Abstract
Porcine circovirus type 2 (PCV2) is the obligate infectious agent in postweaning multisystemic wasting syndrome (PMWS) of pigs. To control PMWS, we vaccinated dams at 4 and 2 weeks before pregnancy and again in the 12th week of gestation with an inactivated PCV2 vaccine (Circovac). Two producer farms run under the control of Swiss Swine Health Organization were selected for the experiment. Previously, in one farm PMWS was diagnosed on pigs after weaning, whereas in the other farm, pigs wasted during the fattening period. For the experiments 113 dams were randomly vaccinated, and 111 dams were sham injected. Vaccination increased serum antibodies in dams 3- to 9-fold, accompanied by serum antibody titer increases in their offspring. In the sixth week of life, progeny from vaccinated dams had about the same IgG antibody titers as progeny of unvaccinated dams at the third day of life. In sera of vaccinated dams only low concentrations of PCV2 DNA were detected, and no progeny developed PMWS. Interestingly, at day 56 four progeny of unvaccinated dams tested positive for anti-PCV2 IgM antibodies, indicating a primary infection with PCV2. Of economic importance is the observation that progeny of vaccinated dams had a significantly higher daily weight gain in the fattening period (farm X, +51 g/day; farm Y, +30 g/day) and thus a shortened fattening period of about 6 days compared to progeny of controls. To our knowledge this is the first demonstration of subclinical circovirus infection and its effects on growth performance of fattening pigs by vaccination of dams.
INTRODUCTION
Postweaning multisystemic wasting syndrome (PMWS) in pigs was first described in Canada (18) and has since been recognized as one of the economically most important swine diseases worldwide (2, 9, 19, 21, 24, 44). PMWS emerged as an epizootic disease in Switzerland in 2003 to 2004 even though cofactors described as important for PMWS development, including porcine reproductive and respiratory syndrome (PRRS), enzootic pneumonia (EP), actinobazillosis, and progressive atrophic rhinitis (pRA), were not present (54).
PMWS is an acute or chronic disease affecting animals at the age of 5 to 16 weeks (1, 11) or exceptionally until 30 weeks of age (37). Typical signs are wasting, profuse diarrhea, and dyspnea, and pigs may have gastric ulcers, enlarged lymph nodes, anemia, icterus, hemorrhages, vasculitis, or edema in various organs (1, 18, 39, 42, 43).
Various porcine circovirus type 2 (PCV2) genotype group members have the potential to be involved in the PMWS etiology (9, 19, 21, 24, 39, 44). Nevertheless, PCV2 can be detected in healthy pigs or isolated from various cells and organs, including peripheral blood, mononuclear cells, dendritic cells, and lymphocytes, and viral antigen is often found in defined lymphatic areas in lymph nodes, tonsils, spleen, and thymus (3, 4) or is scattered in their supporting reticular cells, associated with irregular tissue architecture and in macrophages (39, 49). In other cases, PCV2 was diagnosed in lung, liver, kidney, and the gastrointestinal tract and, in rare cases, in apoptotic vascular endothelial cells of the brain (55).
As PCV2 can replicate in multiple cells of various organs to measurable titers in clinically healthy or diseased animals, the virus may be present in serum or all other body fluids (1, 43) including semen (30, 41). Infection of naïve animals may occur by direct contact with infected animals and their secretions; airborne dissemination must be considered due to high viral loads in large farms (26). In addition, natural vertical transmission was diagnosed in field cases (20, 53) and could be induced experimentally (33, 40). Experimentally infected dams delivered dead and stillborn piglets. PCV2 infection in fetuses was verified and was associated with myocarditis, fibrosis, and degeneration of the myocardium as well as depletion of lymphocytes (32, 38). Recent evidence further suggests that intrauterine infection may have been underestimated at least in some herds (45).
In a retrospective epidemiological study, PCV2 could be traced back to 1979 in Switzerland (54). Nevertheless, the first PMWS case was not confirmed until 2001 (5). However, the epizooty started in late 2003 in areas with large swine populations (52).
PCV2 has been endemic worldwide since the mid-1990s and can be isolated from PMWS-diseased and clinically healthy animals. PCV2-specific antibodies are detected in almost all pigs (1, 16, 29, 36, 48, 51). Another issue is the observation that the profiles of PCV2 serum antibody titers of pigs from PMWS-affected and unaffected herds are almost identical (17, 23). Thus, the presence of PCV2-specific IgG antibodies is of limited diagnostic or prognostic value and should be considered for diagnostics only in conjunction with disease pattern and PCV2 viral load (46).
Until recently, the main effort to reduce PMWS focused on optimizing herd management in general (27, 28) and intensified monitoring of health status such as the program run by the Swiss Swine Health Organization (www.suisag.ch/SGD/Richtlinien). Despite the absence of porcine reproductive and respiratory syndrome, enzootic pneumonia, actinobazillosis, and progressive atrophic rhinitis in Switzerland, PMWS occurred in such herds (54). Hence, other measures had to be taken to control the disease. Vaccination against PCV2 was thus considered.
