for peptides from K88ac major subunit FaeG, F18 minor subunit FedF, and LT toxoid (LT192) A2 and B subunits for a tripartite adhesin-adhesin-toxoid fusion (FaeG-FedF-LT192A2:B). This fusion was used for immunizations in mice and pigs to assess the induction of antiadhesin and antitoxin antibodies. In addition, protection by the elicited antiadhesin and antitoxin antibodies against a porcine ETEC strain was evaluated in a gnotobiotic piglet challenge model. The data showed that this FaeG-FedF-LT192A2:B fusion elicited anti-K88, anti-F18, and anti-LT antibodies in immunized mice and pigs. In addition, the anti-porcine antibodies elicited neutralized cholera toxin and inhibited adherence against both K88 and F18 fimbriae. Moreover, immunized piglets were protected when challenged with ETEC strain 30302 (K88ac/LT/STb) and did not develop clinical disease. In contrast, all control nonvaccinated piglets developed severe diarrhea and dehydration after being challenged with the same ETEC strain. This study clearly demonstrated that this FaeG-FedF-LT192A2:B fusion antigen elicited antibodies that neutralized LT toxin and inhibited the adherence of K88 and F18 fimbrial E. coli strains and that this fusion could serve as an antigen for vaccines against porcine ETEC diarrhea. In addition, the adhesin-toxoid fusion approach used in this study may provide important information for developing effective vaccines against human ETEC diarrhea.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) strains continue to be the most important cause of diarrhea in neonatal and postweaning pigs (12, 31). ETEC diarrhea causes weight loss, slow growth, and death and results in substantial economic losses to swine producers worldwide (1, 11, 27, 28, 30, 31). The key virulence factors of ETEC in diarrhea are bacterial fimbriae and enterotoxins (4, 17, 24, 42, 43). Fimbriae mediate ETEC bacteria for attachment to porcine small intestinal epithelial cells and subsequent colonization, whereas enterotoxins disrupt fluid homeostasis in host small intestinal epithelial cells to cause fluid and electrolyte hypersecretion that leads to diarrhea (20). Fimbriae expressed by ETEC strains isolated from young pigs with diarrhea include K88 (F4), F18, K99 (F5), 987P (F6), and F41 (F7), and toxins produced by porcine ETEC strains are heat-labile (LT), heat-stable type I (STa), heat-stable toxin type II (STb), Shiga toxin 2e (Stx2e), and enteroaggregative heat-stable toxin 1 (EAST1) (9, 44). ETEC strains expressing K88 or F18 fimbriae and LT and ST (STa and STb) toxins are the most commonly associated with diarrhea in weaned pigs (8, 9, 22, 44). In the United States, nearly all porcine postweaning diarrhea cases are associated with ETEC strains expressing K88 or F18 fimbria with one or more toxins (44).
There are no effective vaccines currently available that provide broad protection against porcine postweaning diarrhea caused by ETEC. Common veterinary practice is to immunize pregnant sows for stimulation of maternal antibodies which protect suckling pigs against ETEC diarrhea. However, passively acquired antibodies protect pigs only while they are suckling and are rapidly lost at weaning. Postweaning pigs remain immunologically naïve to ETEC, and they develop diarrhea after ETEC infection. Immunization of weaned pigs with vaccines containing K88 and/or F18 fimbrial antigens induces anti-K88 and/or anti-F18 antibodies (32, 33, 35). However, these products are not likely to fully protect weaned pigs against postweaning diarrhea (10). It becomes clear that effective ETEC vaccines need to induce both antiadhesin immunity to block ETEC adherence and antitoxin immunity to neutralize enterotoxicity (5, 38). New approaches to construct vaccine antigens to stimulate both antiadhesin and antitoxin immunity in hosts are needed for development of effective vaccines against porcine diarrhea.
In this study, we genetically fused nucleotides encoding peptides of K88 FaeG, F18 FedF, and LT toxoid (LT192) for a tripartite adhesin-adhesin-toxin chimeric antigen and evaluated its potential as an ETEC vaccine. FaeG is the major structural subunit for K88 fimbriae (3), and FaeG antigens elicited antibodies blocking K88 fimbrial adherence (26). Experimental vaccines carrying K88 antigens showed some protection against ETEC strains expressing the same fimbriae (25, 34, 36). FedF is a minor subunit of F18 fimbriae, and it plays a critical role in F18 fimbrial adherence (23). However, immunization of purified F18 fimbriae showed no protection to pigs against F18 ETEC infection (35). Interestingly, after its conservative minor subunit FedF was conjugated to K88 fimbriae and coadministered with the strong mucosal adjuvant cholera toxin (CT), the conjugates induced systemic and local anti-F18 immunity that led to reduction of excretion against F18 ETEC infection (29). However, CT (and LT) are potentially toxic and may cause diarrhea in humans and animals, although a few studies indicated that a small dose of up to 100 μg has no effect in weaned pigs (communication from David Francis). LT, a homologue of CT, especially the detoxified LT192 (7), is also a strong antigen and a mucosal adjuvant. The LT192 toxoid can serve as an effective adjuvant and a safe antigen in ETEC vaccine development. Our recent studies demonstrated that genetic fusions of LT192 with ST antigens enhanced anti-ST immunogenicity and elicited protective anti-LT and anti-ST immunity (40, 41). By fusing the F18 FedF antigen to the K88 FaeG and then to the LT192, we constructed a chimeric antigen to induce anti-K88, anti-F18, and anti-LT immunity against ETEC-associated porcine diarrhea.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The strains and plasmids used in this study are listed in Table 1. Porcine ETEC field isolates 3030-2 (K88ac/LT/STb) and 06-20147 (F18/STa/STb/stx2e) were used to PCR amplify the faeG gene (coding for the K88 FaeG major subunit) and the fedF gene (coding for the F18 FedF minor subunit). Strain 3030-2 and an E. coli field isolate, strain 8516, which only expresses F18 fimbriae, were used in antibody adherence inhibition assays. ETEC strain 3030-2 was also used as the challenge strain in the pig challenge study. E. coli strain TOP10 (Invitrogen, Carlsbad, CA) was used as the host strain for fusion protein production. The vector pBAD (Invitrogen) was used to express the chimeric antigen. The constructed E. coli strain was cultured in LB medium supplemented with ampicillin (100 μg/ml).
