Abstract
Mycoplasma genitalium-reactive cervicovaginal IgA and IgG antibodies were detected in 51.9% and 70.4% of 27 infected women and 22.2% and 18.5% of 27 uninfected controls, respectively. The predominance of MgpB- and MgpC-reactive antibodies at the site of infection is consistent with their hypothesized role in selecting antigenic variants during persistent infection.
TEXT
Mycoplasma genitalium is a sexually transmitted pathogen associated with urethritis in men and various inflammatory conditions in women, including cervicitis (reviewed in references 10 and 20). Additionally, M. genitalium is associated with chronic infection, as highlighted by retrospective longitudinal studies of urethritis (7) and cervicitis (3). Such persistence may aggravate the risk of adverse reproductive outcomes, including tubal-factor infertility and preterm birth, conditions associated with this organism (2, 6, 18). Circulating antibodies have been detected in the serum of men with M. genitalium-associated urethritis (19) and women with tubal-factor infertility (2, 18); however, the local antibody response in the lower genital tract remains uncharacterized. To better understand the immunopathogenesis of M. genitalium, we investigated the cervicovaginal antibody response at the initial site of infection.
To detect M. genitalium-reactive antibodies in the lower genital tract by immunoblotting, we used cervical and vaginal samples. These specimens were originally collected in our cross-sectional study of M. genitalium infection in women attending a sexually transmitted disease clinic (5). Infection status was determined using an M. genitalium-specific transcription-mediated amplification (TMA) assay (Gen-Probe, San Diego, CA) (22). In the protocol described below, samples from 27 M. genitalium-positive women were tested for reactivity to whole-cell M. genitalium G37 (ATCC 33530) by immunoblotting, with the next consecutive negative study participant serving as a control. All protocols were approved by the University of Washington Institutional Review Board.
We prepared G37 lysates from bacteria cultured in H broth (11), harvested by centrifugation, washed in phosphate-buffered saline (PBS), and boiled for 5 min in Novex Tris-glycine SDS sample buffer (Invitrogen, Carlsbad, CA) with 0.2 M dithiothreitol (DTT). The lysate (100 μg protein) was separated by electrophoresis using a single-well 7.5% SDS-polyacrylamide gel, and then proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Invitrogen). Membranes were incubated overnight (4°C) in blocking/diluent buffer (5% nonfat milk in PBS-0.1% Tween 20), cut into 5-mm strips, and reacted for 1 h with a 1:50 dilution of the cervical or vaginal specimen (swabs rehydrated in 2-sucrose-phosphate-based transport medium [c2SP]) (22). Membranes were washed (PBS-0.1% Tween 20) and incubated for another hour with peroxidase-conjugated goat anti-human IgG (whole molecule; Sigma-Aldrich, St. Louis, MO), IgA (alpha-chain; Sigma-Aldrich), or IgM (mu-chain; Sigma-Aldrich) diluted 1:10,000 or peroxidase-conjugated mouse anti-human IgG1, IgG2, IgG3, or IgG4 (Invitrogen) diluted 1:500. After another wash, membranes were developed with the Amersham ECL kit (GE Healthcare, Buckinghamshire, United Kingdom) and exposed to Kodak BioMax XAR film (Carestream Health, Rochester, NY); the appearance of bands after 20 min of exposure was interpreted as a positive result. To identify MgpB and MgpC, sera from rabbits immunized with His-tagged recombinant peptides made from fragments of either protein (S. L. Iverson-Cabral and P. A. Totten, unpublished data) were used with peroxidase-conjugated goat anti-rabbit IgG (whole molecule; Sigma-Aldrich), each diluted 1:10,000. Additionally, samples were reacted against lysates of mgpB and mgpC deletion mutants (1).
