Abstract
Potassium homeostasis is crucial for living cells. In the yeast Saccharomyces cerevisiae, the uptake of potassium is driven by the electrochemical gradient generated by the Pma1 H+-ATPase, and this process represents a major consumer of the gradient. We considered that any mutation resulting in an alteration of the electrochemical gradient could give rise to anomalous sensitivity to any cationic drug independently of its toxicity mechanism. Here, we describe a genomewide screen for mutants that present altered tolerance to hygromycin B, spermine, and tetramethylammonium. Two hundred twenty-six mutant strains displayed altered tolerance to all three drugs (202 hypersensitive and 24 hypertolerant), and more than 50% presented a strong or moderate growth defect at a limiting potassium concentration (1 mM). Functional groups such as protein kinases and phosphatases, intracellular trafficking, transcription, or cell cycle and DNA processing were enriched. Essentially, our screen has identified a substantial number of genes that were not previously described to play a direct or indirect role in potassium homeostasis. A subset of 27 representative mutants were selected and subjected to diverse biochemical tests that, in some cases, allowed us to postulate the basis for the observed phenotypes.
INTRODUCTION
In yeast cells, the secondary active transport of inorganic ions and diverse nutrients relies on the existence of an electrochemical gradient of protons across the plasma membrane, which is generated by P2-type, energy-consuming H+-ATPases. In the model yeast Saccharomyces cerevisiae, there is an essential H+-ATPase gene, PMA1, which encodes one of the most abundant proteins in the yeast plasma membrane (45). The electrochemical gradient generated by Pma1 is balanced by the activity of a number of symporters and antiporters, and high-affinity potassium uptake is the principal consumer of the gradient (17, 25). High-affinity potassium uptake, which allows yeast cells to concentrate the cation even when it is present at very low concentrations (<50 μM), is mediated by the plasma membrane transporters Trk1 and Trk2. Several studies have established that the Trk1 transporter, which has a tetra-M1PM2 structure (19), is the most biologically relevant membrane transporter (14, 23, 38). Thus, a trk1 mutant (but not trk2 cells) is deficient for high-affinity potassium uptake and cannot grow when the external potassium concentration is limiting (1 to 2 mM). A trk1 trk2 double mutant strain shows an increased threshold for external potassium. These cells also exhibit low-affinity Rb+ uptake and a hyperpolarized plasma membrane (25). Potassium transport into yeast cells results in a depolarization of the plasma membrane, leading to the stimulation of Pma1 activity and a concomitant cytosolic alkalinization (40).
The regulation of the activities of both Pma1 and Trk1 is fundamental for the modulation of the electrical membrane potential, although the precise regulatory mechanisms involved remain largely unknown. Pma1 can be regulated at the transcriptional level by the transcription factors Rap1 and Grc1, which mediate the increase in PMA1 expression levels triggered by glucose metabolism (39), and by Mcm1, which connects the expression of the Pma1 proton pump with cell cycle regulatory pathways (24). Pma1 activity is regulated by the Ptk2 and Hrk1 protein kinases in response to glucose metabolism. Remarkably, a ptk2 mutant exhibits a pleiotropic phenotype of tolerance to toxic cations, including sodium, lithium, manganese, tetramethylammonium (TMA), hygromycin B, and norspermidine (7, 17). In contrast, the phosphorylation of Pma1 by the Yck1 and Yck2 kinases results in decreased proton pump activity (10).
The molecular mechanisms modulating Trk1 activity have remained largely elusive. Hal4 and Hal5, a pair of partially redundant protein kinases, were shown previously to activate K+ transport in a Trk-dependent manner (31), suggesting the direct regulation of Trk1 activity by phosphorylation. In addition, recent work has revealed that one role of these kinases is to stabilize Trk1 (and other permeases) at the plasma membrane under low-potassium conditions (34), thus underscoring the relevance of protein transport and recycling mechanisms in cation homeostasis. In this context, Arl1, a G protein of the Ras superfamily, was proposed to affect K+ uptake through the regulation of Hal4/Hal5 (32). On the other hand, it is known that Hal3, through the regulation of the protein phosphatase Ppz1, can activate Trk1-mediated potassium transport (6, 12, 51). Although no mechanistic details are available, it was shown previously that Ppz1 physically interacts with Trk1 and that the association between Hal3 and Ppz1 is responsive to changes in the intracellular pH (50). Calcineurin, a calcium-activated phosphatase, was reported previously to be necessary for the transition of Trk1 from the low- to the high-potassium-affinity state in response to sodium stress (30), although this phosphatase may also be required for the regulation of basal activity (3). Finally, the Sky1 protein kinase was reported to affect potassium uptake, although there is some controversy regarding whether or not this occurs in a Trk1,2-dependent manner (8, 9, 13).
The reported evidence suggests that the regulation of the best-characterized components involved in the maintenance of the electrochemical gradient could be a very complex issue, which is far from being solved. In this context, we wished to develop a novel approach to gain further insight into this process. We observed that a common feature of mutations leading to alterations in the electrochemical gradient was a phenotype of abnormal sensitivity to toxic cations. Thus, mutations affecting Pma1 or Trk function (i.e., trk1,2, sky1, hal4,5 ptk2, and ppz1) resulted in increased or decreased tolerance to these compounds (13, 17, 31, 32, 51). According to the concept developed previously by Goossens et al. (17), we considered that any mutant displaying altered tolerance concurrently to diverse toxic cations, differing in the specific uptake transport system and toxicity mechanism(s), would probably reflect a change in the electrochemical gradient and, hence, would identify a relevant component in this fundamental process. Therefore, we screened the S. cerevisiae haploid deletion library for mutants showing enhanced sensitivity/tolerance simultaneously to three toxic cations: hygromycin B, spermine, and TMA. Here, we report the results of this screen, which identified mutants lacking not only many of the expected genes but also a large number of genes encoding proteins previously unrelated to this process. We also present specific examples in which the molecular basis for these phenotypes is investigated.
MATERIALS AND METHODS
Escherichia coli and yeast growth conditions.
Escherichia coli DH5α cells were used as the plasmid DNA host and were grown at 37°C in Luria-Bertani (LB) broth. The LB medium was supplemented with 50 μg/ml ampicillin when plasmid selection was required. Bacterial and yeast cells were transformed by use of standard methods, and recombinant DNA techniques were performed as described elsewhere previously (43).
Unless stated otherwise, yeast cells were grown at 28°C in Translucent medium (catalog number CYN7505; Formedium, United Kingdom), prepared as described previously (33) and containing in each case the specified concentration of KCl. Translucent medium is a yeast nitrogen base (YNB)-based medium that has been reformulated to contain negligible amounts of potassium (usually <15 μM). Yeast extract-peptone-dextrose (YPD) medium contains 1% (wt/vol) yeast extract, 2% (wt/vol) peptone, and 2% (wt/vol) glucose. Arginine-phosphate medium was prepared as described previously (41). For the testing of the growth of yeast mutants under conditions of limiting potassium concentrations, cells were grown in Translucent medium supplemented with 50 mM KCl until saturation. The cells were then diluted to an optical density at 600 nm (OD600) of 0.004 in the same medium supplemented with 50 mM or 1 mM KCl and incubated at 28°C. The OD600 was measured after 16 h of incubation. In all experiments, control samples of the wild-type strain grown in medium supplemented with both 50 mM and 1 mM KCl were analyzed in parallel with the mutants, and the growth percentage values were determined relative to the growth of each mutant in 50 mM KCl. The value for wild-type cells was around 70% for the BY4741 genetic background.