Table 1.
E. coli strains and plasmids used in this study
| Strain or plasmid | Relevant properties | Reference or source |
|---|---|---|
| E. coli strains | ||
| TOP 10 | F−mcrAΔ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139Δ(ara-leu)7697 galU galK rpsL(Strr) endA1 nupG | Invitrogen |
| 8963 | pFaeG-FedF-LT192A2:B in TOP 10 | This study |
| 8752 | p8745 in E. coli BL21 | 15a |
| 3030-2 | Porcine ETEC isolate, K88ac/LT/STb | 40 |
| 06-20147 | Porcine ETEC isolate, F18/STa/STb/stx2e | 44 |
| 8516 | Porcine field isolate, F18 | 44 |
| Plasmids | ||
| p8745 | STa13-LT192 fusion cloned in pET28α at NheI and BamHI sites | 15a |
| p8963 | faeG-fedF-eltA2:B fusion in TA cloning pBAD vector | This study |
Construction of faeG-fedF-eltA2:B chimeric genes.
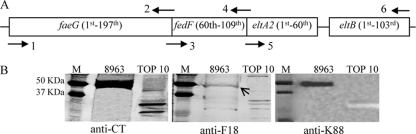
Nucleotide segments coding for the first 197 amino acids of the K88ac FaeG subunit, the 60th to 109th amino acids of the F18 FedF minor subunit, and the last 60 amino acids of the A subunit and the entire B subunit of LT192 were PCR amplified separately and then joined for the fusion antigen gene product (Fig. 1A). We used Pfu polymerase (Strategene, La Jolla, CA) to PCR amplify the faeG gene segment with primers FaeGHindIII-F (5′-GTGGATATCAAGGGGTTAAGCTTT-3′) and K88/F18-R (5′-CATGTCAAAGTGTTTACTCTTTGAATCTGTCCGAG-3′), the fedF gene segment with primers F18/K88-F (5′-CAAAGAGTAAACACTTTGACATGCCAGGCTGGA-3′; underlined nucleotides are complementary to those underlined in the K88/F18-R primer) and F18/LTA2-R (5′-TGCATGATGTGTACCGAATCCTACTTGTG-3′), and the eltAB gene segment with primers LTA2-F (5′-ATTCGGTACACATCATGCACCACAAGGTT-3′; nucleotides in italic are complementary to those in italic in the F18/LTA2-R primer) and LTBBamHI-R (5′-TTGTTATATAGGATCCTAGCATTAC-3′). The amplified faeG segment and the fedF segment were genetically fused for a chimeric gene faeG-fedF in a splicing overlap extension (SOE) PCR, with the aid of the complementary nucleotides, as described previously (40, 41). The LT192A2:B segment was PCR amplified using plasmid p8745 as the template. This p8745 plasmid carries the mutated eltAB genes coding for LT192 toxoid, but it has the eltB gene transmembrane signal nucleotides replaced with nucleotides coding for the Gly-Pro-Gly-Pro linker and the stop codons of the eltA and eltB genes removed. The amplified segment coding for LT192A2:B was fused to the FaeG-FedF fusion gene in another SEO PCR for a single open reading frame coding for FaeG-FedF-LT192A2:B. The FaeG-FedF-LT192A2:B was further PCR amplified using Taq DNA polymerase (Applied Biosystems, Foster City, CA). The amplified products were separated in gel electrophoresis, purified using a QIAquick gel extraction kit (Qiagen, Valencia, CA), and cloned into the pBAD vector using a TOPO TA cloning kit (Invitrogen) to produce the 6×His-tagged fusion proteins in E. coli TOP 10 cells.
Fig. 1.
Construction of the faeG-fedF-eltAB chimeric gene and expression of the FaeG-FedF-LT192A2:B fusion protein. (A) The K88 fimbrial major subunit faeG gene coding for the first 197 amino acids at the N terminus (generated with a PCR using primers 1 and 2), the F18 fimbrial minor subunit fedF gene coding for the 60th to 109th amino acids (produced in another PCR with primers 3 and 4), and the mutated heat-labile gene eltAB coding for the last 60 amino acids of A2 peptide and the B subunit of toxoid LT192 (in a third PCR with primers 5 and 6) were genetically fused with splicing overlap PCRs. Primers 2 and 3 and 4 and 5, respectively, contained complementary nucleotides. Nucleotides coding for the stop codons at the eltA2 and eltB genes and the transmembrane signal peptide of the eltB gene were removed. The chimeric gene was constructed as a single open reading frame. (B) The expressed FaeG-FedF-LT192A2:B fusion protein (extracted with Ni-NTA agarose) was detected by anti-CT (1:3,000; Sigma), anti-F18 (1:2,000, anti-2134), and anti-K88 (1:50; 30/17 and 36/41 MAb hybridoma supernatant) antibodies in standard immunoblot assays. Twenty micrograms of His-tagged recombinant protein extracts were separated in 10% SDS-PAGE, and IRDye-conjugated goat anti-rabbit or anti-mouse IgG (1:5,000) was used as the secondary antibody. Total protein extracts from TOP 10 E. coli cells were used as the negative control. M, size marker.
FaeG-FedF-LT192A2:B fusion expression.
