Abstract
The Saccharomyces cerevisiae general amino acid permease Gap1 (ScGap1) not only mediates the uptake of most amino acids but also functions as a receptor for the activation of protein kinase A (PKA). Fungal pathogens can colonize different niches in the host, each containing various levels of different amino acids and sugars. The Candida albicans genome contains six genes homologous to the S. cerevisiae GAP1. The expression of these six genes in S. cerevisiae showed that the products of all six C. albicans genes differ in their transport capacities. C. albicans Gap2 (CaGap2) is the true orthologue of ScGap1 as it transports all tested amino acids. The other CaGap proteins have narrower substrate specificities though CaGap1 and CaGap6 transport several structurally unrelated amino acids. CaGap1, CaGap2, and CaGap6 also function as sensors. Upon detecting some amino acids, e.g., methionine, they are involved in a rapid activation of trehalase, a downstream target of PKA. Our data show that CaGAP genes can be functionally expressed in S. cerevisiae and that CaGap permeases communicate to the intracellular signal transduction pathway similarly to ScGap1.
INTRODUCTION
Candida albicans is a commensal organism that is carried on the skin and on the mucosal surfaces of the gastrointestinal and urogenital tract of humans and other warm-blooded animals without clinical symptoms (48) and can be detected in up to 71% of the healthy population (41). However, even in healthy people it causes a variety of skin and soft tissue infections (43), and in immunocompromised patients, C. albicans causes more virulent invasive and expanded diseases (9, 13). How this microorganism manages to survive in healthy hosts and also to cause a spectrum of diseases in immunocompromised hosts is currently the subject of considerable biological interest.
Amino acids as a source of nitrogen are essential nutrients that are efficiently transported into C. albicans cells. They are available throughout the host body, as C. albicans possesses a number of similar but independently regulated and functionally distinct secreted proteinases and lipases, which allow the microorganism to break down or decompose almost every tissue of the host into suitable nutrients (27, 41, 42). Amino acids serve as building blocks and energy suppliers that provide maintenance and proliferation. Their catabolism leads to glutamate, ammonia, or glutamine, which are the principal donors of amino groups for the biosynthesis of various compounds containing nitrogen. In order to ensure the efficient uptake and metabolism of amino acids, it is essential that a cell can detect their presence in order to adapt to changing environmental conditions and to express the genes required to proliferate in the given area. There is evidence that amino acid sensing and uptake are very important for C. albicans growth, morphogenesis, and virulence (4, 8, 39) though neither of these phenomena has been studied in detail in this pathogenic yeast.
In the model yeast Saccharomyces cerevisiae, amino acid uptake is mediated by a family of 20 proton-driven permeases with 12 transmembrane domains and cytosolic N- and C-terminal hydrophilic regions. This family is part of a larger family of cation-driven permeases, the amino acid polyamine-choline transporter superfamily (2, 46). Most of the amino acid permeases in S. cerevisiae are specific for one or a few related l-amino acids and exhibit different properties in terms of substrate affinity, specificity, transport capacity, and regulation. In addition to amino acid permeases specific for one or few amino acids, S. cerevisiae cells possess a general amino acid permease Gap1 that has a broad substrate specificity and high capacity and mediates the uptake of most l- and d-amino acids, nonproteic amino acids such as citrulline and ornithine, and a number of amino acid toxic analogues (18, 28, 53).
Amino acids are important nutrients, but on the other hand extensive uptake of some of them can inhibit cell growth (37). It is therefore not surprising that amino acid uptake is controlled. S. cerevisiae Gap1 (ScGap1) is tightly regulated by the nitrogen source at two different levels, transcriptional and posttranslational. While its transcriptional regulation involves predominantly nitrogen catabolite repression (NCR), its nitrogen catabolite inactivation (NCI) is a prime example of posttranslational regulation (25). The transcription of ScGAP1 is controlled by GATA-type transcription factors (Gln3, Nil1, Nil2, and Dal80) in response to the presence of a nitrogen source (54). The ScGAP1 gene is transcribed at high levels, and the synthesized permease accumulates at the plasma membrane in an active and stable form if the cells are growing in medium with poor nitrogen sources, e.g., proline or urea. Upon adding a preferential, rich source of nitrogen (e.g., glutamine or ammonium), ScGAP1 transcription is repressed, and molecules of Gap1 permease present in the plasma membrane are internalized by endocytosis and targeted to the vacuole for degradation (52). Cells growing on glutamate synthesize ScGap1p, but the protein is sent directly to the vacuole and never reaches the plasma membrane (10).
There are at least two plasma membrane sensors for extracellular amino acids in S. cerevisiae. ScSsy1, a unique member of the amino acid permease protein family that is not able to transport amino acids, controls the amino acid uptake capacity of cells by inducing the transcription of genes encoding several amino acid permeases, but not that of ScGap1 (60). Together with the ScPtr3 and ScSsy5 proteins, ScSsy1 forms the plasma membrane SPS nutrient-sensing system (36). The second plasma membrane sensor for extracellular amino acids in S. cerevisiae is the general amino acid permease Gap1. It is required not only for amino acid transport but also for sensing the presence of external amino acids and the subsequent activation of signal transduction pathways that induce many intracellular changes necessary for the optimized use of transported amino acids. The addition of amino acids to S. cerevisiae cells starved of nitrogen in the presence of a fermentable carbon source is detected by the general Gap1 permease, and the fermentable growth medium-induced (FGM) pathway is activated (15). Activation of this pathway causes a rapid cyclic AMP (cAMP)-independent activation of protein kinase A (PKA) targets, e.g., trehalase (56). Under starvation conditions, yeast cells accumulate high levels of trehalose, which protects the cells from a wide range of stress conditions. Upon sensing nutrients, trehalase is activated very rapidly, and this causes the rapid mobilization of trehalose, which is required to resume cell metabolism and adapt it to the changed environment (6).
In this paper, we show that there is a whole family of ScGap1 orthologues in C. albicans that differ in their substrate specificity and transport capacity. When heterologously expressed in S. cerevisiae mutants lacking their own amino acid transporters, putative C. albicans general amino acid permeases (CaGaps) are properly synthesized, become localized in the plasma membrane, and have amino acid transport activity. We show that CaGaps function as amino acid transporters with various substrate specificities and that some of them also play a role as amino acid sensors for rapid activation of the PKA pathway, a pathway that is very important to virulence in many pathogenic fungi.
MATERIALS AND METHODS
Sequence analysis.
BLAST analysis, C. albicans databases (CGD, http://www.candidagenome.org/; CDB, http://genolist.pasteur.fr/CandidaDB/), and the S. cerevisiae database (SGD; http://www.yeastgenome.org/) were used. The phylogenetic tree was created with MEGA (Molecular Evolutionary Genetics Analysis), version 4, software which enables neighbor-joining trees to be created (50).
Strains and growth conditions.
The C. albicans SC5314 strain (30) grown in YPD medium (1% yeast extract, 2% Bacto peptone, 2% glucose) was used as a source of CaGAP genes. All S. cerevisiae strains used in our experiments are listed in Table 1. They were grown in YPD medium for transformations, and for all other experiments we used minimal medium (0.17% yeast nitrogen base without amino acids and ammonium sulfate) supplemented with 2% glucose and various sources of nitrogen (ammonium sulfate or amino acids at the concentrations indicated in the text). To starve the cells of nitrogen, we used nitrogen starvation minimal medium (0.17% yeast nitrogen base without amino acids and ammonium sulfate) supplemented with 4% glucose. For assessments of amino acid uptake or trehalase activation, cells were grown in minimal medium containing 0.5% ammonium sulfate and 0.077% Brent supplement mixture (BSM; Formedia) without uracil. Escherichia coli TOP10 or DH5α cells were used for amplifying constructed plasmids. Cell cultures were grown in Luria-Bertani medium (1% NaCl, 1% Bacto tryptone, 0.5% yeast extract, pH 7.5). For ampicillin resistance selection, ampicillin was added after sterilization to a final concentration of 100 μg/ml.
Table 1.
Saccharomyces cerevisiae strains used in this study
Plasmid construction.
