Abstract
Previously we showed that antibodies specific for the glycan β-1,2-mannotriose [β-(Man)3] on the cell surface of Candida albicans protect mice against disseminated candidiasis (H. Xin, S. Dziadek, D. R. Bundle, and J. E. Cutler, Proc. Natl. Acad. Sci. U. S. A. 105:13526–13531, 2008). Furthermore, six 14-mer peptides that are within the N-terminal portion of C. albicans wall proteins were conjugated to the glycan in an attempt to create immunogenic glycopeptide conjugates. By a dendritic cell (DC)-based immunization approach, all were immunogenic and three of the six conjugates induced a high degree of protection in mice. Interestingly, whereas all six peptides induced antibody responses when used alone to pulse DCs for subsequent immunizations, three peptides induced protection, and one in particular, peptide Fba (derived from fructose-bisphosphate aldolase), induced robust protective responses and is the focus of the current work. Fba peptide is not restricted by the major histocompatibility complex class II (MHC-II), as it induced anti-Fba antibodies in mice of different H-2 haplotypes and in rabbits. Furthermore, the peptide induced protection against disease caused by different C. albicans strains. Partial protection was achieved when alum was used in place of DCs for Fba immunizations. The passive transfer of immune sera from Fba-vaccinated mice, but not immune serum preabsorbed with fungal cells, conferred protection in naïve mice. This result, along with our finding that a monoclonal antibody specific for the peptide, E2-9 (IgM), protected mice against candidiasis, provide strong evidence that antibodies contribute to protection. Our work demonstrates the utility of cell wall peptides alone or as glycopeptides in vaccines designed for the induction of immunity against candidiasis and monoclonal antibodies as a rapid immunoprotective approach against the disease.
INTRODUCTION
The polymorphic fungus Candida albicans is a commensal organism that colonizes the gastrointestinal tract, vagina, and some cutaneous areas of the majority of healthy humans. However, under certain conditions the fungus is able to cause a variety of infections, ranging from mucosal to life-threatening invasive candidiasis (18). C. albicans continues to be the most common cause of various forms of candidiasis (34, 47), but several other Candida spp. are also important agents. Invasive disease is associated with high health care costs and a mortality rate estimated at ∼40% (32, 33). The limited number and toxicity of antifungal agents and, most importantly, the poor outcome of almost half of the number of candidemia patients treated with appropriate antifungal therapy militates in favor of disease prevention, possibly through active and passive immunization strategies (10, 15, 41).
The protective role of antibodies against Candida has been controversial, but the evidence is mounting in favor of this mode of protection. The specificity of protective antibodies may be for C. albicans cell wall polysaccharides, proteins, and peptides (13, 17, 40, 53, 54). As a prevention strategy, protection against disease may be actively or passively acquired by vaccination and the transfer of preformed monoclonal antibodies. As a therapeutic measure, experimental evidence indicates that preformed antibodies enhance the effectiveness of antifungal agents (24, 43).
The first fully synthetic glycopeptide vaccines against C. albicans induced protection against disseminated candidiasis in mice (53). Six putative T-cell peptides found in C. albicans cell wall proteins were conjugated to the protective β-1,2-mannotriose [β-(Man)3] glycan epitope to create glycopeptide conjugates. The six proteins from which the peptides, denoted in parentheses, were derived were selected because of expression during human candidiasis and cell wall association: fructose-bisphosphate aldolase (Fba), methyltetrahydropteroyltriglutamate homocysteine methyltransferase (Met6), hyphal wall protein-1 (Hwp1), enolase (Enol), glyceraldehyde-3-phosphate dehydrogenase (Gap1), and phosphoglycerate kinase (Pgk1). The original intent of this work was that the peptides would serve as T-cell epitopes, promoting protective antibody responses against the glycan part of the glycopeptide conjugates. Thus, our immunization protocols were designed to favor antibody rather than cell-mediated immune responses, and to our surprise antibodies were generated against both the glycan and peptide parts of the various conjugates. That is, by dendritic cell (DC)-based immunization protocols favoring antibody production, the three glycoconjugates β-(Man)3-Fba, β-(Man)3-Met6, and β-(Man)3-Hwp1 induced protection against hematogenous challenge with the fungus as evidenced by mouse survival and low kidney fungal burden. β-(Man)3-Eno1 and β-(Man)3-Gap1 gave moderate protection, and β-(Man)3-Pgk1 slightly enhanced disease. For the β-(Man)3-Fba conjugate, protection was uniquely acquired through immunity against the glycan and the Fba peptide. The native protein fructose-1,6-bisphosphate aldolase (Fba1p), which catalyzes the reversible cleavage of fructose-1,6-bisphosphate to dihydroxyacetone phosphate and glyceraldehyde 3-phosphate, has become an attractive antifungal target for several reasons. First, this key enzyme is required for growth on both fermentative and nonfermentative carbon sources; therefore, it is essential for the viability of C. albicans and other pathogenic fungi (48). Second, fungal fructose-1,6-bisphosphate aldolases are distinct from human fructose-1,6-bisphosphate aldolases. C. albicans Fba1p belongs to the family of class II aldolases found predominantly in fungi and prokaryotes (39). In contrast, the human enzyme belongs to the class I aldolases, and the sequence of human aldolase is significantly different from those of fungal aldolases (39), thus it is reasonable to expect that it is possible to achieve an immunologic response specific only to the fungal enzyme. Indeed, the Fba 14-mer peptide sequence is unique to C. albicans (53).
Peptide Fba, which appears to be an effective carrier for the protective glycan epitope in a glycopeptide vaccine formulation (53), is also a good candidate by itself for vaccine development against candidiasis. In this study, the protective capacity of an Fba peptide vaccine, either formulated with a human-approved adjuvant or by a DC-based immunization approach that favors the production of protective antibody, was assessed in a murine model of human disseminated candidiasis. The vaccine conferred significant protection, and this was associated with the production of anti-Fba peptide antibodies in the sera of immunized mice. Importantly, the Fba peptide is not a major histocompatibility complex class II (MHC-II)-restricted epitope, as it induced anti-Fba antibodies in different strains of mice and rabbits and thus may be expected to be immunogenic in humans as well. Furthermore, a monoclonal antibody (MAb; E2-9) specific for the Fba peptide that was isolated from splenocytes of Fba-immunized mice showed protection against candidiasis in passive transfer experiments.
MATERIALS AND METHODS
Candida strains and culture conditions.
C. albicans 3153A and SC5314, C. krusei (ATCC 6258), C. glabrata (ATCC 2001), and Saccharomyces cerevisiae (ATCC 9463) were grown as stationary-phase yeast cells in glucose-yeast extract-peptone broth at 37°C, washed, suspended to the appropriate cell concentration (5 × 106/ml) in Dulbecco's phosphate-buffered saline (DPBS; Sigma), and used to infect mice intravenously (i.v.) as described previously (25, 29). C. albicans strain 3153A also was used for serum antibody absorption, immunofluorescence staining, and flow-cytometric analysis.
Mice.
BALB/c and C57BL/6 female mice (National Cancer Institute Animal Production Program, Frederick, MD), 5 to 7 weeks old, were used throughout. Mice were maintained in our AAALAC-certified animal facility, and all animal experiments were done in accordance with protocol numbers 120 and 150, approved by the Institutional Animal Care and Use Committee (IACUC) at Children's Hospital Research Institute in New Orleans.
Isolation and culture of DCs from mouse bone marrow.
Dendritic cells (DCs) were generated from mouse bone marrow by a previously described method (49, 53). Briefly, donor mice were euthanized by CO2 asphyxiation, their long bones and tibias were aseptically removed, bone marrow was flushed from the bones by forcibly injecting several ml of RPMI-1640, and clumps were removed or dispersed by gentle pipetting through a sterile 70-mm cell strainer. Red blood cells were lysed (ACK lysing buffer, containing 0.15 M NH4Cl, 1.0 mM KHCO3, 0.1 mM EDTA) for 4 min, and the remaining bone marrow cells were suspended in complete medium (CM; RPMI 1640 supplemented with 10% fetal bovine serum [FBS], 2 mM l-glutamine, 1% nonessential amino acids, 100 U/ml penicillin, and 100 μg/ml streptomycin), adjusted to 2 × 105 cells per ml plated in 6-well plates at 5 ml per well, and cultured for up to 9 days in the presence of 40 ng/ml of recombinant mouse granulocyte-macrophage colony-stimulating factor (rmGM-CSF) and rmIL-4 (R&D Systems) at 37°C and 5% CO2. On days 4 and 7 of culture the same amounts of fresh GM-CSF and IL-4 were added to the wells.
Immunizations with peptide-pulsed dendritic cells.