The expression of the FaeG-FedF-LT192A2:B fusion protein was examined by standard sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The fusion strain was grown overnight with shaking (150 rpm) at 37°C in 5 ml LB medium with ampicillin (100 μg/ml). The overnight growth was added to 500 ml LB medium and incubation continued until the optical density at 600 nm (OD600) reached 0.5. The culture was induced with 0.1% l-arabinose, and the incubation continued for 4 h. The bacterial culture was centrifuged at 5,000 × g for 10 min, and pellets were resuspended into 10 ml bacterial protein extraction reagent (B-PER, in phosphate buffer; Pierce, Rockford, IL) for total protein extraction. The recombinant 6×His-tagged fusion protein was purified from the total protein extracts with Ni-nitrilotriacetic acid (NTA) agarose (Qiagen). Twenty micrograms of the eluted protein were analyzed in 10% SDS-PAGE and a standard immunoblot assay (2). Hybridoma supernatants of anti-K88ac monoclonal antibodies (MAbs) 30/17 and 36/41 (1:50 dilution) (26), rabbit anti-F18 antiserum (anti-2134; 1:2,000), and rabbit anti-CT antiserum (1:3,000; Sigma, St. Louis, MO) were used to detect the fusion proteins. Anti-CT antiserum reacts to both CT and LT and is commonly used to detect LT proteins. IRDye-labeled goat anti-mouse or anti-rabbit IgG (1:5,000; LI-COR, Lincoln, NE) was used as the secondary antibody. The bound proteins were detected using a LI-COR Odyssey premium infrared gel imaging system (LI-COR).
Mouse immunization and anti-mouse antibody titration.
Three female adult BALB/c mice (Harlan, Indianapolis, IN) in a group were immunized intraperitoneally with the extracted 6×His-tagged FaeG-FedF-LT192A2:B fusion protein. Three hundred micrograms of the fusion protein (in 100 μl) in an equal volume of Freund's incomplete adjuvant (Sigma) were injected into each mouse in the group. Two booster injections were administered at biweekly intervals. Blood samples were collected from each mouse before immunization and 14 days after each immunization. The collected blood samples were left at room temperature for 30 min and centrifuged at 8,000 rpm to collect serum. Fecal pellets were also collected and suspended in fecal resuspension buffer (10 mM Tris, 100 mM NaCl, 0.05% Tween 20, 5 mM sodium azide, pH 7.4) with the protease inhibitor phenylmethylsulfonyl fluoride (Sigma) at a ratio of 1 mg per 5 ml (39). Fecal suspensions were centrifuged for 5 min at 13,000 rpm to collect supernatants. In addition, the intestines of each mouse were collected at necropsy and washed with 1 ml phosphate-buffered saline (PBS) by gently rubbing the intestines 2 to 3 times, and the wash samples were collected. Serum, fecal suspension, and intestinal washes were stored at −80°C until use.
Mouse serum, fecal resuspension, and intestinal wash samples were examined for anti-K88, anti-F18, and anti-LT antibodies in enzyme-linked immunosorbent assays (ELISAs) using K88 fimbriae, F18 fimbriae, and LTB (Sigma, St. Louis, MO) as coating antigens, respectively. Anti-LT antibodies were titrated in a GM1 ELISA using GM1 and LTB (not CT, which is typically used in GM1 ELISA) as antigens, as described previously (39, 40, 41). For anti-K88 and anti-F18 antibody titration, each well was coated with 50 ng of heat-extracted K88 fimbriae and F18 fimbriae in a Maxisorb plate (Nunc, Roskilde, Denmark) for ELISA as described previously (39). Mouse serum, fecal suspension, and intestine wash samples (in 1:100 dilution) were used in a binary dilution. All samples were tested in triplicates. Horseradish peroxidase (HRP)-conjugated goat-anti-mouse IgG (1:3,300; Sigma) was used as the secondary antibody. The cutoff OD values in the ELISAs were defined as the A405 background plus 0.4 (15, 39, 40, 41). The dilution that gave OD values above the cutoff was calculated for antibody titers, and titers were expressed in a log10 scale.
Pig immunization.
A group of 3 K88ac receptor-positive gnotobiotic piglets were used in an immunization study. All piglets were delivered with Caesarean section and raised under germ-free conditions, and a fecal swap sample from each gnotobiotic piglet was routinely cultured to verify the absence of E. coli strains. We used gnotobiotic piglets in this study to avoid interference from maternal antibodies. Piglets were housed in isolated gnotobiotic units in a room at 94°F and fed with milk replacer. On day 3, each piglet in the immunized group and the control group was orally inoculated with 1 × 109 CFU of a mixture of several normal porcine gastrointestinal flora strains, including E. coli G58-2, Lactobacillus brevis, and Bacteroides thetaiotaomicron, to activate its naïve immune system. On day 5, 100 μg of purified fusion proteins (in a volume of 100 μl), in an equal volume of Freund's incomplete adjuvant (Sigma), was used to immunize each piglet intramuscularly. Antigen integrity was verified in SDS gel with Coomassie blue staining. One booster at the same dose was administered 2 weeks later. Three K88ac receptor-positive piglets without immunization were used as nonvaccinated controls. Blood and fecal samples were collected from each piglet for serum and fecal suspension before and 14 days after each immunization. In addition, intestine wash samples (obtained by washing an ileal segment of 10 cm in length with 2 ml PBS) were collected from each piglet at necropsy. The collected serum, fecal suspensions, and intestine wash samples were used to titrate anti-LT, anti-K88, and anti-F18 antibodies in ELISAs. HRP-conjugated goat anti-porcine IgA (1:2,500; Thermo Fisher Scientific, Rockford, IL) and IgG (1:2,500; Thermo Fisher Scientific) were used as the secondary antibodies.
Anti-porcine antibody neutralization and adherence inhibition.
