Abstract
Antimicrobial peptides (AMPs) constitute a phylogenetically ancient form of innate immunity that provides host defense at various mucosal surfaces, including the vagina. Recently, we have identified one such AMP, rabbit vaginal fluid hemoglobin alpha peptide (RVFHbαP), from the vaginal lavage of rabbits (Oryctolagus cuniculus). The recent demonstration of a protective role of this peptide in erythrocytes and vaginal cells led us to investigate (i) the lipopolysaccharide (LPS) interactive domain in RVFHbαP and (ii) whether RVFHbαP of rabbit origin modulates the cellular immune responses of another species (humans) in vitro. HeLa-S3, a human vaginal epithelial cell line (hVEC), was exposed to LPS alone (10 μg/ml for 6 h), or LPS-induced cells were treated with RVFHbαP (70.45 μM for 1 h) and cultured for 24 h, and the results obtained were compared with the medium control. We show here that RVFHbαP exerts an anti-inflammatory activity in hVECs, as suggested by the prevention of LPS-induced production of extracellular (supernatant) and intracellular (lysate) levels of cytokines (interleukin 6 [IL-6] and IL-1α) and chemokines (IL-8 and monocyte chemoattractant protein 1 [MCP-1]). The demonstration of Toll-like receptor 4 (TLR4) and NF-κB expression in hVECs and the observations of RVFHbαP suppression of human β-defensin-1 (hBD1) mRNA expression further support the hypothesis of a genomic activity of RVFHbαP. Confocal microscopy and flow cytometry results demonstrate that RVFHbαP inhibits LPS-induced phagocytosis of Escherichia coli by macrophages. The chemotaxis studies performed using the Boyden chamber Transwell method showed the increased migration of U937 cells when supernatants of LPS-induced hVECs were used, and this effect was inhibited by RVFHbαP. In conclusion, our study proposes a novel explanation for the protective role of RVFHbαP in inflammation-associated infections, which not only may provide the new cellular targets for the screening of RVFHbαP ligands acting in the vaginal tissue but also has the potential to develop RVFHbαP as a therapeutic agent for reproductive tract infections.
INTRODUCTION
The mucosal immune system of the female reproductive tract (FRT) represents the third largest epithelial surface in the human body after the gastrointestinal tract (GIT) and the airway tract (10). The vagina is a major portal of entry for the pathogens associated with sexually transmitted infections (STIs), such as those from Neisseria gonorrhoeae, Chlamydia trachomatis, Haemophilus ducreyi, Treponema pallidum, and viruses like human immunodeficiency virus type 1 (HIV-1), herpes simplex virus 1 (HSV-1), and human papillomavirus (HPV), which represent major threats to human health (29, 33). In a large part of bacterial infections, products like lipopolysaccharide (LPS) from Gram-negative bacteria (e.g., Escherichia coli) and lipoteichoic acid (LTA) from Gram-positive bacteria (e.g., Staphylococcus aureus) are involved (5, 43). It has been shown that susceptibility of the vagina to HIV-1 infection increases in the presence of STIs (11, 29), and LPS can drive HIV-1 expression via interaction with Toll-like receptor 4 (TLR4) (36).
The FRT has unique requirements for protecting the host from the pathogens. Central to the protection are the vaginal epithelial cells (VECs), which provide a physical barrier and produce a spectrum of antimicrobials, including complement, lysozyme, lactoferrin, hemoglobin (Hb)-derived peptides, defensins, and secretory leukocyte protease inhibitor (SLPI) (20, 24, 26). Despite being the major transmission route for various infections and therefore of tremendous importance in the prevention and treatment of infections, the innate defense mechanism of the FRT remains severely understudied.
It is documented that if the virulent pathogens invade into the FRT, such pathogens must be held in check by the immune system until a specific immune response is mounted (28). Under such conditions, TLRs play a critical role and trigger a proinflammatory response in the VECs. These cells sense bacterial pathogens through different TLRs, which recognize pathogen-associated molecular patterns (PAMPs) (10, 39). TLR4, the best-characterized member of the TLR family of cell membrane receptor proteins, induces the NF-κB pathway and activates innate and adaptive immune responses (42).
Recent approaches to develop molecules that neutralize LPS have concentrated on characterizing the lipid A binding region from LPS binding peptides and proteins (23, 40, 43). Therefore, identifying the molecule(s) which binds to LPS and neutralizes its activities might have important clinical applications (25, 33). In the past, to prevent LPS-induced inflammatory events in the host, several approaches have been employed (23), including specific antibodies (Abs) directed against LPS. However, these Abs could not recognize natural LPS-carrying polysaccharide components, which include Tachypleus anti-LPS factor (17), Limulus anti-LPS factor (LALF) (13), Scylla serrata anti-LPS factor (SsALF) (43), cathelicidin-derived AMP-18 (CAP-18) (12), and lipopolysaccharide binding protein (LBP) (7).
Further, it has been reported that hemocidins act as the first line of defense against both Gram-negative and Gram-positive pathogens (20, 24, 26). Recently, our group identified epithelial cell-derived broad-spectrum AMP in the vaginal fluid of rabbits (Oryctolagus cuniculus) and named it rabbit vaginal fluid hemoglobin alpha peptide (RVFHbαP) (26). The peptide showed 60% similarity with the human Hb-α subunit. In the same study, it was also shown that RVFHbαP is nontoxic to erythrocytes and human endocervical cells (End1/E6E7).
The objectives of the present study are (i) to predict the LPS interactive domain in RVFHbαP and (ii) to investigate whether RVFHbαP of rabbit origin modulates the inflammatory responses of another species (human).
MATERIALS AND METHODS
Media and reagents.
RVFHbαP and scrambled (nRVFHbαP) peptides were commercially procured from USV Ltd., India. LPS-E. coli 055:B5, N-formyl-methionyl-leucyl-phenylalanine (fMLP), and primary and secondary antibodies were procured from Sigma. Kits for interleukin 6 (IL-6), IL-8, monocyte chemoattractant protein 1 (MCP-1), and IL-1α cytokines/chemokines were obtained from R&D Systems. All the reagents of stock solutions were dissolved in endotoxin-free water. Unless otherwise stated, all other chemicals and media are of high quality and were procured from local suppliers.
Cell lines and transfection. (i) Human vaginal epithelial cells and macrophages.
Human vaginal epithelial cells (HeLa-S3), macrophages, and monocyte-derived U937 cells were procured from the National Center for Cell Sciences (NCCS), Pune, India, and grown in Dulbecco's modified Eagle's medium (for HeLa cells) or RPMI 1640 medium (for macrophages and U937 cells) as per the supplier's instructions.
(ii) Transfection.
HeLa cells were grown in 24-well tissue culture plates in DMEM-10% fetal calf serum (FCS) until 70% confluence was reached (∼24 h). Cells were transiently transfected with MD-2 plasmid (pUNO1-hMD2; InvivoGen) in DMEM-10% FCS by using FuGene 6 (Roche Diagnostics) as described earlier (37).
Structure prediction of RVFAMP by homology modeling.
Amino acid sequence homology of RVFHbαP was used for the selection of the template. Two main criteria were included during the selection of template: (i) the selected protein should possess an authenticated crystal structure and (ii) the template must demonstrate maximum homology with the RVFHbαP amino acid sequence (14). After selecting the template based on the above criteria, its crystal structure was used to model the structure of RVFHbαP. Prior to this, the obtained template was energy minimized using the Swiss-PdbViewer (9) to remove steric clashes from the crystal structure using the What If server (http://swift.cmbi.ru.nl/servers/html/index.html). To predict the overall stability, we determined phi-psi angles and protein structure using a Ramanchandran plot and PROSA software, respectively (35). Finally, the template was validated with the What If server.
Docking studies of RVFHbαP with LPS.
The peptide, RVFHbαP, and the charged LPS were docked using Hex 6.1 software (32). While docking, for correlation type, we selected shape and electrostatic parameters and 3D LITE in the 3-hydroxytetradecanoic acid (FFT) mode. Postprocessing was carried out using molecular modeling (MM) minimization. Of the generated docked structures, the smallest binding energy was selected. Binding interactions of the duo were visualized using Chimera software.
Design and synthesis of peptides (RVFHbαP and nRVFHbαP).
The 15-mer sequence (HKLRVDPVNFKLLSH) obtained by liquid chromatography-mass spectrometry (LC-MS) was subjected to various bioinformatics tools to identify the region which is likely to bind to LPS. Based on the prediction algorithm on AMPs, a 25-mer peptide corresponding to amino acids 88 to 112 (AHKLRVDPVNF KLLSHCLLVTLANH) of the Hb-α subunit was designed and commercially synthesized. To analyze the LPS binding specificity of RVFHbαP, a 25-mer non-LPS binding peptide corresponding to amino acids 18 to 43 of the Hb-α subunit (IGSHGGEYGAEAVERMFLG FPTTKT) was also synthesized (nRVFHbαP), and its LPS binding capacity was assayed along with that of RVFHbαP.