All plasmids used in our experiments are listed in Table 2, and the oligonucleotides used for PCR amplification of coding sequences are listed in Table 3. CaGAP genes amplified by PCR from genomic DNA, isolated as described by Hoffman and Winston (23) were cloned into the pNHA1-985 (31) multicopy vector behind the weak constitutive ScNHA1 promoter by homologous recombination (replacing the original ScNHA1 coding sequence). For the green fluorescent protein (GFP)-tagged versions, the GFP sequence was amplified from the pCK230 vector (21) and cloned by homologous recombination in frame to the 3′ end of the CaGAP genes and ScGAP1 genes in pSc/CaGAPs plasmids. All constructs were verified by diagnostic PCR (used oligonucleotides are listed in Table 3) and by sequencing.
Table 2.
Plasmids used in this study
| Plasmid | Insert | Reference or source |
|---|---|---|
| YEp352 | 22 | |
| pNHA1-985 | ScNHA1 | 31 |
| pScGAP1 | ScGAP1 | This work |
| pCaGAP1 | CaGAP1 | This work |
| pCaGAP2 | CaGAP2 | This work |
| pCaGAP3 | CaGAP3 | This work |
| pCaGAP4 | CaGAP4 | This work |
| pCaGAP5 | CaGAP5 | This work |
| pCaGAP6 | CaGAP6 | This work |
| pScGAP1-GFP | ScGAP1-GFP | This work |
| pCaGAP1-GFP | CaGAP1-GFP | This work |
| pCaGAP2-GFP | CaGAP2-GFP | This work |
| pCaGAP3-GFP | CaGAP3-GFP | This work |
| pCaGAP4-GFP | CaGAP4-GFP | This work |
| pCaGAP5-GFP | CaGAP5-GFP | This work |
| pCaGAP6-GFP | CaGAP6-GFP | This work |
| pCK230 | ScGAP1-GFP | 21 |
Table 3.
Oligonucleotides used in this study
| Oligonucleotidea | Sequence (5′–3′) |
|---|---|
| YEpN-ScGAP1-F | TTTTTTGTACATTATAAAAAAAAATCCTGAACTTAGCTAGATATTATGAGTAATACTTCTTCGTACG |
| YEpN-ScGAP1-R | CACGACGTTGTAAAACGACGGCCAGTGCCAAGCTTGCATGTTAACACCAGAAATTCCAG |
| YEpN-CaGAP1-F | TTTTTTGTACATTATAAAAAAAAATCCTGAACTTAGCTAGATATTATGCTACATAAAAAAGAAAC |
| YEpN-CaGAP1-R | CACGACGTTGTAAAACGACGGCCAGTGCCAAGCTTGCATGTTCACCAAAATCTATAAA |
| YEpN-CaGAP2-F | TTTTTTGTACATTATAAAAAAAAATCCTGAACTTAGCTAGATATTATGCCAGAAAAGGAATTAGA |
| YEpN-CaGAP2-R | CACGACGTTGTAAAACGACGGCCAGTGCCAAGCTTGCATGTCAACACCAAGCATTGTAGAC |
| YEpN-CaGAP3-F | TTTTTTGTACATTATAAAAAAAAATCCTGAACTTAGCTAGATATTATGACTACTAAAGAGAAAG |
| YEpN-CaGAP3-R | CACGACGTTGTAAAACGACGGCCAGTGCCAAGCTTGCATGTTAACACCAATAATTCCATA |
| YEpN-CaGAP4-F | TTTTTTGTACATTATAAAAAAAAATCCTGAACTTAGCTAGATATTATGATATTGAAAAGCAGCAG |
| YEpN-CaGAP4-R | CACGACGTTGTAAAACGACGGCCAGTGCCAAGCTTGCATGTTAGAAGAAAAATTTATAGATACG |
| YEpN-CaGAP5-F | TTTTTTGTACATTATAAAAAAAAATCCTGAACTTAGCTAGATATTATGTCAAAGAAATATAGTGAG |
| YEpN-CaGAP5-R | CACGACGTTGTAAAACGACGGCCAGTGCCAAGCTTGCATGTTAACACCAAAAATGATAAG |
| YEpN-CaGAP6-F | TTTTTTGTACATTATAAAAAAAAATCCTGAACTTAGCTAGATATTATGCCTAAAGAGGCATCTAGC |
| YEpN-CaGAP6-R | CACGACGTTGTAAAACGACGGCCAGTGCCAAGCTTGCATGTTAACACCAGAATTGATAA |
| Sc/CaGAPx-GFP-R | GGTTTTCCCAGTCACGACGTTGTAAAACGACGGCCAGTGCTTATTTGTACAATTCATCCATACC |
| ScGAP1-GFP-F | CACAAAGCCAAGATGGTATAGAATCTGGAATTTCTGGTGTTCTAAAGGTGAAGAATTATTCAC |
| CaGAP1-GFP-F | GAAAAACCATTCTATATTAGAATTTATAGATTTTGGTGCTCTAAAGGTGAAGAATTATTCAC |
| CaGAP2-GFP-F | CTTCTTTGCCATTCTACAGAAGAGTCTACAATGCTTGGTGTTCTAAAGGTGAAGAATTATTCAC |
| CaGAP3-GFP-F | AGCTAAACCATTGTACAAACGGTTATGGAATTATTGGTGTTCTAAAGGTGAAGAATTATTCAC |
| CaGAP4-GFP-F | GAGTAAGCCATTTGTCTATCGTATCTATAAATTTTTCTTCTCTAAAGGTGAAGAATTATTCAC |
| CaGAP5-GFP-F | GAATAAACCATTTTGGTATAGAGCTTATCATTTTTGGTGTTCTAAAGGTGAAGAATTATTCAC |
| CaGAP6-GFP-F | GAGAAGCTTATAAGAGACAGCCATGGTATTACAAGTTTTATCAATTCTGGTCTAAAGGTGAAGAATTATTCAC |
| YEp352-R | GATCGGTGCGGGCCTCTTCG |
| ScNHA1prom-F | GATCACACTTTCTACACACA |
| ScGAP1-Diag-R | GGTACCTGTAGACCCCC |
| CaGAP1-Diag-R | AGAATGCAGCGGTGATAAG |
| CaGAP2-Diag-R | CCGTTATGACACCTTTAGC |
| CaGAP3-Diag-R | CAAAAGCACCATCTTGTTTC |
| CaGAP4-Diag-R | TGTAATACAAATACCAAGAATG |
| CaGAP5-Diag-R | GCAGCTAACACAATTGAAAG |
| CaGAP6-Diag-R | CAGCAGATGTCAACCCCA |
| CaGAP3-387-F | GTTTTATCCGTGGGGAACTCGGCAGTATATGGTTGTTC |
| CaGAP3-387-R | GAACAACCATATACTCGCGAGTTCCCCACGGATAAAAC |
| CaGAP5-324-F | GAATTAACCGGATTAACCTCGGCTGAAGCTGAAAACCCTC |
| CaGAP5-324-R | GAGGGTTTTCAGCTTCAGCCGAGGTTAATCCGGTTAATTC |
F, forward; R, reverse.
Site-directed mutagenesis of CUG codons.
A QuikChange XL Site-Directed Mutagenesis Kit from Stratagene was used to introduce mutations changing the CUG codon into UCG in the CaGAP3 and CaGAP5 genes (cloned as described above) at the positions corresponding to amino acid residue 324 of the CaGap5 protein and 387 in the CaGap3 protein. Used oligonucleotides are listed in Table 3. The introduction of mutations was verified by sequencing.
Growth assays.
The growth of S. cerevisiae strains transformed with YEp352 (empty plasmid; used as a negative control), pScGAP1 (used as a positive control), pCaGAPs, and pSc/CaGAP-GFPs was tested on plates with minimal medium supplemented with ammonium sulfate or amino acids. Each amino acid was tested at various concentrations (20 to 500 μg/ml). When the sensitivity to toxic amino acid analogues was tested, the growth was estimated on minimal medium supplemented with 0.1% proline as the source of nitrogen and various toxic amino acid analogues (listed in Table 4) (all purchased from Sigma) at concentrations ranging from 1 to 500 μg/ml. For growth assays, classical drop tests were used. Fresh cells of each tested strain were resuspended in water (optical density at 600 nm [OD600] adjusted to 1). A series of 10-fold serial dilutions of each suspension was prepared, and 3-μl aliquots of each dilution were spotted on plates. Plates were incubated at 30°C usually for 4 days, and images were obtained using a Nikon Coolpix 4500 digital camera.