The collected porcine serum, fecal suspensions, and intestine wash samples were examined for antibody neutralization against commercial available CT toxin (Sigma) and adherence inhibition against K88 and F18 fimbriae. Neutralization against CT toxin was examined using T-84 cells and a cyclic AMP (cAMP) enzyme immunoassay kit (Assay Design, Ann Arbor, MI) as described previously (39, 41). We used CT instead of LT in neutralization assays in this study. CT is highly homologous to LT, and both stimulate an increase of intracellular cAMP in T-84 cells. Although LT is also commercially available lately, CT is more cost effective. Thus, CT is commonly used in anti-LT antibody titration and neutralization assays. To examine antibody adherence inhibition against K88 and F18 fimbriae, we used the porcine cell line IPEC-J2, expressing K88ac receptors, and the K88ac fimbrial ETEC strain 3030-2 and another porcine cell line, IPEC-1, which is adhered to by F18 fimbrial E. coli cells (14), and a F18 fimbrial E. coli field isolate, 8516, respectively, in a method described previously (39).
Pig challenge.
Two weeks after the booster immunization, the immunized piglets and the control piglets were orally challenged with 3 × 109 CFU of the ETEC strain 3030-2 (K88ac/LT/STb). The challenged piglets were monitored every 4 h and necropsied at 48 h postinoculation. Ileal samples were collected at necropsy and used to examine the colonization of the challenge strain using a quantitative colonization assay as previously described (43). Briefly, 1 g of ileal tissue was ground in 9 ml PBS with a sterile Pyrex glass grinder (Fisher Scientific, Pittsburgh, PA), and the solution was serially diluted and plated on LB plates. Colonies grown overnight were counted and converted to quantitative colonization (CFU/g). All animal studies in this project complied with the Animal Welfare Act by following the 1996 National Research Council guidelines (21) and were approved and supervised by a state veterinarian and South Dakota State University's Institutional Animal Care and Use Committee.
Statistical analysis.
Data were analyzed by using the mixed procedure (SAS for Windows, version 8; SAS Institute, Cary, NC) and the Bonferroni method for multiple comparison. Results are expressed as means ± standard deviations. Student's t test was used to compare the differences between the means for each treatment group. Calculated P values of <0.05 were regarded as significant when treatments were compared at two-tailed distribution and two-sample equal or unequal variance.
RESULTS
Expression of FaeG-FedF-LT192A2:B was verified with anti-K88, anti-F18, and anti-CT antisera.
The genetic fusion consisting of the faeG gene coding for the first 197 amino acids at the N terminus, the fedF gene coding for the 60th to 109th amino acids, and the eltAB genes coding for the last 60 amino acids of the LT192 A subunit and the entire B subunit was confirmed with DNA sequencing, and its expression of the fusion protein was verified in Western blot analysis. A protein at a size close to that predicted for the tripartite fusion antigen (50 kDa) was detected by anti-CT, anti-F18, and anti-K88 antisera (Fig. 1B).
Anti-K88, anti-F18, and anti-LT antibodies were detected in immunized mice.
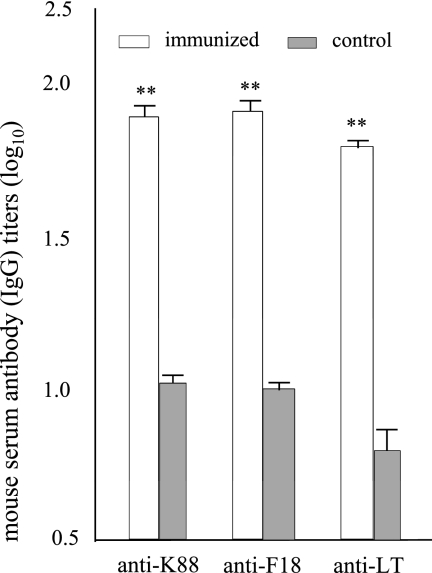
Serum samples from the immunized mice had anti-K88, anti-F18, and anti-LT IgG antibodies detected at titers of 1.90 ± 0.08, 1.92 ± 0.07, and 1.84 ± 0.03 (in log10), respectively. In contrast, serum samples from the control mice showed log10 titers of 1.03 ± 0.06, 1.03 ± 0.04, and 0.78 ± 0.07, which are significantly different than those detected in the immunized mice even after the Bonferroni adjustment (P < 0.01) (Fig. 2). No anti-LT, -K88, or -F18 IgA antibodies were detected from the serum samples, nor did we detect anti-LT, -K88, or -F18 IgG or IgA antibodies from the mouse fecal suspension samples. In addition, we detected no anti-LT, -K88, or -F18 antibodies from the mouse intestine wash samples.
Fig. 2.
Mouse antiserum antibody titration. Serum samples (in binary dilution with an initial dilution of 1:100; performed in triplicate) from mice immunized intraperitoneally with the FaeG-FedF-LT192A2:B fusion protein were titrated for anti-K88, anti-F18, and anti-LT antibodies in ELISAs using purified K88ac fimbriae, F18 fimbriae, and LTB (Sigma) as the coating antigens. HRP-conjugated goat anti-mouse IgG (1:3,300) was used as the secondary antibody. Serum samples collected from the control mice were also included. Antibody titers are expressed at a log10 scale. Bars and error bars represent means and standard deviations. **, P < 0.01.
Anti-K88, anti-F18, and anti-LT antibodies were detected in immunized piglets.
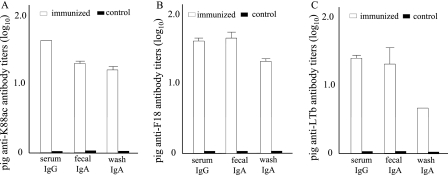
Anti-K88, anti-F18, and anti-LT IgG and IgA antibodies were detected in the serum, fecal suspension, and intestine wash samples of the immunized piglets but not those of the nonvaccinated control piglets (Fig. 3). The mean titers of anti-K88 antibodies were 1.62 ± 0.01 (IgG) in the serum and 1.31 ± 0.03 and 1.22 ± 0.06 (IgA) in the fecal suspension and intestine wash samples of the immunized piglets. Similarly, the mean titers of anti-F18 antibodies were 1.62 ± 0.04 (IgG) in the serum and 1.67 ± 0.11 and 1.36 ± 0.07 (IgA) in the fecal suspension and intestine wash samples of the immunized piglets. Anti-LT antibodies were detected at titers of 1.41 ± 0.06 (IgG) in the serum and 1.3 ± 0.27 and 0.61 ± 0.02 (IgA) in the fecal and intestine wash samples of the immunized piglets.