Two types of vaccines against PCV2 were introduced in Europe. One is used to vaccinate pregnant sows to increase colostral antibody concentration while the other is used to vaccinate piglets. Several field studies have demonstrated that vaccination of nursing piglets is effective in reducing losses caused by PMWS and that maternal antibodies present at the time of vaccination did not interfere with active antibody production (13, 22, 35).
In the present study, dams from two different farms under the control of the Swiss Swine Health Organization and with a PMWS history were immunized. The ubiquitous presence of PCV2 may facilitate intrauterine or perinatal infection (8). Vaccination of dams against PCV2 may decrease overall viral load perinatally, and increasing colostral antibodies may protect offspring within the first days of life. To test the effectiveness of vaccination on reproductive parameters, antibody production of the dams and antibody transfer to piglets, mortality rate, growth performance of offspring, and age of slaughter were analyzed.
MATERIALS AND METHODS
History of the herds.
This study took 14 months. We used Circovac (Merial SA, Lyon, France) in two different farms with a history of recurrent PMWS. In both herds PMWS had been diagnosed before birth of the first litter. PMWS diagnosis was according to Sorden (46) and described by Wiederkehr et al. (54).
Herd X was a breeding herd with 90 Swiss Large White sows. Gilts were raised for replacements within the farm. Breeding dams and weaned pigs were kept in different barns on the same farm. Weaned pigs were sold to a regional finisher at the age of approximately 10 weeks at a body weight of 22 to 27 kg. Only sporadic cases of PMWS had occurred in herd X since 2006, but there were considerable losses in the finishing operations. Ten to 15% of these pigs developed PMWS, as verified by pathological examinations.
Herd Y was a breeding herd with 150 Swiss Large White × Landrace crossbred sows. Gilts were purchased from another farm and added to this herd without quarantine. Here, too, breeding dams and weaned piglets were kept in different barns on the same farm. At 1 to 2 weeks after weaning, 5 to 20% of the piglets showed low daily weight gain and profuse untreatable diarrhea, with a herd mortality rate of 5 to 10% at the weaning stage, indicators of a serious PMWS problem, which was confirmed by laboratory diagnostics. Other pathogens including Escherichia coli, Salmonella spp., Brachyspira spp., Lawsonia intracellularis, and rota- and coronaviruses that normally cause diarrhea were excluded. Nevertheless, the acute PMWS problem had passed with birth of the first litters important to the study. Low PCV2 infections were still measured. Thus, the farm was in a subclinical-infection phase. During the study no changes in management, feeding, or housing were made.
Vaccination protocol.
A total of 224 dams were randomly chosen and split into two groups for either vaccination (n = 113) or sham injection (n = 111) with paraffin adjuvant emulsion as a control group. The study was performed without the vaccination team knowing what was injected. Additionally, data were collected without any knowledge on the part of the investigators as to whether the particular pig was vaccinated or had received just adjuvants. The inactivated PCV2 vaccine Circovac (Merial SA, Lyon, France) was used at a dose of 2 ml and administered deep into the neck musculature using a 1.2- by 40-mm needle at 4 and 2 weeks before artificial insemination and 4 weeks antepartum. One 2 ml-dose contained ≥2.1 log10 PCV2 antigenic units, 0.2 mg of thiomersal, and 500 mg of paraffin as an adjuvant.
Furthermore, for reason of comparison, the dams were categorized as either young (≤3 litters; n = 65) or experienced (>3 litters; n = 159), as indicated in the data sets. Since the progeny of young dams showed an E. coli-associated diarrhea in the first week of life, we excluded their piglets from further analysis.
Blood collection for serum production.
Blood (10 ml) was collected from the jugular vein of dams immediately before the first injection (B0), 4 weeks after the second injection (B1), and 2 weeks after the third injection (B2). Additionally, on farm Y, 2 to 5 ml of blood was collected from each of 101 individually tagged, randomly selected piglets from 17 litters at the age of 3, 10, 31, 42, 56, and 63 days postpartum (pp). Our study was carried out according to Swiss Animal Welfare guidelines (study number 06/07).
Serological examinations.
A competitive enzyme-linked immunosorbent assay ([ELISA] SerELISA PCV2 Ab Mono Blocking Systems; Synbiotics Corporation Europe SAS, Lyon, France) was used for antibody (IgG) detection (14). The completion of the test, data analysis, and transformation of the data into ELISA units (EU) were done according to the manufacturer's instructions and a published reference (14). We supplemented the assay with two additional controls to the serum dilutions suggested by the manufacturer. First, we used additional positive- and negative-control sera to check plate antigen coating homogeneity. Second, we normalized S values (linear s/n ratio [14]) among individual plates with the aid of a known serum. Immunoglobulin M (IgM) was measured using Ingezim Circovirus IgG/IgM (Ingenasa, Madrid), a capture immunoenzymatic assay specific for IgM antibody detection to PCV2.
