Abstract
We produced capsids of Merkel cell polyomavirus (MCPyV) in a baculovirus expression system and developed a virus-like particle (VLP) enzyme-linked immunosorbent assay (ELISA). To determine age-specific seroprevalence, serum samples were collected from 947 individuals attending hospital outpatient clinics and ranging in age from 1 to 93 years. To evaluate the association between exposure to MCPyV and Merkel cell cancer (MCC), plasma samples were obtained from 33 MCC patients and 37 controls. MCPyV seroprevalence was 45% in children under 10 years of age, increased to 60% in the next decade of life, and peaked at 81% among those 60 to 69 years of age. Levels of MCPyV capsid antibodies were positively correlated with age (P = 0.007). Virus specificity of MCPyV seroreactivity was supported by competitive inhibition of reactivity by MCPyV VLPs and not by BK polyomavirus (BKPyV) VLPs. MCPyV seroprevalence was greater among MCC patients (91%) than controls (68%; age-adjusted P value, 0.32); the mean level of MCPyV antibodies was also greater (P = 0.04). The age-specific seroprevalence of MCPyV shares with previously known polyomaviruses, BKPyV and JC polyomavirus (JCPyV), evidence of widespread exposure in human populations beginning early in life. MCPyV age-specific seroprevalence also has unique features. Seroprevalence among children is higher than that of JCPyV but lower than that of BKPyV. Among older adults, MCPyV seroprevalence remains high, while that of BKPyV declines and that of JCPyV continues to rise. In agreement with results from other studies, we found an association between MCPyV seropositivity and MCC, and higher levels of serum MCPyV capsid antibodies in MCC patients than in controls.
INTRODUCTION
Merkel cell polyomavirus (MCPyV), a new human polyomavirus, was recently discovered by molecular techniques in Merkel cell carcinoma (MCC) (11), a rare and aggressive skin tumor (20, 22). Studies from North America and Europe have detected MCPyV DNA by PCR in 69 to 100% of MCC tumors (1, 9, 11, 13, 14, 17, 25). The virus has also been detected in rare instances and in low copy numbers in cutaneous, gastrointestinal, and respiratory tract samples from healthy individuals (2, 11, 15). Little is known about the natural history of MCPyV infection in human populations. Serological assays can reveal the extent of past exposure to a virus and provide insights into its epidemiology. We and others have developed virus-like particle (VLP)-based enzyme-linked immunosorbent assays (ELISAs) to measure antibodies to various human and animal polyomaviruses (10, 27, 31). Polyomavirus VLPs are empty viral capsids produced by expression of the gene for the major capsid protein, VP1, in a eukaryotic expression system. VLPs resemble native virions morphologically and retain their immunological properties, including the ability to bind antiviral capsid antibodies. We now report the development of a VLP-based ELISA to detect antibodies to MCPyV and its application for comparison of the age-specific seroprevalence of MCPyV to those of two other human polyomaviruses initially discovered about 4 decades ago, JC polyomavirus (JCPyV) and BK polyomavirus (BKPyV). We also used the assay to examine the association between prior exposure to MCPyV and MCC in samples from patients and controls.
MATERIALS AND METHODS
Study populations.
For determination of polyomavirus age-specific seroprevalence, serum samples were collected from 947 individuals attending outpatient clinics of the Università degli Studi di Roma La Sapienza, Rome, Italy, between January 2005 and June 2008. Study participants ranged in age from 1 to 93 years and comprised 568 individuals identified as males, 374 individuals identified as females, and 5 individuals whose gender was unknown. The majority of participants (n = 720; 76%) were recruited from general medical, pediatric, infectious disease, and surgical clinics. Smaller numbers were identified through clinics for hematology (n = 93; 9.8%), transplant/dialysis (n = 67; 7.1%), and cystic fibrosis (n = 17, 1.8%) and various subspecialty clinics (n = 50; 5.1%). All procedures for obtaining serum samples were approved by an institutional medical ethics committee.
For evaluation of the association between exposure to MCPyV and MCC, a case-control analysis was conducted using plasma samples obtained from 33 MCC patients and 37 cancer-free controls. The MCC group comprised patients diagnosed with and/or treated for histologically confirmed MCC within the Cutaneous Oncology Program at Moffitt Cancer Center, Tampa, FL, in the period from 2006 to 2008, including 25 males and 8 females (ages 53 to 88 years; median age, 74 years). Fresh frozen MCC tumor tissues were also available from nine of these patients. Controls comprised patients undergoing skin cancer screening exams at Moffitt's Lifetime Cancer Screening facility and/or the University of South Florida Family Medicine Clinic, Tampa. The control subjects had no history of any type of skin cancer and were determined to be negative for all types of skin cancer by a nurse practitioner. All study participants provided informed consent, and all study procedures were approved by the institutional review board at the University of South Florida.
Construction of MCPyV VLPs.