Screen of the systematic deletion library for changes in tolerance to hygromycin B, spermine, and TMA.
The systematic KanMX deletion library constructed in the BY4741 background was grown to saturation in YPD liquid medium supplemented with G418 (150 μg/ml) for 3 to 4 days. The cultures were replicated by using a stainless steel 96-pin replicator (Nalge Nunc International) at a density of 384 clones/plate on Onmigrid plates (Nunc) containing YPD agar or YPD agar supplemented with hygromycin B (40 and 60 μg/ml), spermine (0.6 and 0.7 mM), or TMA (0.5 and 0.6 M). Growth was visually evaluated and recorded after 48 h. Clones that showed strong, weak, or no macroscopic growth in the supplemented media after 48 h were considered putative positive clones. In order to confirm their sensitivity, these clones were recovered from the original 96-well plates, diluted with YPD medium to an OD600 of 0.05, and evaluated for sensitivity by a drop test on YPD plates supplemented with different concentrations of each toxic cation (20, 30, 40, 50, and 60 μg/ml hygromycin B; 0.3, 0.4, 0.5, 0.6, and 0.7 mM spermine; and 0.2, 0.3, 0.4, 0.5, and 0.6 M TMA). The intensity of the phenotype was scored from 1 to 10 (most to least sensitive; for the wild-type strain the value was 6), on the basis of the lowest concentration of each toxic cation at which the strain showed no or marginal growth after 48 h.
Proton efflux.
Proton pumping by whole yeast cells was measured by recording the extracellular pH change after the addition of glucose, as previously described (44). Briefly, cells were grown to the mid-log phase (OD600 of 0.8) in YPD medium, washed three times with distilled water, and stored on ice for 1 to 3 h. After that, cells were pelleted by centrifugation and resuspended in 2 ml of glycyl-glycine solution (10 mM glycyl-glycine and 100 mM KCl [pH 4.5, adjusted with HCl]). Six milliliters of glycyl-glycine solution was mixed with 1.8 ml of cell suspension in a Falcon tube with constant agitation. A pH electrode was suspended in the cell mixture, and the pH was measured until a stable baseline was obtained. Glucose was then added to a final concentration of 20 mM. The pH was read every 10 s with a GLP21 pH meter (Crison) until a new steady state was reached, usually at 20 min. Acidification values were calculated from the linear segment of the curve.
Rubidium transport.
Rb+ was used as a K+ transport analog (41). The time course of Rb+ uptake was studied for cells grown at normal K+ levels and for K+-starved cells. Normal K+ cells were obtained by growing yeasts overnight in Translucent medium supplemented with 50 mM KCl. The cells were then suspended in the same fresh medium at an OD600 of 0.2. After 2 h of incubation 50 mM RbCl was added to the medium (time zero), and samples of cells were withdrawn at the indicated time points. The Rb+ content of cells was determined by collecting the cells onto Millipore filters, which were rapidly washed with 20 mM MgCl2. The cells were then extracted with acid and analyzed by atomic emission spectrophotometry (41). K+-starved cells were prepared by incubating cells grown as mentioned above in Translucent K+-free medium during 2 h. RbCl (0.5 mM) was then added, and samples of cells were taken and treated as mentioned above.
Measurement of relative plasma membrane potential.
The fluorescence assay of the plasma membrane potential was performed as described previously (29). All strains were grown overnight in Translucent medium supplemented with 50 mM KCl to the exponential growth phase. Cells were harvested and washed twice with 10 mM Na2HPO4 (pH 6.0, adjusted with citric acid) and resuspended in the same buffer to a final OD600 of 0.2. A potential-sensitive dye, diS-C3(3) (3,3′-dipropylthiacarbocyanide iodide) (0.1 mM stock solution in ethanol), was added to 3 ml of the cell suspension to a final probe concentration of 0.2 μM. Fluorescence emission spectra were measured by using an ISS PC1 spectrofluorometer. The excitation wavelength was 531 nm, and the emission intensities were measured at 560 and 580 nm. The staining curves (i.e., the dependence of the emission intensity ratio, I580/I560, on the duration of staining, t) were fitted as described previously (26), and the value of the intensity ratio at equilibrium was estimated.
Immunodetection of Pma1 and Trk1.
In order to estimate the relative amounts of Trk1 and Pma1 present in the plasma membrane, each mutant strain was transformed with a plasmid harboring a hemagglutinin (HA) epitope-tagged version of Trk1 described previously (50). Cells were grown to the mid-log phase (0.5 × 107 cells per ml) in selective minimal growth medium. Cells were collected by centrifugation, processed, and analyzed by immunoblotting to detect the epitope-tagged version of Trk1 and endogenous Pma1 as described previously (34).
Other techniques.
For the determination of ENA1 expression, wild-type and mutant strains were transformed with plasmid pKC201, which carries the ENA1 promoter fused to the lacZ gene. Cells were grown in YPD medium and processed for β-galactosidase activity measurements essentially as described previously (46). The distribution of Trk1 in cells grown to mid-log phase (0.5 × 107 cells per ml) in selective minimal growth medium was monitored by confocal microscopy, as described previously (34).
RESULTS
Genomewide mutant screen for changes in tolerance to the toxic cations hygromycin B, spermine, and TMA.
The haploid mutant library in the BY4741 background (∼4,900 clones) was analyzed for changes in tolerance to the toxic cations hygromycin B, spermine, and TMA in comparison to wild-type cells. The initial screen produced 1,174 strains with an altered hygromycin B tolerance (944 more sensitive and 230 hypertolerant), 707 mutants with an abnormal tolerance to spermine (338 sensitive and 369 more tolerant), and 1,935 strains with an anomalous tolerance to TMA (1,610 hypersensitive and 325 with increased tolerance). We concentrated on those mutants that exhibited either hypersensitivity or hypertolerance to all three compounds as possible candidates for an altered electrochemical gradient. The putative positive mutants were retested at 5 different concentrations of each toxic compound to confirm and score the phenotype intensity. To this end, a scale from 1 (most sensitive) to 10 (most tolerant) was defined, where the score for the wild-type strain was 6. At the end of the analysis a final list of 226 mutants with a triple phenotype (202 hypersensitive and 24 hypertolerant) was assembled (see Table S1 in the supplemental material). Because potassium influx is a major consumer of the electrochemical gradient (25), we considered the possibility that some mutations may exhibit growth problems at limiting potassium concentrations. Therefore, all 226 mutants were tested for growth in Translucent medium containing 1 mM K+ in comparison with the same medium containing 50 mM K+. Under these conditions the low/high-K+ growth ratio is about 70% for the wild-type strain (Table S1). In agreement with our hypothesis, 60 mutants showed substantial growth impairment at limiting potassium concentrations (1 mM/50 mM K+ growth ratio equal to or lower than 35%), while 63 strains displayed a moderate growth defect (growth ratio between 35% and 50%). Therefore, more than 50% of the mutants identified in the screen had altered potassium requirements.
