Abstract
Several endemic mycoses cause cross-reactions in the Histoplasma antigen enzyme immunoassay. Herein, a positive Histoplasma antigen result has been recognized in a patient with sporotrichosis.
TEXT
Sporotrichosis is considered a “global health problem” in immunocompromised patients (7). Although Sporothrix schenckii is the main human pathogen, genetic sequencing indicates that the S. schenckii complex is comprised of several species: S. albicans, S. brasiliensis, S. globosa, S. luriei, S. mexicana, and S. schencki (7, 8).
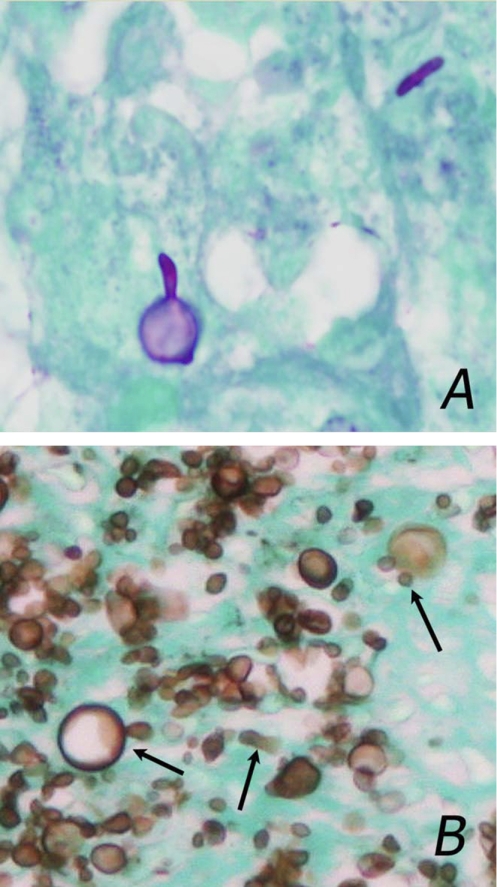
The diagnosis of sporotrichosis can be suspected by demonstration of small yeast cells (3 to 5 μm) with multiple buds, some of which are cigar-shaped or show large spherical bodies (Fig. 1A). While serological diagnosis by detection of antibodies to mycelial antigens has been described, cross-reactions occurred in about one-quarter of patients with histoplasmosis (1). Antigen detection has proven useful for diagnosis of other fungal infections but has not been reported in sporotrichosis. Cross-reactivity in the MVista Histoplasma antigen enzyme immunoassay (EIA) occurs in 70 to 90% of patients with blastomycosis, paracoccidioidomycosis, and penicilliosis marneffei (2) and 59% of patients with coccidioidomycosis (5). Herein, cross-reactivity is reported in a patient with sporotrichosis.
Fig. 1.
(A) Photomicrograph of tissue using periodic acid-Schiff stain with a light green counterstain, showing typical features, including cigar-shaped cells and large spherical bodies. (Shown is a representative image that was not from this case.) (B) Photomicrograph of a skin biopsy specimen from the patient using Gomori methenamine silver stain, showing large numbers of small yeast cells, some demonstrating multiple buds, the cigar shape, and spherical bodies. Arrows point to cells showing these features.
A 34-year-old African-American male from Amarillo, Texas, with untreated AIDS was admitted with a fluctuating of level of consciousness. He had complained of dysphagia, but additional information, including epidemiological history, was not obtainable because of impaired mental status. Examination showed extensive ulcers and nodules on the oral mucosa, face, hands, and chest and impaired mental status. The CD4 count was 11 cells/ml, and the HIV viral load was 110,911 copies/ml. Tests for anti-Blastomyces and anti-Coccidioides antibodies and cryptococcal antigenemia were negative, but Histoplasma antigenuria was detected at 1.7 U (reference range, below 1.0 U). Skin biopsy showed yeast cells, some with multiple buds and spherical bodies (Fig. 1B), and Sporothrix was isolated by culture from the skin and blood. Identification was based upon using lactophenol cotton blue staining of colonies showing narrow hyphae with slender tapering conidiophores at right angles to the hyphae and tear-shaped conidia arranged in rosette-like clusters at the apex of the conidiophores, as well as conversion of the mold to yeast with typical cigar bodies at 37°C. The patient was treated with amphotericin B based upon the skin biopsy findings, but developed multiorgan failure and died after 6 days of treatment. Repeat blood cultures were negative after 24 h of antifungal therapy.
The antigen detected in specimens from patients with histoplasmosis in the MVista Histoplasma antigen EIA is a galactomannan that has (1→6)-α-d-galactofuranosyl side chains (2), which contain the epitopes detected in the antigen assay. A galactomannan with (1→5)-β-d-galactofuranosyl side chains has been described in S. schenckii, but its immunoreactivity was not reported (9). The epitope detected in the Platelia Aspergillus EIA is also (1→5)-β-galactofuranose (6), and low-level cross-reactivity in the Histoplasma antigen EIA has been reported in 8% of patients with aspergillosis (4). Aspergillus antigen testing was not performed for this patient. Four isolates of S. schenckii were incubated at 37°C for 7 days in Histoplasma macrophage medium (12), and culture supernatants were tested in the Platelia Aspergillus EIA and the Histoplasma antigen EIA. Aspergillus galactomannan was detected in the culture supernatants from all four isolates, and Histoplasma antigen was detected in one (Table 1), suggesting weak cross-reactivity, as observed with Aspergillus galactomannan (11).