Fig. 3.
Anti-porcine antibody titration. Serum, fecal resuspension, and intestine wash samples (1:100 dilution) from gnotobiotic piglets immunized intramuscularly with the FaeG-FedF-LT192A2:B fusion protein were titrated for anti-K88, anti-F18, and anti-LT antibodies in ELISAs using purified K88ac fimbriae, F18 fimbriae, and LTB (Sigma) as the coating antigens. HRP-conjugated goat anti-porcine IgG (1:2,500) and IgA (1:2,500) were used as the secondary antibodies. Serum, fecal resuspension, and intestine wash samples collected from piglets of the control group were also titrated. Antibody titers are expressed at a log10 scale. Bars and error bars represent means and standard deviations.
Porcine anti-K88, anti-F18, and anti-LT antibodies inhibited adherence of K88ac fimbriae and F18 fimbriae and neutralized CT toxin.
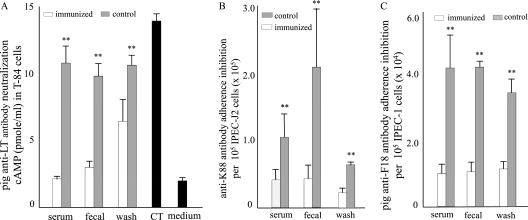
Serum, fecal suspension, and intestine wash samples of the immunized piglets showed neutralizing activity against CT in T-84 cells and adherence inhibition activity against K88ac fimbriae and F18 fimbriae (Fig. 4). After incubation with the serum, fecal suspension, and intestine wash samples from immunized piglets, CT toxin stimulated no or only a limited increase of intracellular cAMP in T-84 cells. The cAMP concentrations in these T-84 cells were 2.13 ± 0.19, 3.2 ± 0.61, and 6.63 ± 2.38 pmol/ml. In contrast, the cAMP concentrations in the T-84 cells incubated with the CT which had been preincubated with the serum, fecal suspension, and intestine wash samples from the control piglets were 10.9 ± 1.41, 9.95 ± 0.99, and 10.85 ± 0.76 pmol/ml, respectively. When T-84 cells were incubated with CT directly, cAMP was detected at 14.1 ± 0.71 pmol/ml, whereas when culture medium was used to incubate T-84 cells, cAMP was measured at a concentration of 2.05 ± 0.21 pmol/ml (Fig. 4A).
Fig. 4.
Anti-porcine antibody neutralization and adherence inhibition. Pig serum, fecal resuspension, and intestine wash samples were examined for neutralizing activity against CT toxin and inhibiting adherence against K88 fimbrial and F18 fimbrial E. coli bacteria. Neutralization against CT was examined using T-84 cells and a cyclic AMP ELISA enzyme immunoassay (EIA) kit (Assay Design). Adherence inhibition against K88ac fimbrial bacteria was evaluated using porcine intestinal cell line IPEC-J2 expressing K88ac receptors and the K88ac fimbrial 3030-2 ETEC strain, whereas inhibition against F18 was examined with porcine intestinal cell line IPEC-1 which expresses F18 receptors and the F18 fimbrial E. coli strain 8516. Bars and error bars represent means and standard deviations. **, P < 0.01.
After incubation with the serum, fecal suspension, and intestine wash samples from immunized piglets, we found a reduction in adherence between 3030-2 bacteria expressing K88ac fimbriae and porcine IPEC-J2 intestine cells which express K88ac receptors (Fig. 4B) and between F18 fimbrial E. coli bacteria and F18 receptor-positive IPEC-1 porcine intestine cells (Fig. 4C). Following preincubation with the serum, fecal suspension, and intestine wash samples of the immunized piglets, the numbers of adherent 3030-2 bacteria (×105) bound to 1 × 105 IPEC-J2 cells were 0.43 ± 0.16, 0.45 ± 0.2, and 0.22 ± 0.09, respectively. These were significantly different than the respective numbers of 3030-2 bound to the same amount of IPEC-J2 cells (P < 0.01) when these 3030-2 bacteria were preinoculated with the serum, fecal suspension and intestine wash samples of the control piglets (1.06 ± 0.41, 2.1 ± 0.9, and 0.63 ± 0.04 [×105]). Similarly, the numbers of the F18 fimbrial strain 8516 adhering to IPEC-1 cells were 1.08 ± 0.33, 1.16 ± 0.37, and 1.18 ± 0.33 (×105), respectively, when incubated with the serum, fecal suspension, and intestine wash samples of the immunized piglets. In contrast, there were 4.18 ± 1.13, 4.19 ± 0.29, and 3.45 ± 0.43 (×105) 8516 cells adhering to the IPEC-1 cells when incubated with the serum, fecal suspension, and intestine wash samples from the control piglets.
Immunized piglets were protected after being challenged with the ETEC strain 3030-2.
After being challenged with the 3030-2 (K88/LT/STb) strain, the immunized piglets remained healthy, did not develop diarrhea, and showed no other signs of clinical disease. In contrast, all three control piglets developed severe diarrhea with signs of dehydration. Moreover, two control piglets died within 18 h postinoculation. When the ileum segments of the small intestines were used for quantitative colonization assays, we found that the immunized piglets had 3.16 ± 2.62 (×108) CFU of 3030-2 bacteria colonized per gram of ileal tissue, which was significantly lower than the level of colonization in the control piglets (15.83 ± 6.38 [×108] CFU per gram of ileum) (P < 0.01). In addition, the total amounts of plasma proteins detected in blood samples of the control piglets after challenge were significantly greater (P < 0.01) than those in the blood samples collected before oral inoculation, suggesting that dehydration occurred in the control piglets after being challenged with the ETEC strain. The expression of K88ac receptors was verified for all piglets in a bacterial brush border adherence assay using brush border vesicles prepared from ileal tissue.