To establish possible functional relationships between the genes whose mutations were responsible for the observed phenotypes, we performed a Gene Ontology analysis. As shown in Table 1, mutants in several cellular processes were enriched in our collection. These mutants included a substantial number of genes encoding protein kinases and protein phosphatases (both catalytic and regulatory subunits) and many genes involved in intracellular trafficking (Golgi compartment, endosome, and vacuolar transport), chromatin modification, and the regulation of transcription. Our screen identified most mutations in genes encoding proteins already known to affect the electrochemical gradient, such as the high-affinity potassium transporter Trk1; the protein kinase Ptk2, which has been proposed to activate the Pma1 proton ATPase; or the protein phosphatase Ppz1, which regulates alkaline cation efflux through the Ena1 ATPase and potassium influx through Trk1. Therefore, the approach appeared to be reliable, consistent, and likely to produce novel findings.
Table 1.
Functional groups enriched in our screen
| Category and function (P value)a | Genes |
|---|---|
| Protein fate (folding, modification, and destination) (9.9E−12) | |
| Modification by phosphorylation, dephosphorylation, and autophosphorylation (3.71E−6) | |
| Protein kinases | SAT4/HAL4, HAL5, PTK2, SKY1, HRK1, PHO85, RTK1 |
| Protein phosphatases | PTC1, SIT4, SAP185, SAP155, PPZ1, YVH1, REG1, RTS1 |
| Cellular transport, transport facilities, and transport routes (3.7E−09) | |
| Vesicular transport (Golgi network, etc.) (2.27E−16) | |
| TRAPP complex | KRE11, GSG1 |
| GARP complex | VPS52, VPS54, VPS51 |
| Membrane-associated retromer complex | VPS29, VPS35, PEP8 |
| CORVET and HOPS complexes | PEP3, PEP5, VPS16, VAM6, VPS41 |
| SNARE proteins | VAM7, SEC22, GOS1, TLG2, PEP12, VAM3 |
| Oligomeric Golgi complex | COG7, COG8, COG6, COG5, RUD3, SNC2 |
| Other proteins related to the Golgi network | VPS15, VPS34, ARL1, ARF1, SYS1, YPT6, GCS1, ERV14, LST7, VPS1 |
| Cell cycle and DNA processing (3.0E−07) | |
| DNA conformation modification (e.g., chromatin) (1.5E−05) | |
| ADA/SAGA/SLIK remodeling complex | GCN5, SPT7, HFI1, SPT20, SGF29, ADA2, NGG1, SGF11, UBP8, DST1 |
| SWR complex | ARP6, SWC5, SWC3, VPS71, HTZ1, VPS72 |
| RSC chromatin-remodeling complex | NPL6, HTL1, RSC1, RSC2, LDB7 |
| Transcription (3.4E−03) | |
| Transcriptional control (2.2E−03) | |
| RNA polymerase II mediator complex | SRB5, MED2, SOH1, MED1, CSE2 |
| Other RNA polymerase II-associated proteins | PAF1, RTF1, RPB9, HPR1, RPB4 |
| Transcription cofactors and related proteins | SWI6, THP1, TAF14, SWI3 |
| Phosphorylation of the RNA polymerase II C-terminal domain | CTK2, SSN3 |
The P value reflects the probability that the observed annotation of the particular GO term to a group of genes occurs by chance.
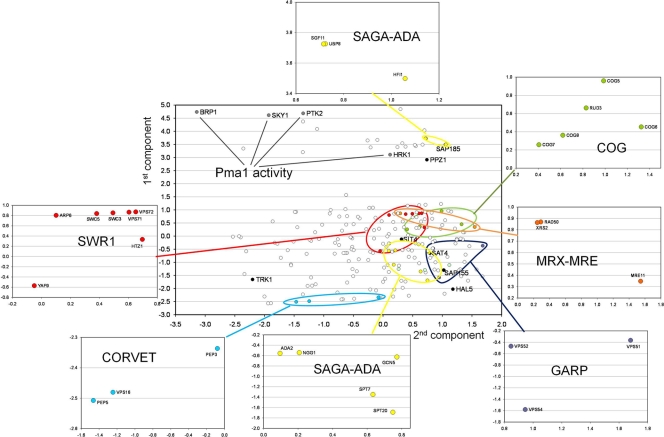
We then carried out a principal component analysis (PCA) in which the first component was a combination of the scores for tolerance to the three drugs and the second component was related to the capacity to grow at limiting potassium concentrations. The results are shown in Fig. 1. We observed that diverse gene mutations clustered together, which is indicative of their functional relationship. For instance, among the triple-sensitive mutants, we identified several members of the conserved oligomeric Golgi (COG) complex, a cytosolic tethering complex that functions to mediate the fusion of transport vesicles to Golgi compartments. Similarly, we recovered three members of the GARP (Golgi-associated retrograde protein) complex, which is required for the recycling of proteins from endosomes to the late Golgi compartment. We also observed a clustering of genes belonging to the SWR complex (ARP6, SWC5, SWC3, VPS71/SWC6, and VPS72/SWC2), required for the ATP-dependent exchange of nucleosomal histone H2A for the minor H2AZ variant, as well as the H2AZ gene itself (HTZ1). Interestingly, while all these mutants displayed diverse degrees of hypersensitivity to all three drugs, they exhibited little or no growth defect at limiting (1 mM) potassium concentrations. In contrast, mutants in members of the CORVET (class C core vacuole/endosome tethering) complex, such as PEP5, VPS16, and PEP3, were highly sensitive to the drugs and grew quite poorly when the potassium concentration was limiting. A remarkable situation was observed when components of the SAGA (Spt-Ada-Gcn5-acetyltransferase) acetylation complex were considered. This group includes not only the GCN5 histone acetyltransferase (the catalytic subunit of the ADA and SAGA complexes) but also diverse members of the complexes (ADA2, NGG1/ADA3, SPT7, and SPT20), whose mutations conferred a marked sensitivity phenotype to all three drugs and moderate growth defects at low potassium concentrations, as well as examples of hypertolerant mutants with no defect at limiting potassium concentrations, such as UBP8, SGF11, and HFI1/ADA1. It is worth noting that whereas the phenotypes of the ubp8 and sgf11 mutants appear to be consistent (Ubp8 is a ubiquitin-specific protease required for the SAGA-mediated deubiquitination of histone H2B, and Sgf11 is required for the Ubp8p association with SAGA), the phenotype of the hfi1/ada1 strain is surprising, since this mutant was reported previously to have phenotypes similar to those of spt20 and spt7 (49).
Fig. 1.
Principal component analysis (PCA) of the drug sensitivity and potassium requirements of the mutants identified in the screen. The positions of specific genes discussed in the main text are also shown in the main graph. Colors denote functional families whose members can be identified in the expansions.