The entire open reading frame (ORF) of the VP1 gene of MCPyV strain MC 339 (GenBank accession number EU375804) with a Kozak consensus sequence and unique restriction sites (EcoRI/NotI) at each end was artificially engineered by PCR-based gene synthesis (performed by GeneScript Inc.) and cloned into a pUC57 vector. The VP1 gene was subcloned between the EcoRI/NotI sites of the pORB baculovirus transfer vector (Orbigen). Spodoptera frugiperda Sf9 cells were cotransfected with the transfer vector and linear baculovirus DNA (DiamondBac; Sigma) using Cellfectin reagent as suggested by the manufacturer (Invitrogen). Five days posttransfection, the recovered recombinant baculovirus was further amplified by large-scale infection of Sf9 cells in TNM-FH (Allele Biotechnology, San Diego, CA)–10% fetal bovine serum (FBS). For large-scale production of VLPs, 108 Trichoplusia ni (High Five) cells (Invitrogen, Carlsbad, CA) grown as adherent cultures in a tissue culture plate (245 by 245 mm; Nunc) were infected with 5 ml of a high-titer recombinant baculovirus stock in 95 ml of Ex-Cell 400 medium (JRH Biosciences) per plate. After 96 h of incubation at 27°C, the cells were harvested and collected by centrifugation at 2,000 rpm in a Sorvall FH18/250 rotor for 5 min. The cell pellet was resuspended in VLP extraction buffer (50 mM Tris, pH 7, 150 mM NaCl, 2 mM MgCl2, 1 mM CaCl2), and the VLPs were released by 3 freeze-thaw cycles. The lysate was clarified by centrifugation at 8,000 × g for 30 min and further delipidated by Freon extraction. The lysate was then loaded onto a cushion of 30% sucrose in VLP buffer and centrifuged in an SW28 rotor at 27,000 rpm for 4 h at 4°C. The resulting pellet was resuspended in VLP buffer with 0.5 M NaCl, loaded onto a discontinuous OptiPrep gradient (26 and 32%), and centrifuged in an SW40 rotor at 37,000 rpm for 4 h at 16°C. The band collected at the 26%/32% interface was diluted 3-fold with VLP buffer, loaded onto a discontinuous CsCl gradient (densities of 1.2 and 1.4 g/ml), and centrifuged in an SW40 rotor at 37,000 rpm for 4 h at 4°C. Bands at the 1.2/1.4 interface were collected and stored at 4°C.
Total particle protein was measured by using the Bio-Rad protein assay kit and immunoglobulin G (IgG) as a standard. The purity of VLPs was assessed by SDS-PAGE, and capsid formation was assessed by electron microscopy. For direct visualization of VLPs by electron microscopy, an aliquot of diluted particles was placed on a 300-mesh Formvar/carbon-coated copper grid (Electron Microscopy Sciences, Hatfield, PA) and the grid was allowed to absorb for 5 min, washed briefly with distilled water (dH2O), and air dried. A drop (10 μl) of 2% phosphotungstic acid (pH 7.0) was placed on the grid for 1 min. The stain was removed, and the grid was allowed to air dry prior to examination by transmission electron microscopy. The microscopy was performed with a JEOL 1200 transmission electron microscope, with micrographs of random sections taken at various magnifications.
VLP ELISAs.
BK and JC polyomavirus VLPs were produced as described previously (31). For ELISAs, MCPyV, BKPyV, and JCPyV particle proteins were diluted, respectively, to 0.25, 0.20, and 0.50 μg per ml in phosphate-buffered saline (PBS; pH 7.4) and 100 μl was added to each well of 96-well polystyrene flat-bottom PolySorp plates (Nunc, Naperville, IL). The plates were incubated overnight at 4°C. After the antigen solution was removed, each well of the plates was blocked for 2 h at room temperature with 300 μl of 0.5% (wt vol−1) polyvinyl alcohol (PVA) at a molecular weight (MW) of 30,000 to 70,000 (Sigma, St. Louis, MO) in Blocker casein in PBS (Pierce). The blocking solution was removed and serum samples, diluted 1:200 in blocking solution, were added to the antigen-coated plates. The plates were incubated at 37°C for 1 h on a microplate shaker and then washed four times with PBS–0.05% Tween 20 in an automatic plate washer (SkanWasher 300; Skatron). Goat anti-human immunoglobulin G conjugated with horseradish peroxidase (HRP; Southern Biotech, Birmingham, AL) was diluted 1:4,000 in a solution of 0.5% PVA, 0.025% Tween 20, and 0.8% (wt vol−1) polyvinylpyrrolidone at an average molecular weight of 360,000 (Sigma) in PBS, and 100 μl of the dilution was added to each well. The plates were incubated at 37°C for 30 min on a microplate shaker and then washed as described above. Freshly prepared 2,2′-azino-di-(3-ethylbenzthiazoline-6-sulfonate) hydrogen peroxide solution (Kirkegaard & Perry, Gaithersburg, MD) prewarmed to 37°C was added at 100 μl per well. The plates were incubated at room temperature in the dark for 20 min. The enzyme reaction was stopped by the addition of 100 μl of 1% sodium dodecyl sulfate to each well of all plates. The plates were read at 405 nm in an automated microtiter plate reader (Molecular Devices, Menlo Park, CA) with a reference wavelength of 490 nm. For the age-specific seroprevalence analysis, optical density (OD) values were classified as seropositive or seronegative as described in “Statistical analyses.”
To test for possible serological cross-reactivity, representative serum samples underwent competitive inhibition (“blocking”) assays. Serum samples were diluted 1:800 in PVA-casein buffer containing 0.05 μg/ml of BKPyV or MCPyV VLP protein or buffer alone. After incubation for 1 h at 37 C, the serum samples were transferred to a MCPyV-coated microtiter plate and the ELISA was completed as described above. Percent inhibition was calculated as follows: 1 OD value of blocking VLP/OD value of buffer control × 100.