DCs were pulsed in vitro with peptide candidate vaccine antigens as described previously (53). Briefly, DCs in culture were pulsed with the peptide antigen (1 μM) on day 6. On day 7, prostaglandin E2 (PGE2) (10−7 M) was added along with lipopolysaccharide (LPS; 2 μg/ml; Sigma) for 24 h. On day 9, antigen-pulsed DCs were washed extensively, and 5 × 105 DCs in 200 μl DPBS were given intraperitoneally (i.p.) as the priming dose to mice. The mice were boosted i.p. at day 14 with fresh antigen-pulsed DCs and boosted a second time at day 28 with antigen (10 μg) emulsified in complete Freund adjuvant (CFA) given subcutaneously (s.c.).
Immunizations with peptide Fba in adjuvants approved for humans.
Fba peptides were administered as either a mixture made with alum (aluminum hydroxide gel; Sigma) or MPL (lipid A; monophosphoryl; Sigma) as adjuvants. Mice were immunized by s.c. injection with 100 μl of 2.5 μg of the Fba peptide with either 50 μg alum or 10 μg MPL on days 1, 21, and 42. Sera from groups of mice given DPBS buffer or adjuvant only were used as negative controls.
Serological assays.
Sera were analyzed by enzyme-linked immunosorbent assay (ELISA) for antibody titers. For DC-based immunization, control groups consisted of mice given DCs alone at the time of priming and the first booster, CFA alone at the time of the second booster, or DPBS alone for all three injections. For Fba peptides administered with alum or MPL, control groups were given adjuvant alone or DPBS buffer. Fba peptide was conjugated to a multiple antigenic peptide (MAP), of which the lysine core displayed approximately eight copies of the Fba peptide epitope. Synthetic Fba-MAP (GenScript) was dissolved at 5 μg/ml in PBS (pH 7.4) and used to coat 96-well ELISA plates for testing duplicate serial 2-fold dilutions of samples of each immune serum and control sera. Color development for each well was achieved by use of secondary antibody (goat anti-mouse polyvalent Ig-horseradish peroxidase [HRP]; Sigma) and substrate (O-phenylenediamine and H2O2), and the optical density at 492 nm (OD492) was determined.
MAbs.
Hybridoma clones producing the MAb E2-9 (IgM) were generated from mice vaccinated with an Fba-DC preparation as described previously (53). Briefly, BALB/c mice were immunized by the injection of synthetic Fba peptide-pulsed DCs to stimulate the production of antibodies against Fba peptide as described above. Ten days after the second booster, serum was taken from each animal to determine animals with the highest anti-Fba titers for subsequent sacrificing, the removal of spleens, and the preparation of single-cell suspensions. Hybridoma clones were established by the polyethylene glycol facilitation of the fusion of spleen cells to an SP2/0-AG14 myeloma cell line by standard protocols. Hybridoma clones were screened by ELISA for the production of a specific anti-Fba antibody; only the highest titers and most rapidly growing clones were selected for subsequent cloning three times or more by limiting dilution. Clone E2-9 produced the MAb designated MAb E2-9 that was reactive with the Fba peptide, as determined by ELISA inhibition with synthetic Fba peptide by methods described below.
The hybridoma cell lines were grown initially in antibiotic-free RPMI 1640 medium (Sigma) supplemented with 10% fetal bovine serum (Invitrogen) and 2 mM l-glutamine (Sigma) at 37°C and in the presence of 5% CO2. For antibody production, the hybridoma clones were grown in antibiotic-free BD cell MAb serum-free medium (but containing 1.1 mg bovine serum albumin/ml) in a CELLine device (BD, Bedford, MA) and concentrated by using centrifugal filter devices (Centricon Plus-80; Millipore Corporation, Bedford, MA). MAb concentrations were determined by measuring the A280 of the sample, and purity was confirmed by analysis on a 10 to 12.5% SDS-PAGE gel.
Inhibition ELISA.
The specificity of MAb E2-9 for Fba peptide was determined by an inhibition ELISA as described previously (52, 53). Briefly, Fba-MAP was dissolved in DPBS (5 μg/ml), and the solution was used to coat 96-well ELISA plates (100 μl; overnight at 4°C). The wells were washed five times with PBST (PBS containing 0.05% [vol/vol] Tween 20) and blocked with 1% bovine serum albumin-PBST. MAb E2-9 was produced as described above and diluted to 1:10,000 for ELISA measurements. MAb E2-9 was mixed with Fba peptide (inhibitor) (dissolved in PBST at a concentration between 0.1 μM and 1 mM), and the resulting solution of each concentration was added to the Fba-MAP-coated microtiter wells (solid phase) in triplicate and incubated at 21 to 23°C for 2 h. The wells were washed three times with PBST, goat anti-mouse heavy chain specific for IgM was HRP conjugated (diluted 1:10,000 in PBST) (Sigma), and 100 μl was added the corresponding wells and incubated for 1 h at 21 to 23°C. The wells were washed five times with PBST, followed by the addition of 100 μl of substrate solution (25 ml of 0.05 M phosphate-citrate buffer [pH 5.0], 200 μl of an aqueous solution of O-phenylenediamine 50 mg/ml [Sigma], and 10 μl of 30% H2O2). Color was allowed to develop for 10 min, stopped by the addition of 100 μl of 2 M H2SO4, and read at 492 nm (microtiter plate reader model 450; Bio-Rad, Richmond, CA). The percent inhibition was calculated relative to levels for wells containing antibody without inhibitor.
SDS-PAGE.
MAb E2-9 was evaluated by SDS-PAGE (12.5% polyacrylamide) analysis under reducing (β-mercaptoethanol) conditions to determine sizes of heavy and light chains of the antibody. The IgM pentamer of E2-9 was shown by the Western blotting of an SDS-PAGE gel under nonreducing SDS-PAGE (2.5% acrylamide/bis [N,N′-methylene-bis-acrylamide] and 0.5% agarose) conditions. Following separation by SDS-PAGE, the proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad). The membrane was blocked for 2 h with 5% nonfat milk dissolved in PBST (pH 7.4). The membrane was washed in PBST (pH 7.4) and then probed with horseradish peroxidase-conjugated secondary Abs. A positive signal was visualized using the ECL system (Perkin Elmer).
Immunofluorescence and flow cytometric analysis.
The distribution of the Fba peptide epitope on yeast cells was determined by indirect immunofluorescence. Two hundred microliters of MAb E2-9 (at 16 μg Ab protein/ml of DPBS) was added to a pellet of C. albicans yeast cells (5 × 106) that was prewashed with DPBS three times. The yeast cells were suspended in the antibody preparation and incubated with shaking by rotation at room temperature (RT; 22 to 24°C) for 1 to 2 h. After incubation, the yeast cells were washed with DPBS three times, suspended in 200 μl of fluorescein-labeled goat anti-mouse IgM (u-chain specific; Sigma) (stock solution, 1 mg/ml; working solution, 20 μg/ml of DPBS), and incubated at RT as described above for 0.5 h. The yeast cells were washed with DPBS three times and suspended in 200 μl of DPBS. The cells were observed by confocal microscopy (LSM 510; Zeiss). The distribution of the Fba peptide epitope on the yeast cell surface was compared to that obtained with yeast cells fluorescently stained for the detection of the MAb B6.1 epitope. Negative controls included the testing of an irrelevant isotype control IgM MAb S-9 (45) and the use of fluorescein-labeled goat anti-mouse secondary antibody only.
For flow-cytometric analysis, following the last incubation cells were washed as described above and suspended in 500 μl DPBS buffer. Flow cytometry was performed using a BD Biosciences FACSVantage SE equipped with an argon laser with excitation at 488 nm. A total of 10,000 cells in each sample were analyzed (CellQuest Pro software).
Fungal challenge and assessment of protection.
Two weeks after the second boost, immune and control mice were infected i.v. with a lethal dose of live C. albicans yeast cells (5 × 105 in 0.1 ml of DPBS) prepared as described above and as before (53). Passively immunized mice (see below) also received the same challenge dose. Protection was evaluated by monitoring animal survival for 80 to 100 days. The mice were monitored for the development of a moribund state, defined as being listless, disinterested in food or water, and nonreactive to finger probing. At the time that a mouse was deemed moribund, it was sacrificed and their kidneys (or, for some experiments, brains) were homogenized in DPBS and plated onto a nutrient agar to determine CFU numbers. After 80 to 100 days, the experiments were terminated and all survivors at that time were sacrificed and their kidneys assessed for numbers of CFU as described above. The lowest limit of detection for the CFU assay was 50 CFU per kidney pair.
Passive transfer of PAbs.