To gain further insight into the molecular basis for the observed phenotypes identified in the screens, we separated the mutants into four different categories. We first separated the tolerant and sensitive mutants, and we then split the mutants in each category into groups based on their growth in medium with a low potassium concentration. Therefore, group A represents sensitive mutants with defects in growth in medium with a low potassium concentration. Group B mutants are sensitive to the three tested cations but grew nearly as well as the wild-type control in low-potassium medium. The tolerant mutants were similarly divided into group C (poor growth in medium with a low potassium concentration) and group D (no defect in medium with a low potassium concentration). We then chose representatives from each category for further analysis and confirmed their phenotypes by creating these mutations de novo in the BY4742 (MATα his3Δ leu2Δ lys2Δ ura3Δ) and, in some cases, in the DBY746 (MATα ura3-52 leu2-3 leu2-112 his3-Δ1 trp1-289) backgrounds. Among the 29 mutants selected from the deletion library, we found that the deletion of the RCK2 and RCY1 genes in the above-mentioned backgrounds did not reproduce the observed phenotypes, so they were not included in further analyses. Table 2 shows the mutants for which we investigated a number of parameters in order to begin to elucidate the molecular basis for these phenotypes. These parameters were related to potassium homeostasis (rubidium uptake and the presence of Trk1 in plasma membrane fractions), proton pumping (measured as the acidification of the medium and the presence of Pma1 in plasma membrane fractions), as well as ENA1 ATPase expression.
Table 2.
Relevant parameters for selected mutantsa
| Group and mutation | Sensitivity score |
% growth at 1 mM K+ | Acidification rate (% vs WT) | Protein score |
% Rb+ uptake |
Membrane potential (% vs WT) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| HYG | SPERM | TMA | Trk1 | Pma1 | 50 mM Rb+ | 0.5 mM Rb+ | ||||
| WT | 6 | 6 | 6 | 70.0 | 100.0 | 5 | 5 | 100.0 | 100.0 | 100.0 |
| A | ||||||||||
| TRK1 | 1 | 1 | 4 | 4.7 | 79.4 | 5 | 5 | ND | 0.0 | 118.4 |
| VAM7 | 3 | 4 | 5 | 2.6 | 95.3 | 4 | 5 | 96.4 | 98.0 | 133.8 |
| VAM3 | 4 | 4 | 4 | 4.5 | 95.5 | 4 | 4 | 107.1 | 103.9 | 136.3 |
| AFG3 | 1 | 3 | 3 | 3.0 | 80.8 | 4 | 4 | 135.7 | 7.8 | 139.0 |
| ERG6 | 1 | 1 | 1 | 7.0 | 99.9 | 4 | 4 | 39.3 | 47.1 | 208.3 |
| LST7 | 3 | 4 | 4 | 13.8 | 109.8 | 5 | 5 | 107.1 | ND | 122.5 |
| PEP5 | 1 | 1 | 1 | 15.0 | 111.7 | 1 | 4 | 92.9 | 100.0 | 223.4 |
| AKR1 | 1 | 2 | 1 | 17.6 | 111.8 | (*)b | (*) | 32.1 | 41.2 | 157.3 |
| REG1 | 1 | 1 | 3 | 30.9 | 95.1 | 7 | 4 | 78.6 | 82.4 | 92.2 |
| PIG1 | 5 | 4 | 5 | 33.7 | 120.8 | 7 | 4 | 53.6 | ND | 101.3 |
| VPS16 | 1 | 1 | 1 | 18.7 | 94.6 | 1 | 2 | 96.4 | 98.0 | 100.5 |
| VPS15 | 2 | 1 | 4 | 21.6 | 70.9 | 1 | 2 | 78.6 | 60.8 | 102.0 |
| B | ||||||||||
| RIC1 | 3 | 3 | 1 | 60.7 | 92.4 | 3 | 5 | 53.6 | 78.4 | 151.0 |
| SNC2 | 2 | 2 | 2 | 63.1 | 81.9 | 5 | 5 | 53.6 | ND | 103.2 |
| VID22 | 1 | 1 | 3 | 63.4 | 94.8 | 5 | 5 | 107.1 | 100.0 | 111.8 |
| NHX1 | 1 | 4 | 5 | 65.5 | 87.7 | 5 | 5 | 96.4 | ND | 122.0 |
| RTS1 | 3 | 2 | 3 | 66.1 | 114.0 | 5 | 5 | 164.3 | 102.0 | 107.4 |
| COG6 | 3 | 4 | 5 | 67.7 | 107.0 | 5 | 5 | 78.6 | ND | 145.0 |
| TLG2 | 1 | 2 | 2 | 69.4 | 105.6 | 4 | 5 | 96.4 | ND | 141.5 |
| VPS54 | 1 | 1 | 3 | 57.1 | 97.4 | 4 | 5 | 75.0 | ND | 156.9 |
| C | ||||||||||
| BRP1 | 9 | 10 | 10 | 2.2 | 79.4 | 5 | 1 | 25.0 | ND | 96.1 |
| SKY1 | 8 | 10 | 10 | 22.3 | 63.3 | 5 | 5 | 78.6 | 82.4 | 112.1 |
| PTK2 | 8 | 10 | 10 | 32.1 | 57.0 | 7 | 4 | 25.0 | ND | 108.5 |
| SUR2 | 8 | 8 | 8 | 47.7 | 101.4 | 5 | 4 | 75.0 | 72.5 | 76.3 |
| D | ||||||||||
| HRK1 | 7 | 8 | 7 | 53.0 | 95.4 | 5 | 3 | 78.6 | 98.0 | 107.3 |
| SGF11 | 8 | 9 | 7 | 64.1 | 95.5 | 5 | 5 | 53.6 | 82.4 | 100.5 |
| SAP185 | 7 | 9 | 7 | 69.4 | 92.4 | 5 | 5 | 39.3 | ND | 99.8 |
Sensitivity to hygromycin (HYG), spermine (SPERM), and TMA ranged from 1 (highly sensitive) to 10 (highly tolerant). The wild type (WT) received a score of 6. Growth at 1 mM K+ was calculated as the ratio of growth at 1 mM/growth at 50 mM KCl (the wild-type ratio was 70.0). The acidification rate for the wild-type strain was 0.362 ± 0.037 μmol/min · mg (wet weight). Rubidium uptake rates for the wild type were 2.8 ± 0.1 and 5.8 ± 0.2 nmol/min · mg (wet weight) at 50 mM and 0.5 mM rubidium, respectively. The membrane potential for the wild-type strain was 1.54 (I580/I560). The amounts of Trk1 and Pma1 were evaluated by immunoblotting and scored (a score <5 indicates lower-than-normal amounts and a score of >5 denotes increased protein levels at the membrane fraction). ND, not determined.
The asterisk denotes that the amounts of Trk1 and Pma1 could not be precisely evaluated in this mutant due to inconsistent protein recovery from the insoluble fraction.
All clones were investigated by Western analysis to determine the levels of both Trk1 and Pma1 in plasma-membrane-containing fractions. As shown in Table 2 and Fig. S1A to S1C in the supplemental material, the group A pep5, vps15, and vps16 mutants showed markedly decreased levels of epitope-tagged Trk1 expressed from a centromeric plasmid. Interestingly, another mutant (akr1, encoding palmitoyl transferase) showed decreased levels of both Trk1 and Pma1. Although further experiments showed that the interpretation of the observed decrease in the amount of Pma1 and Trk1 was impeded due to difficulties in protein recovery from the insoluble fraction, akr1 likely presents lower levels of Trk1 in the insoluble fraction (see Fig. S1D in supplemental material). In fact, this mutant displayed a relative increase in Pma1 activity, as determined by the proton pumping assay; grows poorly in low-potassium medium; and shows defects in low-affinity Rb+ uptake (0.9 ± 0.1 nmol/mg · min, compared to the wild-type strain, at 2.8 ± 0.1 nmol/mg · min), suggesting that this protein may be important to establish and/or maintain Trk1 in an active state in the plasma membrane. Future experiments will be aimed at elucidating the molecular mechanisms involved. Significant differences in Pma1 accumulation were observed only for the brp1 mutant, which is a PMA1 promoter deletion (35). These results identified components of the intracellular trafficking machinery (pep5, vps15, and vps16) as being important for Trk1 delivery to and/or stability in the plasma membrane.