For the analysis of MCC patient and control serum samples, antibody levels were expressed in enzyme immunoassay (EIA) units. Quantitation of antibodies to MCPyV and BKPyV VLPs was performed by incubating serial 2-fold dilutions of test sera and a reference serum in duplicate, beginning with a 1:200 dilution, on VLP-coated plates. From dilutions of the reference serum, a standard curve was constructed using a four-parameter equation as implemented in the software package SoftMax Pro. The four-parameter equation is as follows: A + {(B − A)/[1 + (C/OD)D]}, where A is the minimum asymptote of the curve, B is the maximum asymptote of the curve, C is the midpoint of the curve, and D is the slope of the curve. The highest dilution of the reference serum that gave an OD value greater than twice the mean for PBS controls was arbitrarily assigned as 1 EIA unit. The operational range of the standard curve was from 1 to 4,096 EIA units. The EIA units of serum samples at each dilution were calculated by interpolating off the standard curve and multiplying by the dilution factor above 1:200. The titer for individual serum samples was the mean of EIA units of two dilutions of the serum that fell within the operational range of the reference curve (from approximately 5 to 500 EIA units).
Measurement of MCPyV DNA in MCC tumor tissues.
The presence of MCPyV DNA in the nine fresh frozen MCC tumor tissues was assessed using SYBR green real-time PCR (RT-PCR) for the amplification of the T-antigen region of the MCPyV genome with primers MCV236F (5′-GCA AGC TTT TGG AGA TTG CT-3′) and MCV373R (5′-TCC AAA GGG TGT TCA ATT CC-3′). A standard curve was generated using serial dilutions of T-antigen plasmid, and the same plasmid was used as an MCPyV-positive control for the assay. MCPyV-negative controls included water blanks, human male DNA (Promega), and human papillomavirus type 16 (HPV16)-positive Caski cells. Each primer was diluted to 0.075 μM, and each purified DNA specimen was diluted to 5 ng/μl. SYBR green master mix (AB Applied Biosystems) was diluted to a 1× final concentration. RT-PCR was performed with 17.5 ng of purified genomic DNA using SYBR green in a final reaction volume of 10 μl. Each primer set was evaluated using 40 cycles on the ABI 7900HT real-time analyzer. For the purpose of the present analysis, tumors were considered MCPyV DNA positive if the estimated viral copy number was greater than zero.
Statistical analyses.
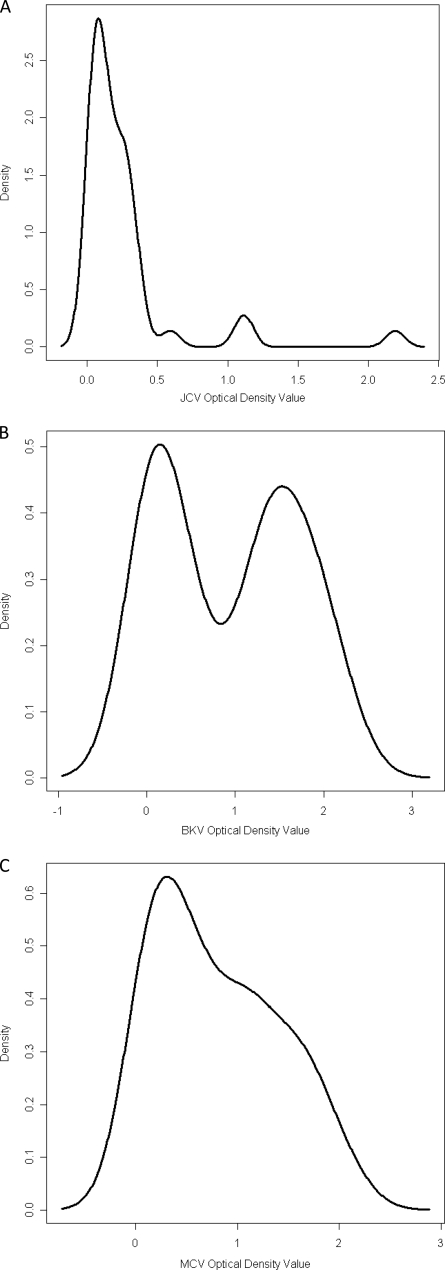
Previous studies have suggested that exposure to human polyomaviruses occurs early in life. Therefore, very young children represent a population with a low likelihood of exposure. We used serum samples from infants and children less than 10 years of age to derive a cutoff point for polyomavirus seropositivity. Histograms were constructed for numbers of serum samples on a continuous scale (referred to as the density; y axis) and OD values on a continuous scale (x axis). The histograms revealed a bimodal age distribution of seroreactivity to the three viruses, although the valley between the distributions was more evident for BKPyV and JCPyV than for MCPyV (Fig. 1). Binary cutoff points for seropositivity were defined as the mean of the lower distribution plus two standard deviations and were equal to 0.367, 0.434, and 0.777 OD units for JCPyV, BKPyV, and MCPyV, respectively. By using these cutoff points, serology results were dichotomized as antibody positive and negative.
Fig. 1.
Histograms of seroreactivities to JCPyV (JCV) (A), BKPyV (BKV) (B), and MCPyV (MPV) (C) VP1 virus-like particles of samples from 42 children less than 10 years of age. The density is the number of serum samples on a continuous scale.