Treatment of hVECs with LPS and peptides.
HeLa hVECs used in this study constitutively express TLR4 and respond to LPS (27, 33). On the day of treatment, cells at 70 to 80% confluence were split by trypsin treatment (0.1%). In our previous study, we showed that RVFHbαP and LPS (26), with the concentrations of 70.45 μM and 10 μg/ml, respectively, did not inhibit the viability of red blood cells (RBCs) and human endocervical cells (End1/E6E7). Therefore, these doses were selected for all the experiments. HeLa cells were seeded at a density of 2 × 106 cells/well in 24-well plates and incubated for 24 h at 37°C in 5% CO2 and 95% air and divided into the following six groups: (i) cells were grown in culture medium for 24 h without any treatment; (ii) cells were treated with RVFHbαP (74.45 μM for 1 h); (iii) cells were treated with scrambled peptide (nRVFHbαP) (74.45 μM for 1 h); (iv) cells were induced with LPS alone (10 μg/ml for 6 h); (v) after being washed, cells were treated with RVFHbαP (74.45 μM for 1 h); or (vi) after being washed, cells were treated with nRVFHbαP (74.45 μM for 1 h). At the end of each treatment, cells were washed twice with phosphate-buffered saline (PBS) (pH 7.4) and cultured for an additional 24 h. At the end of the culture, spent medium along with cells from all the groups were collected for various studies on cytokines/chemokines, TLR4, hBD1, and chemotaxis.
Determination of RVFHbαP binding to LPS.
The ability of RVFHbαP to bind to LPS was determined by the modified method of Ried and colleagues (31). Briefly, a 96-well microtiter plate was coated with 100 μl of LPS (5 μg/ml) dissolved in PBS and incubated for 2 h at 37°C. After blocking with 0.1% gelatin in PBS for 30 min, the plate was incubated for 60 min with 100 μl of 2-fold serially diluted peptides (2.20 to 70.45 μM) dissolved in PBS. After three washes with PBS-Tween 20 (PBS-T), enzyme-linked immunosorbent assay (ELISA) was developed with affinity-purified rat antiserum raised against RVFHbαP (incubation time, 60 min) and a secondary goat anti-rat antibody (Sigma) conjugated to horseradish peroxidase (HRP) (incubation time, 60 min). O-Phenylenediamine (1 mg/ml) was used as a substrate, and the absorbance was measured at 490 nm on a microplate reader (ELX-800; Bio-Tek Instruments). Crab hemocyte-derived Scylla serrata antilipopolysaccharide factor 24 (SsALF24) peptide was used as positive control for LPS binding.
In vitro effect of peptides on HeLa hVEC viability.
The effect of RVFHbαP on HeLa hVEC viability was determined by the KineticBlue assay (Krishgen Biosystems, India). This assay is based on the reduction of resazurin dye into a pink-colored product, resorufin, by dehydrogenase enzymes. Only viable cells having dehydrogenase activities were able to reduce resazurin to resorufin. Briefly, exponentially growing hVECs were seeded into sterile tissue culture 96-well microtiter plates at a density of 106 cells/well and incubated for 24 h at 37°C prior to exposure to peptides. On the day of treatment, DMEM was replaced with fresh medium containing 2-fold serial dilutions of peptides (2.20 to 70.45 μM). Plates were incubated for 6 h before adding KineticBlue reagent as per the manufacturer's instructions. Cells containing scrambled peptide and medium alone with KineticBlue reagent were used as controls, while cells treated with 0.1% Triton X-100 for 6 h served as a positive control for cell lysis. The optical densities at 570 (OD570) and 600 nm were measured on a microplate reader as described previously (30). The results were expressed as means ± standard deviations (SD) from three independent experiments. The minimum effective concentration (MEC) was defined as the lowest concentration that displays 100% reduction in cell viability.
Measurement of cytokine levels by ELISA.
Inflammatory markers, viz., IL-6, IL-8, MCP-1, and IL-1α, were measured in the culture supernatants and cell lysates by ELISA using commercially available human cytokine kits with matched antibodies (R&D Systems) as described earlier (1). The cell pellet was washed with ice-cold PBS and lysed in hypotonic HEPES lysis buffer (pH 7.4). The lysates were cleared of cellular debris by centrifugation at 1,000 × g at 4°C for 10 min and used for the estimation of intracellular (cell-bound) cytokines (IL-6 and IL-8) after determining the total protein levels (4). Compound (LPS and RVFHbαP) interference with cytokine detection was ruled out by spiking known amounts of recombinant IL-6 and IL-8 by measuring the percent cytokine recovery from compound-supplemented medium versus that from the plain medium.
RT-PCR analysis of cytokine/chemokine and hBD1 genes in hVECs.
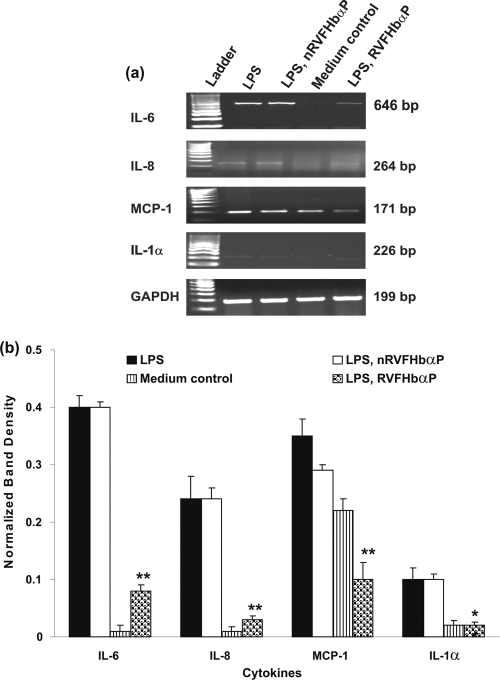
To determine if LPS induces the expression of cytokine/chemokine biomarkers and hBD1 mRNAs, hVECs were seeded at a density of 106 per well in 6-well plates. The treatment groups considered for cytokine/chemokine and hBD1 studies (groups 1, 4, 5, and 6) are given in parentheses below. For hBD1 analysis, we have included an additional group in which the cells were treated for 1 h with TLR4 antibody (2 μg/ml) before inducing with LPS. After being washed, cellular RNA was extracted by TRIzol solution (Invitrogen) according to the manufacturer's protocol. The primer sequences used were IL-6 (645 bp) sense (5′-ATGAACTCCTTCTCCACAAG) and antisense (5′-ACATTTGCCGAAGAGCCCTCAG-3′); IL-8 (264 bp) sense (5′-CTTGGCAGCCTTCCTGATTT-3′) and antisense (5′-CTCAGCCCTCTTCAAAAACT-3′); MCP-1 (171 bp) sense (5′-CCCCAGTCACCTGCTGTTAT-3′) and antisense (5′-TGGAATCCTGAACCCACTTC-3′); IL-1α (226 bp) sense (5′-AATGACGCCCTCAATCAAAG-3′) and antisense (5′-TGGGTATCTCAGGCATCTCC-3′); hBD1 (196 bp) sense (5′-CTCTGCTTGCTGCCATTCTC-3′) and antisense (5′-AATCGTCTGCAAGTACAGGACAC-3′); and GAPDH (199 bp) sense (5′-CCATTCATTGACCTCCACTACA-3′) and antisense (5′-CGTTGCTGACAAT CTTGAGAGA-3′). PCR products were separated on a 2% agarose gel with electrophoresis and visualized by ethidium bromide staining under UV illumination. PCR products of the expected size were generated with each primer pair. The gels were scanned using a gel documentation system (Gel Doc 2000; Bio-Rad Laboratories), and intensities of the bands were quantified by Quantity One software.
Measurement of TLR4 levels in hVECs by ELISA.
To determine whether RVFHbαP competes with LPS at the receptor level or its effects occur downstream from TLR4-LPS signaling, hVECs were induced with LPS (group 4). This ensures TLR-LPS binding in the absence of RVFHbαP. The LPS-induced cells were treated with RVFHbαP for 1 h (group 5). We have included an additional group in which the cells were treated with anti-TLR4 antibody (2 μg/ml for 1 h) before being induced with LPS (10 μg/ml for 6 h). After the treatment, hVECs were lysed with hypotonic HEPES lysis buffer (pH 7.4) and centrifuged at 1,000 × g for 10 min at 4°C. Supernatants were collected, and total protein levels were determined (4) and used for the estimation of TLR4 levels by ELISA as described earlier (33).
Western blot analysis of TLR4 and NF-κB in hVECs.