Production variables of sows.
Parity number, litter weight, number of live- and dead-born piglets, number of mummified pigs, number of piglets weighing below 1 kg, number of piglets lost during the nursing period, and cause of death were recorded for each dam.
Production variables of progeny.
Cross-fostering occurred; however, these piglets were excluded. The parameter average daily weight gain from birth to slaughter (ADWG1) is calculated as live slaughter weight (kg) divided by age in days. The average daily weight gain in the fattening period (ADWG2) is calculated as live slaughter weight (kg) minus the weight at the beginning of the finishing period (kg) divided by the number of finishing days. The carcass weight was considered to represent 78% of the live weight at slaughter and was used to calculate the latter. The number of finishing days was calculated from the dates of weaning and slaughter.
Pathological examinations.
All mummified and stillborn pigs and dead nursing piglets as well as the dead weaning and fattening pigs were examined at the Institute of Veterinary Pathology, Vetsuisse Faculty, University of Zurich. If deemed necessary, histological, bacteriological, and virological examinations were undertaken, too. When PMWS was suspected, tissues were examined immunohistochemically (IHC) and with PCR for PCV2 infection (31, 47, 54).
Statistics.
Statistical calculations were carried out using StatView, version 5.1 (SAS Corporation). Analysis of variance (ANOVA) for repeated measures and unpaired and paired t tests were considered statistically significant at a P value of ≤0.05.
RESULTS
Production parameter of dams.
This study was conducted within a period of 14 months. Dam production parameters, including gestation rate, numbers of mummified, aborted, or weak-born and live-born piglets, and litter size, did not change during the 14 months and did not differ between vaccinated and unvaccinated dams. Also, there were no piglet loss differences observed between the vaccinated and nonvaccinated dam progeny during suckling or weaning time.
Postmortem examination.
Of a total of 2,720 piglets born, 379 (13.9%) died and underwent a postmortem examination. Of these, 176 were from herd X, and 203 were from herd Y. This number includes aborted and stillborn piglets and perinatal losses. The majority of these piglets were crushed by their mothers during the first week of life. Sixty of the dead piglets were examined immunohistochemically for the presence of PCV2 antigen. All were negative. At the weaning stage, 9 of 1,169 weaned pigs died in herd X (0.8%), and 15 of 1,172 weaned pigs died in herd Y (1.3%). In none of these pigs was PMWS diagnosed.
In the fattening period PMWS was diagnosed in 2 of the 112 pigs born to two nonvaccinated sows of herd X (1.8%). Interestingly, no case of PMWS was diagnosed in 311 fattening pigs from herd Y.
Anti-PCV2 IgG antibody titer of dams and PCV2 infection.
At the start of the experiment (time B0), 99% of all dams had antibodies against PCV2. In herd X, the mean titer at B0 of all sows was significantly (P = 0.006) higher than that of herd Y (Table 1). At time points B1 and B2, vaccinated experienced as well as young dams of both herds (Table 1) had significantly higher titers against PCV2 than unvaccinated dams (P ≤ 0.05). Upon vaccination, antibody titers increased up to 9-fold.
Table 1.
Antibody titers of dams in farm X and Y and of young and experienced dams
| Timea | Antibody titer by group (EU)b |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All herd X dams |
All herd Y dams |
Young dams |
Experienced dams |
|||||||||
| Vaccinated (n = 67) | Unvaccinated (n = 68) | P value | Vaccinated (n = 46) | Unvaccinated (n = 43) | P value | Vaccinated (n = 42) | Unvaccinated (n = 24) | P value | Vaccinated (n = 71) | Unvaccinated (n = 88) | P value | |
| B0 | 1,613 ± 306 | 1,893 ± 369 | >0.05 | 887 ± 157 | 1,046 ± 278 | >0.05 | 2,229 ± 781 | 2,229 ± 331 | >0.05 | 874 ± 173 | 564 ± 105 | >0.05 |
| B1 | 5,035 ± 410 | 2,140 ± 339 | <0.05 | 6,303 ± 487 | 1,508 ± 345 | <0.05 | 6,820 ± 511 | 3,255 ± 929 | <0.05 | 5,176 ± 431 | 1,360 ± 184 | <0.05 |
| B2 | 5,156 ± 358 | 1,670 ± 344 | <0.05 | 5,661 ± 358 | 1,140 ± 193 | <0.05 | 6,404 ± 475 | 2,068 ± 531 | <0.05 | 4,895 ± 422 | 1,157 ± 193 | <0.05 |
B0, before first vaccination; B1, 4 weeks after second injection; B2, 2 weeks after third injection.
Values are mean antibody titers and standard deviations.