DISCUSSION
Data from this study indicated that peptides from the K88ac FaeG major subunit, F18 FedF minor subunit, and LT192 toxoid could be genetically fused to form a tripartite adhesin-adhesin-toxoid fusion protein and that this fusion protein simultaneously induced protective antiadhesin and antitoxin immunity. This FaeG-FedF-LT192A2:B fusion antigen elicited antibodies causing 2- to 5-fold reductions in adherence by both K88ac fimbriae and F18 fimbriae and also neutralizing enterotoxin activity in vitro. Moreover, piglets immunized with this tripartite fusion antigen were protected against infection by an ETEC strain expressing K88ac fimbriae and LT and STb toxins. Porcine ETEC-associated diarrhea, especially postweaning diarrhea, continues to be a major problem for swine producers worldwide. Neonatal piglets can be protected by suckling colostrial antibodies from immunized mothers or sows recently infected with ETEC strains (13, 18, 19). However, maternal protection does not extend beyond the suckling period, as passively acquired antibodies are rapidly cleared and do not stimulate local mucosal immunity in piglets. Therefore, postweaning piglets are naïve to ETEC infection unless they are immunized during the suckling time to induce actively acquired anti-ETEC immunity (10, 25). As adhesins and enterotoxins are the critical virulence determinants of ETEC in porcine diarrhea, vaccines inducing antiadhesin combined with antitoxin immunity are the optimal approach for protecting against ETEC diarrhea (5, 38).
Vaccines carrying K88 and F18 adhesin antigens and LT and ST antigens would provide effective protection against porcine postweaning diarrhea, as the vast majority of ETEC strains causing postweaning diarrhea express K88 or F18 adhesin and LT and/or ST toxins (6, 8, 9, 16, 22, 37, 44). Data from our recent studies indicated that the LT192 toxoid and STb fusion antigen (LT192-STb) elicited protective anti-LT and anti-STb antibodies in pigs (40), and a live K88ac fimbrial E. coli strain expressing this fusion antigen induced anti-K88, anti-LT, and anti-STb antibodies in young pigs (unpublished data). Moreover, weaned piglets, following oral immunization with this experimental live attenuated vaccine strain during the suckling period, were protected against infection from 3030-2 or other K88ac+ LT+ STb+ ETEC strains (unpublished data). However, this K88ac/LT192-STb strain is not expected to protect weaned pigs against F18 ETEC strains, as it does not induce cross-protective anti-F18 immunity. The current study was designed to explore a multivalent vaccine strategy to induce antiadhesin immunity against multiple fimbriae and antitoxin immunity against LT enterotoxin. The results from this study clearly demonstrated that the FaeG-FedF-LT192A2:B fusion can induce protective anti-K88, anti-F18, and anti-LT immunity simultaneously. If an STa toxoid and/or STb peptide is linked at the C terminus of LTB of this fusion, the resultant quadruple or pentagonal fusion antigens would probably also induce anti-ST immunity. However, additional experiments are necessary to determine its broader protection against ETEC diarrhea. In the present report, induced antiadhesin immunity was only examined for protection against K88ac fimbrial ETEC infection. We were unable to test the protective efficacy against F18 fimbrial ETEC strains due to the difficulty in experimentally reproducing F18 ETEC diarrhea in weaned pigs. Future studies using F18-susceptible piglets and optimizing the challenge model will help us to thoroughly assess the vaccine candidacy of this tripartite fusion antigen.
To induce anti-F18 antiadhesin immunity in this study, we selected the FedF minor subunit as the antigen. Unlike purified K88 fimbriae, which induce protective anti-K88 antibodies (32, 33), purified F18 fimbriae do not induce protective antibodies against infection by F18 fimbrial ETEC strains (34). However, when the conserved F18 minor fimbrial subunit FedF was produced as a fusion protein with maltose-binding protein (MBP) and conjugated to K88 fimbriae, protective anti-F18 immunity was induced (29). Therefore, it was suggested that the FedF antigen should be conjugated with K88 fimbriae for developing vaccines against porcine ETEC postweaning diarrhea (8, 29). Data from the current study showed that genetic fusion of the FedF peptide with the K88 FaeG major subunit (and LT192A2:B) induced antibodies inhibiting F18 fimbrial adherence. In this study, we did not use the entire FedF minor subunit. Instead, we only included a peptide of 50 amino acids. This peptide domain was suggested to play a key role in F18 fimbrial adherence activity because truncation of these 50 amino acids from the FedF subunit results in loss of F18 fimbriae binding to F18 receptor-positive enterocytes (23). The current study clearly showed that antibodies to this 50-amino-acid peptide significantly inhibited the adherence of an F18 fimbrial E. coli strain to F18 receptor-positive intestinal cells (Fig. 4C). In the antibody adherence inhibition assays, we used an E. coli isolate (strain 8516) that only expresses F18 fimbriae, because the wild-type F18 ETEC strain 06-20147 expresses nonfimbrial adhesins (44). Further studies using additional F18 ETEC strains expressing F18 fimbriae and toxins may perhaps be useful for further assessing elicited antibodies for inhibiting adherence in vitro. Additional challenge studies using F18-susceptible pigs will unambiguously evaluate anti-F18 immunity induced by the tripartite fusion in protection against F18 ETEC infection.