For the Italian study population, age-specific seroprevalence was calculated for 10-year intervals from <10 years of age to >70 years of age. To examine differences in age-adjusted seroprevalences by gender, logistic regression was used, including seropositivity as the dependent variable and gender and age (as a continuous variable) as independent variables. The P value corresponding to the gender coefficient was used to determine the statistical significance of gender-associated differences in age-adjusted seroprevalences. A similar approach was used to compare age-adjusted seroprevalences between patients recruited from specialty clinics and those recruited from general clinics.
To compare MCPyV and BKPyV antibody levels between MCC patients and controls, antibody levels were first log transformed to achieve a normal distribution. Log-transformed values were then compared between MCC patients and controls using a t test. Adjustment for age was conducted using logistic regression, with case-control status serving as the dependent variable and age and log-transformed antibody values serving as continuous independent variables. With the binary cutoff points defined above, MCPyV and BKPyV seropositivity statuses were first compared between MCC patients and controls using Fisher's exact test. Age adjustment was conducted using logistic regression, with seropositivity and age as continuous independent variables.
RESULTS
Production of MCPyV VLPs.
The VP1 gene of MCPyV was expressed in insect cells from a recombinant baculovirus, and the cell lysate was subjected to a protocol we have used previously to prepare VLPs of human polyomaviruses and papillomaviruses. A band was detected at the 26%/32% OptiPrep interface, where VLPs are normally found, and the sample collected at the OptiPrep interface banded in CsCl at the 1.2/1.4 interface, consistent with the expected density of a polyomavirus VLP. Upon SDS-PAGE, the purified MCPyV particle protein yielded a major band of ∼45 kDa, a slightly higher molecular mass than those of the ∼41-kDa VP1 proteins of BKPyV and JCPyV (Fig. 2). For all the VLP preparations, a lighter higher-molecular-mass band corresponding to a dimer of VP1 was also visible, and for JCPyV, a faint band the size of a trimer was visible. Analysis of the purified MCPyV preparation by electron microscopy showed the presence of fully assembled VLPs with the approximate size of 45 nm (Fig. 3).
Fig. 2.
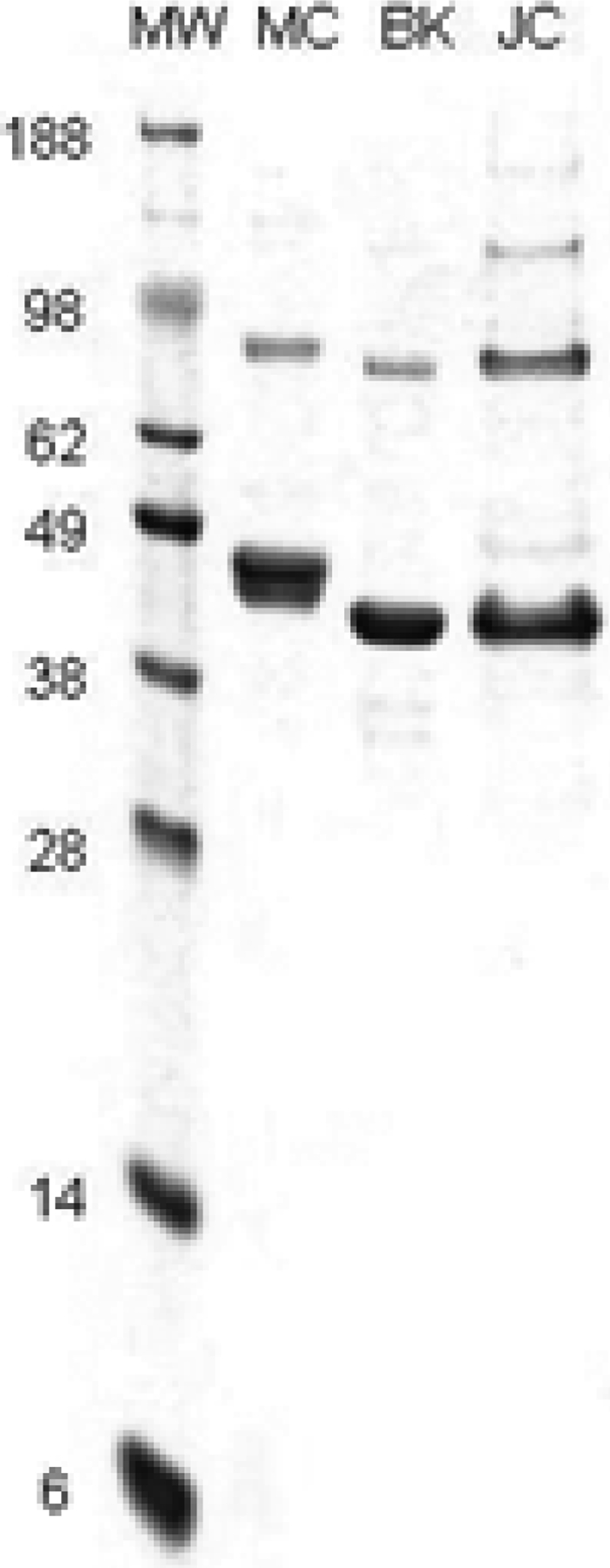
SDS-PAGE analysis of Merkel cell polyomavirus, BK virus, and JC virus VP1 virus-like particles. Five-microgram samples of gradient-purified MCPyV (MC), BKPyV (BK), or JCPyV (JC) VP1 virus-like particles produced in insect cells from recombinant baculoviruses were subjected to SDS-PAGE. Molecular weight markers (MW) are shown in lane 1, with the sizes of markers (103) indicated to the left of the gel.