Interestingly, the majority of the selected mutants did not present important differences in the levels of either Trk1 or Pma1. Thus, we went on to investigate the activities of these two proteins. As a first step, we measured low-affinity rubidium uptake as an indication of Trk1 activity (Table 2). As expected, most group A mutants (sensitive to all three compounds and presenting a growth defect in low-potassium medium) showed a reduction in Rb+ uptake. However, there are several interesting exceptions. For instance, the lst7 mutant, lacking a protein required for the transport of the nitrogen-regulated amino acid permease Gap1 from the Golgi compartment to the cell membrane, exhibited quite poor growth in Translucent medium containing 1 mM K+ but did not display obvious defects in Trk1 or Pma1 membrane localization or Rb+ uptake (Table 2). We observed that this mutant grew similarly to the wild-type strain in arginine-phosphate medium (which does not contain ammonium) in the presence of 1 mM KCl. However, the addition of 30 mM ammonium sulfate resulted in a marked growth defect similar to that observed for the wild-type strain in the same medium. It must be noted that a previous report using a chemostat model described that ammonium becomes toxic for yeast cells growing under conditions of potassium limitation (20). Therefore, it can be hypothesized that this phenotype observed for the lst7 mutant cells is related to a toxic effect of ammonium, which is detectable only at low potassium concentrations.
Vam3 and Vam7 are syntaxin and SNAP-25 homologs, respectively, that work in a SNARE complex implicated in vacuolar protein sorting. Both mutants exhibit a plasma membrane potential slightly higher than that of the wild-type strain, which may contribute to their sensitivity to the three toxic cations, but they grow very poorly in low-potassium media despite normal Trk1 protein levels and Rb+ transport. Similar to lst7 strain, ammonium seems to exert a toxic effect on vam3 and vam7 mutants but less intense than that observed for the lst7 mutant (Fig. 2).
Fig. 2.
Effects of ammonia and low potassium concentrations on the growth of trk1, lst7, pig1, vam3, and vam7 mutants. (A) Exponentially growing cultures of each strain were diluted to an OD600 of 0.004 in Translucent medium supplemented with 50 mM or 1 mM KCl and incubated at 28°C for 16 h. Final OD600 values were measured, and growth percentage values were calculated relative to the growth of each mutant in 50 mM KCl. (B) Growth of the same strains in arginine-phosphate medium supplemented with 50 mM KC1 (+) or 1 mM KCl (−) and with (closed bars) or without (open bars) ammonium sulfate (30 mM). Exponentially growing cultures of each strain were diluted to an OD600 of 0.008 in arginine phosphate medium supplemented as indicated and incubated at 28°C for 16 h. Growth percentage values are relative to the growth of each mutant in 50 mM KCl without ammonium sulfate.
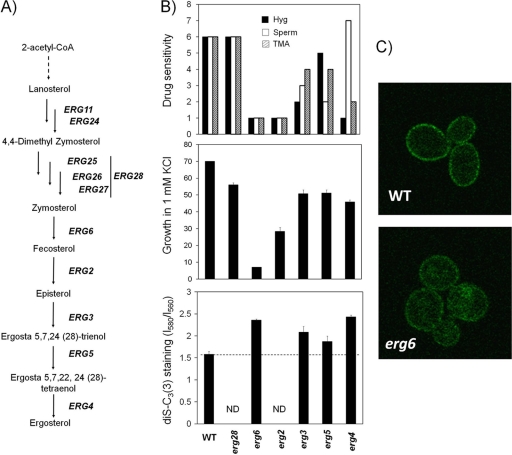
Although not quantitatively overrepresented in the screen, we recovered four mutants (erg6, erg2, erg3, and erg5) corresponding to the last steps in biosynthesis of ergosterol. We selected erg6, lacking a Δ(24)-sterol C-methyltransferase, for further analysis. The erg6 mutant grew very poorly in low-potassium medium and presented a significant defect in Rb+ uptake (approximately a 70% reduction) and a marked increase in membrane potential. Therefore, we tested other mutants of the ergosterol synthesis pathway. As shown in Fig. 3, the erg28 strain showed wild-type-like phenotypes for drugs and a moderate sensitivity to low potassium concentrations. In contrast, erg2 displayed a strong sensitivity to drugs and poor growth at low potassium concentrations. These defects were less dramatic for mutants in the next two steps, erg3 and erg5. The determination of high-affinity Rb+ uptake showed decreased values for all mutants, particularly in the case of erg6 (data not shown).
Fig. 3.
Characterization of erg mutants for K+ homeostasis-associated attributes. (A) The last steps in the ergosterol biosynthetic pathway from acetyl coenzyme A (CoA) to ergosterol. (B, top) Phenotypes for drug sensitivity. The scale is described in the text. (Middle) Growth in limiting-potassium (1 mM) medium, expressed as a percentage for the 1 mM/50 mM KCl ratio. (Bottom) Estimation of relative membrane potential values for the indicated mutants. ND, not determined. For the middle and bottom panels, data are means ± standard deviations (SD) of data from 3 to 4 independent experiments. (C) Confocal microscopy analysis of the Trk1-GFP distribution in wild-type and erg6 cells.
Curiously, erg4, the mutant in the last step of the pathway, was highly sensitive to hygromycin B and TMA but moderately tolerant to spermine and exhibited a moderate growth defect at limiting potassium concentrations. Most of these mutants exhibited higher levels of staining with the potentiometric probe, which might reflect a hyperpolarized plasma membrane relating quite well to their sensitivity to drugs.
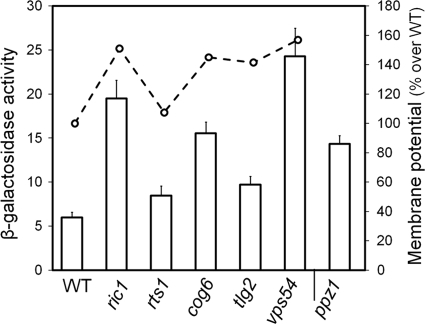
In the case of the group B mutants, these strains were all sensitive to the three compounds tested but showed no growth defect in low-potassium medium, and they expressed near-normal levels of Pma1 or Trk1. In accordance with the observed sensitivity phenotypes, these mutants displayed various degrees of increased membrane potential (Table 2). Seven of the eight mutants are involved in different aspects of vesicle trafficking, thus suggesting that this cellular process is important for the establishment and maintenance of membrane potential through Pma1/Trk1-independent mechanisms. We considered the possibility that some of these mutants, particularly those exhibiting a higher-than-normal plasma membrane potential, may suffer a deregulation of the expression of the ENA1 Na+-K+-ATPase. Therefore, we tested the expression of the activity of the ENA1 promoter by using a reporter plasmid carrying a translational fusion of the promoter to the lacZ gene (Fig. 4). Interestingly, mutants with high plasma membrane potential, such a ric1, cog6, tlg2, or vps54 mutant, also exhibited an expression level from the ENA1 promoter clearly higher than that of the wild-type strain, roughly in the same range as that of the ppz1 mutant, which has been known for years to have abnormally increased basal ENA1 expression levels (36, 42). However, although it is tempting to speculate that the increased membrane potential is the result of exacerbated ENA1 activity, additional studies will be required to establish the potential link between both phenotypes.