To determine whether LPS induces the expression of TLR4, hVECs were seeded at a density of 106 per well in 6-well plates. The treatment groups 1, 2, 4, and 5 were considered for TLR4 and NF-κB determination by Western blotting as described earlier (11). Briefly, equal amounts of cellular protein (20 μg/lane) were loaded on 10% SDS-PAGE gels and transferred onto nitrocellulose membranes (Amersham). Membranes were then blocked for 1 h at room temperature with 5% nonfat dry milk in PBS containing 0.1% PBS-Tween 20 (PBS-T). The membranes were rinsed with PBS-T and incubated for 1 h at room temperature with mouse monoclonal anti-TLR4 antibody (1/100) or anti-β-actin antibody (1/1,000). For NF-κB activity, phosphor-NF-κB p65 (Ser536) antibody (1:1,000, clone no. 3034) (Cell Signaling) was used. Blots were washed with 0.5% PBS-T and incubated for 1 h at RT in HRP-conjugated goat anti-human secondary antibody (Sigma) at a dilution of 1/1,000. After being washed, bands were detected using the 3′-3′ diaminobenzidine (DAB) substrate. Intensities of the bands were quantified using Quantity One software by densitometry scanning (Bio-Rad). Relative expression ratios were calculated and normalized to the values obtained with β-actin control (TLR4 band volume/β-actin band volume).
Measurement of phospho-NF-κB p65 levels in hVECs by ELISA.
To evaluate the effect of RVFHbαP in LPS-induced NF-κB, levels in hVECs were determined using phospho-NF-κB p65 (Ser536) sandwich ELISA kit number 7174 (Cell Signaling). The treatment details are the same as those described above for NF-κB Western blot analysis. After treatment, hVECs were lysed with hypotonic HEPES lysis buffer (pH 7.4) and centrifuged at 1,000 × g for 10 min at 4°C, and supernatants were collected and used for the estimation of NF-κB p65 as described earlier (33).
Macrophage phagocytic assay and FITC labeling of bacteria (E. coli).
Gram-negative bacteria (E. coli) were labeled with fluorescein isothiocyanate (FITC) as described earlier (2). Briefly, E. coli cells were suspended in Dulbecco PBS (DPBS) (pH 7.4) and centrifuged at room temperature for 5 min. The cell pellet was resuspended in 0.5 ml FITC solution (1 mg FITC/ml of buffer containing 50 mM sodium carbonate, 100 mM NaCl). The mixture was wrapped in aluminum foil to prevent bleaching by light and incubated at room temperature for 20 min. E. coli cells were washed three times with PBS containing 3 mM glucose, 0.5 mg/ml human serum albumin (HSA), and 0.2 unit/ml aprotinin. The concentration of FITC-labeled E. coli cells was adjusted to 2 × 108 CFU/ml (OD of 0.1 at 600 nm). E. coli cells were stored at 4°C in the dark for immediate use.
Macrophages (1 × 106) were stimulated with LPS (10 μg/ml for 1 h). After being washed twice with RPMI, they were treated with RVFHbαP or nRVFHbαP (70.45 μM for 1 h). After two washings in RPMI to remove peptides, cells were incubated at 37°C with the cell suspensions of FITC-labeled E. coli cells (ratio of macrophages for E. coli was 1:20) in a total volume of 1 ml in siliconized glass tubes. Macrophages not exposed to E. coli were handled similarly to determine the background. After 30 min of incubation, 1 ml of ice-cold complete RPMI medium per ml was added, and cells were centrifuged (110 × g, 8 min) to separate phagocytic cells from free bacteria. Cells were washed twice in complete RPMI. The internalized bacteria and surface-bound bacteria were visualized under FITC optics using a confocal laser scanning microscope (CLSM; Zeiss 510 Meta; Germany). The number of internalized labeled E. coli cells was determined as the phagocytic index (PCI). The PCI was defined as the number of bacterium-containing macrophages per a confluent ×63-magnification high-power field divided by the total number of cells in the field, expressed as a percentage. For quantification of the PCI for a given condition, at least 25 fields were sequentially examined. Detection of labeled E. coli in macrophages was carried out, and the results were confirmed by flow cytometry, using the central equipment facility of NIRRH, Mumbai, India.
Flow cytometric analysis of E. coli phagocytosis by macrophages.
To confirm the above-described microscopic observations, semiquantitative analysis of macrophages that had internalized FITC-labeled E. coli cells was performed on a FACS vantage flow cytometer (Becton Dickinson). The fluorescence signals for each E. coli cell present within macrophages were detected through a 520-nm argon-ion laser. We included plain macrophages as the appropriate negative control to rule out any nonspecific activity. The percentage of labeled bacteria engulfed by macrophages in the presence of RVFHbαP was calculated using Cell Quest software (http://facs.scripps.edu).
Assay for chemotaxis.
RVFHbαP bioactivity was assessed by chemotaxis assay using cyclic AMP (cAMP)-activated U937 cells as described earlier (39). For this study, cultures of groups 1 to 6 were used, and the treatment protocol was the same as that discussed for cell culture and treatment. LPS-induced cells of group 5 were treated with increased concentrations of RVFHbαP (2.20, 17.60, and 70.45 μM for 1 h). Cells treated with 700 μl of 0.1 mM chemotactic peptide (N-formyl-methionyl-leucyl-phenylalanine [fMLP]) in PBS-bovine serum albumin (BSA) were placed in the lower chamber and considered a positive control for cell migration.
Conditioned medium was harvested 24 h later from all the treatment and control groups and used for chemotaxis of U937 cells. Briefly, for this experiment we used 24-well plates assembled with Boyden chamber Transwell membranes (0.4-μM-pore-size polycarbonate membranes). Before the cells were seeded, Transwell permeable supports and cells were preincubated for 30 min. U937 cells (5 × 104 cells in 500 μl of serum-free medium) were loaded into the upper chamber of the Transwell membranes. Lower chambers were loaded with spent media obtained from treated and scrambled control groups. Three concentrations of RVFHbαP (70.45, 17.60, and 2.20 μM) were tested and added to the lower chamber. The chambers were then incubated in a humidified CO2 incubator at 37°C for 3 h. Nonmigrated U937 cells remained on the upper chamber of the insert and were removed by placing the insert into a sterile 24-well plate, and cells migrating across the membrane were fixed, stained with crystal violet, and counted directly under a phase-contrast microscope (×40 magnification). The results were expressed as the percentage of chemotaxis obtained in response to a maximal stimulation with the fMLP chemoattractant (100%).
Data analysis.
Data are presented as means ± standard deviations (SD) from triplicate samples and are representative of at least three independently performed experiments. Analysis of variance (ANOVA) was performed to evaluate statistical significance of differences between experimental groups with the post hoc Bonferroni test (38). The level of statistical significance was assigned to P values of <0.05.
RESULTS
Structure prediction of RVFHbαP by docking with LPS.
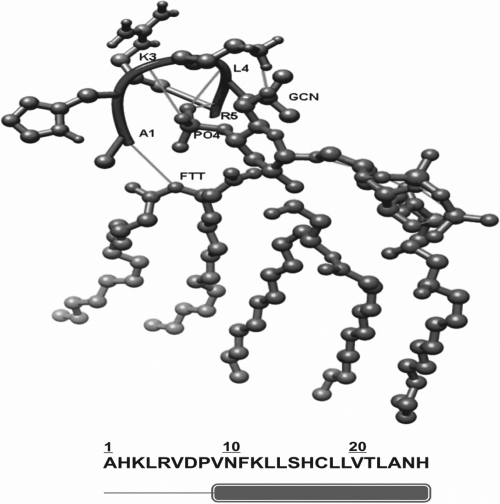
To elucidate the structural basis of the identified peptide (RVFHbαP) interaction with LPS, herein we report the predicted structure of RVFHbαP binding to LPS. The amino acid sequence of RVFHbαP was blasted using Blastp software using the Protein Data Bank (PDB) as the search database (www.ncbi.nlm.nih.gov/blast). The results demonstrated a significant sequence homology (>90%) with the rabbit Hb-α subunit. The three-dimensional structure of this subunit was used as a template for modeling RVFHbαP using Modeler software. Amino acids 1 to 8 (Ala-Pro) were in the loop region, and amino acids 9 to 25 (Val-His) were in the helix. The N-terminal region is found to be hydrophilic utill the 9th residue (hydropathy plot; http://www.vivo.colostate.edu/molkit/hydropathy/). The peptide and its ligand were docked using Hex 6.1 software.
In the docking parameters, the correlation type was comprised of shape and electrostatics. The binding energy of the RVFHbαP with LPS was predicted to be −670.9 kcal/mol. This region showed interaction with phosphate ion (PO4), GCN (3-deoxy-d-glucosamine), and FTT (3-hydroxy-tetradacanoic acid), which were observed mainly between the basic residues and the phosphate ion. Ala1 showed interaction with FTT, whereas Lys3, Leu4, and Arg5 showed interaction with PO4. Lys3 interacted with GCN. Even though this short peptide comprises both the helix and loop region, the binding is confined to only the loop region with the basic residues. The negatively charged phosphate ion showed interactions with the positively charged residues, such as Lys3 and Arg5, in RVFHbαP (Fig. 1).
Fig. 1.