Fig. 3.
Electron micrograph of MCPyV VP1 virus-like particles at a magnification of ×105,000. The bar corresponds to 100 nm.
Age-specific seroprevalence.
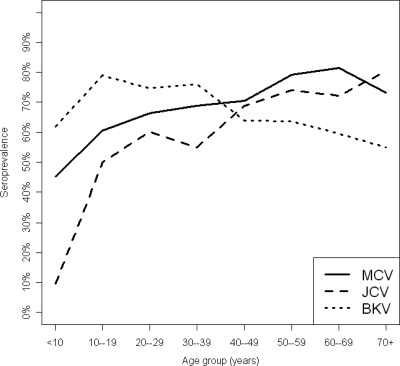
The age-specific seroprevalences of MCPyV, BKPyV, and JCPyV are shown in Fig. 4 and Table 1. For JCPyV, seroprevalence increased with age, the steepest increase being observed between children <10 years of age (9.5%) and those 10 to 19 years of age (50%). JCPyV seroprevalence continued to increase after age 20, peaking at 80% in those >70 years of age. BKPyV seroprevalence among those <10 years of age was 62%, and it peaked in the second decade of life (79%) and then held steady during the third and fourth decades of life and began to gradually decline in those 40 to 49 years of age (64%), reaching a low point of 55% in those >70 years of age. MCPyV seroprevalence fell between that of BKPyV and JCPyV in those <10 years of age (45%), rose to 60% in those 10 to 19 years of age, and eventually surpassed those of both JCPyV and BKPyV by the fifth decade of life (70%). MCPyV seroprevalence peaked in those 60 to 69 years of age (81%) and fell slightly among those >70 years of age (73%). MCPyV titers expressed in OD values were positively associated with age (Spearman correlation, r = 0.103; P = 0.007), and BKPyV titers were negatively associated with age (r = −0.12; P = 0.003); age was not associated with JCPyV titers (r = 0.047; P = 0.247). Similar age-related patterns in polyomavirus seroprevalence were observed in males and females. There were no significant differences in age-adjusted MCPyV, BKPyV, or JCPyV seroprevalences between patients attending general medical clinics and those attending subspecialty clinics (data not shown).
Fig. 4.
Age-specific seroprevalence of Merkel cell polyomavirus, BK virus, and JC virus among 945 individuals recruited from hospital-based general and subspecialty outpatient clinics. Serum samples were tested at a 1:200 dilution in VLP-based ELISAs. The distribution of reactivities of serum samples from children less than 10 years of age was used to set cutoff points for seropositivity, and results are displayed as the percent positive in 10-year age groups. Numbers used to construct the graph are shown in Table 1.
Table 1.
MCPyV, JCPyV, and BKPyV age-specific seroprevalencea
| Subject age (yrs) | Total no. of samples | No. (%) of MCPyV-seropositive samples | No. (%) of JCPyV-seropositive samples | No. (%) of BKPyV-seropositive samples |
|---|---|---|---|---|
| <10 | 42 | 19 (45.2) | 4 (9.5) | 26 (61.9) |
| 10–19 | 38 | 23 (60.5) | 19 (50.0) | 30 (78.9) |
| 20–29 | 83 | 55 (66.3) | 50 (60.2) | 62 (74.7) |
| 30–39 | 109 | 75 (68.8) | 60 (55.0) | 83 (76.1) |
| 40–49 | 247 | 174 (70.4) | 170 (68.8) | 158 (64.0) |
| 50–59 | 193 | 153 (79.3) | 143 (74.1) | 123 (63.7) |
| 60–69 | 151 | 123 (81.5) | 109 (72.2) | 90 (59.6) |
| ≥70 | 82 | 60 (73.2) | 66 (80.5) | 45 (54.9) |
Two samples were excluded from the analysis because the ages of the participants were not available.
Specificity of MCPyV seroreactivity.
There were no correlations between levels of antibodies to MCPyV, JCPyV, and BKPyV (MCPyV versus JCPyV, r = 0.043; MCPyV versus BKPyV, r = −0.061; and BKPyV versus JCPyV, r = −0.043), indicating little or no cross-reactivity among the viruses. The specificity of MCPyV seroreactivity was assessed by competitive inhibition assays. Reactivity of 74 serum samples in the MCPyV ELISA was strongly inhibited by preincubation with MCPyV VLPs (median percent inhibition, 94.8%; interquartile range [IQR], 91.5 to 97.4%; minimum inhibition, 57.6%) and was minimally inhibited by BKPyV VLPs (median percent inhibition, 0.4%; IQR, 0.0 to 2.2%; maximum inhibition, 10.5%).
Seroreactivity of samples from MCC patients and controls.
The prevalence of MCPyV capsid antibodies was greater among MCC patients (91%) than controls (68%; P = 0.02; P = 0.32 after age adjustment) (Table 2). The mean level of MCPyV antibodies was also greater (P = 0.005; P = 0.04 after age adjustment). In contrast to the MCPyV results, there were no case-control differences in BKPyV capsid IgG seroprevalence or antibody levels. Of the nine patients for whom MCC tumor tissue was also available, MCPyV DNA was detected in six (67%), with MCPyV copies per cell equivalent ranging from 8.0 to 15.6 (median, 10.5). The six DNA-positive MCC patients were all MCPyV seropositive, compared to two of three DNA-negative MCC patients. The mean level of MCPyV antibodies was greater among the six patients with MCPyV DNA-positive tumors than among the three MCPyV DNA-negative patients; however, these differences did not reach statistical significance (Table 3). In contrast, there were no differences in BKPyV seroprevalence or antibody levels between MCPyV DNA-positive and MCPyV DNA-negative patients.