Nevertheless, data from this study proved that multiple adhesin antigens and, probably, multiple toxin antigens can be expressed by a single protein, and such adhesin-toxin antigen fusions can elicit antiadhesin immunity against multiple adhesins and antitoxin immunity against an individual toxin. The expression of this tripartite antigen by a nonpathogenic E. coli field isolate could lead to the development of a live attenuated vaccine strain against porcine ETEC. Protecting weaned pigs from ETEC infection may require vaccination while piglets are suckling colostrum or milk that can contain antibodies to ETEC antigens in the vaccine. It is possible that neutralizing antibodies in colostrum or milk might prevent active mucosal immune responses in suckling piglets. Additional experiments are required to determine whether vaccination with the tripartite antigen reported here can result in protective mucosal immunity in the presence of maternal colostral antibodies. The timing and dosage of vaccination may be critical, but it may be possible to elicit a protective response later in the suckling period when maternal antibodies wane. Regardless, the results presented here clearly show that the K88-F18-LT fusion antigen has the potential for eliciting protection from ETEC infection and preventing postweaning diarrhea. This study could be another step leading toward the development of multivalent vaccines against ETEC-associated porcine diarrhea, a disease caused by ETEC strains expressing multiple immunogenically distinctive virulence factors. In addition, ETEC strains causing diarrhea in humans also produce enterotoxins, including LT and STa, which are highly homologous to the LT and STa produced by porcine ETEC strains, and heterogenic adhesins. Therefore, the approach in constructing the adhesin-toxin fusion used in this study could be adapted in developing multivalent vaccines for protection against ETEC diarrhea in humans.
ACKNOWLEDGMENTS
We thank Chengxian Zhang for technical assistance, Ashley Hanson and Diane Baker for their assistance in animal care, David Knudsen and Steven Lawson for help in the immunization study, and Radhey Kaushik for providing the porcine cell lines.
Financial support for this study was provided by NIH grant AI083897 (W. Zhang) and the South Dakota Agricultural Experiment Station.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Alexander T. J. L. 1994. Neonatal diarrhoea in pigs, p. 151–170In Gyles C. L. (ed.) Escherichia coli in domestic animals and human. CAB International, Willingford, United Kingdom [Google Scholar]
- 2. Ausubel F. M., et al. 1999. Short protocols in molecular biology, 4th ed John Wiley & Sons, Inc., New York, NY [Google Scholar]
- 3. Bakker D., Willemsen P. T. J., Simons L. H., van Zijderveld F. G., de Graaf F. K. 1992. Characterization of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol. Microb. 6:247–255 [DOI] [PubMed] [Google Scholar]
- 4. Berberov E. M., et al. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 72:3914–3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boedeker E. C. 2005. Vaccines for enterotoxigenic Escherichia coli: current status. Curr. Opin. Gastroenterol. 21:15–19 [PubMed] [Google Scholar]
- 6. Cheng D., Sun H., Xu J., Gao S. 2006. PCR detection of virulence factor genes in Escherichia coli isolates from weaned piglets with edema disease and/or diarrhea in China. Vet. Microbiol. 115:320–328 [DOI] [PubMed] [Google Scholar]
- 7. Clements J. D. 1990. Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect. Immun. 58:1159–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. De la Fe Rodriguez P. Y., et al. 2011. High prevalence of F4+ and F18+ Escherichia coli in Cuban piggeries as determined by serological survey. Trop. Anim. Health Prod. 43:937–946 doi:10.1007/s11250-011-9786-4 [DOI] [PubMed] [Google Scholar]
- 9. Frydendahl K. 2002. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 85:169–182 [DOI] [PubMed] [Google Scholar]
- 10. Haesebrouck F., et al. 2004. Efficacy of vaccines against bacterial diseases in swine: what can we expect? Vet. Microbiol. 100:255–268 [DOI] [PubMed] [Google Scholar]
- 11. Hampson D. J. 1994. Postweaning Escherichia coli diarrhoea in pigs, p. 171–191In Gyle C. L. (ed.), Escherichia coli in domestic animals and humans. CAB International, Oxon, United Kingdom [Google Scholar]
- 12. Harvey R. B., Anderson R. C., Genovese K. J., Callaway T. R., Nisbet D. J. 2005. Use of competitive exclusion to control enterotoxigenic strains of Escherichia coli in weaned pigs. J. Anim. Sci. 83:44–47 [Google Scholar]
- 13. Isaacson R. E., Dean E. A., Morgan R. L., Moon H. W. 1980. Immunization of suckling pigs against enterotoxigenic Escherichia coli-induced diarrheal disease by vaccinating dams with purified K99 or 987P pili: antibody production in response to vaccination. Infect. Immun. 29:824–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koh S. Y., George S., Brozel V., Francis D. H., Kaushik R. S. 2008. Porcine epithelial cell lines as an in vitro model for studying pathogenesis of enterotoxigenic Escherichia coli. Vet. Microbiol. 130:191–197 [DOI] [PubMed] [Google Scholar]
- 15. Lapa J. A., et al. 2008. Randomized clinical trial assessing the safety and immunogenicity of oral microencapsulated enterotoxigenic Escherichia coli surface antigen 6 with or without heat-labile enterotoxin with mutation R192G. Clin. Vaccine Immunol. 15:1222–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a. Liu M., et al. 2011. Heat-labile (LT) and heat-stable (STa) toxoid-fusions (LTR192G-STaP13F) of human enterotoxigenic Escherichia coli elicit neutralising antitoxin antibodies. Infect. Immun. 79:4002–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Madoroba E., et al. 2009. Prevalence of enterotoxigenic Escherichia coli virulence genes from scouring piglets in Zimbabwe. Trop. Anim. Health Prod. 41:1539–1547 [DOI] [PubMed] [Google Scholar]
- 17. Moon H. W. 1978. Mechanisms in the pathogenesis of diarrhea: a review. J. Am. Vet. Med. Assoc. 172:443–448 [PubMed] [Google Scholar]