We noted a fluctuation in titers over time in the unvaccinated dams that may have been due to subclinical infections. The dams of herd Y had a lower titer at time point B0 than herd X (Table 1). The unvaccinated controls showed a mild but significant increase in titer (herd X, P = 0.03; herd Y, P = 0.04) between time points B0 and B1 and a decrease between B1 and B2.
A closer analysis of the titers of young and experienced dams revealed some interesting observations (Table 1). Young dams had a 3.5-fold higher baseline titer (B0) than experienced dams (P < 0.0001). At B1, vaccinated young dams had the highest titer (6,820 EU) measured during the entire study. Only low concentrations of PCV2 DNA (<5 × 105 copies/ml) were detected by PCR in some sera (data not shown).
Offspring anti-PCV2 IgG and IgM antibody titers.
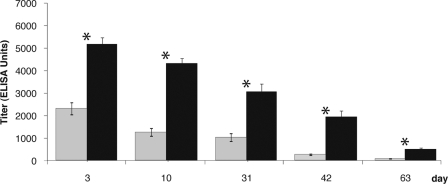
For experiments determining the anti-PCV2 IgG and IgM antibody titers of offspring, sera from 101 randomly selected offspring of vaccinated and unvaccinated dams were collected in herd Y. At all times, the piglets from vaccinated dams had significantly higher PCV2 antibody titers than piglets from unvaccinated dams (P < 0.001) (Table 2 and Figure 1). From day 3 postpartum (pp) to day 63 pp, the antibody titer in the vaccinated group decreased gradually from 5,173 EU to 511 EU. In the piglets from the unvaccinated dams, the antibody level decreased from 2,323 EU at day 3 pp to 86 EU at day 63 pp. The decrease was gradual until day 63. At no time point was a seroconversion noted, as previously described (12). At this stage, this is paralleled by the absence of any case of PMWS.
Table 2.
Antibody titers of piglets from vaccinated and unvaccinated dams of herd Y
| No. of days postpartum | Antibody titer (EU)a |
P value | |
|---|---|---|---|
| Vaccinated (n = 53) | Unvaccinated (n = 48) | ||
| 3 | 5,173 ± 322 | 2,323 ± 308 | <0.05 |
| 10 | 4,319 ± 248 | 1,266 ± 203 | <0.05 |
| 31 | 3,062 ± 359 | 1,037 ± 206 | <0.05 |
| 42 | 1,943 ± 296 | 279 ± 69 | <0.05 |
| 63 | 511 ± 81 | 86 ± 31 | <0.05 |
Values are mean antibody titers and standard deviations.
Fig. 1.
Piglet anti-PCV2 IgG antibody titers. Blood (2 to 5 ml) was collected from offspring of vaccinated (filled black bars) or unvaccinated (filled gray bars) dams at the age of 3, 10, 31, 42, and 63 days postpartum (pp). Anti-PCV2 IgG concentrations were determined by ELISA. At all times, the piglets from vaccinated dams had significantly higher anti-PCV2 antibody titers than piglets from unvaccinated dams (*, P < 0.001).
To detect potential subclinical infections, sera of all piglets from unvaccinated and vaccinated dams collected at days 10, 31, 42, 56, and 63 were examined for IgM antibody against PCV2. Four piglets from unvaccinated sows in herd Y were positive at day 56, indicative of subclinical infections.
Offspring production parameters.
During the fattening period, a total of 423 randomly selected pigs from 218 litters were monitored until slaughter (112 of herd X and 311 of herd Y). Offspring of vaccinated dams from both herds X and Y had significantly greater average daily weight gains from birth to slaughter (ADWG1s) than the controls of unvaccinated dams (Table 3). The difference in ADWG1 values between progeny from vaccinated and unvaccinated dams was 33 g/day in herd X and 20 g/day in herd Y. The difference in the average daily weight gains in the fattening period (ADWG2s) between offspring of vaccinated and unvaccinated dams was 51 g/day in farm X and 30 g/day in farm Y. The age at slaughter of pigs from vaccinated dams was reduced by 6.7 days in herd X (P = 0.03) and by 5.5 days in herd Y (P = 0.02). Therefore, the increased antibody titers in dams were transmitted by colostrum and were associated with a possible impact on progeny health status, as reflected by increased weight gain in the fattening period.
Table 3.
Production parameters of offspring from vaccinated and unvaccinated dams at farm X and farm Y
| Parametera | Value for the parameter by group |
|||||
|---|---|---|---|---|---|---|
| Farm X progeny (n = 112) |
Farm Y progeny (n = 311) |
|||||
| Vaccinated (n = 57) | Unvaccinated (n = 55) | P value | Vaccinated (n = 182) | Unvaccinated (n = 119) | P value | |
| ADWG1 (g/day) | 626 ± 0.01 | 593 ± 0.01 | <0.05 | 585 ± 0.01 | 565 ± 0.01 | <0.05 |
| ADWG2 (g/day) | 785 ± 0.02 | 734 ± 0.02 | <0.05 | 726 ± 0.01 | 696 ± 0.01 | <0.05 |
| Age at slaughter (days) | 170 ± 1.9 | 176 ± 2.2 | <0.05 | 183 ± 1.8 | 189 ± 1.4 | <0.05 |
ADWG1, average daily weight gain, calculated as live slaughter weight (kg) divided by age in days; ADWG2, average daily weight gain in the fattening period, calculated as live slaughter weight (kg) minus weight at the beginning of the finishing period (kg) divided by the number of finishing days.