Docking of RVFHbαP with LPS. Residues 1 to 8 (Ala-Pro) are in a loop region and residues 9 to 25 (Val-His) are in the helix. Ala1 showed interaction with FTT (3-hydroxy-tetradacanoic acid), Leu4 and Arg5 with PO4, and Lys3 with GCN (3-deoxy-d-glucosamine). The N-terminal region is found to be hydrophilic utill the 9th residue. This region showed interactions with PO4, GCN, and FTT, which were observed mainly between the basic residues and the phosphate ion.
Peptide synthesis.
A bioinformatic analysis of the peptide sequences was performed using the AMP database and prediction algorithm on AMPs (http://aps.unmc.edu/AP/prediction/prediction_main.php). Peptides RVFHbαP and nRVFHbαP were designed using the template of an Hbα. Each of the peptides is comprised of 25 residues. The results indicated that the presence of positively charged residues in RVFHbαP constitutes a net positive charge of +5 and a hydrophobic ratio of 52%. A non-LPS binding region of the protein corresponding to amino acids 18 to 43 of the Hb-α subunit was also synthesized and used as the scrambled peptide (nRVFHbαP). nRVFHbαP exhibited a hydrophobic ratio of 36% but with zero charge. The amino acid region corresponding to amino acids 88 to 122 (containing the LPS binding domain) of the Hb-α subunit was synthesized in linear form (Fig. 2). The purity of the peptide (>90%) was confirmed by mass spectrometry and amino acid analysis.
Fig. 2.
Translation of the rabbit Hb-α coding sequence. Hb-α, corresponding to amino acids 90 to 104, was originally identified in the rabbit vaginal fluid by LC-MS analysis, and amino acids 88 to 112 correspond to the peptide RVFHbαP (K. V. R. Reddy, 19 August 2010, Indian Patent Office). The predicted amino acid sequence which interacts with LPS and the scrambled peptide, nRVFHbαP, corresponding to amino acids 18 to 43, are shown. (Underlining with dotted lines means amino acids are interactive with LPS).
In vitro effect of peptide on HeLa cell viability.
The cells were incubated with 2-fold serial dilutions of the peptide (2.20 to 70.45 μM for 6 h). Most of the AMPs are cationic in nature and cause cell membrane disruption, and the use of such peptides becomes problematic. Therefore, the effect of RVFHbαP on HeLa hVEC viability was analyzed using the KineticBlue assay. The results revealed that RVFHbαP did not interfere with HeLa cell viability even at a high concentration (70.45 μM) compared to that of the scrambled peptide, nRVFHbαP (data not shown).
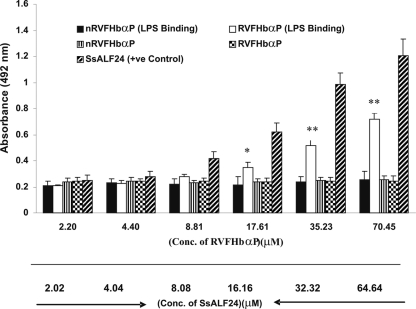
LPS-neutralizing potential of RVFHbαP.
Here, we examined the LPS-neutralizing ability of RVFHbαP in vitro using ELISA. The results demonstrate that RVFHbαP binds to LPS in a dose-dependent manner. As expected, the scrambled peptide, nRVFHbαP, failed to interact with LPS, whereas the known positive-control peptide, SsALF24, significantly bound to LPS (Fig. 3). Based on these results, RVFHbαP was further investigated to see its effect on the synthesis of the inflammatory mediators in LPS-induced hVECs.
Fig. 3.
Determination of RVFHbαP binding to LPS. RVFHbαP bound to LPS in a dose-dependent manner. No binding of nRVFHbαP to LPS was observed. SsALF24 peptide was used as a positive control for binding, which showed significant binding in a dose-dependent manner. Values represent the means ± SD of triplicate determinations performed on different days. Levels of significance (*, P < 0.05; **, P < 0.001; compared with the nRVFHbαP-treated group) were calculated by an ANOVA test followed by a post hoc Bonferroni analysis.
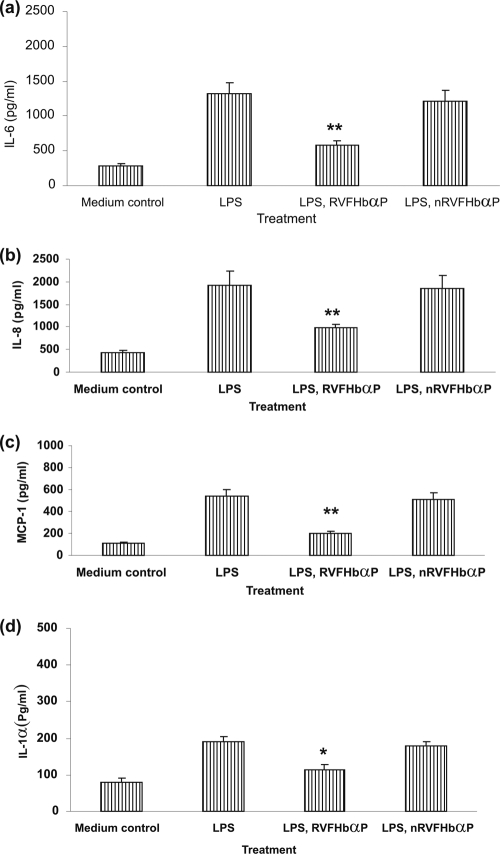
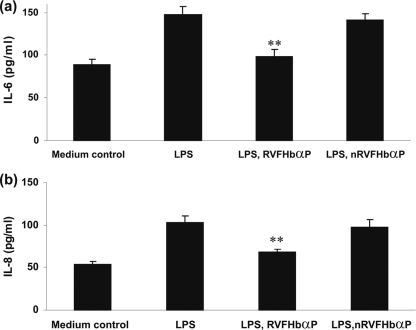
RVFHbαP prevents LPS-induced cytokine/chemokine synthesis of hVECs.
To investigate whether RVFHbαP has any effect on the LPS-induced synthesis/release of cytokines/chemokines (group 4), we quantified these mediators by ELISA in the culture supernatants (Fig. 4) and cell lysates (Fig. 5) as specified in Materials and Methods. The results reveal that a significant increase (P < 0.05) of extracellular (supernatant) as well as intracellular (lysate) levels of cytokines (IL-6 and IL-1α) and chemokines (IL-8 and MCP-1) in hVECs when stimulated with a nontoxic dose range of LPS (10 μg/ml for 6 h) (Fig. 4a to d). In contrast, when the LPS stimulated cells treated with RVFHbαP (group 5), the levels were significantly downregulated in both supernatants and cell lysates and reached almost to the baseline level compared to that of the medium control (group 1) (Fig. 5a and b). The observed decrease of these mediators is not due to cytotoxicity, since RVFHbαP, nRVFHbαP, and LPS were nontoxic in the immunosuppressive dose range. Known concentrations of IL-8 and MCP-1 spikes were fully recovered (data not shown); thus, observed results may not be due to the assay interference.
Fig. 4.
Cytokine/chemokine levels in the supernatants of HeLa hVECs. HeLa cells were seeded at a density of 106/well in 24-well plates and treated with LPS (10 μg/ml for 6 h), or LPS-induced (10 μg/ml for 6 h) cells were treated with RVFHbαP (70.45 μM for 1 h) or scrambled peptide nRVFHbαP (70.45 μM for 1 h) as detailed in Materials and Methods. At the end of treatment, supernatants were collected and analyzed for inflammatory mediators by ELISA. RVFHbαP attenuates LPS-induced production of these mediators compared to the cells that were treated with scrambled peptide. Values represent the means ± SD of triplicate determinations performed on different days. Levels of significance (*, P < 0.05; **, P < 0.001; compared with the LPS- and LPS-nRVFHbαP-treated groups) were calculated by an ANOVA test followed by a Bonferroni analysis.
Fig. 5.
Determination of intracellular IL-6 and IL-8 levels in HeLa hVECs. HeLa cells were seeded at a density of 106/well in 24-well plates and treated with LPS (10 μg/ml for 6 h), or LPS-induced (10 μg/ml for 6 h) cells were treated with RVFHbαP (70.45 μM for 1 h) or scrambled peptide (70.45 μM for 1 h). At the end of the treatment, cells were collected, lysed, and used for the determination of IL-6 (a) and IL-8 (b) levels by ELISA as detailed in Materials and Methods. RVFHbαP suppressed these inflammatory mediators induced by LPS compared to the cells that were treated with scrambled peptide. Values represent the means ± SD of triplicate determinations performed on different days. Levels of significance (**, P < 0.001 compared with the LPS- and LPS-nRVFHbαP-treated groups) were calculated by an ANOVA test followed by a Bonferroni analysis.
RVFHbαP downregulates LPS-induced cytokine gene expression in hVECs.