To determine if antibodies in the sera from vaccinated mice were responsible for the protection induced by active immunization, polyclonal antibodies (PAbs) were obtained from vaccinated mice and pooled. The titers of pooled immune sera from Fba-pulsed DC-immunized mice were determined by ELISA as described above. The pooled PAbs were stored at −20°C or absorbed with C. albicans yeast cells and stored. For the transfer of rabbit anti-Fba sera, one mouse group that received preimmune rabbit serum was used as an additional control. For testing, mice received 0.5 ml i.p. full-strength immune serum or control serum. Four hours later, all mice were challenged i.v. with C. albicans (5 × 105 yeast cells). All animals received a second dose (100 to 200 μl) of serum or buffer i.p. 24 h after the first dose.
Passive transfer of MAbs by i.p. route.
The preventive effect of MAb E2-9 was examined by the same injection schedules as those described above for experiments on PAbs. Control mice received equivalent volumes of the DPBS diluent. The MAb E2-9 was appropriately diluted in DPBS (16 μg/ml) to give a 10,000 ELISA titer against Fba-MAP peptide coated on the plate. Prior to mouse injections, antibody solutions were spun at 15,000 × g for 15 min to remove possible antibody aggregates. The negative-control materials tested in mice were MAbs absorbed with C. albicans yeast cells and DPBS. For each condition, 6- to 8-week-old female BALB/c mice (Jackson Laboratories) were given 0.5 ml of test MAb or control materials intraperitoneally, followed 4 h later by 0.1 ml intravenously of a suspension containing 5 × 106 yeast cells per milliliter of DPBS.
Statistical analysis.
Survival times were statistically evaluated by Kaplan-Meier (GraphPad Prism, version 4). In all analyses, there were five mice per group (n = 5) and a two-tailed t test was used.
RESULTS
Vaccination with three synthetic peptides induced antibody production and protection against disseminated candidiasis in mice.
C. albicans carrier peptides were selected from previously identified cell wall proteins that are expressed during the pathogenesis of human disseminated candidiasis (11, 46). We selected six candidate carriers, each of which was comprised of 14 amino acids located near the N terminus of their respective complete protein, presumed to be located in the cell wall of the fungus (46). By convention, the N terminus would be expected to be toward the exterior of the fungal cell wall and most likely accessible to the host immune system. The six candidate carrier peptides were derived from the proteins Fba, Met6, Hwp1, Enol, Gap1, and Pgk1. Each synthetic peptide was chemically conjugated to the synthetic glycan β-(Man)3 to produce the six glycopeptide vaccine constructs for immunogenic testing. Three of the glycopeptide conjugates, β-(Man)3-Fba, β-(Man)3-Met6, and β-(Man)3-Hwp1, induced a high degree of protection, as evidenced by survival and low kidney fungal burden following challenge with a lethal dose of C. albicans (53). These results led us to consider whether protection also was contributed by responses to the carrier peptides. By an antigen-pulsed dendritic cell (DC)-based vaccine strategy favoring the production of antibodies as described before (49, 53), all six peptides were immunogenic by themselves, as shown by high titers of specific antibody to each (Fig. 1A). Peptide controls of random sequences were used, and they never induced detectable specific antibody responses (data not shown). In addition, tests were negative for cross-reactivity between each of the various immune sera and the respective unrelated peptides (data not shown). Also, each of the six antisera, but not negative-control sera, reacted directly with yeast and hyphal forms of the fungus, as evidenced by indirect immunofluorescence microscopy (data not shown).
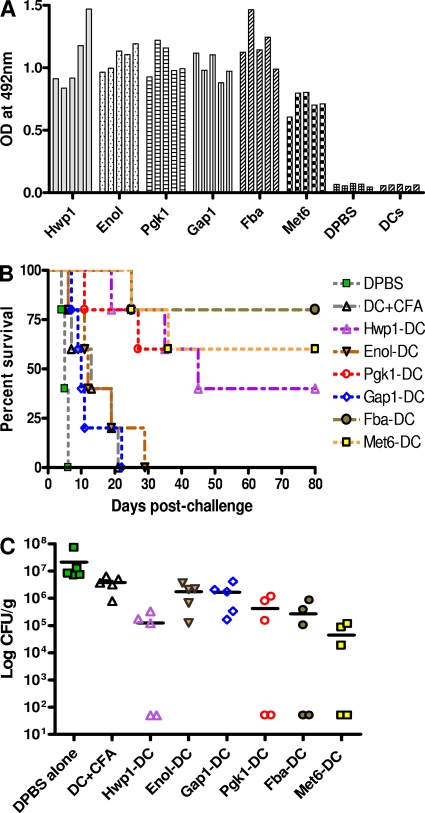
Fig. 1.
Antibody responses in BALB/c mice against peptide-pulsed DC vaccines. Sera from mice immunized with six synthetic peptide carriers presented on dendritic cells (DCs) for the priming and first booster immunizations and emulsified in complete Freund adjuvant (CFA) for the second booster were tested by ELISA for antibody responses against the peptides. Control groups consisted of mice given the same regimen for priming and boosters, except that the peptides were omitted (DC+CFA) or only DPBS was used at each injection time. (A) Serum samples were collected from each of the five mice per group of vaccinated animals and each of the five mice in the two control groups 14 days after the second booster immunization, diluted 1:100, and tested by ELISA. All peptides were able to induce antibody responses in mice. (B) Peptide vaccines induced protective responses in mice against disseminated candidiasis. Following challenge with a lethal dose of live C. albicans, mice vaccinated with peptides Fba, Hwp1, and Met6 survived significantly longer than either of the control groups (P < 0.05). (C) Mice immunized with Fba, Hwp1, and Met6 had greatly reduced or nondetectable (ND) viable fungal CFU per kidney pairs compared to levels for either of the control groups (P < 0.001). Mice immunized with Pgk1 also had reduced or nondetectable CFU in their kidneys compared to those of controls. The bars of each panel indicate means of CFU per kidney pair for each group. The lowest limit of detection for the CFU assay is 50 CFU per kidney pair.
Interestingly, the three carrier peptides Fba, Met6, and Hwp1 induced a high degree of protection, as evidenced by survival and low kidney fungal burden in mice challenged with the fungus (Fig. 1B and C). The immunized groups that received the Fba, Met6, or Hwp1 peptide vaccine showed 40 to 80% survival throughout the 80-day postchallenge observation period and survived significantly longer than DPBS or adjuvant-only controls following i.v. challenge with a lethal dose of live fungal cells. This conclusion was further strengthened by an extended observation in which neither of two other peptides, Gap1 and Eno1, induced protection against disseminated candidiasis (Fig. 1B). Importantly, the survivors in groups immunized against Fba, Met6, and Hwp1 had low or even nondetectable viable fungal units (CFU) in kidney (Fig. 1C), a target organ in disseminated candidiasis, compared to numbers for animals that succumbed (P < 0.001). Mice immunized with Gap1 or Enol1 alone resulted in a slightly lower fungal burden in the kidneys compared to those of nonimmune mice, but the differences were not significant. Surprisingly, Pgk1 alone also induced some protection, and the immunized mice also had low or nondetectable CFU numbers in their kidneys compared to those of controls. We didn't expect the protection by Pgk1, since β-(Man)3-Pgk1 immunization actually enhanced disease (53); further investigation will answer whether different host response characteristics are associated with the β-(Man)3-Pgk1 conjugate that account for disease enhancement.
Fba peptide is not an MHC-II-restricted epitope.
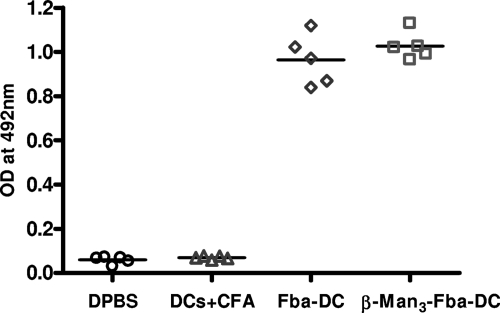
Fba peptide has been our focus point for vaccination development, because by itself this carrier peptide is a good candidate for vaccines, so it is critical to test if the Fba peptide is class II restricted. BALB/c mice have been used extensively as our mouse model of disseminated candidiasis (25–27, 30). In this study, we tested the Fba peptide in C57BL/6 mice, another common inbred mouse strain, but with an MHC haplotype and immunophenotype distinct from those of BALB/c mice (35). These two strains also differ in their resistance or susceptibility to experimental disseminated candidiasis (2–4). In addition to mouse strain differences with respect to a Th1 (C57BL/6) or Th2 (BALB/c) bias, the strains differ in their macrophages' ability to be activated (6). By the same DC-based immunization approach used for the BALB/c mice, C57BL/6 mice responded well by making antibodies against Fba when the peptide was given alone or as a glycoconjugate compared to responses of the control groups of mice injected with DPBS buffer or DC plus CFA (Fig. 2). Following the first booster immunization, an isotype switch from IgM to IgG of Fba-specific antibodies was observed in the sera from immunized mice (data not shown).