DISCUSSION
Neither farm showed any reproductive disorders due to PCV2. The perinatal loss of some 14% was not affected by vaccination, and this value is in the range of small to mid-sized pig farms surveyed by the Swiss Swine Health Organization (50). Therefore, the existing natural immune responses of the dams against PCV2 enhanced by vaccination appeared sufficient to prevent fatal intrauterine infection. This is not surprising as PCV2-caused reproductive disorders are infrequent (16). Furthermore, all collected sera from adult animals showed low concentrations of PCV2 DNA (<5 × 105 copies/ml). Also, with the first litter important for this study, we did not find any indication of PCV2-associated diseases in 379 analyzed dead pigs. In contrast, the historical loss of 5 to 10% of pigs after weaning and in the early fattening period compared to data points of interest had improved 6- to 12-fold. Only 2 of the 423 evaluated fattening pigs died of PMWS during the experiment in the early finishing period. Interestingly, the two pigs in question were born to unvaccinated dams. Therefore, we suggest that the study be conducted in a postepizootic period of the PCV2 disease cycle.
As expected due to previous epidemiological and serological examinations, all dams had antibodies from natural exposure to PCV2 before vaccination (23, 26). In contrast to other investigators (34, 35), we opted to vaccinate twice before and once during pregnancy to compensate for a potential loss of serum antibodies into the colostrum (7) and to improve reproduction parameters. Antibody titers were boosted 3- to 9-fold after two vaccinations, and these values either leveled off or decreased in spite of the third immunization. Decreased antibody titers were also observed in unvaccinated dams. This is an independent indication that the serum antibody decline may be attributed to colostrum antibody supplementation that could not be overcome by additional vaccination.
Right after birth, PCV2-specific antibody titers in sera from piglets of vaccinated dams were very similar to those of their mothers, as previously described (7). The antibody titers in these piglets decreased gradually about 10-fold within 60 days and was 2.5 to 6 times higher than that of the controls at the same point of time. Antibody titers against PCV2 in neonates of unvaccinated dams reflected those of their mothers in mid-gestation (7), but the decrease in the mean titers in these animals until day 63 was 30-fold. The steeper proportional antibody loss in the control piglets might be because their anti-PCV2 antibodies are less mature than the anti-PCV2 antibodies elicited by vaccination.
It is very difficult to induce a protective immune response in mammals right after birth. Therefore, piglets are vaccinated against PCV2 at the weaning stage. In the first weeks of life, piglets are protected by maternal antibodies that may vary depending on the immune status of the dam. Thus, piglets will be exposed to the PCV2 viral pressure present in the herd, with potentially devastating effects. The vaccination of dams appeared to decrease the infective pressure generally and provided increased antibodies against PCV2 in colostrum to protect piglets within the first days of life. At this age colostral antibody is the only specific immune mediator available to the piglets until they can generate their own. We calculated by linear regression of anti-PCV2 IgG antibody concentrations that progeny antibody titers from vaccinated dams at day 49 pp were about the same as the titers of the controls at day 14 pp. Interestingly enough, we found that 4 of 48 pigs from the unvaccinated group contained IgM antibodies against PCV2 at the age of 56 days pp. Based on the IgM antibodies observed, we concluded either that these four piglets were newly PCV2 infected, probably around day 49 pp, or that the antibodies of this isotype were not detected in previous testing (data not shown). Nevertheless, we could not detect PCV2 DNA in blood of these piglets (data not shown). The protective antibody titers at day 14 pp compare to values from the literature as PMWS is not found before 3 weeks of age.
The general health status improvement of pigs from vaccinated dams was further observed in the significantly high average daily weight gain and decreased time to slaughter compared to offspring from unvaccinated dams. It is tempting to speculate that the improved immune status of vaccinated dams and the increased colostral antibodies taken up by their offspring decreased the overall infectious pressure and enhanced overall viability of the progeny. Yet a direct comparison of results in an industrial setting is difficult. Vaccination of dams as done here, rather than on 2- to 3-week-old piglets (10, 12, 22), might prove advantageous. The perinatal virus load of gilts or piglets may have been underestimated at least in some herds (15, 45). PCV2 can infect a variety of cells and preferentially replicates in dividing cells (49). Dividing cells are very important to the process of reaching adult stage cell numbers; they are crucial for the development of the architecture of organs of the immune system, the gastrointestinal tract, and the central nervous system. Failure or delayed maturation of the immune system may favor secondary infections or growth retardation due to inadequate food intake or inadequate hormonal regulation of body growth (6). The weight gain of young animals may thus be considered a mirror reflecting the combined effect of a virus on rapidly dividing cells necessary for individual development.