Table 1.
Aspergillus galactomannan and Histoplasma antigen results in culture supernatants from three isolates of S. schenckii grown in Histoplasma macrophage medium
| Specimen | Result by: |
|
|---|---|---|
| Platelia Aspergillus EIA (index units)a | MVista Histoplasma antigen EIA (U)b | |
| Isolate 1 | ||
| Undiluted | 6.6 | Negative |
| 1:10 dilution | 3.0 | Negative |
| Isolate 2 | ||
| Undiluted | 6.5 | Negative |
| 1:10 dilution | 3.2 | Negative |
| Isolate 3 | ||
| Undiluted | 5.2 | Negative |
| 1:10 dilution | 1.4 | Negative |
| Isolate 4 | ||
| Undiluted | 11.2 | 4.1 |
| 1:10 dilution | 2.0 | 1.2 |
| Positive control | 4.9 | 36.8 |
| Histoplasma macrophage medium | 0.1 | Negative |
Results of 0.5 U or higher are positive.
Results of 1.0 U or higher are positive.
False-positive Histoplasma antigen results in patients without fungal infections and cross-reactions in aspergillosis are usually at low concentrations: 1 to 4 U in the second generation (10) and 2 ng/ml or less in the quantitative assay (2). In this case, the fungal burden was high, considering the extensive mucocutaneous involvement, large number of yeast cells in the skin lesions, fungemia, multiorgan failure, and rapid death. In patients with severe histoplasmosis, the antigen concentration is low in only 5% of cases (3). Thus, a low-positive Histoplasma antigen result, as in this case, in a severely ill patient suggests an etiology other than histoplasmosis. Raising the cutoff would reduce false-positive results and cross-reactions caused by fungi with weakly related epitopes, but at the cost of lower sensitivity for diagnosis of histoplasmosis: low-positive results occur in 15% of disseminated histoplasmosis (3) and should not be disregarded. Instead, false-positive results and cross-reactivity caused by fungi with weakly cross-reactive antigens, including Sporothrix spp., should be suspected in severely ill patients with low-positive results. The magnitude of cross-reactivity in the Histoplasma antigen assay and the occurrence of cross-reactivity in the Platelia Aspergillus EIA in specimens from patients with sporotrichosis must be determined by testing a larger number of cases.
Acknowledgments
We thank Nancy Kountz and Thomas R. Kluzak, Via Christi Hospitals and University of Kansas School of Medicine, and Stephen D. Allen, Indiana University School of Medicine, for providing photomicrographs and Melinda Smedema and Samantha Swartzentruber, MiraVista Diagnostics, for testing culture supernatants of Sporothrix spp. with the Platelia Aspergillus EIA and Histoplasma antigen EIA.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Almeida-Paes R., et al. 2007. Use of mycelial-phase Sporothrix schenckii exoantigens in an enzyme-linked immunosorbent assay for diagnosis of sporotrichosis by antibody detection. Clin. Vaccine Immunol. 14:244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Connolly P. A., Durkin M. M., LeMonte A. M., Hackett E. J., Wheat L. J. 2007. Detection of histoplasma antigen by a quantitative enzyme immunoassay. Clin. Vaccine Immunol. 14:1587–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hage C. A., et al. A multicenter evaluation of tests for diagnosis of histoplasmosis. Clin. Infect. Dis., in press [DOI] [PubMed] [Google Scholar]
- 4. Hage C. A., et al. 2010. Diagnosis of histoplasmosis by antigen detection in BAL fluid. Chest 137:623–628 [DOI] [PubMed] [Google Scholar]
- 5. Kuberski T., et al. 2007. Diagnosis of coccidioidomycosis by antigen detection using cross-reaction with a Histoplasma antigen. Clin. Infect. Dis. 44:e50–e54 [DOI] [PubMed] [Google Scholar]
- 6. Latge J. P., et al. 1994. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect. Immun. 62:5424–5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lopez-Romero E., et al. 2011. Sporothrix schenckii complex and sporotrichosis, an emerging health problem. Future Microbiol. 6:85–102 [DOI] [PubMed] [Google Scholar]
- 8. Marimon R., et al. 2007. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 45:3198–3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mendonca-Previato L., Gorin P. A., Travassos L. R. 1980. Galactose-containing polysaccharides from the human pathogens Sporothrix schenckii and Ceratocystis stenoceras. Infect. Immun. 29:934–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wheat L. J., Witt J., III, Durkin M., Connolly P. 2007. Reduction in false antigenemia in the second generation Histoplasma antigen assay. Med. Mycol. 45:169–171 [DOI] [PubMed] [Google Scholar]
- 11. Wheat L. J., et al. 2007. Histoplasmosis-associated cross-reactivity in the BioRad Platelia Aspergillus enzyme immunoassay. Clin. Vaccine Immunol. 14:638–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Worsham P. L., Goldman W. E. 1988. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J. Med. Vet. Mycol. 26:137–143 [PubMed] [Google Scholar]