Table 4.
Toxic amino acid analogues used in this study
| Name | Amino acid analogue | Tested final concn (μg/ml) |
|---|---|---|
| Canavanine | Arginine | 1–20 |
| Thialysine | Lysine | 1–60 |
| Ethionine | Methionine | 0.5–5 |
| p-Fluoro-l-phenylalanine | Phenylalanine | 5–25 |
| 3-Hydroxynorvaline | Valine | 100–500 |
| Azaleucine | Leucine | 50–500 |
| 5-Fluoro-dl-tryptophan | Tryptophan | 3–10 |
| 4-Methyl-dl-tryptophan | Tyrosine | 3–20 |
Fluorescence microscopy.
Strain M4584 transformed with the pSc/CaGAPs-GFP plasmids was pregrown overnight in minimal medium with ammonium sulfate. Cells were transferred to minimal medium (without ammonium sulfate) with amino acids (100 μg/ml). After 4 h of incubation, cell suspensions were spotted on microscopy slides and viewed in an Olympus AX70 microscope equipped with an Olympus DP70 digital camera. Nomarski optics were used for whole-cell images, and a U-MWB fluorescent cube with excitation filter of 450 to 480 nm and a barrier filter of 515 nm was used for visualizing the GFP signal.
Estimation of transport activity.
Cells for amino acid uptake measurements were grown in minimal medium with ammonium sulfate and Brent supplement mixture to an OD600 of ∼1.5 to 2, harvested by centrifugation, resuspended in minimal nitrogen starvation medium, and further cultivated for 24 h. Nitrogen-starved cells were cooled on ice for 20 min, harvested by centrifugation, and resuspended at a cell density of 80 mg (wet weight)/ml in fresh nitrogen starvation medium. Forty microliters of this cell suspension was incubated at 30°C in a shaking water bath for 10 min; then a 10-μl mixture of unlabeled (2 mM final concentration) and 14C-labeled l-amino acid (3,000 cpm/nmol) was added, and the reaction was stopped by the addition of 4 ml of cold water after 60 s. The suspension was filtered through a glass microfiber filter soaked with unlabeled amino acid solution and immediately washed twice with 4 ml of ice-cold water. The radioactivity on the filter was counted in a liquid scintillation counter (Beckman Coulter LS6500). Five hundred microliters of the cell suspension was used to determine the protein content using the Bradford method (7). The transport activity was calculated from the measured radioactivity and estimated protein content in the samples and was expressed as a percentage relative to the transport observed in the strain expressing ScGAP1 (100%).
Monitoring of trehalase activity.
S. cerevisiae cells were grown in minimal medium with ammonium sulfate and Brent supplement mixture to an OD600 of ∼1.5 to 2, harvested, resuspended in fresh minimal nitrogen starvation medium, and further cultivated for 24 h. To follow the effect of the added amino acids on trehalase activation, nitrogen-starved glucose-repressed cells were cooled on ice for 30 min, harvested by centrifugation, washed twice, and resuspended in ice-cold nitrogen starvation medium with 4% glucose at a concentration of 25 mg of wet weight/ml. Subsequently, cells were incubated in a shaking water bath at 30°C for 10 min, and then the sampling began at 10-min intervals. After 30 min, the amino acid was added (10 mM final concentration), and sampling continued for the next 60 min. Each withdrawn sample (suspension with 50 mg of cells) was immediately added to 40 ml of ice-cold water. After centrifugation, the cell pellet was resuspended in 500 μl of extraction buffer (50 mM morpholineethanesulfonic acid [MES]-KOH, pH 7, 5 μM CaCl2); crude cell extracts were prepared as described previously (45) and dialyzed overnight at 4°C against 2 liters of dialysis buffer (10 mM MES-KOH, pH 7, 5 μM CaCl2). Trehalase activity in dialyzed cell extracts was determined by a previously described method (45), adapted for microtiter plates. The amount of protein in samples was determined using the Lowry method with bovine serum albumin (BSA) as a standard (38). Specific trehalase activity was expressed as nmols of glucose liberated per min and mg of protein. Each experiment was repeated at least three times, and results from representative experiments are shown.
RESULTS
The Candida genome encodes six ScGap1 orthologues.
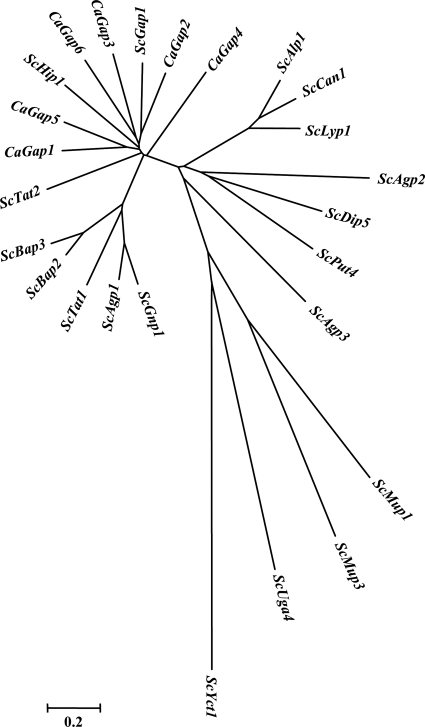
Our first search in databases revealed that the C. albicans genome contains approximately 27 genes encoding putative amino acid permeases similar to those of S. cerevisiae. A detailed BLAST analysis using the ScGap1 protein sequence as a query against the two available C. albicans databases, CGD (3) and CDB (47), resulted in the identification of six putative orthologues. A reciprocal BLAST of these six putative CaGap protein sequences against the S. cerevisiae SGD database (11) always found the ScGap1 permease to be the closest orthologue. As the six genes are all denoted GAP genes but with different numbers in the two databases, we propose a new and unified numbering (Table 5). Phylogenetic analysis of the six CaGap protein sequences with all members of the S. cerevisiae amino acid permease gene family (Fig. 1) clearly shows that the six CaGap proteins are most closely related to ScGap1. CaGap2 constitutes the closest ScGap1 orthologue, and CaGap4 is more distant but still belongs to the same group as ScGap1 and other CaGaps. ScHip1 (a histidine-specific permease) also clusters in the group of Gap permeases. Nevertheless, a detailed comparison of C. albicans Gaps with ScGap1 or ScHip1 always showed a higher level of identity between ScGap1 and CaGaps than between ScHip1 and any of the CaGaps.
Table 5.
Numbering of the CaGAP genes used in this study
| Gene | Systematic name | Allele name | CGD name(s)a | CDB nameb |
|---|---|---|---|---|
| CaGAP1 | orf19.4304 | orf19.11780 | GAP1 | GAP3 |
| CaGAP2 | orf19.6993 | orf19.6993 | GAP2, GAP13 | GAP2 |
| CaGAP3 | orf19.3195 | orf19.10706 | HIP1, GAP3, GAP21 | GAP7 |
| CaGAP4 | orf19.4456 | orf19.11936 | GAP4, GAP31 | GAP5 |
| CaGAP5 | orf19.1799 | orf19.9365 | GAP6, GAP12 | GAP4 |
| CaGAP6 | orf19.6659 | orf19.6659 | GAP6, GAP5, GAP22 | GAP6 |
CGD, Candida Genome Database.
CDB, Candida Database.
Fig. 1.
Phylogenetic analysis of all known amino acid transporters in S. cerevisiae and of the six-member Gap family in C. albicans by means of MEGA (Molecular Evolutionary Genetics Analysis), version 4, software. Distances between the permeases are related to the degree of divergence between sequences.
Heterologous expression of the six CaGAP genes in S. cerevisiae results in correct targeting of their products to the plasma membrane.
An important question that we wanted to answer was whether these six CaGap proteins are all genuine general amino acid permeases, are specific amino acid transporters, or have developed a function independent of amino acid transport. We are currently unable to characterize the transport properties of the six putative CaGaps directly in C. albicans as there is no strain available in which only one CaGAP gene is expressed and the other five CaGAPs are deleted together with the genes encoding other types of amino acid permeases. As a result, we used S. cerevisiae as a heterologous host to characterize the transport properties, substrate specificity, and potential signaling function of the six C. albicans Gap proteins. S. cerevisiae mutant strains lacking their own transport systems have already served for the characterization of various C. albicans plasma membrane transporters including amino acid permeases, e.g., CaCan1, a permease for basic amino acids (40), the CaCnh1 Na+/H+ antiporter (32), or the CaCdr1 ATPase that exports drugs (51). To characterize the CaGap permeases' properties, we expressed them in several S. cerevisiae mutant strains lacking various combinations of their own amino acid transport systems and thus unable to transport one or more amino acids.