Table 2.
Antibodies to MCPyV and BKPyV in MCC patients and controls
| Antibody | Total no. of samples from MCC patients | No. (%) of seropositive samples | Total no. of samples from controls | No. (%) of seropositive samples |
P value |
Mean antibody units (SD) in: |
P value |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Crudea | Age adjustedb | MCC patient samples | Control samples | Crudec | Age adjustedd | |||||
| MCPyV capsid IgG | 33 | 30 (90.9) | 36 | 25 (67.6) | 0.02 | 0.32 | 1,876.0 (4,000.6) | 1,521.5 (4,889.0) | 0.005 | 0.04 |
| BKPyV capsid IgG | 31 | 20 (64.5) | 37 | 29 (78.4) | 0.28 | 0.99 | 215.9 (461.1) | 199.2 (583.8) | 0.93 | 0.32 |
Determined by Fisher's exact test.
Determined by logistic regression including seropositivity and age as continuous variables.
Determined by a t test based on log-transformed antibody levels.
Determined by logistic regression including log-transformed antibody levels and age as continuous variables.
Table 3.
MCPyV and BKPyV seroreactivity among MCC patients with and without MCPyV DNA in corresponding tumor tissues
| Antibody | Total no. of samples from MCPyV DNA-positive MCC patients | No. (%) of seropositive samples | Total no. of samples from MCPyV DNA-negative MCC patients | No. (%) of seropositive samples | P valuea | Mean antibody units (SD) in: |
P valueb | |
|---|---|---|---|---|---|---|---|---|
| MCPyV DNA-positive MCC patients | MCPyV DNA-negative MCC patients | |||||||
| MCPyV capsid IgG | 6 | 6 (100%) | 3 | 2 (67%) | 0.33 | 1,040.4 (1,210.1) | 384.3 (493.7) | 0.52 |
| BKPyV capsid IgG | 6 | 3 (50%) | 2 | 1 (50%) | 1.00 | 71.4 (122.9) | 72.5 (85.6) | 0.62 |
Determined by Fisher's exact test.
Determined by the Wilcoxon rank sum test.
DISCUSSION
Using a newly developed VLP-based ELISA, we determined the age-specific seroprevalence of Merkel cell polyomavirus, a recently discovered human polyomavirus implicated in the etiology of Merkel cell cancer, and compared its seroprevalence to those of the first two human polyomaviruses to be discovered, BKPyV and JCPyV, both isolated in 1971. MCPyV seroprevalence was 45% in children less than 10 years of age and rose steeply during the subsequent decade of life to 60.5%. Serological evidence of exposure during childhood is characteristic of human polyomaviruses and has been well documented for BKPyV and JCPyV (5, 8, 12, 19, 27). In our population, seroprevalence of MCPyV during the first 2 decades of life was lower than that of BKPyV, 79% at 10 to 19 years of age, and higher than that of JCPyV, 50% at 10 to 19 years of age. Differences in seroprevalence among these viruses may be due to different efficiencies of transmission, differences in the route of transmission, the frequency of perinatal transmission, or the extent of intra- versus interfamilial spread of the viruses. The mode of transmission of MCPyV is unknown. The virus has been detected in urban sewage, which indicates that it may be disseminated through fecal-urine contamination of water and spread by fecal/oral transmission (3). In support of fecal/oral transmission, the virus has been detected in the upper aerodigestive tract, digestive system, and saliva (21). MCPyV has also been detected in tonsillar tissue, nasopharyngeal aspirates, and nasal swabs and thus could be spread by the respiratory route (2, 15, 16). MCC is a cutaneous cancer, and MCPyV has been recovered from normal skin of up to 40% of healthy adult volunteers, which would support cutaneous transmission of the virus (13, 26). MCPyV was not detected in 535 fetal autopsy samples, and thus vertical transmission from mother to fetus does not occur or is very rare (24). However, this does not exclude the possibility of perinatal horizontal transmission at the time of birth. For BKPyV and JCPyV, we have previously observed serological evidence of possible perinatal infection manifested by a rising IgG titer and IgM seropositivity (4). Serological evidence of exposure to MCPyV in childhood has been reported previously. Chen et al. observed a seroprevalence of 35% in children 4 to 13 years of age (7), Kean et al. reported a seroprevalence of 34% in subjects under the age of 21 (18), and Tolstov et al. described a seroprevalence of 50% in children 15 years of age and younger (28). Chen et al. and Tolstov et al. used VLP ELISAs similar in design to our assay, while Kean et al. used an ELISA with N-terminally glutathione S-transferase-tagged VP1 capsomeres as the solid-phase antigen. The higher seroprevalence in our study most likely reflects differences in populations, although technical differences between assays cannot be excluded as an explanation. In our adult population, the seroprevalence ranged from approximately 66 to 81% in subjects 20 to greater than 70 years of age, with a trend of rising seroprevalence with increasing age. This finding is consistent with reports in the literature for other adult populations, where seroprevalences have ranged from 46 to 88% (6, 18, 23, 28, 29). The increase in seroprevalence with age suggests that transmission may occur throughout life.