The above-described results demonstrate that RVFHbαP decreases the production of inflammatory mediators induced by LPS in hVECs. Next, to evaluate whether this activity involves a direct effect of the peptide on gene transcription, we performed a reverse transcription (RT)-PCR to detect IL-6, IL-8, IL-1α, and MCP-1 mRNA expression in hVECs (Fig. 6 a and b). With this experiment, we were able to show that hVECs express all the four inflammatory biomarkers. By comparing the quantitative data of these markers with that of the GAPDH gene, a housekeeping gene, we could observe that the expression of these biomarkers was upregulated in the presence of LPS (group 4) and attenuated in LPS-induced cells after the treatment with RVFHbαP (group 5) compared with that of the medium control (group 1).
Fig. 6.
Expression of cytokine/chemokine mRNAs in the cell lysates of HeLa hVECs. HeLa cells were seeded at a density of 106/well in 24-well plates and treated with LPS (10 μg/ml for 6 h) or LPS-induced (10 μg/ml for 6 h) cells treated with RVFHbαP (70.45 μM for 1 h) or scrambled peptide (70.45 μM for 1 h). At the end of treatment, cells were collected, and lysates were prepared and analyzed for inflammatory mediators and GAPDH mRNA transcription by RT-PCR as detailed in Materials and Methods. (a) Representative image of RT-PCR analysis of cytokine/chemokine mRNA expression is shown. GAPDH blots confirmed roughly equivalent loading of RNA samples. Expression of cytokine genes was upregulated in LPS-induced cells and was significantly suppressed following the treatment of LPS-induced cells with RVFHbαP in respect to control values and is calculated as the mean ± SD of triplicate determinations performed on different days. (b) A quantitative assessment of the intensity of each band was determined by densitometry. Level of significance (**, P < 0.001 compared with the LPS- and LPS-nRVFHbαP-treated groups) was calculated by an ANOVA test followed by a Bonferroni analysis.
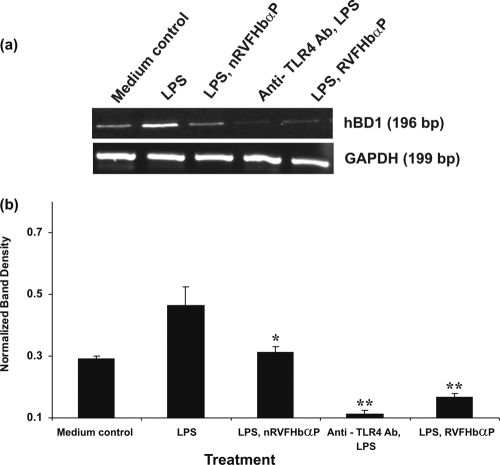
LPS-induced hBD1 mRNA expression is blocked by RVFHbαP.
The above-described study demonstrated that induction of hVECs by LPS led to the upregulation of inflammatory mediator genes. Next, we examined whether the increased expression of inflammatory biomarkers (IL-6, IL-8, IL-1α, and MCP-1) and their genes are associated with cellular immune functions related to the defense pathway. For this, we chose human-β-defensin 1 (hBD1), a known marker for vaginal protection against pathogens, and determined its mRNA expression after LPS induction in hVECs by RT-PCR. The results show that hVECs express hBD1 mRNA (Fig. 7 a and b). By comparing these levels with that of GAPDH in LPS-induced hVECs, we could say that hBD1 mRNA is increased by ∼50%, whereas this upregulation is significantly reduced in hVECs treated with RVFHbαP (group 5) compared with that of the medium control (group 1).
Fig. 7.
RT-PCR analysis of hBD1 gene expression in HeLa hVECs. Cells were seeded at a density of 106/well in 24-well plates and treated with LPS (10 μg/ml for 6 h), or LPS-induced (10 μg/ml for 6 h) cells were treated with RVFHbαP (70.45 μM for 1 h) or scrambled peptides (70.45 μM for 1 h). At the end of treatment, cells were collected and lysates were prepared and analyzed for hBD1 and GAPDH mRNAs by RT-PCR as detailed in Materials and Methods. (a) Expression of hBD1 mRNA was upregulated in LPS-induced cells and attenuated following the treatment of LPS-induced cells with RVFHbαP in respect to the scrambled peptide. Values were calculated as the means ± SD of triplicate determinations and are representative of at least three separate experiments performed on different days. Complete inhibition of hBD1 was observed when cells were treated with anti-TLR4 antibody before LPS induction. A representative image of RT-PCR analysis of hBD1 mRNA expression is shown. GAPDH blots confirmed roughly equivalent loading of RNA samples. (b) A quantitative assessment of the intensity of each band was determined by densitometry. Levels of significance (*, P < 0.05 compared with the LPS; **, P < 0.001 compared with the LPS- and LPS-nRVFHbαP-treated groups) were calculated by an ANOVA test followed by a Bonferroni analysis.
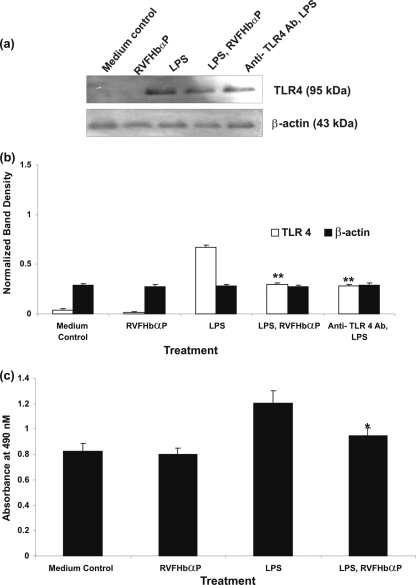
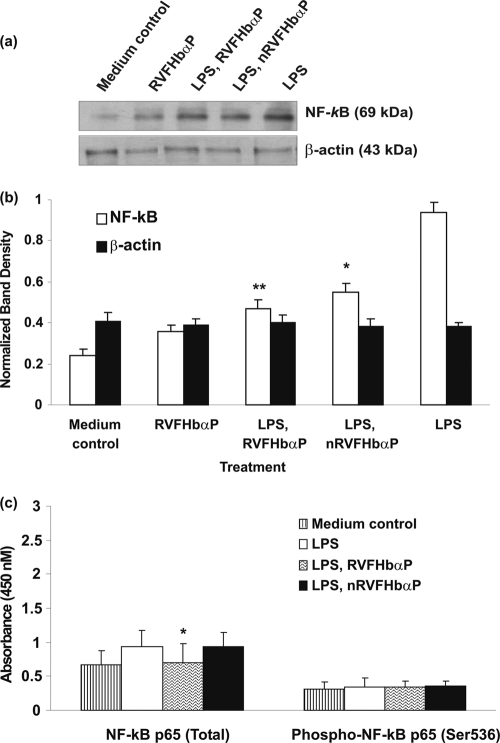
LPS-induced TLR4 and NF-κB expression is downregulated by RVFHbαP.
LPS is a potent agonist of the innate immune system and mediates its effects via the CD14/TLR4 receptor complex. The induction of hVECs by LPS leads to the upregulation of cytokine and hBD1 gene expression. We then extended our observations to investigate whether the inhibitory effects of RVFHbαP on LPS-triggered cytokine/hBD1 responses are the result of its competition with LPS for the TLR4 receptor or whether its effects occurred downstream from TLR4-LPS binding. To accomplish this task, hVECs were stimulated with LPS (group 4); this ensured interaction between TLR4 and LPS in the absence of RVFHbαP. LPS-stimulated cells are treated with RVFHbαP (group 5). Cell lysates of LPS, RVFHbαP, and the anti-TLR4 antibody (cells treated with anti-TLR4 antibody before LPS induction) group were analyzed for TLR4 and NF-κB expression.
ELISA and Western blot results with anti-TLR4 and anti-NF-κB antibodies revealed that both TLR4 and NF-κB were expressed in the hVECs; treatment with LPS (group 4) led to the activation of expression of these proteins. Interestingly, treatment of hVECs with either RVFHbαP (for TLR4 and NF-κB before LPS induction) or anti-TLR4 antibody (for TLR4 before LPS induction) shows a significant decrease in TLR4 and NF-κB expression in comparison with the medium control. A quantitative densitometry assessment of TLR4 and NF-κB revealed that by comparing the levels of TLR4 and NF-κB with that of β-actin in LPS-induced hVECs, we could say that the expression of TLR4 and NF-κB was upregulated by 60 to 70%. However, after RVFHbαP treatment, the induction is drastically reduced and reached to the level of the medium control (group 1) (Fig. 8 a to c and 9 a to c).
Fig. 8.