This is the first evidence of effective vaccination in a PCV2 subclinical infection as determined by daily weight increase of pigs during the fattening period. Dam vaccination was associated with offspring health status improvement at the weaning stage, which became obvious in the fattening period in PMWS postepizootic farms. Offspring of vaccinated dams outperformed pigs from unvaccinated dams in terms of weight gain and decreased maturity time for slaughter in the fattening period. Even in the absence of overt PCV2-associated diseases, small amounts of PCV2 might interfere with the maturation of individuals, possibly by interfering with rapidly diving cells. We associated these economic benefits with low PCV2 viral pressure due to vaccination of dams and their transfer of colostral antibodies to progeny in particular.
ACKNOWLEDGMENTS
We thank all those who contributed to this study. We are truly indebted to the farmers who provided the pig herds for this study. We thank Biokema SA (Crissier-Lausanne, Switzerland) for the vaccine and Synbiotics Corporation Europe SAS (Lyon, France) for their support and the serological tests. We are especially grateful to Roseline Weilenmann for her dutiful laboratory work and to Irene Schweizer for the preparation of the tables and the figure.
Footnotes
Published ahead of print on 18 August 2011.
REFERENCES
- 1. Allan G. M., Ellis J. A. 2000. Porcine circoviruses: a review. J. Vet. Diagn. Invest. 12:3–14 [DOI] [PubMed] [Google Scholar]
- 2. Allan G. M., et al. 1998. Novel porcine circoviruses from pigs with wasting disease syndromes. Vet. Rec. 142:467–468 [PubMed] [Google Scholar]
- 3. Allan G. M., et al. 1998. Isolation of porcine circovirus-like viruses from pigs with a wasting disease in the U.S.A. and Europe. J. Vet. Diagn. Invest. 10:3–10 [DOI] [PubMed] [Google Scholar]
- 4. Allan G. M., et al. 1995. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet. Microbiol. 44:49–64 [DOI] [PubMed] [Google Scholar]
- 5. Borel N., et al. 2001. Three cases of postweaning multisystemic wasting syndrome (PMWS) due to porcine circovirus type 2 (PCV 2) in Switzerland. Schweiz. Arch. Tierheilkd. 143:249–255[In German.] [PubMed] [Google Scholar]
- 6. Buonomo F. C., Bailer C. A. 1991. Influence of nutritional deprivation on insulin-like growth factor I, somatotropin, and metabolic hormones in swine. J. Anim. Sci. 69:755–760 [DOI] [PubMed] [Google Scholar]
- 7. Butler J. E., Zhao Y., Sinkora M., Wertz N., Kacskovics I. 2009. Immunoglobulins, antibody repertoire and B cell development. Dev. Comp. Immunol. 33:321–333 [DOI] [PubMed] [Google Scholar]
- 8. Chae C. 2005. A review of porcine circovirus 2-associated syndromes and diseases. Vet. J. 169:326–336 [DOI] [PubMed] [Google Scholar]
- 9. Choi C., Chae C., Clark E. 2000. Porcine postweaning multisystemic wasting syndrome in Korean pig: detection of porcine circovirus 2 infection by immunohistochemistry and polymerase chain reaction. J. Vet. Diagn. Invest. 12:151–153 [DOI] [PubMed] [Google Scholar]
- 10. Desrosiers R., Clark E., Tremblay D., Polson D. 2007. Results obtained with a novel PCV2-vaccine to protect multiple ages of pigs against PCVD, p. 121 In Proceedings of the 5th International Symposium on Emerging and Re-emerging Pig Diseases, Krakow, Poland, 24 to 27 June 2007 [Google Scholar]
- 11. Ellis J., et al. 1998. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 39:44–51 [PMC free article] [PubMed] [Google Scholar]
- 12. Fachinger V., Bischoff R., Judicial S. B., Saalmuller A., Elbers K. 2008. The effect of vaccination against porcine circovirus type 2 in pigs suffering from porcine respiratory disease complex. Vaccine 26:1488–1499 [DOI] [PubMed] [Google Scholar]
- 13. Fort M., et al. 2009. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 27:4031–4037 [DOI] [PubMed] [Google Scholar]
- 14. Guillossou S., Lebon E., Mieli L., Bonnard M., Thomsen C. 2008. Development of a quantification method to specific anti-ORF2 antibody using a blocking ELISA, vol. 2, p. 402 In Proceedings of the 20th Int. Pig Vet. Soc. Congress, 22 to 26 June 2008 Durban, South Africa [Google Scholar]