Fig. 2.
Antibody responses in C57BL/6 mice against the Fba peptide vaccines. Fourteen days after the second booster, immune sera were tested by ELISA for antibody responses against the Fba peptide. ELISA titers, done on plates coated with the synthetic peptide Fba-MAP, showed relatively strong specific antibody responses against the peptide compared to the responses of two control groups, which consisted of mice given DPBS or DC+CFA only.
To obtain enough antisera for passive transfer experiments and to determine whether the Fba peptide is immunologically restricted in other animal species, we obtained rabbit antisera against the peptide from a commercial source (Genscript). Fba was conjugated to keyhole limpet hemocyanin (KLH) prior to the rabbit immunizations. Titers of 512,000 of anti-Fba peptide immune sera from each of two rabbits were obtained (data not shown), which offer additional evidence that the Fba peptide is not an MHC-restricted epitope and most likely will be immunogenic in humans.
Fba-DC vaccination protects mice challenged with different C. albicans strains.
C. albicans strain 3153A is readily available from the ATCC and has been extensively used in our previous studies (52, 53). To test if vaccination with the Fba peptide-pulsed DCs protects mice challenged with a different C. albicans strain, we challenged immunized mice with C. albicans strain SC5314, which is also available from the ATCC and is the most commonly used clinical isolate of C. albicans for research purposes. As a positive control, a second group of mice immunized at the same time was challenged with strain 3153A. Similar protection patterns were observed in both groups of mice regardless of the challenge strain (Fig. 3A and B for challenge with 3153A and SC5314, respectively). In addition to prolonged survival times, each group had reduced or nondetectable CFU numbers in their kidneys compared to those of nonimmune mice (Fig. 3).
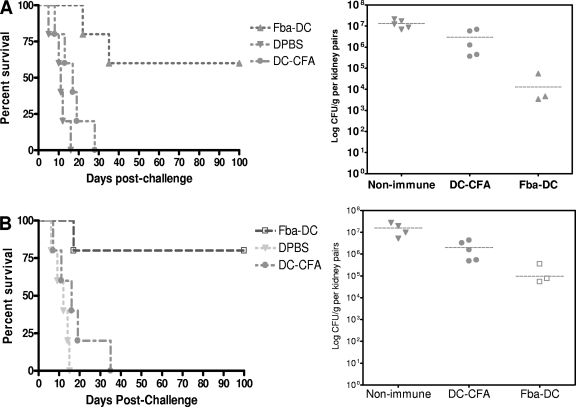
Fig. 3.
Fba peptide vaccines induce protective responses in BALB/c mice against disseminated candidiasis caused by different C. albicans strains. Vaccination with Fba peptide by the dendritic cell approach induced significant protection against experimental disseminated candidiasis in mice, regardless of the fungal strain. (A) Vaccinated mice challenged with a lethal dose of a prototypical strain of C. albicans (SC5314) had a significantly prolonged survival time compared to that of control mice that received DC+CFA or DPBS only (P < 0.01). Consistently with survival data, immunized mice had greatly reduced or nondetectable numbers of CFU in their kidneys (P < 0.01) compared to those of control mice. (B) Similar results were obtained when immunized mice were challenged with C. albicans strain 3153A.
Vaccine efficacy in BALB/c and C57BL/6 mice: antibody titers and survival studies.
As indicated above (Fig. 2), the Fba peptide also induced strong antibody responses in C57BL/6 mice. C57BL/6 mice are more prone to Th1 responses and supposedly are more resistant to disseminated candidiasis than are BALB/c mice that are more prone to Th2 and, hence, antibody responses. Thus, in an effort to determine the general efficacy of the Fba vaccine, we decided to test whether immunized C57BL/6 mice are protected against disseminated candidiasis. Indeed, vaccination with Fba peptide-pulsed DCs induced protection against experimental disseminated candidiasis in this mouse strain (Fig. 4A), as was observed for the BALB/c mouse (53). Specifically, Fba peptide vaccination resulted in a prolongation of the survival of both BALB/c (53) and C57BL/6 mice compared to that of DPBS controls or adjuvant-only vaccination. Consistently with survival data, immune mice that survived the observation period had greatly reduced or nondetectable live fungal units in their kidneys and brains compared to numbers for controls that were sacrificed when they became moribund following i.v. challenge with the fungus (Fig. 4B and C).
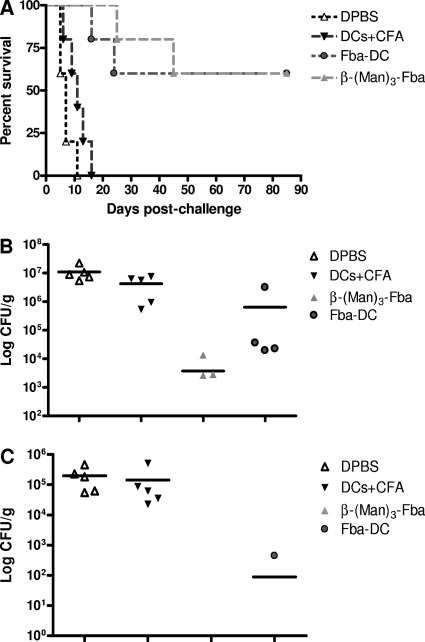
Fig. 4.
Fba peptide vaccines induce protective responses in C57BL/6 mice against experimental disseminated candidiasis. (A) Mice immunized against either the Fba peptide-DC vaccine or β-(Man)3-Fba-pulsed DCs survived significantly longer (P < 0.01) than DPBS and DC+CFA control mice. (B and C) The immunized mice that survived the experiment were sacrificed and found to have greatly reduced or nondetectable live CFU in their kidneys (P < 0.01) and brains (P < 0.001) compared to those of controls. Bars in each panel indicate mean CFU for each group. Each CFU value represents each individual mouse. There are no data points for the third and fourth column because the CFU numbers of these vaccinated mice were beneath the detectable level.
Anti-Fba peptide immune sera provide passive protection for naïve mice.
To answer whether induced anti-Fba antibody responses are responsible for the protection, antisera were collected from immunized mice and transferred i.p. to naïve mice 4 h before i.v. challenge with a lethal dose of C. albicans. Control groups were given either immune sera absorbed with live C. albicans yeast cells or DPBS buffer prior to the challenge. Immune serum donors, which were immunized with Fba peptide-pulsed DCs, were used as a positive control for protection. After challenge, immunized mice and mice treated with the antiserum had prolonged survival times compared to those of the two control groups (Fig. 5A), and consistently, mice that received the antiserum had significantly reduced fungal counts in their kidneys (Fig. 5B). The data provided strong evidence that anti-Fba peptide antibodies are at least partially, if not entirely, responsible for the protection against a lethal challenge with the fungus.
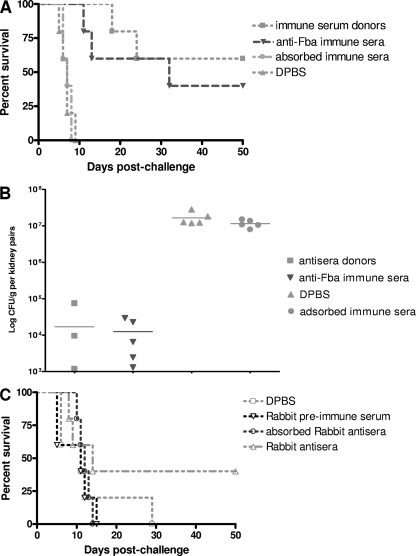
Fig. 5.
Antibody is responsible for protection against disseminated candidiasis. Serum was collected from mice immunized by the dendritic cell approach against Fba peptide 14 days postimmunization. Immune sera were pooled and tested for the passive protection of naive mice against experimental disseminated candidiasis as described in Materials and Methods. (A) Enhanced protection against disseminated C. albicans infection was observed in mice that received serum from mice immunized with the Fba peptide compared to that of animals that received control materials. Note that the donor mice used as positive controls for protection had survival curves similar to those of the naïve mice that received the immune serum. Importantly, absorption with C. albicans before transfer also removed the protective value of immune serum. (B) Immunized mice and mice that received antiserum had significantly fewer (P < 0.0001) fungal counts or nondetectable CFU in kidneys compared to those of the control groups that received either the same sera but were preadsorbed with C. albicans yeast cells or DPBS buffer only. (C) Rabbit immune sera containing specific anti-Fba polyclonal antibodies (PAbs) also provided some protection to mice against disseminated candidiasis. The rabbit immune serum-treated group had prolonged survival times compared to those of the control groups that received adsorbed rabbit sera, rabbit preimmune serum only, or DPBS buffer only.