(a) Western blot analysis of TLR4 expression in HeLa hVEC. Cells were seeded at a density of 106/well in 24-well plates and treated with LPS (10 μg/ml for 6 h), or LPS-induced (10 μg/ml for 6 h) cells were treated with RVFHbαP (70.45 μM for 1 h) or scrambled peptides (70.45 μM for 1 h). At the end of treatment, cells were collected and lysates were prepared and analyzed for TLR4 and β-actin by Western blotting as detailed in Materials and Methods. Expression of TLR4 was upregulated in LPS-induced cells and significantly blocked following the treatment of LPS-induced cells with RVFHbαP in respect to the scrambled peptide. Values are calculated as the means ± SD of triplicate determinations and are representative of at least three separate experiments performed on different days. Similar inhibition of TLR4 was observed when hVECs were treated with anti-TLR4 antibody before being induced with LPS. A representative image of Western blot analysis of TLR4 expression is shown. β-Actin blotting confirmed roughly equivalent loading of protein samples. (b) A quantitative assessment of the intensity of each band was determined by densitometry. (c) Protein ELISA was carried out to confirm the data obtained by Western blot analysis, and the results are in agreement with Western blotting results. Levels of significance (**, P < 0.001 compared with the LPS- and LPS-nRVFHbαP-treated groups) were calculated by an ANOVA test followed by a Bonferroni analysis.
Fig. 9.
Western blot analysis of NF-κB expression in HeLa hVECs. Cells were seeded at a density of 106/well in 24-well plates and treated with LPS (10 μg/ml for 6 h), or LPS-induced (10 μg/ml for 6 h) cells were treated with RVFHbαP (70.45 μM for 1 h) or scrambled peptide (70.45 μM for 1 h). At the end of treatment, cells were collected and lysates were prepared and analyzed for NF-κB and β-actin expression by Western blotting as detailed in Materials and Methods. Expression of NF-κB was upregulated in LPS-induced cells and significantly attenuated following the treatment of LPS-induced cells with RVFHbαP in respect to the scrambled peptide. Values were calculated as the means ± SD of triplicate determinations and are representative of at least three separate experiments performed on different days. Representative image of Western blot analysis of NF-κB expression is shown. β-Actin blot confirmed roughly equivalent loading of protein samples. (b) A quantitative assessment of the intensity of each band was determined by densitometry. (c) Protein ELISA was carried out to confirm the data obtained by Western blot analysis, and the results are in agreement with Western blot results. Levels of significance (*, P < 0.05; **, P < 0.001; compared with the LPS-induced group) were calculated by an ANOVA test followed by a Bonferroni analysis.
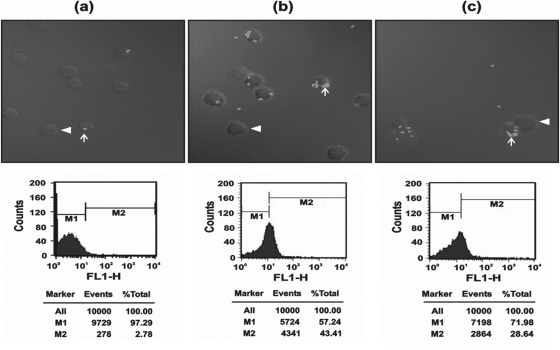
RVFHbαP suppresses LPS-induced macrophage phagocytosis.
We next investigated whether RVFHbαP modulates the LPS-stimulated phagocytic activity of macrophages. As shown in Fig. 10, when the macrophages were treated with LPS (10 μg/ml for 1 h), a higher number of E. coli cells are internalized in the cytoplasm of the macrophages. The phagocytosis index (PCI) of the medium control is ∼2.9 ± 0.20% compared to 5.20 ± 0.93% in LPS-stimulated cells. When LPS activated macrophages treated with RVFHbαP, the PCI is significantly reduced (P < 0.05). These results were also confirmed by flow cytometry and are in agreement with PCI data.
Fig. 10.
(a) Laser scanning confocal images of macrophage phagocytosis. (b) Medium control showing very few internalized FITC-labeled E. coli cells within the macrophages (×63 magnification). When macrophages were induced with LPS, phagocytosis was significantly increased compared to that of the scrambled peptide. (c) In LPS-induced macrophages treated with RVFHbαP (70.45 μM), the number of E. coli cells internalized within macrophages was significantly inhibited. Also shown are positions of plasma membranes (arrowheads) and labeled bacteria internalized within the macrophages (arrows). The images are representative of one of three identical experiments performed on three different days.
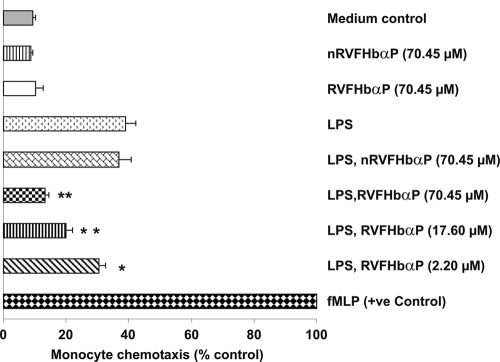
LPS-induced chemotaxis of U937 cells is inhibited by RVFHbαP.
The effect of RVFHbαP on LPS-triggered macrophage chemotaxis was investigated in vitro using the Boyden chamber Transwell method. The results demonstrated that infiltration of U937 cells was found to be significantly higher (P < 0.001) toward the lower compartment, which contained conditioned medium obtained from LPS-induced hVECs, than that of the medium control or cells treated with RVFHbαP and nRVFHbαP. Treatment with an increased concentration of RVFHbαP reduces infiltration of U937 cells compared with that of LPS treatment. This effect was found to be directly proportional with the concentration of RVFHbαP. An ∼28% reduction was observed with as low as 2.20 μM RVFHbαP, whereas the maximum effect (∼68%) was seen with 70.45 μM (Fig. 11). RVFHbαP or nRVFHbαP alone did not affect the chemotaxis. N-Formyl-Met-Leu-Phe was used as a positive control, and chemoattraction was considered 100%.
Fig. 11.
Determination of chemotaxis in U937 cells. When culture supernatants of HeLa hVECs that were induced with LPS were used in the lower Transwell chamber, U937 cell migration was significantly increased compared to that of the scrambled peptide. When supernatants were obtained from LPS-induced cells treated with increased concentrations of RVFHbαP (70.45, 17.60, and 2.20 μM) and used in the lower Transwell chamber, the migration of U937 cells was inhibited in a dose-dependent manner. RVFHbαP at a concentration of 70.45 μM resulted in maximum inhibition of U937 cell migration. As expected, the scrambled peptide nRVFHbαP did not interfere with LPS-induced chemotaxis. The data were normalized to U937 chemotaxis in response to 0.1 mM fMLP in PBS-BSA, which was used to stimulate cell migration (100%). Each value is the mean ± SD from six individual observations obtained from three independent experiments. Levels of significance (*, P < 0.05; **, P < 0.001; compared with the LPS- and LPS-nRVFHbαP-treated groups) were calculated by an ANOVA test followed by a Bonferroni analysis.
DISCUSSION
Innate and adoptive immune functions of the FRT to pathogens has gained a substantial interest in recent years, and molecules involved in the maintenance of vaginal tissue homeostasis are just beginning to be identified. In women of child-bearing age, the majority of FRT complications (e.g., tubal infertility and endometriosis) occur owing to bacterial infections (1). LPS, the major constituent of the outer membrane of Gram-negative bacteria, is an important protein against the permeability of bactericidal agents, including AMPs. However, the structural determinants of AMPs for LPS recognition are not clearly understood (35). Therefore, identifying molecules that bind and neutralize the toxic effects of LPS may have clinical application as a therapy for the treatment of reproductive complications that may arise due to pathogenic bacteria.
Several natural and synthetic inhibitors of LPS-induced inflammatory responses in mammals have been tested in vivo (22). These molecules fall into two broad categories: receptor antagonists and LPS-neutralizing molecules. Among the first are LPS-like molecules isolated from bacteria (e.g., Helicobacter pylori), synthetic lipid A analogues, certain cationic peptides (18), and LPS-neutralizing molecules, such as recombinant bactericidal permeability-increasing protein 23 (rBPI23) (21). Recently, we have identified one such molecule, RVFHbαP, in the vaginal lavage of rabbits (26). Using various bioinformatic tools and immunologic approaches, this study describes for the first time the role of RVFHbαP in LPS-induced cellular immune functions in hVECs.
Using Moeller software, the structure of RVFHbαP was acquired. Studies suggested that the interaction between LPS and the amphipathic loop of the Hb-α subunit is of an electrostatic nature, whereby the positive charges of the peptide is assumed to bind to the negatively charged groups of LPS (phosphates and carboxylates) (Fig. 1 and 2). Further, our results demonstrated that RVFHbαP is able to recognize and bind to LPS in a concentration-dependent fashion, indicating that the peptide may act at the extracellular level by complexing with LPS and neutralizing its activity (Fig. 3). Contrary to RVFHbαP, scrambled peptide (nRVFHbαP) did not show binding to LPS, attributing to the weak interaction between the basic residues of the peptide with negatively charged residues of the LPS. These observations led us to speculate that, in contrast to the several LPS inhibitors, RVFHbαP is devoid of any agonistic activity even at high concentrations and acts as a pure LPS antagonist. Collectively, the results described here are consistent with the notion that RVFHbαP can bind specifically to LPS.