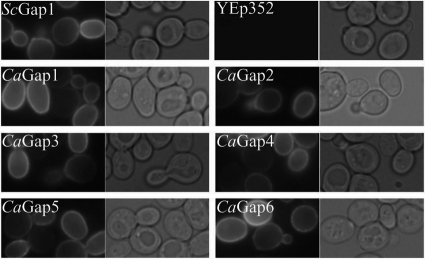
To express the C. albicans genes, we used the multicopy YEp352 vector and the S. cerevisiae NHA1 weak and constitutive promoter as we know from our previous work that this combination allows an optimal level of synthesis and proper targeting of heterologous transporters to the S. cerevisiae plasma membrane. To verify that the level of overexpression is not toxic to cells and that synthesized proteins reach the plasma membrane, we prepared a series of plasmids in which the CaGAP open reading frames (ORFs) were tagged at their 3′ termini with the GFP sequence. Growth of all strains was similar to that of the controls; i.e., the heterologous protein expression was not toxic, and as shown in Fig. 2, all six GFP-tagged C. albicans Gap permeases were localized to the cell periphery, as was the S. cerevisiae Gap1p. In this and all subsequent experiments, the ScGap1 permease (expressed under the same conditions as CaGaps) and the empty YEp352 vector served as positive and negative controls, respectively.
Fig. 2.
Expression of the six C. albicans Gap permeases in the S. cerevisiae M4584 strain. Cells transformed with pCaGAPs-GFP plasmids or pScGAP-GFP and empty YEp352, as positive and negative controls, respectively, were first grown to early exponential phase in minimal medium with ammonium sulfate and then transferred to minimal medium supplemented with 100 μg/ml of amino acids and observed in microscope after 4 h of cultivation. Leucine was used for cells transformed with pScGAP1-GFP and pCaGAP6-GFP; phenylalanine was used for cells transformed with pCaGAP2-GFP, pCaGAP3-GFP, and pCaGAP5-GFP; and arginine was used for cells transformed with pCaGAP1-GFP and pCaGAP4-GFP. GFP fluorescence and cells viewed by Nomarski optics are shown.
The six Candida Gap permeases differ in their substrate specificities and only CaGap2 should be considered to be a general amino acid permease.
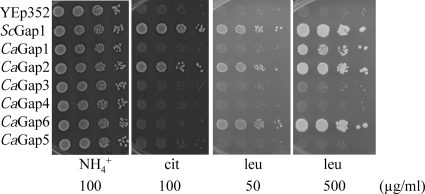
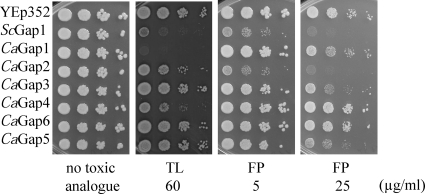
To determine the transport properties of the six putative CaGap proteins, they were expressed in S. cerevisiae strains deficient in the transport of various amino acids (Table 1). The HS100-3c strain does not transport arginine, lysine, and citrulline; the P3 strain is unable to grow on proline or citrulline as a sole source of nitrogen; and the M4584 strain is unable to use valine, leucine, isoleucine, phenylalanine, tryptophan, tyrosine, threonine, citrulline, or methionine as the sole source of nitrogen. We employed two methods to test the transport capacity and substrate specificity of the CaGap proteins. In the first approach, single amino acids as the sole source of nitrogen in minimal medium were used, and the growth of cells was screened. The second approach was based on the inhibitory effect of toxic amino acid analogues that enter the cells via amino acid permeases; i.e., the sensitivity of cells to such a toxic compound suggests that the tested permease transports this toxic analogue and probably also the corresponding amino acid. The second approach was used to confirm the results from the first approach, and in some cases where an amino acid could not be used as a sole source of nitrogen by S. cerevisiae cells (e.g., lysine), it served as an indicator of substrate specificity. To avoid possible nitrogen catabolite inactivation of the permeases, the experiments with toxic analogues were performed in minimal medium containing proline as a source of nitrogen. All of the toxic amino acid analogues inhibited the growth of cells expressing ScGAP1. Using the two approaches, we were able to directly test 11 amino acids and 8 toxic amino acid analogues (Table 4). The results obtained in growth experiments, i.e., the amino acids that are the substrates of the individual CaGaps, are summarized in Tables 6 and 7; examples of drop tests with amino acids as nitrogen sources or with toxic analogues are shown in Fig. 3 and 4, and the remaining results are shown in Fig. S1 in the supplemental material.
Table 6.
Substrate specificity of CaGap permeases
| Nitrogen source (amino acid) | Relative growth of cells with the indicated permeasea |
||||||
|---|---|---|---|---|---|---|---|
| ScGap1 | CaGap1 | CaGap2 | CaGap3 | CaGap4 | CaGap5 | CaGap6 | |
| Arg | ••• | •• | ••• | − | •• | − | − |
| Pro | ••• | − | •• | − | − | − | ••• |
| Met | ••• | • | ••• | − | − | − | ••• |
| Leu | ••• | • | ••• | − | − | − | ••• |
| Val | ••• | − | ••• | − | − | − | ••• |
| Ile | ••• | − | ••• | − | − | − | ••• |
| Phe | ••• | • | ••• | − | − | − | ••• |
| Trp | ••• | − | ••• | − | − | − | ••• |
| Thr | ••• | − | ••• | − | − | − | ••• |
| Tyr | ••• | − | ••• | − | − | − | ••• |
| Cit | ••• | − | ••• | − | − | − | − |
The table summarizes growth experiments (Fig. 3; see also Fig. S1 in the supplemental material) on minimal medium with amino acids as the sole sources of nitrogen. The number of dots corresponds to the relative level of growth. −, no growth.
Table 7.
Sensitivity of CaGap permeases to toxic amino acid analogues
| Toxic amino acid analogue | Relative growth inhibition of cells with the indicated permeasea |
||||||
|---|---|---|---|---|---|---|---|
| ScGap1 | CaGap1 | CaGap2 | CaGap3 | CaGap4 | CaGap5 | CaGap6 | |
| Canavanine | ○○○○○ | ○○○ | ○○○○ | − | ○○ | ○ | − |
| Thialysine | ○○○ | ○○○○○ | ○ | − | ○○ | − | − |
| Ethionine | ○○○ | − | ○○○ | ○ | ○ | ○○ | ○○ |
| Azaleucine | ○○○ | − | ○○○ | − | − | − | − |
| 3-Hydroxynorvaline | ○○○ | − | ○○ | − | − | − | ○○○○ |
| p-Fluoro-l-phenylalanine | ○○○ | − | ○○○ | ○○ | − | ○○ | ○ |
| 5-Fluoro-dl-tryptophan | ○○○ | − | ○○○ | ○○ | ○ | ○○ | − |
| 4-Methyl-dl-tryptophan | ○○○ | − | ○○ | ○ | − | ○ | − |
The table summarizes growth experiments (Fig. 4; see also Fig. S1 in the supplemental material) on minimal medium with proline as a source of nitrogen (100 μg/ml) and with various toxic amino acid analogues. The number of circles corresponds to the relative sensitivity to the indicated analogue (growth inhibition). −, not sensitive.
Fig. 3.
Growth of S. cerevisiae M4584 expressing CaGap permeases on minimal medium with three sources of nitrogen. Tenfold serial dilutions of yeast cell suspensions were prepared, 3 μl was spotted, and plates were incubated for 4 days at 30°C. Cells with empty YEp352 vector and cells expressing S. cerevisiae Gap1 served as negative and positive controls, respectively.
Fig. 4.
Growth of S. cerevisiae cells expressing CaGap permeases on minimal medium with proline (100 μg/ml) and toxic amino acid analogues. Tenfold serial dilutions of yeast cell suspensions were prepared, 3 μl was spotted, and plates were incubated for 4 days at 30°C. Cells with empty YEp352 vector and cells expressing S. cerevisiae Gap1 served as negative and positive controls, respectively. FP, p-fluoro-l-phenylalanine, toxic analogue of phenylalanine; TL, thialysine, toxic analogue of lysine. The M4584 strain was used for experiments with FP, and HS100-3C was used with TL.