- 18. Moon H. W. 1981. Protection against enteric colibacillosis in pigs suckling orally vaccinated dams: evidence for pili as protective antigens. Am. J. Vet. Res. 42:173–177 [PubMed] [Google Scholar]
- 19. Nagy B., Moon H. W., Isaacson R. E., To C. C., Brinton C. C. 1978. Immunization of suckling pigs against enteric enterotoxigenic Escherichia coli infection by vaccinating dams with purified pili. Infect. Immun. 21:269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nataro J. P., Kaper J. B. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Research Council 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 22. Oanh T. K. N., Nguyen V. K., Do T. N., Goddeeries B. M., De Greve H. 2010. Escherichia coli strains causing edema disease in northern Vietnam share an identical verotoxin 2e. Trop. Anim. Health Prod. 42:1797–1804 [DOI] [PubMed] [Google Scholar]
- 23. Smeds A., et al. 2003. Mapping the binding domain of the F18 fimbrial adhesin. Infect. Immun. 71:2163–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith H. W., Linggood M. A. 1971. Observation on the pathogenic properties of the K88, HIY and ENT plasmids of Escherichia coli with particular reference to porcine diarrhea. J. Med. Microbiol. 4:467–485 [DOI] [PubMed] [Google Scholar]
- 25. Snoeck V., et al. 2003. Enteric-coated pellets of F4 fimbriae for oral vaccination of suckling piglets against enterotoxigenic Escherichia coli infection. Vet. Immunol. Immunopathol. 96:219–227 [DOI] [PubMed] [Google Scholar]
- 26. Sun R., Anderson T. J., Erickson A. K., Nelson E. A., Francis D. H. 2000. Inhibition of adhesion of Escherichia coli K88ac fimbria to its receptor, intestinal mucin-type glycoproteins, by a monoclonal antibody directed against a variable domain of the fimbria. Infect. Immun. 68:3509–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Svensmark B., Jorsal S. E., Nielsen K., Willeberg P. 1989. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. I. Pre-weaning diarrhoea. Acta Vet. Scand. 30:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Svensmark B., Nielsen K., Willeberg P., Jorsal S. E. 1989. Epidemiological studies of piglet diarrhoea in intensively managed Danish sow herds. II. Post-weaning diarrhoea. Acta Vet. Scand. 30:55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tiels P., Verdonck F., Coddens A., Goddeeris B., Cox E. 2008. The excretion of F18+ E. coli is reduced after oral immunization of pigs with a FedF and F4 fimbriae conjugate. Vaccine 26:2154–2163 [DOI] [PubMed] [Google Scholar]
- 30. Tubb R. C., Hurd H. S., Dargatz N., Hill G. 1993. Preweaning morbidity and mortality in the United States swine herd. Swine Health Prod. 1:21–28 [Google Scholar]
- 31. USDA 2002. Reference for swine health and health management in the United States, 2000. Part II, p. 7-25. #355.0202. National Animal Health Monitoring System, USDA, Fort Collins, CO [Google Scholar]
- 32. Van den Broeck W., Cox E., Goddeeris B. M. 1999. Induction of immune responses in pigs following oral administration of purified F4 fimbriae. Vaccine 17:2020–2029 [DOI] [PubMed] [Google Scholar]
- 33. Van den Broeck W., Cox E., Goddeeris B. M. 1999. Receptor-dependent immune responses in pigs after oral immunization with F4 fimbriae. Infect. Immun. 67:520–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Verdonck F., Cox E., van der Stede Y., Goddeeris B. M. 2004. Oral immunization of piglets with recombinant F4 fimbrial adhesin FaeG monomers induces a mucosal and systemic F4-specific immune response. Vaccine 22:4291–4299 [DOI] [PubMed] [Google Scholar]
- 35. Verdonck F., et al. 2007. Mucosal immunization of piglets with purified F18 fimbriae does not protect against F18+ Escherichia coli infection. Vet. Immunol. Immunopathol. 120:69–79 [DOI] [PubMed] [Google Scholar]
- 36. Verfaillie T., et al. 2004. Priming of piglets against enterotoxigenic E. coli F4 fimbriae by immunization with FaeG DNA. Vaccine 22:1640–1647 [DOI] [PubMed] [Google Scholar]
- 37. Vidotto M. C., et al. 2009. Frequency of virulence genes in Escherichia coli strains isolated from piglets with diarrhea in the North Parana State, Brazil. Braz. J. Microbiol. 40:199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Walker R. I. 2005. Considerations for development of whole cell bacterial vaccines to prevent diarrheal diseases in children in developing countries. Vaccine 23:3369–3385 [DOI] [PubMed] [Google Scholar]
- 39. Zhang C., Zhang W. 2010. Escherichia coli K88ac fimbriae expressing heat-labile and heat-stable (STa) toxin epitopes elicit antibodies that neutralize cholera toxin and STa toxin and inhibit adherence of K88ac fimbrial E. coli. Clin. Vaccine Immunol. 17:1859–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang W., Francis D. H. 2010. Genetic fusions of heat-labile toxoid (LT) and heat-stable toxin b (STb) of porcine enterotoxigenic Escherichia coli elicit protective anti-LT and anti-STb antibodies. Clin. Vaccine Immunol. 17:1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang W., et al. 2010. Genetic fusions of heat-labile (LT) and heat-stable (ST) toxoids of porcine enterotoxigenic Escherichia coli elicit neutralizing anti-LT and anti-STa antibodies. Infect. Immun. 78:316–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang W., et al. 2008. Escherichia coli constructs expressing human or porcine enterotoxins induce an identical diarrheal disease in a piglet infection model. Appl. Environ. Microbiol. 74:5832–5837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang W., et al. 2006. Significance of heat-stable and heat-labile enterotoxins in porcine colibacillosis in an additive model for pathogenicity studies. Infect. Immun. 74:3107–3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang W., Zhao M., Ruesch L., Omot A., Francis D. 2007. Prevalence of virulence genes in Escherichia coli strains isolated from young pigs with diarrhea in North Central U.S. Vet. Microbiol. 123:145–152 [DOI] [PubMed] [Google Scholar]