Given the diverse effects of LPS in the inflammation process, studies were extended to delineate the role of RVFHbαP in LPS-induced effects on inflammatory mediators of hVECs. We here demonstrated that RVFHbαP prevents hVEC activation and inhibits the LPS-induced production of cytokines/chemokines (IL-6, IL-8, MCP-1, and IL-1α). These results suggest that LPS activates hVECs through the interactions with TLR4, which in turn transfers the signal into the nuclei through the NF-κB pathway. RVFHbαP as a soluble LPS-recognizing peptide binds to LPS through its lipid binding domain (LBD) and activates NF-κB. Our unpublished observations revealed that when the LPS-RVFHbαP complex was added to the cells, the complex failed to stimulate cytokine/chemokine production, probably due to the failure of TLR4 in recognizing LPS (neutralized by coincubation with LPS). Similar results were observed when the cells were treated with RVFHbαP alone or when these treated cells were induced with LPS. This clearly suggests that LPS binding to its receptor, TLR4, plays a key role in deciding the RVFHbαP activity. It is possible that LPS-TLR4 binding might activate other cellular targets (cell surface or intracellular) other than TLR4, through which RVFHbαP exerts its effect. However, at present it is not known what the other cellular targets are for RVFHbαP. We also do not know at present whether the LPS-TLR4 complex might activate other cell surface receptors, and RVFHbαP could be interacting through these receptors, thereby inhibiting cytokine production. The other possibility is that RVFHbαP might be getting internalized upon activation by LPS. We assumed that TLR4-bound LPS would expose the RVFHbαP to cellular targets other than LPS on hVECs to transfer the signals intracellularly.
Further, to clarify how RVFHbαP acts after LPS had interacted with the TLR4 receptor on hVECs and suppress LPS-induced proinflammatory cytokines/chemokines. This striking and somewhat unexpected result with respect to anti-LPS activity of RVFHbαP prompted us to speculate concerning alternative mechanisms of peptide-mediated protection unrelated to the LPS binding to TLR4. One possible starting point for such a speculation is that our previous results (M. S. Patgaonkar, Bashir Tahir, and K. V. R. Reddy, unpublished data) reveal that RVFHbαP inhibited protein kinase C (PKC), a key intracellular signal-transducing molecule implicated in cell activation. This PKC inhibitory activity correlates with RVFHbαP-mediated inhibition of LPS-induced cytokine/chemokine production by hVECs, suggesting anti-LPS activities of the intracellularly produced RVFHbαP. Further studies in this direction are required, and this is the current interest of our laboratory.
These results support the observations obtained by RT-PCR, wherein we show a significant elevation of IL-6, IL-8, MCP-1, and IL-1α mRNA transcription in response to LPS induction in hVECs (Fig. 6), suggesting that RVFHbαP inhibits production of inflammatory mediators by affecting gene transcription. These results are consistent with the previous findings, which showed that posttranscriptional mechanisms play a major role in the regulation of cytokine gene expression in LPS-stimulated cells (23). Therefore, we believe that RVFHbαP behaves as an inhibitor of LPS-induced cytokine/chemokine synthesis at the level of mRNA in hVECs. The mechanism by which RVFHbαP exerts its immunosuppressive influence on hVECs has remained enigmatic. Interestingly, we observed that correct timing and concentration of RVFHbαP and LPS are necessary to observe the effects reported in this paper, revealing that RVFHbαP is active when inflammatory reaction is already ongoing. The effect of RVFHbαP may also depend on the situation, and the local environment may play a role in the control of vaginal infection, but the mechanisms and the key players participating in such an event are largely unknown.
There have been a series of reports demonstrating that LPS-TLR4 can modulate the synthesis of defense molecules. The present results show that RVFHbαP inhibited LPS-induced expression of hBD1 mRNA of hVECs. Induced expression of hBD1 mRNA in response to LPS stimuli represents an immediate and dynamic response by hVECs to potential infection.
Also, there is a growing body of evidence which suggests that the vaginal epithelium is one of the critical interfaces with the external environment and VECs synthesize and release a number of Hb-derived peptides into the lumen which act against invading pathogens (6, 19, 24). Indeed, Hb-derived peptide-regulated pathways have been identified in VECs (20, 24), and cell survival (26) and inflammatory responses (6, 16) have been shown to be modulated by Hb-derived peptides. These studies were further strengthened by recent studies, which showed that virulent bacterial proteins (e.g., hemolysin) lyse RBCs and release Hb, which then get oxidized into methemoglobin (metHb). The oxidized Hb, although no longer carrying O2 transport, is active against pathogens (6, 16). Our previous studies indicate that RVFHbαP is synthesized by rabbit VECs, shows over 90% sequence homology with the Hbα chain, and also possesses antibacterial activities (26). Our present demonstration of RVFHbαP expression and activity in hVECs strongly supports these views.
The inhibitory effects of RVFHbαP on cytokine/chemokine protein and gene expression has prompted us to investigate the expression of TLR4 in hVECs. The results demonstrated that the hVECs respond to pathogens and associated membrane proteins (e.g., LPS). This process is mediated at least in part by increasing TLR4 expression in hVECs. In the presence of RVFHbαP, the inflammatory activity of LPS is significantly inhibited due to the neutralization of LPS by the peptide, thereby lowering the activation of TLR4. It remains to be seen how LPS-induced TLR4 expression is inhibited by RVFHbαP, which led to the inactivation of TLR4 in hVECs. These results are supported by recent studies, where it was shown that hemoglobin-derived peptides can bind to LPS and potentially prevent the interaction between LPS and TLRs (3). A similar inhibitory effect of Scylla serrata antilipopolysaccharide factor 24 (SsALF24) on cytokine levels has been recently reported (33).
Vaginal epithelium is exposed to a large number of pathogenic bacteria and their secreted proteins, and the interaction between LPS and TLR4 is important for the mediation of vaginal inflammatory responses that are NF-κB dependent that occur downstream from NF-κB gene transactivation (23, 41). Therefore, binding of RVFHbαP to LPS through the activation of TLR4 constitutes a major pathway by which NF-κB is upregulated in hVECs, which can favor host survival following bacterial infection. Thus, we expected that RVFHbαP inhibited phagocytosis of E. coli by macrophages through interaction with the surface receptors of E. coli. Phagocytosis of E. coli is an essential component of host defense against microbial pathogenesis that requires LPS-TLR4 activation (8, 15, 34, 35). We also demonstrated that the culture supernatants of LPS-induced hVECs show enhanced chemotaxis of U937 cells. In contrast, LPS-stimulated cells treated with RVFHbαP led to the inhibition of LPS-induced migration of U937 cells in a dose-dependent manner, suggesting a protective role for RVFHbαP on hVECs (Fig. 11). RVFHbαP is a nonmyeloid cell-derived protein, and its involvement in host defense has not been demonstrated previously. To the best of our knowledge, this is the first report to document a potential role for RVFHbαP in cellular immune functions.
The aforementioned studies strongly reveal that RVFHbαP is capable of protecting vaginal cells from LPS-mediated insults and may be explored as a therapeutic against reproductive tract infections. RVFHbαP, which is inherently present in the VECs, therefore, may not cause damage to the vaginal mucosa. Exploring such molecules for vaginal application has certain advantages over chemical-based drugs. By identifying such novel molecules and cellular targets for RVFHbαP action, our results might be relevant for the screening of RVFHbαP ligands to be used in the prevention of vaginal infections. However, we are far from understanding how the three-tier system, i.e., RVFHbαP-LPS-TLR4, mediates the innate cellular immune functions. Given the nascent state of knowledge concerning this important area, it is clear that more studies are needed to provide valuable insight into the immunobiology of RVFHbαP.
ACKNOWLEDGMENTS
We are grateful to our director for giving encouragement in carrying out this study. We are thankful to A. R. Passi for statistical analysis of the data and Mangesh Malvankar for secretarial assistance.
This study was partially supported by grants from the International Partnership on Microbicides (IPM) and Indian Council of Medical Research (ICMR) (NIRRH/MS/27/2010). We thank IPM for providing a research fellowship to M.S.P.
Footnotes
Published ahead of print on 24 August 2011.