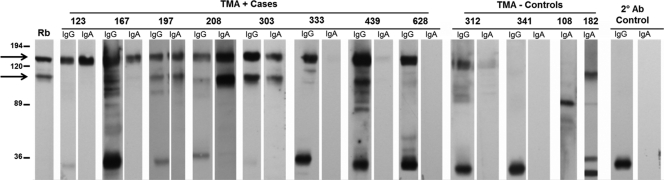
Infected women exhibited a cervicovaginal anti-M. genitalium response. M. genitalium-positive women were more likely to exhibit M. genitalium-reactive IgG and IgA than uninfected controls (P = <0.001 and 0.047, respectively) (Table 1). While we were unable to identify M. genitalium-reactive IgM in cases or controls (data not shown), the IgG and IgA responses were pronounced (Fig. 1). The concordance for these isotypes among cases was relatively high, with both IgG and IgA detected in 74% and 50% of immunoblot-positive cervical and vaginal samples, respectively. To characterize the IgG response further, we isotyped a subset of the strongly IgG-positive specimens for which adequate sample volume was available: 11 (57.9%) and 7 (36.8%) of the 19 cervical and 3 (37.5%) and 1 (12.5%) of the eight vaginal samples tested were positive for IgG1 and IgG3, respectively (Fig. 2). Beyond the nonspecific binding of the IgG2 secondary antibody (Fig. 2), there was no evidence of an IgG2 or IgG4 response.
Table 1.
Cervicovaginal immune response to M. genitalium among cases and controls
| Immunoglobulin isotype | Sample site | No. (%) with reactivity (n = 27) |
P valuec | |
|---|---|---|---|---|
| Casesa | Controlsb | |||
| IgG | Cervix | 19 (70.4) | 4 (14.8) | <0.001 |
| Vagina | 12 (44.4) | 3 (11.1) | 0.014 | |
| Either sited | 19 (70.4) | 5 (18.5) | <0.001 | |
| IgA | Cervix | 14 (51.9) | 6 (22.2) | 0.047 |
| Vagina | 9 (33.3) | 0 (0.0) | 0.002 | |
| Either sitee | 14 (51.9) | 6 (22.2) | 0.047 | |
Women with M. genitalium DNA detected in both urine and vagina by TMA.
Women who were TMA-negative for M. genitalium DNA in the urine and vagina.
P values calculated by Fisher's exact two-tailed test.
Among cases, 63% were IgG positive in the cervix and vagina and 37% in the cervix alone. Among controls, 50% were IgG positive in the cervix and vagina, 25% in the cervix alone, and 25% in the vagina alone.
Among cases, 64% were IgA positive in the cervix and vagina and 36% in the cervix alone. All of the controls were IgA positive in the cervix only.
Fig. 1.
Immunoblot analysis of M. genitalium cervical antibodies. Cervical results from representative cases and controls (study participant identification numbers listed above) demonstrating the range of reactivity observed against whole-cell M. genitalium G37 lysates. The locations of the immunodominant MgpB (140-kDa) and MgpC (110-kDa) proteins (arrows) were identified using a combination of sera from rabbits (designated “Rb”) immunized with His-tagged recombinant proteins spanning regions of MgpB (amino acids [aa] 1092 to 1209; GenBank accession number AAA25420) or MgpC (aa 36 to 308; GenBank accession number AAA25421). Membranes were exposed for 20 min; however, strongly IgG (participants 123, 197, 208, 303, and 629)- and IgA (participants 123 and 303)-reactive samples were exposed for 2 min to visualize individual bands. Secondary antibody controls in the absence of primary antibody indicated that an unknown M. genitalium protein (∼36 kDa) reacts nonspecifically with anti-human IgG.
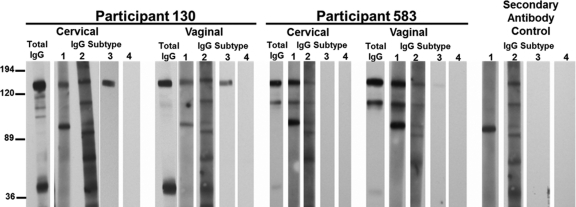
Fig. 2.
M. genitalium infection elicits IgG1 and IgG3 subtypes. A subset of strongly IgG-positive samples was characterized further using subtype-specific secondary antibodies. The results from two IgG-positive cases are shown. Cervical and vaginal samples for participant 130 have IgG1 and IgG3 antibodies against MgpB, while participant 583 has a cervical and vaginal IgG1 response against MgpB and MgpC. Results using secondary antibody in the absence of primary antibody are included as a control showing the nonspecific cross-reactivity of anti-human IgG1 and IgG2.