For the commercial rabbit anti-Fba sera, both the anti-Fba immune serum and preimmune serum were absorbed by mouse splenocytes to remove possible rabbit natural anti-mouse cytotoxic antibodies. As a negative control, after the splenocyte absorption rabbit anti-Fba immune serum was absorbed again, but this time with live C. albicans yeast cells to remove Fba-specific antibodies. We tested anti-Fba antibody titers by ELISA before and after adsorption with the fungal cells and showed that the Fba antibodies were no longer detectable following the adsorption (data not shown). The mice treated with rabbit antiserum had 40% survival at the end of the experiment and overall prolonged survival times compared to those of the control groups (Fig. 5C), which again supported a protective role for anti-Fba antibodies against candidiasis. The reason for the relatively weak protection by rabbit immune sera compared to that of mouse anti-Fba sera is not known but may well be due to the lower efficiency of FcR effector function of rabbit antibodies within the mouse system (1).
Fba peptide administered along with alum induces modest protection against candidiasis.
By the use of the DC-based immunization approach, the synthetic Fba peptide vaccine provided protection against disseminated candidiasis in normal mice. However, an important limitation of the vaccine is that the use of DCs, followed by a booster of Fba emulsified in CFA, is inappropriate for human use. To resolve this problem, Fba peptide was administered as a mixture with alum or MPL adjuvant, both of which may be used in humans. Mice were immunized by s.c. injection of 0.2 ml containing 2.5 μg of Fba peptide mixed with either 50 μg alum or 10 μg MPL on days 1, 21, and 42. Negative-control groups of mice were given a similar volume of DPBS buffer or adjuvant only. Serum samples were collected 14 days after immunization, diluted 1:100, and tested by ELISA on plates coated with synthetic Fba-MAP peptide. After the first booster, immune sera from mice immunized with Fba peptide prepared in either alum or MPL showed that antibody responses to either preparation peptide were more than 5- to 8-fold higher than the background level for sera obtained from mice that received DPBS or adjuvant only (Fig. 6A). Following the second booster immunization, an isotype switch from IgM to IgG of Fba-specific antibodies was observed in the sera from immunized mice (data not shown), which suggested the induction of an immune memory response. In addition, the vaccinated groups had prolonged survival times compared to those of two control groups after challenge with a lethal dose of C. albicans cells (Fig. 6B), although the protection was not as strong as that which was induced by the DC-plus-CFA approach. Mice immunized with Fba peptide administered along with alum had 40% survival; however, Fba with MPL only induced slight protection compared to that of the control groups. Along with prolonged survival times, immunized mice had reduced live fungal cells in their kidneys as expected (Fig. 6C).
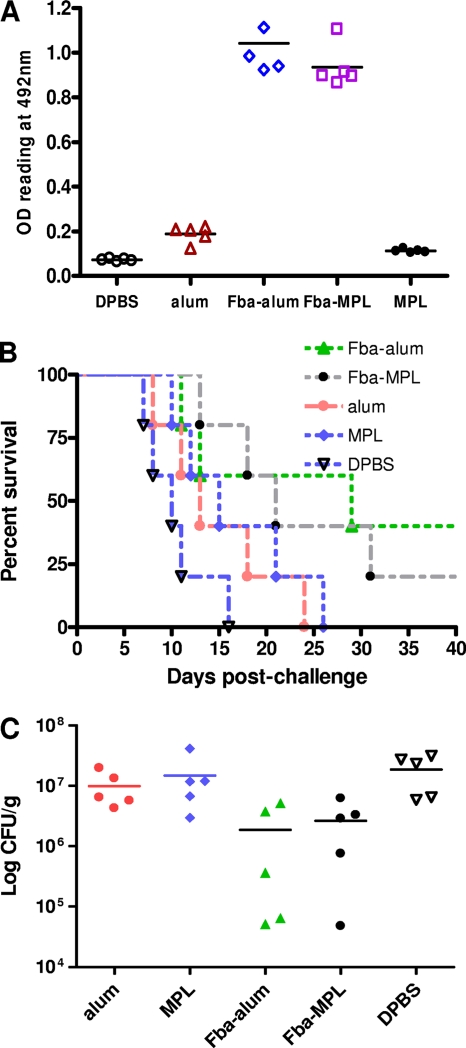
Fig. 6.
Fba peptide vaccines administered along with the approved adjuvant alum (approved for humans) induced protective responses in mice against disseminated candidiasis. (A) Serum samples were collected 14 days after immunization, diluted 1:100, and tested by ELISA on plates coated with synthetic Fba-MAP. After the first booster, immune serum from mice immunized with Fba peptide prepared in either alum or MPL showed that antibody responses to the Fba peptide were more than 5- to 8-fold greater than those of sera from groups that received DPBS or adjuvant only. (B) Compared to levels for DPBS-treated or unimmunized controls, moderate protective immunity was induced by Fba peptide when alum was used as the adjuvant, and slight protection was observed when MPL was used as the adjuvant. The survival also was slightly extended in mice that received only alum or MPL compared to those receiving only DPBS, but the differences were not statistically significant. (C) Immunized mice had reduced numbers of viable fungal units (CFU) per kidney pair compared to those of DPBS control or adjuvant-only groups (P < 0.01).
Fba MAb binds to the fungal cell surface.
MAbs specific for Fba peptide were obtained by the use of standard hybridoma techniques. After cell fusion, specific antibody-producing hybridomas were cloned four times by limiting dilution, of which we selected 48 anti-Fba IgM clones and 12 anti-Fba IgG clones.
Clones that produced MAbs E2-9, B7-18, and B7-22, isotyped as IgM, were selected and expanded in BD serum-free culture medium. Under reducing conditions and polypeptide chain confirmation by Western blotting developed with goat anti-mouse IgGAM(H+L)-HRP antibody, heavy and light chains of E2-9 and B7-18 showed the correct corresponding sizes of 50 and 25 kDa, respectively (Fig. 7 A). The IgM pentamers (∼900 kDa) of MAbs were detected by Western blot analysis developed with goat anti-mouse IgGMA-HRP antibody from nonreducing SDS gels (Fig. 7B). Clone E2-9 grew well and proliferated faster than clones B7-18 and B7-22 and was a consistently high producer of high-titer anti-Fba antibodies. Therefore, we chose to work on E2-9 for further studies. The reaction of E2-9 for the Fba peptide was determined by an inhibition ELISA (Fig. 7C), in which soluble synthetic peptide Fba effectively inhibited the reactivity of MAb E2-9 with solid-phase Fba.
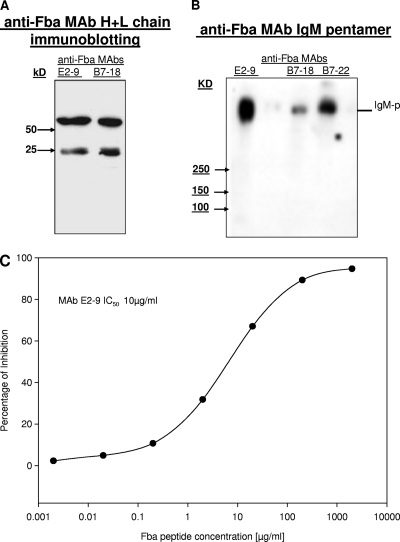
Fig. 7.
MAbs were detected by SDS-PAGE, and the specificity of Fba reaction with MAb E-29 was determined by inhibition ELISA. (A) Clones producing IgM MAbs (E2-9, B7-18, and B7-22) were selected and expanded in BD serum-free culture. Heavy and light chains of E2-9 and B7-18 with corresponding sizes were shown on 12.5% SDS page gels under reducing conditions. (B) The isotypes of E2-9, B7-18, and B7-22 were confirmed as IgM by SDS-PAGE analysis, which gave a whole molecular size consistent with that of the IgM molecule; the putative IgM pentamer was observed by Western blotting of a 10% SDS-PAGE gel run under nonreducing conditions, and the molecular mass of the purified anti-Fba IgM MAb (E2-9) was estimated at 900 kDa. (C) ELISA inhibition data for the anti-Fba peptide MAb E2-9. Synthetic Fba peptide was used as an inhibitor to determine the reaction and binding affinity of MAb E2-9 with Fba peptide. Each point is the means from three determinations, and the data shown are from a typical experiment of four independent experiments, all producing similar results. The concentration of inhibitor (Fba peptide) required to achieve 50% inhibitory concentration was ∼10 μg/ml.