These results clearly show that all six C. albicans Gap orthologues are able to transport amino acids though only one of them fulfils the criteria of being a general amino acid permease. Only CaGap2 transports all tested amino acids, including citrulline, which is transported uniquely by the Gap1 permease in S. cerevisiae (18, 46). This confirms the close relationship between the two permeases found by the phylogenetic analysis (Fig. 1). Also, CaGap6 transports the majority of tested amino acids, except for basic ones and citrulline, and can therefore be considered to be an amino acid transporter with rather broad substrate specificity. Similarly, CaGap1 is an amino acid permease capable of transporting several structurally unrelated amino acids, though probably with a lower capacity than the CaGap2 and CaGap6 permeases, as was shown for leucine and phenylalanine (Fig. 3; see also Fig. S1A in the supplemental material). The presence of a CaGap4 permease enabled the growth of S. cerevisiae cells only on arginine, and cells expressing the CaGap5 or CaGap3 permease were unable to grow on any of the 11 amino acids tested (Table 6). Nevertheless the second approach, evaluating the sensitivity of cells to toxic amino acid analogues, revealed that these three C. albicans permeases probably also transport more than one amino acid (Table 7 and Fig. 4; see also Fig. S1B). The use of thialysine revealed that three C. albicans Gap proteins, CaGap1, CaGap2, and CaGap4, are able to transport this compound and thus probably also lysine, and the different levels of sensitivity suggested that these permeases transport thialysine with different capacities (Table 7). Similarly, the use of p-fluoro-l-phenylalanine, a toxic analogue of phenylalanine, confirmed the results obtained with phenylalanine, but additionally the growth of strains expressing either CaGap3 or CaGap5 became sensitive at a higher dose, suggesting that these transporters are also able to transport phenylalanine.
It is worth noting that all the above-described experiments with untagged CaGap protein versions were repeated with cells expressing GFP-tagged proteins, and the same results were obtained, showing that the C-terminally GFP-tagged permeases are functional and that the attachment of GFP does not change their substrate specificities (data not shown).
Taken together, our data obtained in growth experiments suggested that all six CaGap proteins are functional amino acid permeases with different substrate specificities and that CaGap2 can be considered to be the closest homologue of ScGap1, both structurally and functionally.
Amino acid transport measurements confirm results obtained from growth experiments.
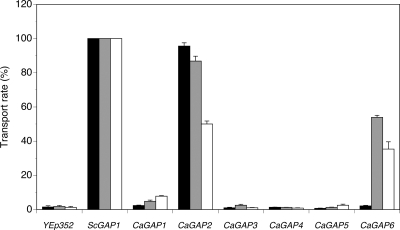
To confirm the results obtained in the growth experiments with amino acids and their toxic analogues, we measured the rate of uptake of 2 mM amino acids into nitrogen-starved cells. The transport of phenylalanine and leucine was followed in the same strain used in the growth experiments (M4584), and the transport activity for citrulline was analyzed in the 25.969b strain. In both strains, the results obtained with the negative control (cells transformed with an empty vector) confirmed the absence of transport activity for the tested amino acids, and positive-control cells expressing ScGap1 exhibited a relatively high transport capacity for all tested amino acids (Fig. 5). As proline seemed to be transported less via CaGap2 than via CaGap6 (see Fig. S1B in the supplemental material), the uptake of 2 mM proline was measured in strains expressing these two permeases and in the strain with ScGap1. Obtained results confirmed the prediction from drop tests that the proline uptake via CaGap2 was significantly lower than via ScGap1 and CaGap6 (10 nmol/min/mg of protein versus 30 and 23 nmol/min/mg of protein, respectively). The uptake measurements confirmed the results obtained in the growth assays and again clearly showed that CaGap2p is the true orthologue of ScGap1 permease, whereas the CaGap1 and CaGap6 proteins transport leucine, proline, and phenylalanine but not citrulline.
Fig. 5.
Uptake of amino acids into S. cerevisiae gap1Δ cells expressing CaGap permeases. Transport of 2 mM l-citrulline (black bars) was measured in 25.969b cells. Uptake of 2 mM l-leucine (gray bars) and 2 mM l-phenylalanine (white bars) was measured in M4584 cells. The level of transport is expressed as a percentage relative to the transport measured for cells expressing ScGap1.
Mistranslation of CUG codons in S. cerevisiae is not the reason for the lack of functionality of CaGap3 and CaGap5.
It is well known that CUG, a universal leucine codon, is, in most cases, translated into serine in C. albicans (44). In our experiments we used S. cerevisiae as a host for the functional characterization of C. albicans proteins, and therefore proteins that contain CUG codons could be mistranslated. CaGAP1, CaGAP4, and CaGAP6 genes do not contain any CUG codons and are therefore translated correctly in S. cerevisiae. CaGAP2 has one CUG codon at position 194; CaGAP3 has three at positions 13 (N terminus), 80 (N terminus), and 387 (8th transmembrane domain); and CaGAP5 has two at positions 55 (N terminus) and 324 (6th transmembrane domain). The putative positions of these residues within the structure of the CaGaps were estimated according to the analyzed structure of ScGap1 (17, 33). Whereas the Ser residues at the N termini of the Gap permeases are not significantly conserved and as the hydrophilic N terminus itself has never been shown to be very important for the transport capacity of ScGap1, the Ser residues localized within the transmembrane domains might play an important function in the binding and transport of amino acids or cotransported protons. Alignment of the two transmembrane domains containing Ser residues encoded by the CUG codons in CaGap3 and CaGap5 proteins (Fig. 6) revealed a certain level of conservation in the 6th domain and a highly conserved seryl residue in the 8th transmembrane domain.
Fig. 6.
Comparison of the amino acid sequences of the 6th and 8th transmembrane domains of general amino acid permeases.
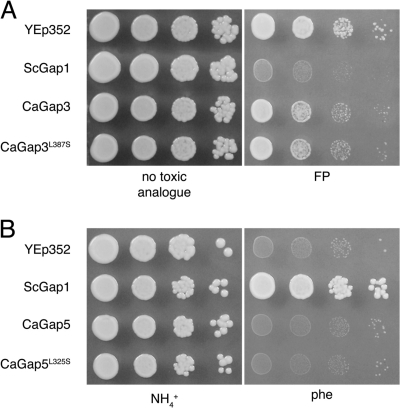
According to the results described above (e.g., Tables 6 and 7 and Fig. 3 to 5), the CUG codon present in CaGAP2 probably does not influence the activity of this permease in S. cerevisiae. On the other hand, the expression of CaGAP3 or CaGAP5 in S. cerevisiae did not result in a clear transport activity. To determine the importance of CUG-encoded Ser residues in transmembrane domains 6 and 8 for the functionality of CaGap3 and CaGap5, we mutated the CUG to UCG (translated as serine in S. cerevisiae) at the positions of the genes (cloned in pCaGAP3 and pCaGAP5 plasmids) corresponding to amino acid residue 387 for CaGap3 and 324 for CaGap5 (Fig. 6). Growth experiments with various amino acids as the sole sources of nitrogen or with toxic amino acid analogues performed as described for Fig. 3 and 4 did not exhibit any differences between the original CaGaps and the mutated versions, CaGap3 with the L387S mutation [CaGap3(L387S)] and CaGap5(L324S) (examples are shown in Fig. 7). We can thus conclude that neither of the two seryl residues encoded by the CUG codons and located within the putative transmembrane domains of CaGap3 and CaGap5 are crucial for their permease activity.
Fig. 7.
Growth of S. cerevisiae M4584 cells expressing CaGap3 permease and its mutated L387S version on minimal medium with proline (100 μg/ml) and a toxic analogue of phenylalanine (FP, p-fluoro-l-phenylalanine; 25 μg/ml) (A) and of cells expressing CaGap5 permease and its mutated L325S version on minimal medium with phenylalanine (500 μg/ml) as a nitrogen source (B). Tenfold serial dilutions of yeast cell suspensions were prepared, 3 μl was spotted, and plates were incubated for 4 days at 30°C. Cells with empty YEp352 vector and cells expressing S. cerevisiae Gap1 served as negative and positive controls, respectively.