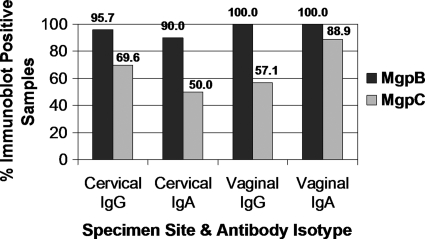
Although various M. genitalium proteins were antigenic (Fig. 1), antibodies against the 140-kDa MgpB and 110-kDa MgpC proteins were predominantly detected. These observations are consistent with previous immunologic studies of circulating antibodies in M. genitalium-infected individuals (2, 18, 19). Immunoblotting against lysates of mgpB and mgpC deletion mutants confirmed that these antigens were indeed MgpB and MgpC (Fig. 3). Among all immunoblot-positive women (including cases and controls), 92.3% (24/26) and 69.2% (18/26) had antibodies that recognized MgpB and MgpC, respectively; in fact, only two cervical samples from control women failed to react with MgpB and instead contained antibodies that targeted other, unknown M. genitalium antigens (participants 108 and 182) (Fig. 1). More detailed data regarding the antigenicity of MgpB and MgpC by sample site and antibody isotype are shown in Fig. 4.
Fig. 3.
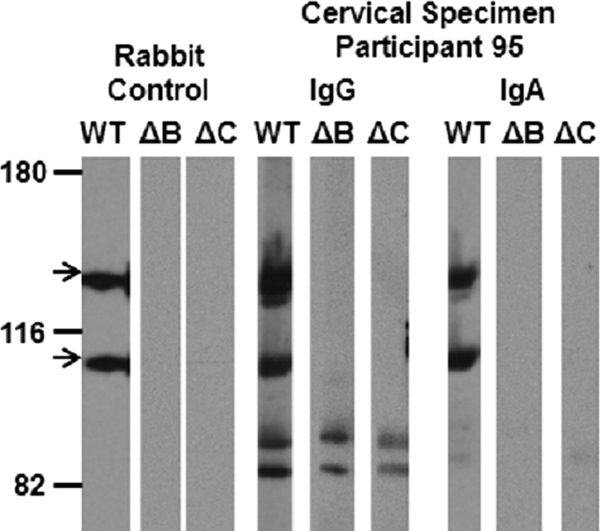
Confirmation that the dominant M. genitalium antigens are the MgpB and MgpC proteins. The reactivities of MgpB and MgpC (arrows) were confirmed among a subset of immunoblot-positive women by reacting specimens with lysates of wild-type G37 (WT) or mgpB (ΔB) and mgpC (ΔC) deletion mutants. Immunoblot-positive samples failed to react with either antigen in the mutant lysates, because the MgpB and MgpC proteins are reciprocally stabilized; the mgpB mutant has reduced levels of MgpC, and the mgpC mutant has reduced levels of MgpB (1). Representative results are shown above, with the reactivity of participant 95 compared with that of the rabbit anti-MgpB and anti-MgpC control.
Fig. 4.
Reactivity of immunoblot-positive specimens to the MgpB and MgpC proteins. The majority of immunoblot-positive samples contain antibodies that recognized MgpB and MgpC. All immunoblot-positive samples that demonstrated reactivity to M. genitalium G37 among cases and controls were used in this analysis, including 23 cervical and 14 vaginal IgG-positive samples and 20 cervical and 9 vaginal IgA-positive specimens. The percentage of samples with antibodies to MgpB (black) and MgpC (gray) is presented above. Among all immunoblot-positive specimens, only two fail to recognize MgpB (participants 108 and 182) (Fig. 1).
To our knowledge, this is the first documentation of the antibody response to M. genitalium in the lower genital tract. Our observations indicate that M. genitalium infection elicits a cervicovaginal IgA and IgG response, including IgG1 and IgG3 subtypes, with the MgpB and MgpC proteins immunogenic. Furthermore, the association between M. genitalium infection and the detection of IgG and IgA was significant. Differences in antibody response among infected women may reflect the site or duration of infection, previous exposure to this pathogen, or hormonal influences (12). The reactivity of some control specimens is likely due to prior M. genitalium infection or to nonspecific or cross-reactive antibodies, as likely observed with participants 108 and 182 (Fig. 2).