MAb E2-9 also was detected by an indirect immunofluorescence antibody test to confirm its specific reactivity with Fba peptide on the cell surface of C. albicans (Fig. 8A), which was readily apparent on both fresh and formaldehyde-fixed C. albicans yeast cells and hyphae. MAb B6.1, which binds to an abundantly expressed cell surface epitope, β-1,2-mannotriose, and protects mice against disseminated candidiasis (25, 28), was used as a positive control for IgM antibody binding to the fungal cell surface. No cross-reactivity was detected with other Candida species (data not shown), which was expected because the Fba 14-mer amino acid peptide should be unique to C. albicans, as we previously reported (53). The reactivity of MAb E2-9 with the cell surface of C. albicans also was demonstrated by flow-cytometric analysis (Fig. 8B). MAb B6.1 also was used as a positive control for antibody binding to C. albicans yeast cell surfaces. Although MAb E2-9 reacted with wild-type C. albicans 3153A, as expected the antibody did not label isolates of other Candida species (C. glabrata and C. krusei) or S. cerevisiae (Fig. 8C).
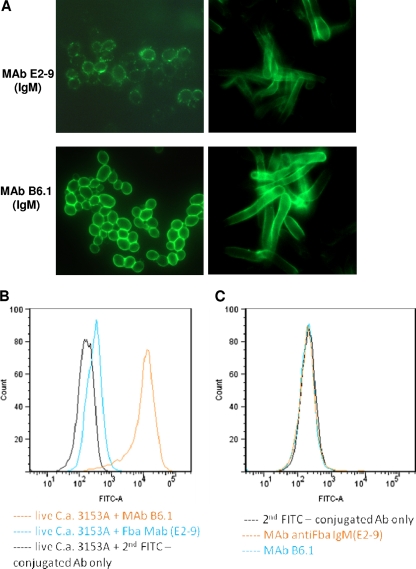
Fig. 8.
Fba peptide epitope is expressed on C. albicans cell surface as detected by MAb E2-9. (A) Use of MAb E2-9 in confocal microscopic analyses showed that the peptide Fba is expressed on the fungal surface and E2-9 can directly bind to both yeast and hyphal forms of the fungus. MAb B6.1, which is specific to β-(Man)3, was used as a positive control. (B) MAb E2-9 binding to the Fba peptide expressed on the C. albicans cell surface was further confirmed by the use of flow-cytometric analysis. Compared to MAb B6.1, which is specific for the C. albicans cell surface epitope β-trimannose, the reactivity of MAb E2-9 with live C. albicans cells was relatively slight. (C) As an important additional negative control, we tested the binding of both MAbs E2-9 and B6.1 to live Saccharomyces cerevisiae, which should not express either Fba or the β-trimannose epitope. Neither MAb bound to the cell surface of S. cerevisiae.
IgM MAb (E2-9) conferred enhanced protection against systemic candidiasis in passive transfer experiments.
We showed the protective potential of anti-Fba antibodies induced by either the Fba-DC vaccine approach or by immunization with the peptide suspended in alum adjuvant. The development of monoclonal antibodies specific for Fba peptide not only affords us important probes to study the fungal expression of the peptide but also provides us with the possibility of producing an unlimited supply of protective antibody for in vivo applications. MAb E2-9 was selected for study and was tested as described above in passive transfer experiments showing protection by polyclonal antiserum. BALB/c mice given an i.p. dose of MAb E2-9 4 h before hematogenous challenge with a lethal dose of C. albicans 3153A had prolonged survival compared to that of control animals (Fig. 9A). In addition, surviving animals that received the antibody had reduced fungal burden in their kidneys (Fig. 9B). Importantly, passive protection was prevented by the removal of the MAbs by absorption with Candida cells before transfer, which provided strong additional evidence for the protection being due to the MAb.
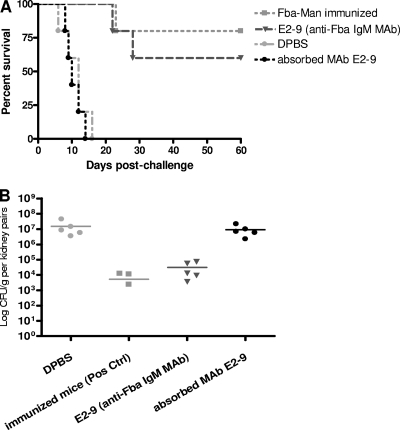
Fig. 9.
Anti-Fba IgM MAb (E2-9) protects mice against disseminated candidiasis. To confirm that MAb E2-9 produced by cell culture was able to protect mice against experimental disseminated candidiasis, MAb E2-9 (16 μg/ml; 0.5 ml) was given to naïve mice 4 h before i.v. C. albicans challenge, and another 0.2 ml of MAb E2-9 was given 24 h after the first dose. Mice that were immunized with the β-(Man)3-Fba conjugate were used as a positive control for survival, and DPBS and MAb E2-9 absorbed by C. albicans yeast cells was given to naïve mice as a negative control. (A) The mice that received MAb E2-9 had a prolonged survival time that was similar to that of the positive-control group that was actively immunized with β-(Man)3-Fba but not DPBS or the absorbed MAb E2-9 negative-control group (P < 0.001). (B) Passive transfer of MAb E2-9 to naïve mice reduced the kidney fungal burden to a level similar to that of survivors that were actively immunized compared to the fungal burden in mice that were given DPBS or absorbed MAb E2-9 (P < 0.001).
DISCUSSION
We originally began an investigation into the use of C. albicans cell wall-derived peptides to serve as T-cell epitopes when conjugated to the low-molecular-weight glycan β-1,2-mannotriose, which we had described as a cell wall epitope protected by antibodies against disseminated candidiasis. Surprisingly, we showed in this study that all six derived peptides induced antibody responses when used alone to pulse DCs for subsequent immunizations, and three peptides, Fba, Hwp1, and Met6, induced a high degree of protection, as evidenced by survival and low kidney fungal burden in mice challenged with the fungus. Vaccinated mice obviously had less fungal burden in kidneys than did nonsurviving controls; in fact, the CFU burden was not detectable in the kidneys of some of the vaccinated mice, whereas this never happened in control groups. The combination of prolonged survival with a significant reduction or clearance of kidney CFU provided strong evidence of protection induced by vaccines. One particular peptide, Fba, which induced robust antibody responses and the best protection among the three protective peptides, became the focus of the current work, in which the Fba peptide was further characterized as a novel vaccine candidate. The Fba peptide epitope alone is not MHC II restricted, as it is immunogenic as demonstrated by specific antibody production in different strains of mice and rabbits. The hyperimmunized animals produced both IgM and IgG classes of antibodies, suggesting the production of memory cells and long-term immunologic responsiveness.
We defined a vaccine composition that provides protection against disseminated candidiasis with the limitation of the incorporation of an adjuvant inappropriate for human use (53). That is, the use of DC vaccination followed by a booster with CFA is impractical or not allowed for human use, respectively. However, CFA contains dead mycobacteria, which enhances its adjuvanticity over that of alum or MPL. To move the vaccine to a more acceptable formulation for human use, we tested alum as the adjuvant in place of DCs and CFA. Mice immunized with a combination of the Fba peptide and alum showed protection against disseminated candidiasis that was statistically significant in terms of lower kidney CFU and increased survival compared to that of nonimmune controls. These results are encouraging and have prompted us to continue to investigate vaccine design not dependent on expensive or inappropriate adjuvants.
Antibody activity specific for the Fba peptide appears to be responsible at least in part for the anti-Candida protection, as was demonstrated by experiments involving the passive transfer of whole immune serum, which conferred protection to naïve mice. Such protection was not conferred by control prebled normal mouse serum, and the protection was abrogated when immune serum was preabsorbed by fungal cells prior to the passive transfer protection test. The protective ability against disseminated candidiasis of MAb E2-9, which is specific for the Fba epitope, also was demonstrated in vivo. When administered at a dose of 0.5 ml (16 μg MAb/ml) 4 h before lethal challenge with C. albicans and 0.2 ml (16 μg/ml) 24 h after challenge, MAb E2-9 produced a statistically significant reduction in the number of CFU in kidney from infected mice compared to numbers for mice that received buffered saline in place of the MAb (P < 0.0001). In conclusion, the loss of the protective capacity of immune sera upon absorption with fungal cells and the passive protection conferred by MAb E2-9 provide strong evidence that anti-Fba antibodies are responsible for the vaccine-induced protection.
We are in the throes of determining the mechanism by which antibodies to the Fba peptide are protective. This issue is complex, because antibodies against Candida may exert their protection by a variety of different actions. Classical mechanisms of antibody action against infectious diseases in general include direct effects, such as toxin and viral neutralization, and indirect effects mediated by effector cells, such as enhancement of phagocytosis by opsonization and complement fixation and antibody-dependent cellular cytotoxicity (9). Recently, additional mechanisms of antibody-mediated immunity against fungi have been revealed, including the inhibition of adherence (17, 19, 51), growth inhibition (17, 19), the inhibition of germination (5, 50, 51), and direct antifungal effects (50).