Our study and others that have examined age-specific seroprevalence of BKPyV have generally shown that seropositivity is very common in infants and children, reaches peak prevalence in older children or young adults, and declines in older individuals (10, 19, 27). The peak seroprevalence of 79% observed in adults in the present study is consistent with data from previous studies that observed peak prevalences ranging from approximately 65 to 95%. The decline in BKPyV seropositivity with age may be due to waning of antibody levels over time and suggests that exogenous or endogenous re-exposure to BKPyV is less common later in life. In contrast, the maintenance of high seroprevalence of MCPyV even in older individuals suggests that there is a source of continued antigenic stimulation for MCPyV. In support of possible age-related differences in antigenic stimulation of BKPyV and MCPyV antibodies, we observed a positive correlation between age and MCPyV antibody levels and a negative correlation between age and BKPyV antibody levels. Although seroprevalence of JCPyV also increased with age, there was a null association between age and JCPyV antibody levels. The positive correlation of age and antibody titer for MCPyV may reflect unique features of immune surveillance of MCPyV and deserves further study. The age-related seroprevalence profile that we and others (10, 18, 19, 27) have observed for JCPyV differs from those for MCPyV and BKPyV. Of the three polyomaviruses, JCPyV had the lowest seroprevalence among children less than 10 years of age (9.5%) and the highest among adults over 70 years of age (80.5%). Similar to that of MCPyV, the increasing seroprevalence of JCPyV with age suggests that transmission occurs throughout life. A higher seroprevalence in older adults could be interpreted as a cohort effect. While this possibility cannot be entirely excluded for MCPyV, a cohort effect is unlikely to explain the age-related increase in seroprevalence of JCPyV because smaller studies from the 1970s reported similar trends.
For viruses within the same family, serological cross-reactivity is always possible. We have previously addressed this question for BKPyV and JCPyV using competitive inhibition assays and found no evidence of serological cross-reactivity between the major capsid proteins of these two viruses (30). In the present study, competitive inhibition assays with MCPyV and BKPyV support the specificity of the responses, although we cannot rule out cross-reactivity with other known human polyomaviruses. In support of the specificity of seroreactivity, we also found no evidence of a correlation between seroreactivity to MCPyV and that to BKPyV or JCPyV. Kean et al. (18) showed that MCPyV seroreactivity cannot be blocked by preincubation with soluble VP1 protein of the phylogenetically closely related polyomavirus lymphotropic polyomavirus. Tolstov et al. showed that the MCPyV reactivity of 4 serum samples was not blocked by preincubation with BKPyV capsids. Pseudovirion neutralization assays for MCPyV also support the species specificity of MCPyV seroreactivity (23, 28).
Seroepidemiological studies provide important evidence for an etiological role of a virus in a human cancer by demonstrating a higher rate of exposure in cancer patients than in controls. Similar to researchers in other studies, we found a higher seroprevalence of MCPyV in MCC patients than in controls (6, 28), although the association was attenuated after age adjustment, likely due to the high seroprevalence among controls (68%) and the small sample size. The lack of association between MCC and BKPyV seropositivity indicates that the tumor does not cause generalized antibody reactivity to polyomaviruses. The findings support an etiological role for MCPyV in MCC but also indicate that other factors play an important role in the development of what is a rare cancer occurring in a very small subset of individuals exposed to a nearly ubiquitous virus. We also found that the level of MCPyV antibodies was higher in MCC patients than controls, even after adjustment for age, as has been reported in other serological studies of MCC (6, 23, 28). The high levels in patients are unlikely to be due to antigen stimulation from tumor cells because truncating mutations in the large T-antigen gene are expected to block viral replication and production of capsids. However, high levels of antibody could be due to a high viral burden at the time of initial exposure or subsequent reactivation and could be a risk factor for development of MCC.
The age-specific seroprevalence of the newly discovered MCPyV has in common with those of previously known polyomaviruses, BKPyV and JCPyV, evidence of widespread exposure in human populations beginning early in life. However, the pattern of MCPyV age-specific seroprevalence also has unique features compared to those of the other two polyomaviruses. Seroprevalence among children is higher than that of JCPyV but lower than that of BKPyV. Among older adults, MCPyV seroprevalence remains high while that of BKPyV declines and that of JCPyV continues to rise. Although our study included a small number of subjects, we found an association between MCPyV seropositivity and MCC and higher levels of serum MCPyV capsid antibodies in MCC patients than in controls, as reported previously by other investigators.
ACKNOWLEDGMENTS
This study was supported by a generous donation from the Campbell family to the Moffitt Cancer Center Foundation for support of Merkel cell cancer research and by NIH grant RO-1 AI 51227.
Footnotes
Published ahead of print on 31 August 2011.