- 15. Hansen M. S., et al. 2010. Selection of method is crucial for the diagnosis of porcine circovirus type 2 associated reproductive failures. Vet. Microbiol. 144:203–209 [DOI] [PubMed] [Google Scholar]
- 16. Harding J. 2004. The clinical expression and emergence of porcine circovirus 2. Vet. Microbiol. 98:131–135 [DOI] [PubMed] [Google Scholar]
- 17. Harding J., Clark E., Ellis J. 1999. The clinical expression of porcine circovirus, p. 252–254In Proceedings of the Allen D. Leman Swine Conference, St. Paul, MN [Google Scholar]
- 18. Harding J., Clark E. 1997. Recognizing and diagnosis postweaning multisystemic wasting syndrome. Swine Health Prod. 5:201–203 [Google Scholar]
- 19. Hinrichs U. V. F., Pesch S., Wang L. 1999. Erster Nachweis einer Infektion mit dem porzinen Circovirus Typ 2 in Deutschland. Tierärztl. Umsch. 54:255–258 [Google Scholar]
- 20. Kim J., Jung K., Chae C. 2004. Prevalence of porcine circovirus type 2 in aborted fetuses and stillborn piglets. Vet. Rec. 155:489–492 [DOI] [PubMed] [Google Scholar]
- 21. Kiupel M., Stevenson G. W., Mittal S. K., Clark E. G., Haines D. M. 1998. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet. Pathol. 35:303–307 [DOI] [PubMed] [Google Scholar]
- 22. Kixmöller M., et al. 2008. Reduction of PMWS-associated clinical signs and co-infections by vaccination against PCV2. Vaccine 26:3443–3451 [DOI] [PubMed] [Google Scholar]
- 23. Larochelle R., Magar R., D'Allaire S. 2003. Comparative serologic and virologic study of commercial swine herds with and without postweaning multisystemic wasting syndrome. Can. J. Vet. Res. 67:114–120 [PMC free article] [PubMed] [Google Scholar]
- 24. LeCann P., Albina E., Madec F., Cariolet R., Jestin A. 1997. Piglet wasting disease. Vet. Rec. 141:660. [PubMed] [Google Scholar]
- 25. Reference deleted.
- 26. Lopez-Soria S., et al. 2005. An exploratory study on risk factors for postweaning multisystemic wasting syndrome (PMWS) in Spain. Prev. Vet. Med. 69:97–107 [DOI] [PubMed] [Google Scholar]
- 27. Madec F., Waddilove J. 2002. Control PCV2 or control other factors? Several approaches to a complex problem, p. 45–53In PMWS and PCV2 diseases: beyond the debate. Proceedings of the Merial Symposium, International Pig Veterinary Society Congress, Ames, IA [Google Scholar]
- 28. Madec F., et al. 2000. Post-weaning multisystemic wasting syndrome (PMWS) in pigs in France: clinical observations from follow-up studies on affected farms. Livestock Prod. Sci. 63:223–233 [Google Scholar]
- 29. Magar R., Muller P., Larochelle R. 2000. Retrospective serological survey of antibodies to porcine circovirus type 1 and type 2. Can. J. Vet. Res. 64:184–186 [PMC free article] [PubMed] [Google Scholar]
- 30. McIntosh K. A., Harding J., Parker S., Ellis J. A., Appleyard G. D. 2006. Nested polymerase chain reaction detection and duration of porcine circovirus type 2 in semen with sperm morphological analysis from naturally infected boars. J. Vet. Diagn. Invest. 18:380–384 [DOI] [PubMed] [Google Scholar]
- 31. McNeilly F., et al. 2001. Production, characterisation and applications of monoclonal antibodies to porcine circovirus 2. Arch. Virol. 146:909–922 [DOI] [PubMed] [Google Scholar]
- 32. Nielsen J., Ladekjaer-Mikkelsen A. S., Bille-Hansen V., Lohse L., Botner A. 2004. PCV2-associated disease following intrauterine infection, p. 14 In Proceedings of the 18th International Pig Veterinary Society Congress, Hamburg, Germany, 27 June to 1 July 2004 [Google Scholar]
- 33. O'Connor B., et al. 2001. Multiple porcine circovirus 2-associated abortions and reproductive failure in a multisite swine production unit. Can. Vet. J. 42:551–553 [PMC free article] [PubMed] [Google Scholar]
- 34. Opriessnig T., et al. 2010. Comparison of the effectiveness of passive (dam) versus active (piglet) immunization against porcine circovirus type 2 (PCV2) and impact of passively derived PCV2 vaccine-induced immunity on vaccination. Vet. Microbiol. 142:177–183 [DOI] [PubMed] [Google Scholar]