Since the adhesion of C. albicans to tissues of the host represents an important first step in pathogenesis, the inhibition of this event should prevent infection. A number of antibodies have been demonstrated to inhibit the adhesion of C. albicans and other Candida species to different host surfaces by blocking cell wall antigens such as Als3, enolase, mannan, and other adhesins (5, 17, 42, 51). These antibodies include conventional antibodies (IgM, IgG1, or IgG2b) as well as single-chain variable fragment (scFv) fragments (7). We showed that the Fba peptide epitope is expressed on the fungal cell surface and is accessible by antibodies, thus the blockage of adhesion is a reasonable mechanism to investigate.
We found that the Fba epitope is distributed on the surface of C. albicans but is not nearly as abundant as the epitope that is recognized by our MAb B6.1, which is specific for the glycan β-1,2-mannotriose, a major surface marker as indicated by confluent distribution patterns revealed by immunofluorescence (26, 29). Microscopic observations after immunofluorescence staining with both the anti-Fba polyclonal serum and MAb E2-9 showed similar patterns of C. albicans cell surface distribution, indicating that the Fba epitope is accessible to antibodies on C. albicans cell surfaces. The fluorescence was not distributed uniformly but rather partial and punctate along the yeast cells; however, its labeling was relatively more uniform on the hyphae, indicating a more abundant exposure of the Fba epitope on the cell surface of hyphae than on yeast cells. An important step in the pathogenesis of C. albicans infections is the production of germ tubes. Grappel and Calderone were the first to demonstrate that antibodies present in a rabbit hyperimmune serum suppressed filamentation in C. albicans (22); a similar effect was reported with MAb 2G8 (IgG2b) reacting with β-1,3 glucan (50, 51). Since both anti-Fba immune sera and MAb E2-9 bind to yeast and hyphal forms of the fungus cell, we thought that the interference with hyphal growth was a mechanism of protection by anti-Fba antibodies. Our growth inhibition assays, however, have shown that MAb E2-9 exerted only slight but not remarkable inhibition of Candida hyphal growth in vitro (10 to 15% inhibition in three independent experiments; data not shown), which was well below the 80% afforded by MAb 2G8 (51). Further investigation will be needed to confirm the inhibitory function by the antibody.
Antibodies may exert indirect activities that lead to the killing of C. albicans through the participation of complement and/or phagocytic cells. MAbs B6.1 and C3.1, both of which protect against disseminated candidiasis (27), augmented the candidacidal activity of neutrophils in a complement-dependent manner (27), suggesting that complement fixation is required for the mechanism of protection by these antibodies. Our findings show that the Fba peptide epitope is present on fungal cell surfaces and is accessible to antibodies, which could opsonize the cells and facilitate phagocyte ingestion and killing of the fungus. This classical series of events initiated by antibody binding to an infectious disease agent may play an important role in the control of Candida infection by MAb E2-9.
One of the most surprising biological activities of a few antibodies elicited against C. albicans is their direct candidacidal activity (7). Without the contribution of any host effector cell, direct killing makes these antibodies appealing for the prevention and even treatment of Candida infections in immunocompromised patients. A variety of antibodies, including classical antibodies (IgM, IgG2b, and IgA) and antibody derivatives such as peptides of the CDR domains of MAbs, have been reported to have a direct candidacidal effect (5, 22, 36, 42, 50). Interestingly, these antibodies recognize different fungal epitopes, ranging from polysaccharides to proteins. We asked whether the anti-Fba antibodies could exert a direct anti-Candida effect. We tested the in vitro candidacidal activity of MAb E2-9 by following the method described by Magliani et al. (38). In preliminary investigations in which we tested only several concentrations of antibody and fungal cells, the differences in the candidacidal assays between the tested MAb E2-9 groups and controls were not significant. In future experiments we will expand this testing to include a multitude of antibody/fungal cell ratios at different time points to assess if direct candidacidal activity contributes to the protection by MAb E2-9.
Fba1p is a key enzyme required for growth on both fermentative and nonfermentative carbon sources, and this enzyme is essential for the viability of C. albicans and other Candida spp. (48). However, Fba1p must be depleted to below 5% of wild-type levels before growth is blocked (48). Fba1p depletion appears to exert static rather than lethal effects upon C. albicans. Therefore, even though the role of Fba1p on the cell surface is unknown, it is possible that antibodies against Fba1p prevent the growth of C. albicans infecting a patient. Thus, peptide-specific MAb E2-9 may have the potential for use as an immunotherapy against disseminated candidiasis when combined with other antifungal drugs. A possible limitation to the use of vaccines in immunosuppressed patients is that these patients may not mount protective responses, but passive immunization with protective antibodies may well be a rapid and effective preventive or even therapeutic measure. The efficacy of this immunoprophylaxis can be augmented when used in combination with conventional antifungal therapy, as it has been shown with Mycograb and amphotericin B (Amp B) in patients with invasive candidiasis (44). MAb B6.1 also was demonstrated to enhance the therapeutic efficacy of Amp B, which may lead to a reduction of the amount of the antifungal agent needed for treatment to nontoxic levels (24).
Regardless of the mechanism of protection afforded by antibodies specific to the Fba peptide, the incorporation of this peptide into a vaccine also comprised of the glycan β-1,2-manntriose will provide the host with two chemically distinct epitope targets to mount an immunologic response. Although we have not found C. albicans isolates to be nonproducers of the glycan epitope, others have shown that beta-linked oligomannosides linked to the alpha-mannan through a phosphodiester link of this fungus are not produced at low pH (37), and mutants without the production of these kinds of oligomannosides are viable (31), which raises the possibility that a patient is infected with a strain of the fungus not producing β-1,2-mannotriose. However, a vaccine composed of the Fba peptide in addition to the glycan should induce protection in this particular instance.
In this study, we have assessed Fba peptide for its ability to induce vaccine-dependent and/or antibody-dependent protection in mice. The increasing importance of severe invasive fungal infections and the need for new treatment options for these infections have fuelled interest in the development of immunoprophylaxis approaches for the prevention of invasive mycoses. In the case of invasive Candida infections, there is considerable experimental evidence of the usefulness of active immunization with vaccines and passive immunization with antibodies (7, 8). The demonstration that vaccine-induced antibodies and MAb E2-9, which are specific for a defined cell wall peptide and confer protection against invasive candidiasis in animal models, will be the foundation for novel strategies for the control of this disease in humans.
ACKNOWLEDGMENTS
We thank David Bundle (University of Alberta) for the synthetic β-trimannose used in one of the experiments. We thank P. Lambert for technical assistance in providing cell wall extracts of C. albicans.
We thank the Research Institute for Children (RIC) at the Children's Hospital in New Orleans for supporting our research.
Footnotes
Published ahead of print on 10 August 2011.
REFERENCES
- 1. Antonsson A., Johansson P. J. H. 2001. Binding of human and animal immunoglobulins to the IgG Fc receptor induced by human cytomegalovirus. J. Gen. Virol. 82:1137–1145 [DOI] [PubMed] [Google Scholar]
- 2. Ashman R. B. 1987. Mouse candidiasis. II. Host responses are T-cell dependent and regulated by genes in the major histocompatibility complex. Immunogenetics 25:200–203 [DOI] [PubMed] [Google Scholar]
- 3. Ashman R. B. 1990. Murine candidiasis: susceptibility is associated with the induction of T cell-mediated, strain-specific autoreactivity. Immunol. Cell Biol. 68:179–185 [DOI] [PubMed] [Google Scholar]
- 4. Ashman R. B., Papadimitriou J. M. 1987. Murine candidiasis. Pathogenesis and host responses in genetically distinct inbred mice. Immunol. Cell Biol. 65:163–171 [DOI] [PubMed] [Google Scholar]
- 5. Brena S., et al. 2007. Fungicidal monoclonal antibody C7 binds to Candida albicans. Infect. Immun. 75:3680–3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brett S. J., Butler R. 1988. Macrophage activity in resistant and susceptible mouse strains infected with Mycobacterium lepraemurium. Immunology 63:701–706 [PMC free article] [PubMed] [Google Scholar]
- 7. Cabezas J., et al. 2010. Potential of anti-Candida antibodies in immunoprophylaxis. Immunotherapy 2:171–183 [DOI] [PubMed] [Google Scholar]
- 8. Casadevall A., Dadachova E., Pirofski L.-A. 2004. Passive antibody therapy for infectious diseases. Nat. Rev. 2:695–703 [DOI] [PubMed] [Google Scholar]
- 9. Casadevall A., Pirofski L.-A. 2007. Antibody-mediated protection through cross-reactivity introduces a fungal heresy into immunological dogma. Infect. Immun. 75:5074–5078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cassone A., De Bernardis F., Torosantucci A. 2005. An outline of the role of anti-Candida antibodies within the context of passive immunization and protection from candidiasis. Curr. Mol. Med. 5:377–382 [DOI] [PubMed] [Google Scholar]
- 11. Clancy C. J., et al. 2008. Immunoglobulin G responses to a panel of Candida albicans antigens as accurate and early markers for the presence of systemic candidiasis. J. Clin. Microbiol. 46:1647–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reference deleted.