REFERENCES
- 1. Aranha C. C., Gupta S. M., Reddy K. V. R. 2008. Assessment of cervicovaginal cytokine levels following exposure to microbicide Nisin gel in rabbits. Cytokine 43:63–70 [DOI] [PubMed] [Google Scholar]
- 2. Bavoil P. M. 1994. Measurement of nonopsonic phagocytic killing by human and mouse phagocytes, p. 107 In Abelson J. N., Clark V. L., Simon M. I. (ed.), Methods in enzymology. Bacterial pathogenesis, part B: interaction of pathogenic bacteria with host cells. Academic press, San Diego, CA [Google Scholar]
- 3. Bowdish D. M., Davidson D. J., Scott M. G., Hancock R. E. 2005. Immunomodulatory activities of small host defense peptides. Antimicrob. Agents Chemother. 49:1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 5. Cole A. E. 2001. Invited review: bacterial lipopolysaccharides and innate immunity. J. Endot. Res. 7:167–202 [PubMed] [Google Scholar]
- 6. Du R., Ho B., Ding J. K. 2010. Rapid reprogramming of haemoglobin structure-function exposes multiple dual-antimicrobial potencies. The EMBO J. 29:632–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Galanos C., et al. 1984. Immunogenic properties of lipid A. Rev. Infect. Dis. 6:546–551 [DOI] [PubMed] [Google Scholar]
- 8. Greenberg S., Silverstein S. C. 1993. Phagocytosis, p. 941–964 In Paul W. E. (ed.), Fundamental immunology. Raven Press, Inc., New York, NY [Google Scholar]
- 9. Guex N., Peitsch M. C. 1997. SWISS-MODEL and the Swiss-dbViewer: an environment for comparative protein modeling. Electrophoresis 18:2714–2723 [DOI] [PubMed] [Google Scholar]
- 10. Gupta S. M., Aranha C. C., Mohanty M. C., Reddy K. V. R. 2008. Toll-like receptors and cytokines as surrogate biomarkers for evaluating vaginal immune response following microbicide administration. Mediators Inflamm. 23:45–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guthrie B. L., et al. 2009. Sexually transmitted infections among HIV-1-discordant couples. PLoS One 4:e8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirata M., Shimomura Y., Yoshida M., Wright S. C., Larrick J. W. 1994. Endotoxin-binding synthetic peptides with endotoxin-neutralizing, antibacterial and anticoagulant activities. Prog. Clin. Biol. Res. 388:147–153 [PubMed] [Google Scholar]
- 13. Hoess A., Schneider-Mergener J., Liddington R. C. 1995. Identification of the LPS-binding domain of an endotoxin neutralising protein, Limulus anti-LPS factor. Prog. Clin. Biol. Res. 392:327–334 [PubMed] [Google Scholar]
- 14. Howe J., et al. 2008. Structural investigations into the interaction of hemoglobin and part structures with bacterial endotoxins. Innate Immun. 14:39–49 [DOI] [PubMed] [Google Scholar]
- 15. Huffnagle G. B., et al. 1995. The role of monocyte chemotactic protein-1 (MCP-1) in the recruitment of monocytes and CD4+ T cells during a pulmonary Cryptococcus neoformans infection. J. Immunol. 155:4790–4795 [PubMed] [Google Scholar]
- 16. Kaca R., Roth I., Levin J. 1994. Hemoglobin, a newly recognized lipopolysaccharide (LPS)-binding protein that enhances LPS biological activity. J. Biol. Chem. 269:25078–25084 [PubMed] [Google Scholar]
- 17. Kloczewiak M., et al. 1994. Synthetic peptides that mimic the binding site of horseshoe crab antilipopolysaccharide factor. J. Infect. Dis. 170:1490. [DOI] [PubMed] [Google Scholar]
- 18. Lepper P. M., Triantafilou M., Schumann C., Schneider E. M., Triantafilou K. 2005. Lipopolysaccharides from Helicobacter pylori can act as antagonists for Toll-like receptor 4. Cell. Microbiol. 7:519–528 [DOI] [PubMed] [Google Scholar]
- 19. Li M., et al. 2007. Purification of antimicrobial factors from human cervical mucus. Hum. Reprod. 22:1810–1815 [DOI] [PubMed] [Google Scholar]
- 20. Liepke C., et al. 2003. Human hemoglobin-derived peptides exhibit antimicrobial activity: a class of host defense peptides. J. Chromatogr. 791:345–356 [DOI] [PubMed] [Google Scholar]
- 21. Little R. G., Kelner D. N., Lim E., Burke D. J., Conlon P. J. 1994. Functional domains of recombinant bactericidal/permeability increasing protein (rBPI23). J. Biol. Chem. 269:1865. [PubMed] [Google Scholar]
- 22. Lynn M., et al. 2003. Blocking of responses to endotoxin by E5564 in healthy volunteers with experimental endotoxemia. J. Infect. Dis. 187:631–639 [DOI] [PubMed] [Google Scholar]
- 23. Macagno A., et al. 2006. A cyanobacterial LPS antagonist prevents endotoxin shock and blocks sustained TLR4 stimulation required for cytokine expression. J. Exp. Med. 203:1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mak P. 2008. Hemocidins: a functional and structural context of human antimicrobial peptides. Front. Biosci. 13:6859–6871 [DOI] [PubMed] [Google Scholar]
- 25. Morita T., et al. 1985. Isolation and biological activities of Limulus anticoagulant (anti-LPS factor) which interacts with lipopolysaccharide (LPS). J. Biochem. 97:1611–1620 [DOI] [PubMed] [Google Scholar]
- 26. Patgaonkar M. S., Aranha C. C., Bhonde G., Reddy K. V. R. 2011. Identification and characterization of anti-microbial peptides from rabbit. Vet. Immunol. Immunopathol. 139:176–186 [DOI] [PubMed] [Google Scholar]
- 27. Pridmore A. C., et al. 2003. Activation of Toll-like receptor 2 (TLR2) and TLR4/MD2 by Neisseria is independent of capsule and lipooligosaccharide (LOS) sialylation but varies widely among LOS from different strains. Infect. Immun. 71:3901–3908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Quayle A. J. 2002. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J. Reprod. Immunol. 57:61–79 [DOI] [PubMed] [Google Scholar]
- 29. Reddy K. V. R., Yedery R. D., Aranha C. C. 2004. Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Peptides 24:536–547 [DOI] [PubMed] [Google Scholar]
- 30. Reddy K. V. R., Aranha C. C., Gupta S. M., Yedery R. D. 2004. Evaluation of antimicrobial peptide, Nisin, as a safe vaginal contraceptive agent in rabbits: in vitro and in vivo studies. Reproduction 128:126–137 [DOI] [PubMed] [Google Scholar]
- 31. Ried C., et al. 1996. High affinity endotoxin-binding and neutralizing peptides based on the crystal structure of recombinant Limulus anti-LPS factor. J. Biol. Chem. 271:28120–28127 [DOI] [PubMed] [Google Scholar]
- 32. Ritchie D. W., Kemp G. J. L. 2000. Protein docking using spherical polar Fourier correlations. Proteins 39:178–194 [PubMed] [Google Scholar]
- 33. Sharma S., Yedery R. D., Patgaonkar M. S., Selvaakumar C., Reddy K. V. R. 2011. Antibacterial activity of a synthetic peptide that mimics the LPS binding domain of Indian mud crab, Scylla serrata anti-lipopolysaccharide factor (SsALF), also involved in the modulation of vaginal immune functions through NF-κB signaling. Microb. Pathog. 50:179–191 [DOI] [PubMed] [Google Scholar]
- 34. Shiratsuchi A., Watanabe I., Takeuchi O., Akira S., Nakanishi Y. 2004. Inhibitory effect of Toll-like receptor 4 on fusion between phagosomes and endosomes/lysosomes in macrophages. J. Immunol. 172:2039–2047 [DOI] [PubMed] [Google Scholar]
- 35. Sippl M. J. 1993. Recognition of errors in three-dimensional structures of proteins. Proteins 17:355–356 [DOI] [PubMed] [Google Scholar]
- 36. Spear G. T., John S. E., Zariffard M. R. 2007. Bacterial vaginosis and human immunodeficiency virus infection. AIDS Res. Ther. 4:25–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Steeghs L., et al. 2008. Differential activation of human and mouse Toll-like receptor 4 by the adjuvant candidate LpxL1 of Neisseria meningitides. Infect. Immun. 76:3801–3807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stoline M. R. 1981. The status of multiple comparisons: simultaneous estimation of all pairwise comparisons in one-way ANOVA designs. Am. Stat. Assoc. 35:134–141 [Google Scholar]
- 39. Takeda K., Akira S. 2005. Toll-like receptors in innate immunity. Int. Immunol. 17:1–14 [DOI] [PubMed] [Google Scholar]
- 40. Taylor A. H., et al. 1995. Lipopolysaccharide (LPS) neutralizing peptides reveal a lipid A binding site of LPS binding protein. J. Biol. Chem. 270:17934–17938 [DOI] [PubMed] [Google Scholar]
- 41. Trifonova T., Pasicznyk J. M., Fichorova R. N. 2006. Biocompatibility of solid-dosage forms of anti-human immunodeficiency virus type 1 microbicides with the human cervicovaginal mucosa modeled ex vivo. Antimicrob. Agents Chemother. 50:4005–4010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wira C. R., Fahey J. V., Sentman C. L., Pioli P. A., Shen L. 2005. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol. Rev. 206:306–335 [DOI] [PubMed] [Google Scholar]
- 43. Yedery R. D., Reddy K. V. R. 2009. Identification, cloning, characterization and recombinant expression of an anti-lipopolysaccharide factor from the hemocytes of Indian mud crab, Scylla serrata. Fish Shellfish Immunol. 27:275–284 [DOI] [PubMed] [Google Scholar]