The biologic activity of M. genitalium-specific antibodies remains unknown. The isotypes identified in this study suggest that cervicovaginal antibodies may be involved in attachment-inhibition, complement-mediated killing, and/or opsonophagocytosis. IgG and IgA play an important role in mucosal defense and have different effector functions that work in concert to protect the host from infection. IgA serves as a first line of defense by preventing binding (17), interfering with motility, and enhancing opsonization through agglutination (21). The dominant isotype in cervicovaginal secretions is IgG (12, 14). Of the four isotypes, IgG1 and IgG3 are the most biologically active and proinflammatory through complement activation (15). These subtypes help control bacterial infections with opsonization and enhanced phagocytosis via the Fc receptor (4). In contrast, IgG4 lacks such effector functions (16), and IgG2 has low levels of complement activation (17), which is interesting given our negative results with these subtypes. We also failed to detect an IgM response, which may be explained by the low levels of IgM in genital secretions (14). Alternatively, these observations could be influenced by the timing of sample collection in the course of infection, as IgM is a short-lived isotype typically produced early in infection (17). Testing specimens collected closer to the onset of infection or selectively removing potentially interfering IgG from the sample may allow the detection of cervicovaginal IgM antibodies in future experiments.
Despite the induction of an antibody response at the initial site of infection, M. genitalium persists (3, 7), suggesting that this organism may have a mechanism(s) of immune evasion. It is noteworthy that the dominant antigens, MgpB and MgpC, are encoded by genes that exhibit extensive sequence diversity. Portions of mgpB and mgpC vary over time within M. genitalium G37 cultured in vitro and in samples collected longitudinally from individuals chronically infected with a single strain (8, 9, 13). We hypothesize that the evolution of sequence heterogeneity corresponds to changes in MgpB and MgpC antigenicity, allowing bacteria to escape the biologic activity of antibodies elicited by infection. Future studies are needed to characterize the biologic role of M. genitalium-specific antibodies and to correlate the development of antigenic diversity with the induction of these antibodies.
(These data were presented, in part, at the 108th General Meeting of the American Society for Microbiology, Boston, MA, 1 to 5 June 2008.)
Acknowledgments
This study was supported by grants R01 AI/4634, R56 A107115, and NIH U19 A131448 and the University of Washington STD/AIDS Research Training Grant NIH/HIAID T32 AI7140-33 (S.L.I.).
We thank Anna Wald, Gwen Wood, and Raul Burgos for critical reading of the manuscript, Laura DeMaster for assisting in preliminary experiments, and Jaume Piñol for providing us with the deletion mutant strains.
Footnotes
Published ahead of print on 3 August 2011.