- 13. Cutler J. E. 2005. Defining criteria for anti-mannan antibodies to protect against candidiasis. Curr. Mol. Med. 5:383–392 [DOI] [PubMed] [Google Scholar]
- 14. Reference deleted.
- 15. Cutler J. E., Deepe G. S., Jr., Klein B. S. 2007. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 5:13–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reference deleted.
- 17. De Bernardis F., et al. 1997. Protective role of antimannan and anti-aspartyl proteinase antibodies in an experimental model of Candida albicans vaginitis in rats. Infect. Immun. 65:3399–3405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Eggimann P., Garbino J., Pittet D. 2003. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect. Dis. 3:685–702 [DOI] [PubMed] [Google Scholar]
- 19. Elguezabal N., Maza J. L., Moragues M. D., Pontón J. 2009. Monoclonal antibody-mediated inhibition of adhesion of Candida albicans and Candida dubliniensis to human epithelial cells. Eur. J. Oral Sci. 117:474–478 [DOI] [PubMed] [Google Scholar]
- 20. Reference deleted.
- 21. Reference deleted.
- 22. Grappel S. F., Calderone R. A. 1976. Effect of antibodies on the respiration and morphology of Candida albicans. S. Afr. Med. J. 14:51–60 [DOI] [PubMed] [Google Scholar]
- 23. Reference deleted.
- 24. Han Y. 2010. Efficacy of combination immunotherapy of IgM MAb B6.1 and amphotericin B against disseminated candidiasis. Int. Immunopharmacol. 10:1526–1531 [DOI] [PubMed] [Google Scholar]
- 25. Han Y., Cutler J. E. 1995. Antibody response that protects against disseminated candidiasis. Infect. Immun. 63:2714–2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Han Y., Kanbe T., Cherniak R., Cutler J. E. 1997. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect. Immun. 65:4100–4107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han Y., et al. 2001. Complement is essential for protection by an IgM and an IgG3 monoclonal antibody against experimental hematogenously disseminated candidiasis. J. Immunol. 167:1550–1557 [DOI] [PubMed] [Google Scholar]
- 28. Han Y., Morrison R. P., Cutler J. E. 1998. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect. Immun. 66:5771–5776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han Y., Riesselman M. H., Cutler J. E. 2000. Protection against candidiasis by an immunoglobulin G3 (IgG3) monoclonal antibody specific for the same mannotriose as an IgM protective antibody. Infect. Immun. 68:1649–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han Y., Ulrich M. A., Cutler J. E. 1999. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J. Infect. Dis. 179:1477–1484 [DOI] [PubMed] [Google Scholar]
- 31. Hobson R. P., et al. 2004. Loss of cell wall mannosylphosphate in Candida albicans does not influence macrophage recognition. J. Biol. Chem. 279:39628–39635 [DOI] [PubMed] [Google Scholar]
- 32. Horn D. L., et al. 2009. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 48:1695–1703 [DOI] [PubMed] [Google Scholar]
- 33. Hsu F. C., Lin P. C., Chi C. Y., Ho M. W., Wang J. H. 2009. Prognostic factors for patients with culture-positive Candida infection undergoing abdominal surgery. J. Microbiol. Immunol. Infect. 42:378–384 [PubMed] [Google Scholar]
- 34. Jarvis W. R., Martone W. J. 1992. Predominant pathogens in hospital infections. J. Antimicrob. Chemother. 29:19–24 [DOI] [PubMed] [Google Scholar]
- 35. JAX Mice and Services 1988. Mice, H-2 haplotypes of mice from Jackson Laboratory colonies, pamphlet. JAX Mice and Services, Jackson Laboratory, Bar Harbor, ME [Google Scholar]
- 36. Kavishwar A., Shukla P. K. 2006. Candidacidal activity of a monoclonal antibody that binds with glycosyl moieties of proteins of Candida albicans. Med. Mycol. 44:159–167 [DOI] [PubMed] [Google Scholar]
- 37. Kobayashi H., et al. 1991. Candida albicans serotype A strains grow in yeast extract-added Sabouraud liquid medium at pH 2.0, elaborating mannans without β-1,2 linkage and phosphate group. Biochem. Biophys. Res. Commun. 175:1003–1009 [DOI] [PubMed] [Google Scholar]
- 38. Magliani W., Conti S., Giovati L., Maffei D., Polonelli L. 2008. Anti-beta-glucan-like immunoprotective candidacidal antiidiotypic antibodies. Front. Biosci. 13:6920–6937 [DOI] [PubMed] [Google Scholar]
- 39. Marsh J. J., Lebherz H. G. 1992. Fructose-bisphosphate aldolases: an evolutionary history. Trends Biochem. Sci. 17:110–113 [DOI] [PubMed] [Google Scholar]
- 40. Matthews R. C., et al. 2003. Preclinical assessment of the efficacy of mycograb, a human recombinant antibody against fungal HSP90. Antimicrob. Agents Chemother. 47:2208–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mochon A. B., Cutler J. E. 2005. Is a vaccine needed against Candida albicans? Med. Mycol. 43:97–115 [DOI] [PubMed] [Google Scholar]
- 42. Moragues M. D., et al. 2003. A monoclonal antibody directed against a Candida albicans cell wall mannoprotein exerts three anti-C. albicans activities. Infect. Immun. 71:5273–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nooney L. M., Matthews R. C., Burnie J. P. 2005. Evaluation of Mycograb, amphotericin B, caspofungin, and fluconazole in combination against Cryptococcus neoformans by checkerboard and time-kill methodologies. Diagn. Microbiol. Infect. Dis. 51:19–29 [DOI] [PubMed] [Google Scholar]
- 44. Pachl J., et al. 2006. A randomized, blinded, multicenter trial of lipid-associated amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 42:1404–1413 [DOI] [PubMed] [Google Scholar]
- 45. Pincus S. H., Shigeoka A. O., Moe A. A., Ewing L. P., Hill H. R. 1988. Protective efficacy of IgM monoclonal antibodies in experimental group B streptococcal infection is a function of antibody. J. Immunol. 140:2779–2785 [PubMed] [Google Scholar]
- 46. Pitarch A., et al. 2004. Proteomics-based identification of novel Candida albicans antigens for diagnosis of systemic candidiasis in patients with underlying hematological malignancies. Proteomics 4:3084–3106 [DOI] [PubMed] [Google Scholar]
- 47. Richards M. J., Edwards J. R., Culver D. H., Gaynes R. P., National Nosocomial Infections Surveillance System 1998. Nosocomial infections in coronary care units in the United States. Am. J. Cardiol. 82:789–793 [DOI] [PubMed] [Google Scholar]
- 48. Rodaki A., Young T., Brown A. J. 2006. Effects of depleting the essential central metabolic enzyme fructose-1,6-bisphosphate aldolase on the growth and viability of Candida albicans: implications for antifungal drug target discovery. Eukaryot. Cell 5:1371–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Son Y.-I., et al. 2002. A novel bulk-culture method for generating mature dendritic cells from mouse bone marrow cells. J. Immunol. Methods 262:145–157 [DOI] [PubMed] [Google Scholar]
- 50. Torosantucci A., et al. 2005. A novel glyco-conjugate vaccine against fungal pathogens. J. Exp. Med. 202:597–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Torosantucci A., et al. 2009. Protection by anti-beta-glucan antibodies is associated with restricted beta-1,3 glucan binding specificity and inhibition of fungal growth and adherence. PLoS One 4:e5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xin H., Cutler J. E. 2006. Hybricoma passage in vitro may result in reduced ability of antimannan antibody to protect against disseminated candidiasis. Infect. Immun. 74:4310–4321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Xin H., Dziadek S., Bundle D. R., Cutler J. E. 2008. Synthetic glycopeptide vaccines combining β-mannan and peptide epitopes induce protection against candidiasis. Proc. Natl. Acad. Sci. U. S. A. 105:13526–13531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang Q., et al. 2005. Prophylactic vaccination with phage-displayed epitope of C. albicans elicits protective immune responses against systemic candidiasis in C57Bl/6 mice. Vaccine 23:4088–4096 [DOI] [PubMed] [Google Scholar]