Fig. 4.
Activity of the ENA1 promoter and relative plasma membrane potential in selected members of group B mutants. The β-galactosidase activity was measured as indicated in Materials and Methods, and data are the means ± standard errors of the means (SEM) of data from five independent experiments performed in triplicate. Plasma membrane potential values, generated from at least three independent experiments, are expressed as a percentage relative to the wild-type value, which was set as 100%.
Interestingly, we observed marked decreases in Rb+ uptake in three group C and D mutants: the brp1, ptk2, and sap185 mutants. Both the brp1 and ptk2 mutants have reduced Pma1 activity (and expression, in the case of brp1). This reduction in Pma1 activity, which is known to be required for Trk1 function, is likely to explain these results and would also reduce the relative accumulation of these toxic cations. The case of sap185 is quite interesting and may be related to its function in Nha1 regulation (Fig. 1 and Table 2, and see Discussion). Sky1 was previously suggested to be involved in salt tolerance and plasma membrane potential through the regulation of Trk1 (13), and the mutant is also hypertolerant to diverse polyamines (8). In agreement with that study, we observed that the sky1 mutant was resistant to all three toxic cations tested here.
The fourth group C mutant, sur2 (encoding a sphinganine C4-hydroxylase involved in sphingolipid biosynthesis), did not show defects in Pma1 activity but displayed a significant decrease in membrane potential. This decrease in the plasma membrane potential would explain the observed tolerance phenotypes and suggests that this mutant affects the activities of proteins other than Pma1 and Trk1, which are important for the determination of plasma membrane potential.
DISCUSSION
We have performed a screen of the haploid deletion library for changes in tolerance to three drugs that, having different toxicity mechanisms, share the characteristic of being cations at the pH of standard growth media. The output for this screen has been the identification of 226 mutants with altered tolerance, most of which are hypersensitive strains. Our results show substantial differences from data from previous multidrug deletant screens. For instance, Hillenmeyer and coworkers (21) analyzed the homozygous diploid mutant collection for hypersensitivity to 178 different drugs (including hygromycin B but not spermine or TMA). It is worth noting that our screen has uncovered far more hygromycin B-sensitive mutants than the above-mentioned work. The difference can be attributed to the distinct experimental approaches used and the fact that we used higher concentrations of hygromycin B in our screen. The report by Hillenmeyer and coworkers defined a list of 510 multidrug resistance (MDR) genes. Interestingly, only 57 of our mutants are included in this list, and among them, 8 are hypertolerant to all three drugs. In addition, we did not find multidrug efflux transporters, such as PDR5, SNQ2, PDR10, or functionally related genes. This suggests that most of our findings do not reflect a general drug toxicity effect, but instead, we have selected for a more specific subset of genes. A functional survey of the coincident genes reveals that this subset is largely enriched in vesicular transport (5.7E−17), which is in agreement with the notion that intracellular traffic is a key function for multidrug tolerance (21, 48). In contrast, the list of noncoincident genes is enriched in DNA modification and transcription as well as protein fate and modification (mainly protein phospho-dephosphorylation) activities. As far as DNA modification and transcription are concerned, components of the SAGA and ADA complexes have been identified as being important for drug tolerance, as these complexes are involved in both the positive and negative transcriptional regulation of numerous genes, particularly under conditions of cellular stress. Different subunits of SAGA have been genetically and biochemically defined, and usually, the mutation of its components (when viable) results in sensitivity to drugs. However, although we observed that the mutations affecting Gcn5/Ada2/Ngg1 (histone acetyltransferase module) do result in drug sensitivity, mutations affecting the deubiquitination module (22), such as that of UBP8, the ubiquitin-specific protease required for the SAGA-mediated deubiquitination of histone H2B, and that of SGF11, required for the Ubp8 association with SAGA, are similarly tolerant (Fig. 1). The sus1 mutant, lacking the third component of the module, was absent in our library.
The identification of several protein kinases and phosphatases in our screen suggests that diverse signaling pathways are key elements in tolerance to cationic drugs. An interesting example is the Sky kinase. SKY1 was considered an MDR gene in the study mentioned above (21). However, we observed a marked tolerance to all three drugs, accompanied by a significant defect in growth in low-potassium medium, a slight defect in low-affinity Rb+ uptake, and a pronounced decrease in the proton-pumping activity. Thus, although previous reports suggested a Trk1-dependent role for Sky1 in salt tolerance and plasma membrane potential (13) and that the deletion or overexpression of SKY1 does not significantly affect Pma1 activity (9), our results suggest that, at least in this genetic background, the tolerance of the sky1 mutant could be due primarily to a decrease in Pma1 activity. Sky1 was found to phosphorylate Npl3, an RNA-binding protein that carries poly(A)+ mRNA from the nucleus into the cytoplasm. The phosphorylation of Npl3p by the cytoplasmically localized Sky1 is required for the efficient release of mRNA upon the termination of export, and this phosphorylation-and-dephosphorylation cycle allows Npl3 to disengage from the mRNA and shuttle back into the nuclear compartment (16). Interestingly, we have found a drug-hypersensitive phenotype for the npl3 mutant (see Table S1 in the supplemental material). Therefore, it is tempting to speculate that an alteration in mRNA export is the underlying cause for the observed drug tolerance. Another example of the likely involvement of protein kinases in the maintenance of the electrochemical gradient is revealed by the evaluation of the akr1 mutation. Akr1 is known to palmitoylate the type 1 casein kinases Yck1 and Yck2, an event required for kinase localization to the plasma membrane (11). Yck1 and Yck2 were shown previously to phosphorylate and inhibit the Pma1 H+-ATPase (10). Therefore, it is conceivable that the lack of Akr1 could result in increased Pma1 activity, in agreement with our proton-pumping activity data. This increased Pma1 activity would justify, at least in part, the drug sensitivity of this mutant. However, it would not explain the impaired rubidium transport and the observed incapacity to grow in low-K+ medium. However, Yck kinases are known to modulate the activity and/or turnover of several membrane transporters, such as the maltose permease (15) and the monocarboxylate transporter Jen1 (15). It is tempting to speculate that the Trk1 potassium transporter could also be affected in this mutant, and this hypothesis is supported by our analysis of the amount of Trk1 present in plasma membrane-enriched fractions (see Fig. S1D in the supplemental material). Unfortunately, as mentioned above, the quantification of membrane proteins in this strain is not a straightforward task.