REFERENCES
- 1. Burgos R., et al. 2006. Mycoplasma genitalium P140 and P110 cytadhesins are reciprocally stabilized and required for cell adhesion and terminal-organelle development. J. Bacteriol. 188:8627–8637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Clausen H. F., et al. 2001. Serological investigation of Mycoplasma genitalium in infertile women. Hum. Reprod. 16:1866–1874 [DOI] [PubMed] [Google Scholar]
- 3. Cohen C. R., et al. 2007. Mycoplasma genitalium infection and persistence in a cohort of female sex workers in Nairobi, Kenya. Sex. Transm. Dis. 34:274–279 [DOI] [PubMed] [Google Scholar]
- 4. Hamilton R. G. 1987. Human IgG subclass measurements in the clinical laboratory. Clin. Chem. 33:1707–1725 [PubMed] [Google Scholar]
- 5. Hancock E. B., et al. 2010. Comprehensive assessment of sociodemographic and behavioral risk factors for Mycoplasma genitalium infection in women. Sex. Transm. Dis. 37:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hitti J., et al. 2010. Correlates of cervical Mycoplasma genitalium and risk of preterm birth among Peruvian women. Sex. Transm. Dis. 37:81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horner P., Thomas B., Gilroy C. B., Egger M., Taylor-Robinson D. 2001. Role of Mycoplasma genitalium and Ureaplasma urealyticum in acute and chronic nongonococcal urethritis. Clin. Infect. Dis. 32:995–1003 [DOI] [PubMed] [Google Scholar]
- 8. Iverson-Cabral S. L., Astete S. G., Cohen C. R., Rocha E. P., Totten P. A. 2006. Intrastrain heterogeneity of the mgpB gene in Mycoplasma genitalium is extensive in vitro and in vivo and suggests that variation is generated via recombination with repetitive chromosomal sequences. Infect. Immun. 74:3715–3726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iverson-Cabral S. L., Astete S. G., Cohen C. R., Totten P. A. 2007. mgpB and mgpC sequence diversity in Mycoplasma genitalium is generated by segmental reciprocal recombination with repetitive chromosomal sequences. Mol. Microbiol. 66:55–73 [DOI] [PubMed] [Google Scholar]
- 10. Jensen J. S. 2004. Mycoplasma genitalium: the aetiological agent of urethritis and other sexually transmitted diseases. J. Eur. Acad. Dermatol. Venereol. 18:1–11 [DOI] [PubMed] [Google Scholar]
- 11. Kenny G. E. 1985. Mycoplasmas, p. 407–411In Lennette E. H., Balows A., Hausler W. J., Jr., Shadomy H. J. (ed.), Manual of clinical microbiology, 4th ed. American Society for Microbiology, Washington, DC [Google Scholar]
- 12. Kutteh W. H., Prince S. J., Hammond K. R., Kutteh C. C., Mestecky J. 1996. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin. Exp. Immunol. 104:538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ma L., et al. 2007. Mycoplasma genitalium: an efficient strategy to generate genetic variation from a minimal genome. Mol. Microbiol. 66:220–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mestecky J., Alexander R. C., Wei Q., Moldoveanu Z. 2011. Methods for evaluation of humoral immune responses in human genital tract secretions. Am. J. Reprod. Immunol. 65:361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mestecky J., Raska M., Novak J., Alexander R. C., Moldoveanu Z. 2010. Antibody-mediated protection and the mucosal immune system of the genital tract: relevance to vaccine design. J. Reprod. Immunol. 85:81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nirula A., Glaser S. M., Kalled S. L., Taylor F. R. 2011. What is IgG4? A review of the biology of a unique immunoglobulin subtype. Curr. Opin. Rheumatol. 23:119–124 [DOI] [PubMed] [Google Scholar]
- 17. Schroeder H. W., Jr., Cavacini L. 2010. Structure and function of immunoglobulins. J. Allergy Clin. Immunol. 125:S41–S52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Svenstrup H. F., et al. 2008. Mycoplasma genitalium, Chlamydia trachomatis, and tubal factor infertility—a prospective study. Fertil. Steril. 90:513–520 [DOI] [PubMed] [Google Scholar]
- 19. Svenstrup H. F., Jensen J. S., Gevaert K., Birkelund S., Christiansen G. 2006. Identification and characterization of immunogenic proteins of Mycoplasma genitalium. Clin. Vaccine Immunol. 13:913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Totten P., Taylor-Robinson D., Jensen J. S. 2008. Genital mycoplasmas, p. 709–736In Holmes P. S. K., Stamm W., Wasserheit J., Corey L., Cohen M., Watts H.(ed.), Sexually transmitted diseases, 4th ed. McGraw-Hill, New York, NY [Google Scholar]
- 21. van Egmond M., et al. 2001. IgA and the IgA Fc receptor. Trends Immunol. 22:205–211 [DOI] [PubMed] [Google Scholar]
- 22. Wroblewski J. K., Manhart L. E., Dickey K. A., Hudspeth M. K., Totten P. A. 2006. Comparison of transcription-mediated amplification and PCR assay results for various genital specimen types for detection of Mycoplasma genitalium. J. Clin. Microbiol. 44:3306–3312 [DOI] [PMC free article] [PubMed] [Google Scholar]