Three of six CaGap permeases participate in amino acid-induced activation of trehalase in S. cerevisiae lacking ScGAP1.
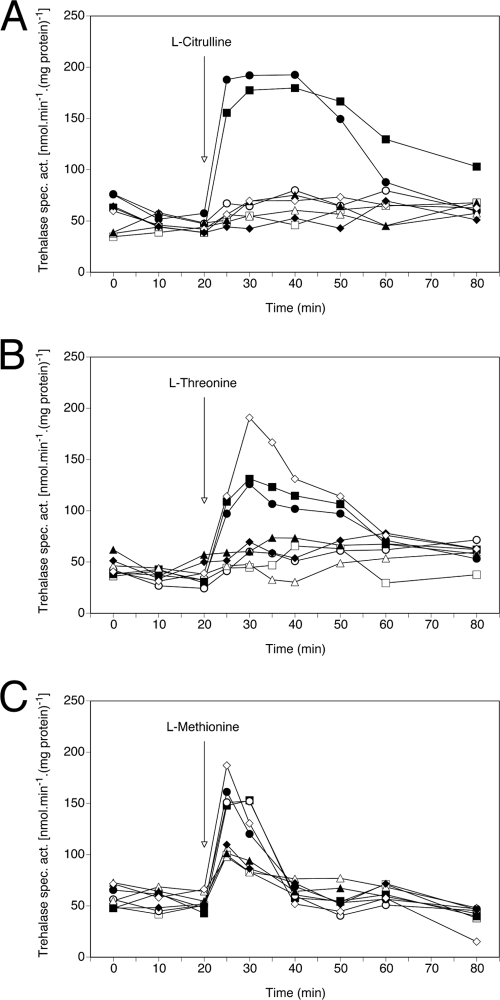
As mentioned above, ScGap1 functions as a transceptor; i.e., it serves both as an amino acid transporter and an amino acid sensor activating protein kinase A (15, 59). For C. albicans it would be extremely important if a similar sensing system was present as it is well known that the PKA pathway is important for fungal morphogenesis and virulence (5, 24), and amino acid induction of this pathway would constitute a new input signal. Again, as we do not yet have C. albicans strains expressing only one of the GAP genes, we tested whether the CaGap proteins also function as transceptors when they are expressed in S. cerevisiae cells lacking their own Gap1 permease (strain 25.969b). The signaling function was followed as an amino acid-induced activation of one of the PKA pathway targets, neutral trehalase (15). Figure 8 A shows that CaGap2p is the only permease (of the six C. albicans Gap proteins) able to activate trehalase after the addition of citrulline. This is in agreement with the observed substrate specificities (Table 6 and Fig. 3). The level of activation via CaGap2p is similar to that mediated by ScGap1p. Two of the C. albicans Gap proteins, CaGap2 and CaGap6, are involved in trehalase activation by the addition of threonine to nitrogen-starved cells (Fig. 8B). The addition of methionine results in a rapid and significant increase in trehalase activity in four tested strains. In addition to ScGap1, CaGap2, and CaGap6, CaGap1 also seems to be involved in signaling of the presence of this amino acid (Fig. 8C). With methionine, however, a certain level of trehalase activation is also visible in cells expressing the other three C. albicans permeases and even in cells transformed with an empty vector (Fig. 8C). This observation suggests that apart from ScGap1, another of the S. cerevisiae amino acid permeases (active under nitrogen starvation conditions in the 25.969b strain) may also be involved in signaling the presence of methionine, e.g., the methionine-specific permease Mup1. This ScGap1-independent activation of trehalase upon adding methionine has been previously observed (G. Van Zeebroeck, unpublished results).
Fig. 8.
Three CaGap proteins activate the PKA target trehalase in S. cerevisiae. Activation of trehalase by the addition of 10 mM citrulline (A), threonine (B), and methionine (C) to 25.969b cells expressing C. albicans general amino acid permeases and nitrogen starved for 24 h. ▪, ScGap1 (positive control); □, YEp352 (negative control); ○, CaGap1; •, CaGap2; ▵, CaGap3; ▴, CaGap4; ⧫, CaGap5; ⋄, CaGap6.
In summary, we show that the S. cerevisiae general amino acid permease (that served as a positive control in all our experiments) is involved in the activation of PKA targets upon adding various (three tested in our experiments) amino acids to nitrogen-starved cells. Taken together, the observed activation of S. cerevisiae trehalase by three C. albicans permeases suggests that similar mechanisms of amino acid sensing and signaling targets (upon adding amino acids to cells starved for nitrogen on a glucose-containing medium) may exist in C. albicans.
DISCUSSION
In this work, we analyzed the activity and substrate specificity of six members of the C. albicans amino acid permease family that are the closest orthologues of the S. cerevisiae general amino acid permease Gap1. As the construction of C. albicans deletion mutants lacking individual GAP genes has not fully elucidated the substrate specificity and uptake capacity of putative Gap permeases (L. Kraidlova, unpublished results), the properties of individual CaGap proteins were studied upon expression in S. cerevisiae. Previous work dealing with C. albicans GAP genes concentrated on their transcriptional regulation under various conditions (1, 35, 61), and with one exception (see below), the transport properties and localization of encoded proteins have never been tested. Our results showed that all six proteins are targeted to the plasma membrane of S. cerevisiae cells, and all of them demonstrate at least some activity features typical of amino acid permeases; their presence either improves the growth of cells on amino acids used as sole nitrogen sources or increases the sensitivity of cells to amino acid toxic analogues that enter cells via these permeases.
Though the six C. albicans Gap proteins share a high level of sequence identity (approximately 55%), they differ substantially in their substrate specificities and transport capacities. The only one whose performance highly resembles that of S. cerevisiae Gap1 is CaGap2. It efficiently transports all tested amino acids, including the ScGap1-specific substrate citrulline. All five other CaGaps have narrower substrate specificities. Surprisingly, we did not observe any significant involvement of the CaGap1 permease in citrulline uptake, either in growth tests or in direct transport measurements. This is different from a previous study, where it was shown that the expression of CaGap1 in another yeast gap1 mutant did increase the citrulline uptake by a factor of 2.5 (4). In contrast, in our experiments, the expression of CaGap2 increased the citrulline uptake rate by an approximate factor of 50 compared to the negative control.
Besides CaGap2, only CaGap1 and CaGap4 transport basic amino acids, and CaGap4 seems to be specific for them (Tables 6 and 7). It is worth noting that there are five other genes encoding putative basic amino acid-specific permeases (three CAN1 genes, LYP1 and ALP1 [Kraidlova, unpublished]) in the C. albicans genome. The multiplication of genes encoding permeases for basic amino acids in C. albicans cells (in total, at least eight compared to four in S. cerevisiae) suggests that arginine and lysine might be among their preferred amino acids. CaGap6, on the other hand, has rather broad substrate specificity and transports various structurally unrelated amino acids, but not basic ones or citrulline.
CaGap2 and CaGap6 also transport leucine, valine, and isoleucine, similarly to ScGap1, though ScGap1 is not a high-affinity permease for branched-chain amino acids (57). In S. cerevisiae, there are Bap permeases specialized in the uptake of branched-chain amino acids (12, 14, 57). Bap permeases transport leucine, valine, and isoleucine in S. cerevisiae with high affinity, and the main one, ScBap2, has a much higher transport capacity for leucine than for valine and isoleucine (57). Surprisingly, CaGap1 transports only leucine and no other branched-chain amino acids, at least not with a moderate or high affinity. The existence of three Gaps able to transport leucine might reflect the absence of BAP genes in the C. albicans genome (Kraidlova, unpublished). This absence found in our in silico search was confirmed by the decreased ability of a C. albicans strain lacking the GAP2 gene to use leucine as a source of nitrogen (Kraidlova, unpublished).
Three of the studied CaGaps were not very efficient transporters of the tested amino acids (Table 6). They might transport other amino acids that could not be tested in our strains (since these strains have efficient permeases for them), e.g., His, Glu, Gln, Asp, Asn, Gly, and Ala. To confirm this presumption, a new S. cerevisiae strain (lacking, compared to the M4584 mutant, an additional 7 to 8 genes for amino acid permeases) will need to be constructed and transformed with plasmids bearing CaGAP genes, and similar tests as described above should be performed in order to determine their substrate specificities and check for trehalase activation as we cannot exclude the possibility that the observed inability of these three permeases to activate trehalase was due to the use of inappropriate substrates.