It is known that the mutation of sap185 involves the inhibition of the Nha1 Na+-K+/H+ antiporter (28), which would reduce the potassium loss. This would imply reduced potassium uptake requirements and may explain the decreased rubidium influx observed for this mutant. This mutant is likely to contain higher-than-normal intracellular potassium levels, as documented previously for the nha1 mutant (1), which would explain its normal growth at limiting potassium concentrations and its moderate hypertolerance to the drugs. On the contrary, we observed that a sap155 mutant, which has an activated Nha1 antiporter (28), is hypersensitive to the drugs and presents a moderate growth defect at limiting potassium concentrations. Since the acidification rate in this mutant is normal (not shown), increased potassium efflux could augment the electrochemical gradient, thus explaining the hypersensitive phenotype.
Ergosterol is one of the principal components of the plasma membrane in yeast, and its synthesis, specifically the function of Erg4 and Erg6, has been shown to be important for the delivery of proteins to the plasma membrane (37). For instance, the inhibition of ergosterol synthesis or the mutation of erg6 or erg2 promotes the abnormal sorting of the tryptophan permease Tat2 to the plasma membrane (4, 5). However, at least in the case of erg6, although we observed a significant decrease in potassium uptake, which likely explains the growth defect in low-potassium medium, the amount of Trk1 at the plasma membrane was not drastically reduced. In this regard, it was reported previously that the heterologously expressed hexose-H+ symporter Hup1, although present in the cell membrane when expressed in an erg6 mutant, showed significantly decreased catalytic activity (18). Therefore, one might speculate that, even if properly delivered to the membrane, the ERG6 mutation could affect the activity of the Trk1 transporter. In fact, Trk1 was shown previously to be associated with plasma membrane “rafts” (50), a kind of structure that, in many cases, depends on proper sterol biosynthesis (27). Accordingly, the pattern of fluorescence of Trk1-green fluorescent protein (GFP) is notably altered in this mutant, thus providing support for this hypothesis (Fig. 3C). In addition, a very recent report described that the treatment of wild-type cells with antifungals presumed to block sterol biosynthesis results in increased potassium efflux (2). Those authors also detected an increase in the plasma membrane potential, similar to what we found for our erg mutants. Although we have not determined the potassium efflux in these mutants, it is conceivable that this effect may contribute to the plasma membrane hyperpolarization described here.
In conclusion, our screen has identified a substantial number of genes which were not previously described to play a direct or indirect role in potassium homeostasis. For a number of cases, it is possible, in the context of our current knowledge, to provide an explanation for the observed phenotypes. Thus, it appears that most mutants belonging to group A may suffer a defect in Trk1 function, whereas the phenotypes of group B strains would be independent of Trk1 and Pma1. In contrast, decreased levels of Pma1 expression or activity, which could indirectly affect Trk1 function, may explain the behavior of group C mutants. Finally, the basis for group D phenotypes is not obvious, although it may involve, at least in some cases, a modulation of Nha1 activity. We stress that our screen has revealed coherent phenotypes in many cases for different mutations affecting a given function. For instance, among the mutations conferring drug sensitivity, we have identified not only YPT6, which encodes a GTPase involved in the secretory pathway required for the fusion of endosome-derived vesicles with the late Golgi compartment, but also RGP1 and RIC1, encoding the exchange factor that catalyzes nucleotide exchange on Ypt6; SYS1, which encodes a multicopy suppressor of the ypt6 null mutation; and even VPS63, a dubious open reading frame, which overlaps with YPT6. Therefore, we are confident that further exploration of the data generated in this work will lead to the identification of functional associations currently unknown.
Supplementary Material
ACKNOWLEDGMENTS
We thank all members of the Translucent Consortium (http://www.translucent-network.org/index.php) for stimulating discussions.
This work was supported by grants BFU2008-04188-C03-01, GEN2006-27748-C2-1-E/SYS (SysMo ERA-NET), and EUI2009-04147 (SysMo2 ERA-NET) to J.A.; GEN2006-27748-C2-2-E/SYS (SysMo ERA-NET) and BFU2008-04188-C03-03 to J.R.; and BFU2008-04188-C03-03 to L.Y. (Ministry of Science and Innovation, Spain, and FEDER). Work in the laboratory of the Institute of Physiology in Prague was supported by grants MSMT LC531, GA AS CR IAA500110801, and AV0Z 50110509. J.A. was the recipient of an Ajut 2009SGR-1091 and an ICREA academia award (Generalitat de Catalunya).
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 1 July 2011.
REFERENCES
- 1. Banuelos M. A., et al. 2002. Role of the Nha1 antiporter in regulating K(+) influx in Saccharomyces cerevisiae. Yeast 19:9–15 [DOI] [PubMed] [Google Scholar]
- 2. Calahorra M., Lozano C., Sanchez N. S., Pena A. 2011. Ketoconazole and miconazole alter potassium homeostasis in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1808:433–445 [DOI] [PubMed] [Google Scholar]
- 3. Casado C., et al. 2010. Regulation of Trk-dependent potassium transport by the calcineurin pathway involves the Hal5 kinase. FEBS Lett. 584:2415–2420 [DOI] [PubMed] [Google Scholar]
- 4. Daicho K., et al. 2009. Sorting defects of the tryptophan permease Tat2 in an erg2 yeast mutant. FEMS Microbiol. Lett. 298:218–227 [DOI] [PubMed] [Google Scholar]
- 5. Daicho K., et al. 2007. The ergosterol biosynthesis inhibitor zaragozic acid promotes vacuolar degradation of the tryptophan permease Tat2p in yeast. Biochim. Biophys. Acta 1768:1681–1690 [DOI] [PubMed] [Google Scholar]
- 6. de Nadal E., et al. 1998. The yeast halotolerance determinant Hal3p is an inhibitory subunit of the Ppz1p Ser/Thr protein phosphatase. Proc. Natl. Acad. Sci. U. S. A. 95:7357–7362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eraso P., Mazon M. J., Portillo F. 2006. Yeast protein kinase Ptk2 localizes at the plasma membrane and phosphorylates in vitro the C-terminal peptide of the H+-ATPase. Biochim. Biophys. Acta 1758:164–170 [DOI] [PubMed] [Google Scholar]
- 8. Erez O., Kahana C. 2001. Screening for modulators of spermine tolerance identifies Sky1, the SR protein kinase of Saccharomyces cerevisiae, as a regulator of polyamine transport and ion homeostasis. Mol. Cell. Biol. 21:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erez O., Kahana C. 2002. Deletions of SKY1 or PTK2 in the Saccharomyces cerevisiae trk1Deltatrk2Delta mutant cells exert dual effect on ion homeostasis. Biochem. Biophys. Res. Commun. 295:1142–1149 [DOI] [PubMed] [Google Scholar]
- 10. Estrada E., et al. 1996. Phosphorylation of yeast plasma membrane H+-ATPase by casein kinase I. J. Biol. Chem. 271:32064–32072 [DOI] [PubMed] [Google Scholar]
- 11. Feng Y., Davis N. G. 2000. Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane. Mol. Cell. Biol. 20:5350–5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferrando A., Kron S. J., Rios G., Fink G. R., Serrano R. 1995. Regulation of cation transport in Saccharomyces cerevisiae by the salt tolerance gene HAL3. Mol. Cell. Biol. 15:5470–5481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forment J., Mulet J. M., Vicente O., Serrano R. 2002. The yeast SR protein kinase Sky1p modulates salt tolerance, membrane potential and the Trk1,2 potassium transporter. Biochim. Biophys. Acta 1565:36–40 [DOI] [PubMed] [Google Scholar]