- 35. Opriessnig T., et al. 2008. Effect of porcine circovirus type 2 (PCV2) vaccination on porcine reproductive and respiratory syndrome virus (PRRSV) and PCV2 coinfection. Vet. Microbiol. 131:103–114 [DOI] [PubMed] [Google Scholar]
- 36. Opriessnig T., Yu S., Thacker E., Halbur P. G. 2004. Derivation of porcine circovirus type 2-negative pigs from positive breeding herds. J. Swine Health Prod. 12:186–191 [Google Scholar]
- 37. Pallares F. J., et al. 2002. Porcine circovirus type 2 (PCV-2) coinfections in US field cases of postweaning multisystemic wasting syndrome (PMWS). J. Vet. Diagn. Invest. 14:515–519 [DOI] [PubMed] [Google Scholar]
- 38. Park J. S., et al. 2005. Birth abnormalities in pregnant sows infected intranasally with porcine circovirus 2. J. Comp. Pathol. 132:139–144 [DOI] [PubMed] [Google Scholar]
- 39. Rosell C., et al. 1999. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 120:59–78 [DOI] [PubMed] [Google Scholar]
- 40. Sanchez R. E., Jr., Nauwynck H. J., McNeilly F., Allan G. M., Pensaert M. B. 2001. Porcine circovirus 2 infection in swine foetuses inoculated at different stages of gestation. Vet. Microbiol. 83:169–176 [DOI] [PubMed] [Google Scholar]
- 41. Schmoll F., Lang C., Steinrigl A. S., Schulze K., Kauffold J. 2008. Prevalence of PCV2 in Austrian and German boars and semen used for artificial insemination. Theriogenology 69:814–821 [DOI] [PubMed] [Google Scholar]
- 42. Seeliger F. A., et al. 2007. Porcine circovirus type 2-associated cerebellar vasculitis in postweaning multisystemic wasting syndrome (PMWS)-affected pigs. Vet. Pathol. 44:621–634 [DOI] [PubMed] [Google Scholar]
- 43. Segales J., Domingo M. 2002. Postweaning multisystemic wasting syndrome (PMWS) in pigs. Rev. Vet. Q. 24:109–124 [DOI] [PubMed] [Google Scholar]
- 44. Segales J., et al. 1997. First report of post-weaning multisystemic wasting syndrome in pigs in Spain. Vet. Rec. 141:600–601 [PubMed] [Google Scholar]
- 45. Shen H., Wang C., Madson D. M., Opriessnig T. 2010. High prevalence of porcine circovirus viremia in newborn piglets in five clinically normal swine breeding herds in North America. Prev. Vet. Med. 97:228–236 [DOI] [PubMed] [Google Scholar]
- 46. Sorden S. D. 2000. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 8:133–136 [Google Scholar]
- 47. Staebler S., et al. 2005. PMWS: an emerging disease identified in archived porcine tissues. Vet. J. 170:132–134 [DOI] [PubMed] [Google Scholar]
- 48. Staebler S., et al. 2004. Porcine circovirus as a possible cause of postweaning wasting in pigs in Switzerland. Schweiz. Arch. Tierheilkd. 146:461–468 [DOI] [PubMed] [Google Scholar]
- 49. Steiner E., Balmelli C., Herrmann B., Summerfield A., McCullough K. 2008. Porcine circovirus type 2 displays pluripotency in cell targeting. Virology 378:311–322 [DOI] [PubMed] [Google Scholar]
- 50. Swiss Swine Health Organization 2011. Geschäftsbericht und Anhang Zahlen und Projekte 2010. Swiss Swine Health Organization, Sempach, Switzerland: http://www.suisag.ch/UberSUISAG/Geschaftsbericht/tabid/144/Default.aspx [Google Scholar]
- 51. Tischer I., Bode L., Peters D., Pociuli S., Germann B. 1995. Distribution of antibodies to porcine circovirus in swine populations of different breeding farms. Arch. Virol. 140:737–743 [DOI] [PubMed] [Google Scholar]
- 52. Welti S., Sydler T., Buergi E., Brugnera E., Sidler X. 2010. PDNS was not a homogenous entity in the Swiss PMWS epizooty from 2003-2006, p. 455 In Proceedings of the 21st International Pig Veterinary Society Congress, Vancouver, British Columbia, Canada, 18 to 21 July 2010 [Google Scholar]
- 53. West K. H., et al. 1999. Myocarditis and abortion associated with intrauterine infection of sows with porcine circovirus 2. J. Vet. Diagn. Invest. 11:530–532 [DOI] [PubMed] [Google Scholar]
- 54. Wiederkehr D. D., et al. 2009. A new emerging genotype subgroup within PCV-2b dominates the PMWS epizooty in Switzerland. Vet. Microbiol. 136:27–35 [DOI] [PubMed] [Google Scholar]
- 55. Yu S., et al. 2007. Porcine circovirus type 2 (PCV2) distribution and replication in tissues and immune cells in early infected pigs. Vet. Immunol. Immunopathol. 115:261–272 [DOI] [PubMed] [Google Scholar]