In general, the use of the toxic amino acid analogues confirmed the results from the growth experiments on media with amino acids as the sole sources of nitrogen. Nevertheless, in a few cases, the growth in the presence of certain amino acids did not correspond to the absence of growth when their toxic counterparts were tested and vice versa. For example, upon expression in S. cerevisiae, the CaGap1 permease transports methionine, phenylalanine, and leucine but apparently not their toxic analogues since the strain expressing this transporter still grows in the presence of the highest-used concentrations of the corresponding toxic analogues. In contrast, CaGap3 cells do not grow on any tested media with amino acids as the sole sources of nitrogen, but they are sensitive to toxic analogues of methionine, phenylalanine, tryptophan, and tyrosine. (see Fig. 3 and 4 and Fig. S1 in the supplemental material). This apparent discrepancy could probably be explained as a high difference in the affinities of these permeases to an amino acid and its corresponding analogue, phenomena observed already for S. cerevisiae amino acid permeases (25).
In S. cerevisiae, Gap1 functions as a transceptor activating PKA targets upon detecting amino acids and in a cAMP-independent way (15). An important target of the PKA pathway in yeast is the yeast-to-pseudohypha transition mediated by Flo11 (49, 58). The addition of serum or amino acids to C. albicans cells also results in morphogenesis (19). This is, e.g., the case when cells are grown on Lee medium, a medium rich in amino acids (34). The underlying mechanism of this amino acid-induced morphogenesis is not understood. Here, we show that some C. albicans amino acid transporters have the ability to rapidly activate trehalase, a target of PKA, upon expression in S. cerevisiae, and so we may have discovered the link between the detection of amino acids and morphogenesis. That such links may exist in C. albicans was previously shown using microarray and mutation analyses. The induction of morphogenesis by the addition of serum results in the upregulation of CaGAP4 in a cAMP-independent way (20). The deletion of CaGAP1 results in a morphogenesis defect on solid synthetic low-ammonium dextrose (SLAD) medium (4).
Whereas the C. albicans Gap transporters (or at least some of them) may function as transceptors for the activation of the PKA pathway, another amino acid sensor was described previously as being involved in morphogenesis. The CaCsy1 sensor is required not only for the expression of amino acid permeases and the increased uptake of some amino acids but also for morphogenesis on amino acid-containing medium such as solid serum or Lee medium (8). It has been shown that the expression of CaGap1 and CaGap2 is under the control of CaCsy1 (8). Whether the expression of the other four GAP genes is also under the control of Csy1 remains to be established. These results clearly point toward a possible network of amino acid-sensing mechanisms, with CaCsy1 responsible for the amino acid-induced expression of some CaGAP genes that are then responsible for the activation of PKA and subsequent induction of morphogenesis. We are currently investigating whether this is indeed the case in C. albicans cells.
Since Gap permeases are involved in amino acid-induced morphogenesis, they are potentially interesting targets for antifungals, especially as there are no close homologues in humans. The fact that there are six general amino acid permeases in C. albicans (in addition to 21 probably more specific amino acid permeases) also points toward a possible important virulence factor. Many gene families that have been described in C. albicans and of which there are only one or two gene copies in S. cerevisiae have been shown to be involved in virulence. Examples are the families of genes encoding phospholipases, lipases, adhesins, or secreted aspartyl proteases.
Taken together, our results clearly show that at least three of the six studied genes (CaGAP1, CaGAP2, and CaGAP6) encode functional and efficient amino acid permeases with rather broad but not completely overlapping substrate specificities and the ability to participate in amino acid sensing. Our future work will focus on characterizing their roles in C. albicans physiology and on elucidating the transport properties and roles of the CaGap3, CaGap4, and CaGap5 permeases.
Supplementary Material
ACKNOWLEDGMENTS
We thank B. André for the P3 strain, B. Regenberg for the M4584 strain, and M. Rubio-Texeira for the pCK230 plasmid.
This work was supported by the MSMT LC531 and AV0Z 50110509 grants, by the Flanders-Czech Republic bilateral project BIL05/54, and by the Fund for Scientific Research Flanders (G.0242.04).
Footnotes
Supplemental material for this article may be found at http://ec.asm.org/.
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Alvarez F. J., Konopka J. B. 2007. Identification of an N-acetylglucosamine transporter that mediates hyphal induction in Candida albicans. Mol. Biol. Cell 18:965–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andre B. 1995. An overview of membrane transport proteins in Saccharomyces cerevisiae. Yeast 11:1575–1611 [DOI] [PubMed] [Google Scholar]
- 3. Arnaud M. B., et al. 2007. Sequence resources at the Candida Genome Database. Nucleic Acids Res. 35:D452–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Biswas S., Roy M., Datta A. 2003. N-acetylglucosamine-inducible CaGAP1 encodes a general amino acid permease which co-ordinates external nitrogen source response and morphogenesis in Candida albicans. Microbiology 149:2597–2608 [DOI] [PubMed] [Google Scholar]
- 5. Biswas S., Van Dijck P., Datta A. 2007. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 71:348–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonini B. M., Van Dijck P., Thevelein J. M. 2003. Uncoupling of the glucose growth defect and the deregulation of glycolysis in Saccharomyces cerevisiae Tps1 mutants expressing trehalose-6-phosphate-insensitive hexokinase from Schizosaccharomyces pombe. Biochim. Biophys. Acta 1606:83–93 [DOI] [PubMed] [Google Scholar]
- 7. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 8. Brega E., Zufferey R., Mamoun C. B. 2004. Candida albicans Csy1p is a nutrient sensor important for activation of amino acid uptake and hyphal morphogenesis. Eukaryot. Cell 3:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cannon R. D., et al. 2009. Efflux-mediated antifungal drug resistance. Clin. Microbiol. Rev. 22:291–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen E. J., Kaiser C. A. 2002. Amino acids regulate the intracellular trafficking of the general amino acid permease of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U. S. A. 99:14837–14842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cherry J. M., et al. 1997. Genetic and physical maps of Saccharomyces cerevisiae. Nature 387:67–73 [PMC free article] [PubMed] [Google Scholar]
- 12. De Boer M., et al. 1998. Regulation of expression of the amino acid transporter gene BAP3 in Saccharomyces cerevisiae. Mol. Microbiol. 30:603–613 [DOI] [PubMed] [Google Scholar]
- 13. De Rosa F. G., Garazzino S., Pasero D., Di Perri G., Ranieri V. M. 2009. Invasive candidiasis and candidemia: new guidelines. Minerva Anestesiol. 75:453–458 [PubMed] [Google Scholar]
- 14. Didion T., Grauslund M., Kielland-Brandt M. C., Andersen H. A. 1996. Amino acids induce expression of BAP2, a branched-chain amino acid permease gene in Saccharomyces cerevisiae. J. Bacteriol. 178:2025–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Donaton M. C., et al. 2003. The Gap1 general amino acid permease acts as an amino acid sensor for activation of protein kinase A targets in the yeast Saccharomyces cerevisiae. Mol. Microbiol. 50:911–929 [DOI] [PubMed] [Google Scholar]
- 16. During-Olsen L., Regenberg B., Gjermansen C., Kielland-Brandt M. C., Hansen J. 1999. Cysteine uptake by Saccharomyces cerevisiae is accomplished by multiple permeases. Curr. Genet. 35:609–617 [DOI] [PubMed] [Google Scholar]
- 17. Gilstring C. F., Ljungdahl P. O. 2000. A method for determining the in vivo topology of yeast polytopic membrane proteins demonstrates that Gap1p fully integrates into the membrane independently of Shr3p. J. Biol. Chem. 275:31488–31495 [DOI] [PubMed] [Google Scholar]
- 18. Grenson M., Hou C., Crabeel M. 1970. Multiplicity of the amino acid permeases in Saccharomyces cerevisiae. IV. Evidence for a general amino acid permease. J. Bacteriol. 103:770–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall R. A., Muhlschlegel F. A. 2010. A multi-protein complex controls cAMP signalling and filamentation in the fungal pathogen Candida albicans. Mol. Microbiol. 75:534–537 [DOI] [PubMed] [Google Scholar]
- 20. Harcus D., Nantel A., Marcil A., Rigby T., Whiteway M. 2004. Transcription profiling of cyclic AMP signaling in Candida albicans. Mol. Biol. Cell 15:4490–4499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Helliwell S. B., Losko S., Kaiser C. A. 2001. Components of a ubiquitin ligase complex specify polyubiquitination and intracellular trafficking of the general amino acid permease. J. Cell Biol. 153:649–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hill J. E., Myers A. M., Koerner T. J., Tzagoloff A. 1986. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast 2:163–167 [DOI] [PubMed] [Google Scholar]
- 23. Hoffman C. S., Winston F. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267–272 [DOI] [PubMed] [Google Scholar]
- 24. Hogan D. A., Sundstrom P. 2009. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans. Future Microbiol. 4:1263–1270 [DOI] [PubMed] [Google Scholar]
- 25. Horak J. 1997. Yeast nutrient transporters. Biochim. Biophys. Acta 1331:41–79 [DOI] [PubMed] [Google Scholar]
- 26.Reference deleted.