- 14. Gaber R. F., Styles C. A., Fink G. R. 1988. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 8:2848–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gadura N., Robinson L. C., Michels C. A. 2006. Glc7-Reg1 phosphatase signals to Yck1,2 casein kinase 1 to regulate transport activity and glucose-induced inactivation of Saccharomyces maltose permease. Genetics 172:1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gilbert W., Siebel C. W., Guthrie C. 2001. Phosphorylation by Sky1p promotes Npl3p shuttling and mRNA dissociation. RNA 7:302–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goossens A., de La Fuente N., Forment J., Serrano R., Portillo F. 2000. Regulation of yeast H(+)-ATPase by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell. Biol. 20:7654–7661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grossmann G., Opekarova M., Novakova L., Stolz J., Tanner W. 2006. Lipid raft-based membrane compartmentation of a plant transport protein expressed in Saccharomyces cerevisiae. Eukaryot. Cell 5:945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haro R., Rodriguez-Navarro A. 2002. Molecular analysis of the mechanism of potassium uptake through the TRK1 transporter of Saccharomyces cerevisiae. Biochim. Biophys. Acta 1564:114–122 [DOI] [PubMed] [Google Scholar]
- 20. Hess D. C., Lu W., Rabinowitz J. D., Botstein D. 2006. Ammonium toxicity and potassium limitation in yeast. PLoS Biol. 4:e351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hillenmeyer M. E., et al. 2008. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science 320:362–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ingvarsdottir K., et al. 2005. H2B ubiquitin protease Ubp8 and Sgf11 constitute a discrete functional module within the Saccharomyces cerevisiae SAGA complex. Mol. Cell. Biol. 25:1162–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ko C. H., Gaber R. F. 1991. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 11:4266–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuo M. H., Grayhack E. 1994. A library of yeast genomic MCM1 binding sites contains genes involved in cell cycle control, cell wall and membrane structure, and metabolism. Mol. Cell. Biol. 14:348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Madrid R., Gomez M. J., Ramos J., Rodriguez-Navarro A. 1998. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J. Biol. Chem. 273:14838–14844 [DOI] [PubMed] [Google Scholar]
- 26. Malac J., Urbankova E., Sigler K., Gaskova D. 2005. Activity of yeast multidrug resistance pumps during growth is controlled by carbon source and the composition of growth-depleted medium: DiS-C3(3) fluorescence assay. Int. J. Biochem. Cell Biol. 37:2536–2543 [DOI] [PubMed] [Google Scholar]
- 27. Malinsky J., Opekarova M., Tanner W. 2010. The lateral compartmentation of the yeast plasma membrane. Yeast 27:473–478 [DOI] [PubMed] [Google Scholar]
- 28. Manlandro C. M., Haydon D. H., Rosenwald A. G. 2005. Ability of Sit4p to promote K+ efflux via Nha1p is modulated by Sap155p and Sap185p. Eukaryot. Cell 4:1041–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maresova L., Muend S., Zhang Y. Q., Sychrova H., Rao R. 2009. Membrane hyperpolarization drives cation influx and fungicidal activity of amiodarone. J. Biol. Chem. 284:2795–2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mendoza I., Rubio F., Rodriguez-Navarro A., Pardo J. M. 1994. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. J. Biol. Chem. 269:8792–8796 [PubMed] [Google Scholar]
- 31. Mulet J. M., et al. 1999. A novel mechanism of ion homeostasis and salt tolerance in yeast: the Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 19:3328–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munson A. M., et al. 2004. Yeast ARL1 encodes a regulator of K+ influx. J. Cell Sci. 117:2309–2320 [DOI] [PubMed] [Google Scholar]
- 33. Navarrete C., et al. 2010. Lack of main K+ uptake systems in Saccharomyces cerevisiae cells affects yeast performance both in potassium sufficient and limiting conditions. FEMS Yeast Res. 10:508–517 [DOI] [PubMed] [Google Scholar]
- 34. Perez-Valle J., et al. 2007. Key role for intracellular K+ and protein kinases Sat4/Hal4 and Hal5 in the plasma membrane stabilization of yeast nutrient transporters. Mol. Cell. Biol. 27:5725–5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Porat Z., Wender N., Erez O., Kahana C. 2005. Mechanism of polyamine tolerance in yeast: novel regulators and insights. Cell. Mol. Life Sci. 62:3106–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Posas F., Camps M., Arino J. 1995. The PPZ protein phosphatases are important determinants of salt tolerance in yeast cells. J. Biol. Chem. 270:13036–13041 [DOI] [PubMed] [Google Scholar]
- 37. Proszynski T. J., et al. 2005. A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc. Natl. Acad. Sci. U. S. A. 102:17981–17986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ramos J., Alijo R., Haro R., Rodriguez-Navarro A. 1994. TRK2 is not a low-affinity potassium transporter in Saccharomyces cerevisiae. J. Bacteriol. 176:249–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rao R., Drummond-Barbosa D., Slayman C. W. 1993. Transcriptional regulation by glucose of the yeast PMA1 gene encoding the plasma membrane H(+)-ATPase. Yeast 9:1075–1084 [DOI] [PubMed] [Google Scholar]
- 40. Rodriguez-Navarro A. 2000. Potassium transport in fungi and plants. Biochim. Biophys. Acta 1469:1–30 [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez-Navarro A., Ramos J. 1984. Dual system for potassium transport in Saccharomyces cerevisiae. J. Bacteriol. 159:940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ruiz A., Yenush L., Arino J. 2003. Regulation of ENA1 Na(+)-ATPase gene expression by the Ppz1 protein phosphatase is mediated by the calcineurin pathway. Eukaryot. Cell 2:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 44. Serrano R. 1980. Effect of ATPase inhibitors on the proton pump of respiratory-deficient yeast. Eur. J. Biochem. 105:419–424 [DOI] [PubMed] [Google Scholar]
- 45. Serrano R., Kielland-Brandt M. C., Fink G. R. 1986. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature 319:689–693 [DOI] [PubMed] [Google Scholar]
- 46. Serrano R., Ruiz A., Bernal D., Chambers J. R., Arino J. 2002. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Mol. Microbiol. 46:1319–1333 [DOI] [PubMed] [Google Scholar]
- 47.Reference deleted.
- 48. Venancio T. M., Balaji S., Aravind L. 2010. High-confidence mapping of chemical compounds and protein complexes reveals novel aspects of chemical stress response in yeast. Mol. Biosyst. 6:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu P. Y., Winston F. 2002. Analysis of Spt7 function in the Saccharomyces cerevisiae SAGA coactivator complex. Mol. Cell. Biol. 22:5367–5379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yenush L., Merchan S., Holmes J., Serrano R. 2005. pH-responsive, posttranslational regulation of the Trk1 potassium transporter by the type 1-related Ppz1 phosphatase. Mol. Cell. Biol. 25:8683–8692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yenush L., Mulet J. M., Arino J., Serrano R. 2002. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 21:920–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.