REFERENCES
- 1. Becker J. C., et al. 2009. MC polyomavirus is frequently present in Merkel cell carcinoma of European patients. J. Invest. Dermatol. 129:248–250 [DOI] [PubMed] [Google Scholar]
- 2. Bialasiewicz S., Lambert S. B., Whiley D. M., Nissen M. D., Sloots T. P. 2009. Merkel cell polyomavirus DNA in respiratory specimens from children and adults. Emerg. Infect. Dis. 15:492–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bofill-Mas S., Rodriguez-Manzano J., Calgua B., Carratala A., Girones R. 2010. Newly described human polyomaviruses Merkel cell, KI and WU are present in urban sewage and may represent potential environmental contaminants. Virol. J. 7:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Boldorini R., et al. 2011. Serological evidence of vertical transmission of JC and BK polyomaviruses in humans. J. Gen. Virol. 92:1044–1050 [DOI] [PubMed] [Google Scholar]
- 5. Brown D. W., Gardner S. D., Gibson P. E., Field A. M. 1984. BK virus specific IgM responses in cord sera, young children and healthy adults detected by RIA. Arch. Virol. 82:149–160 [DOI] [PubMed] [Google Scholar]
- 6. Carter J. J., et al. 2009. Association of Merkel cell polyomavirus-specific antibodies with Merkel cell carcinoma. J. Natl. Cancer Inst. 101:1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen T., et al. 2011. Serological evidence of Merkel cell polyomavirus primary infections in childhood. J. Clin. Virol. 50:125–129 [DOI] [PubMed] [Google Scholar]
- 8. Dei R., et al. 1982. Age-related changes in the prevalence of precipitating antibodies to BK virus in infants and children. J. Med. Microbiol. 15:285–291 [DOI] [PubMed] [Google Scholar]
- 9. Duncavage E. J., Zehnbauer B. A., Pfeifer J. D. 2009. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod. Pathol. 22:516–521 [DOI] [PubMed] [Google Scholar]
- 10. Egli A., et al. 2009. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J. Infect. Dis. 199:837–846 [DOI] [PubMed] [Google Scholar]
- 11. Feng H., Shuda M., Chang Y., Moore P. S. 2008. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 319:1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Flaegstad T., Traavik T., Kristiansen B. E. 1986. Age-dependent prevalence of BK virus IgG and IgM antibodies measured by enzyme-linked immunosorbent assays (ELISA). J. Hyg. (Lond.) 96:523–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Foulongne V., et al. 2008. Merkel cell polyomavirus and Merkel cell carcinoma, France. Emerg. Infect. Dis. 14:1491–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garneski K. M., et al. 2009. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J. Invest. Dermatol. 129:246–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goh S., Lindau C., Tiveljung-Lindell A., Allander T. 2009. Merkel cell polyomavirus in respiratory tract secretions. Emerg. Infect. Dis. 15:489–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kantola K., et al. 2009. Merkel cell polyomavirus DNA in tumor-free tonsillar tissues and upper respiratory tract samples: implications for respiratory transmission and latency. J. Clin. Virol. 45:292–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kassem A., et al. 2008. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 68:5009–5013 [DOI] [PubMed] [Google Scholar]
- 18. Kean J. M., Rao S., Wang M., Garcea R. L. 2009. Seroepidemiology of human polyomaviruses. PLoS Pathog. 5:e1000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Knowles W. A., et al. 2003. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J. Med. Virol. 71:115–123 [DOI] [PubMed] [Google Scholar]
- 20. Lemos B. D., et al. 2010. Pathologic nodal evaluation improves prognostic accuracy in Merkel cell carcinoma: analysis of 5823 cases as the basis of the first consensus staging system. J. Am. Acad. Dermatol. 63:751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loyo M., et al. 2010. Quantitative detection of Merkel cell virus in human tissues and possible mode of transmission. Int. J. Cancer 126:2991–2996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. McCardle T. W., Sondak V. K., Zager J., Messina J. L. 2010. Merkel cell carcinoma: pathologic findings and prognostic factors. Curr. Probl. Cancer 34:47–64 [DOI] [PubMed] [Google Scholar]
- 23. Pastrana D. V., et al. 2009. Quantitation of human seroresponsiveness to Merkel cell polyomavirus. PLoS Pathog. 5:e1000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadeghi M., et al. 2010. Newly discovered KI, WU, and Merkel cell polyomaviruses: no evidence of mother-to-fetus transmission. Virol. J. 7:251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sastre-Garau X., et al. 2009. Merkel cell carcinoma of the skin: pathological and molecular evidence for a causative role of MCV in oncogenesis. J. Pathol. 218:48–56 [DOI] [PubMed] [Google Scholar]
- 26. Schowalter R. M., Pastrana D. V., Pumphrey K. A., Moyer A. L., Buck C. B. 2010. Merkel cell polyomavirus and two previously unknown polyomaviruses are chronically shed from human skin. Cell Host Microbe 7:509–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stolt A., Sasnauskas K., Koskela P., Lehtinen M., Dillner J. 2003. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 84:1499–1504 [DOI] [PubMed] [Google Scholar]
- 28. Tolstov Y. L., et al. 2009. Human Merkel cell polyomavirus infection. II. MCV is a common human infection that can be detected by conformational capsid epitope immunoassays. Int. J. Cancer 125:1250–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Touze A., et al. 2011. High levels of antibodies against Merkel cell polyomavirus identify a subset of patients with Merkel cell carcinoma with better clinical outcome. J. Clin. Oncol. 29:1612–1619 [DOI] [PubMed] [Google Scholar]
- 30. Viscidi R. P., Clayman B. 2006. Serological cross reactivity between polyomavirus capsids. Adv. Exp. Med. Biol. 577:73–84 [DOI] [PubMed] [Google Scholar]
- 31. Viscidi R. P., et al. 2003. Serological cross-reactivities between antibodies to simian virus 40, BK virus, and JC virus assessed by virus-like-particle-based enzyme immunoassays. Clin. Diagn. Lab. Immunol. 10:278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]