- 27. Hruskova-Heidingsfeldova O. 2008. Secreted proteins of Candida albicans. Front. Biosci. 13:7227–7242 [DOI] [PubMed] [Google Scholar]
- 28. Jauniaux J. C., Grenson M. 1990. GAP1, the general amino acid permease gene of Saccharomyces cerevisiae. Nucleotide sequence, protein similarity with the other bakers yeast amino acid permeases, and nitrogen catabolite repression. Eur. J. Biochem. 190:39–44 [DOI] [PubMed] [Google Scholar]
- 29.Reference deleted.
- 30. Jones T., et al. 2004. The diploid genome sequence of Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 101:7329–7334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kinclova O., Ramos J., Potier S., Sychrova H. 2001. Functional study of the Saccharomyces cerevisiae Nha1p C-terminus. Mol. Microbiol. 40:656–668 [DOI] [PubMed] [Google Scholar]
- 32. Kinclova-Zimmermannova O., Sychrova H. 2007. Plasma-membrane Cnh1 Na+/H+ antiporter regulates potassium homeostasis in Candida albicans. Microbiology 153:2603–2612 [DOI] [PubMed] [Google Scholar]
- 33. Kota J., Gilstring C. F., Ljungdahl P. O. 2007. Membrane chaperone Shr3 assists in folding amino acid permeases preventing precocious ERAD. J. Cell Biol. 176:617–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee K. L., Buckley H. R., Campbell C. C. 1975. An amino acid liquid synthetic medium for the development of mycelial and yeast forms of Candida albicans. Sabouraudia 13:148–153 [DOI] [PubMed] [Google Scholar]
- 35. Liao W. L., Ramon A. M., Fonzi W. A. 2008. GLN3 encodes a global regulator of nitrogen metabolism and virulence of C. albicans. Fungal Genet. Biol. 45:514–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ljungdahl P. O. 2009. Amino-acid-induced signalling via the SPS-sensing pathway in yeast. Biochem. Soc. Trans. 37:242–247 [DOI] [PubMed] [Google Scholar]
- 37. Ljungdahl P. O., Gimeno C. J., Styles C. A., Fink G. R. 1992. SHR3: a novel component of the secretory pathway specifically required for localization of amino acid permeases in yeast. Cell 71:463–478 [DOI] [PubMed] [Google Scholar]
- 38. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. 1951. Protein measurement with the folin phenol reagent. J. Biol. Chem. 193:265–275 [PubMed] [Google Scholar]
- 39. Martinez P., Ljungdahl P. O. 2004. An ER packaging chaperone determines the amino acid uptake capacity and virulence of Candida albicans. Mol. Microbiol. 51:371–384 [DOI] [PubMed] [Google Scholar]
- 40. Matejckova A., Sychrova H. 1996. Properties of Candida albicans CAN1 permease expressed in Saccharomyces cerevisiae. Folia Microbiol. 41:107–109 [DOI] [PubMed] [Google Scholar]
- 41. Naglik J., Albrecht A., Bader O., Hube B. 2004. Candida albicans proteinases and host/pathogen interactions. Cell Microbiol. 6:915–926 [DOI] [PubMed] [Google Scholar]
- 42. Naglik J. R., Challacombe S. J., Hube B. 2003. Candida albicans secreted aspartyl proteinases in virulence and pathogenesis. Microbiol. Mol. Biol. Rev. 67:400–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Noble S. M., Johnson A. D. 2007. Genetics of Candida albicans, a diploid human fungal pathogen. Annu. Rev. Genet. 41:193–211 [DOI] [PubMed] [Google Scholar]
- 44. Ohama T., et al. 1993. Non-universal decoding of the leucine codon CUG in several Candida species. Nucleic Acids Res. 21:4039–4045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pernambuco M. B., et al. 1996. Glucose-triggered signalling in Saccharomyces cerevisiae: different requirements for sugar phosphorylation between cells grown on glucose and those grown on non-fermentable carbon sources. Microbiology 142:1775–1782 [DOI] [PubMed] [Google Scholar]
- 46. Regenberg B., Kielland-Brandt M. C. 2001. Amino acid residues important for substrate specificity of the amino acid permeases Can1p and Gnp1p in Saccharomyces cerevisiae. Yeast 18:1429–1440 [DOI] [PubMed] [Google Scholar]
- 47. Rossignol T., et al. 2008. CandidaDB: a multi-genome database for Candida species and related Saccharomycotina. Nucleic Acids Res. 36:D557–D561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rupp S. 2004. Proteomics on its way to study host-pathogen interaction in Candida albicans. Curr. Opin. Microbiol. 7:330–335 [DOI] [PubMed] [Google Scholar]
- 49. Rupp S., Summers E., Lo H. J., Madhani H., Fink G. 1999. MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 18:1257–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Saitou N., Nei M. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406–425 [DOI] [PubMed] [Google Scholar]
- 51. Shukla S., et al. 2003. Functional characterization of Candida albicans ABC transporter Cdr1p. Eukaryot. Cell 2:1361–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Soetens O., De Craene J. O., Andre B. 2001. Ubiquitin is required for sorting to the vacuole of the yeast general amino acid permease, Gap1. J. Biol. Chem. 276:43949–43957 [DOI] [PubMed] [Google Scholar]
- 53. Sophianopoulou V., Diallinas G. 1995. Amino acid transporters of lower eukaryotes: regulation, structure and topogenesis. FEMS Microbiol. Rev. 16:53–75 [DOI] [PubMed] [Google Scholar]
- 54. Stanbrough M., Magasanik B. 1995. Transcriptional and posttranslational regulation of the general amino acid permease of Saccharomyces cerevisiae. J. Bacteriol. 177:94–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sychrova H., Chevallier M. R. 1993. Cloning and sequencing of the Saccharomyces cerevisiae gene LYP1 coding for a lysine-specific permease. Yeast 9:771–782 [DOI] [PubMed] [Google Scholar]
- 56. Thevelein J. M. 1994. Signal transduction in yeast. Yeast 10:1753–1790 [DOI] [PubMed] [Google Scholar]
- 57. Tullin S., Gjermansen C., Kielland-Brandt M. C. 1991. A high-affinity uptake system for branched-chain amino acids in Saccharomyces cerevisiae. Yeast 7:933–941 [DOI] [PubMed] [Google Scholar]
- 58. van Dyk D., Pretorius I. S., Bauer F. F. 2005. Mss11p is a central element of the regulatory network that controls FLO11 expression and invasive growth in Saccharomyces cerevisiae. Genetics 169:91–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Zeebroeck G., Bonini B. M., Versele M., Thevelein J. M. 2009. Transport and signaling via the amino acid binding site of the yeast Gap1 amino acid transceptor. Nat. Chem. Biol. 5:45–52 [DOI] [PubMed] [Google Scholar]
- 60. Zaman S., Lippman S. I., Zhao X., Broach J. R. 2008. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 42:27–81 [DOI] [PubMed] [Google Scholar]
- 61. Zeng Y. B., Qian Y. S., Ma L., Gu H. N. 2007. Genome-wide expression profiling of the response to terbinafine in Candida albicans using a cDNA microarray analysis. Chin Med. J. 120:807–813 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.