Abstract
Many types of cells migrate directionally in direct current (DC) electric fields (EFs), a phenomenon termed galvanotaxis or electrotaxis. The directional sensing mechanisms responsible for this response to EFs, however, remain unknown. Exposing cells to an EF causes changes in plasma membrane potentials (Vm). Exploiting the ability of Dictyostelium cells to tolerate drastic Vm changes, we investigated the role of Vm in electrotaxis and, in parallel, in chemotaxis. We used three independent factors to control Vm: extracellular pH, extracellular [K+], and electroporation. Changes in Vm were monitored with microelectrode recording techniques. Depolarized Vm was observed under acidic (pH 5.0) and alkaline (pH 9.0) conditions as well as under higher extracellular [K+] conditions. Electroporation permeabilized the cell membrane and significantly reduced the Vm, which gradually recovered over 40 min. We then recorded the electrotactic behaviors of Dictyostelium cells with a defined Vm using these three techniques. The directionality (directedness of electrotaxis) was quantified and compared to that of chemotaxis (chemotactic index). We found that a reduced Vm significantly impaired electrotaxis without significantly affecting random motility or chemotaxis. We conclude that extracellular pH, [K+], and electroporation all significantly affected electrotaxis, which appeared to be mediated by the changes in Vm. The initial directional sensing mechanisms for electrotaxis therefore differ from those of chemotaxis and may be mediated by changes in resting Vm.
INTRODUCTION
Cells migrate directionally in response to many extracellular cues including chemical gradients (chemotaxis), topography, mechanical forces (mechanotaxis/durataxis), and electrical fields (EFs) (electrotaxis/galvanotaxis) (1, 3, 8, 15, 27). Electric fields have long been suggested to be a candidate directional signal for cell migration in development, wound healing, and regeneration. The mechanisms used by cells to sense the weak direct current (DC) EFs, however, have remained very poorly understood.
One of the immediate effects felt by a cell upon exposure to an EF is a change in the cell membrane potentials (Vm). In an EF, the plasma membrane facing the cathode depolarizes while the membrane facing the anode hyperpolarizes (17, 18). It has been proposed that the changes in Vm may underlie electrotaxis. In a cell with negligible voltage-gated conductance, the hyperpolarized membrane facing the anode attracts Ca2+ by passive electrochemical diffusion. This side of the cell then contracts, thereby propelling the cell toward the cathode. In a cell with voltage-gated Ca2+ channels, channels near the cathodal (depolarized) side open, thereby allowing Ca2+ influx. Intracellular Ca2+ levels will rise both on the anodal side and on the cathodal side in such a cell. The direction of cell movement in this situation will depend on the balance between the opposing contractile forces (17). The role of Vm in electrotaxis has not yet been directly tested.
In this report, we used Dictyostelium cells to test this directly. Dictyostelium cells show robust electrotaxis and tolerate significant changes in Vm while maintaining good motility under conditions of different extracellular pH values and ion concentrations and even following electroporation (20, 25, 29). These features make Dictyostelium cells a unique testing model. We quantified electrotaxis and chemotaxis of cells with well-controlled Vms by varying three independent factors. We found that the Vm indeed regulated electrotaxis while having no effect on chemotaxis. We thus identified a contrasting role of Vm between electrotaxis and chemotaxis which may underlie the mechanisms used by cells to sense weak dc EFs.
MATERIALS AND METHODS
Cell culture and development.
Dictyostelium discoideum AX3 cells were grown axenically in HL5 medium. Vegetative cells were washed and starved in development buffer (DB) and then were pulsed with 50 nM cyclic AMP (cAMP) every 6 min for an additional 4 h (29). All procedures were carried out at room temperature (∼22°C).
Micropipette chemotaxis assay.
Chemotaxis experiments were performed as reported (4, 10). Briefly, 20 μl of cells (1 × 105 to 4 × 105 cells/ml) in DB were seeded onto a coverslip chamber. Bathing solutions with different pH values or different K+ concentrations were then introduced. A Femtotip microinjection needle filled with 10 μM cAMP was placed into the field, and a positive pressure of 25 lb/in2 was applied via a connected microinjector. Chemotaxis was recorded by time-lapse video using an inverted microscope (CKX41; Olympus) with a 10× objective lens. Images were taken every 30 s for 30 min.
Electrotaxis assay.
Electrotaxis experiments were carried out as described previously (21, 28, 29). Developed cells were seeded into an electrotactic chamber. After 10 min of incubation, unattached cells were removed by gently washing with DB. Cells were then bathed in defined buffers, as indicated, with different pH values or different K+ concentrations in parallel with the chemotaxis assay. For cells treated with electroporation, normal DB (pH 6.5, 5 mM K+) was used, and the EF was switched on 10 min after seeding.
The applied EF was maintained at 12 V/cm for 30 min. Time-lapse images of cell migration were acquired using an inverted microscope (Axiovert 40; Carl Zeiss) equipped with a charge-coupled-device (CCD) camera (C4742-95; Hamamatsu Corporation) and a motorized XYZ stage (BioPoint 2; Ludl Electronic Products, Ltd.), and controlled by Simple PCI, version 5.3, imaging software.
Quantitative analysis of electrotaxis and chemotaxis.
Chemotaxis and electrotaxis were analyzed as previously described (4, 29). The chemotactic index and electrotactic index (directedness) were used to quantify how, directionally, cells migrated toward cAMP or in response to an EF, respectively. To calculate the chemotactic index or electrotactic index, the cosine of the angle between the direction of movement and the direction of the chemoattractant gradient or electric vector was determined (29). For migration speed, we used trajectory and displacement speeds (29). Persistency was further calculated as the shortest linear distance between the start and endpoints of the migration path divided by the total distance traveled by a cell. All motile isolated cells were analyzed. At least 30 cells from three independent experiments were analyzed.
Membrane potential (Vm) measurements.
Cells were seeded on a sterile glass coverslip and observed with a 60× objective. Vm measurements were conducted using fine-tipped glass pipette microelectrodes. Two types of recording, transient and continuous, were used to verify each other. The pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL) using a PP-830 pipette puller (Narishige International, Inc., New York, NY), and the resistance was ∼20 MΩ when the pipettes were filled with 3 M KCl solution, as measured in the DB. Recordings were performed using a GeneClamp 500 amplifier (Axon Instrument/Molecular Devices, Union City, CA). The signals were digitally filtered at 1 kHz and digitized at 2 kHz using a Digidata 1322A digitizer and pClamp, version 9.0, software (Axon Instrument/Molecular Devices).
DB solution was used as the standard recording solution. The solutions with higher or lower pH values were obtained by adding HCl or NaOH. The solutions with different K+ concentrations were made by using 3 M KCl and normal DB. All experiments were conducted at room temperature, and the recording was repeated in 16 or more cells.
Both transient impolement recording and continuous recording yielded similar Vm, which were consistent with previously published results (24).
Modulation of membrane potential (Vm).
The first method used to control Vm was to maintain cells in four bathing solutions with pH values of 5.0, 6.5, 7.5, and 9.0. All solutions were autoclaved and stored at room temperature until use. Before measurement of Vm or chemotaxis and electrotaxis experiments, cells were bathed in a defined solution for ∼10 min. The second method modulated Vm by adjusting the recording buffer [K+] at three concentrations (5 mM, 25 mM, and 50 mM). The K+ concentrations were verified with an ion-selective probe. All solutions were autoclaved. The third method was electroporation, which was carried out as previously described (7, 11). Electroporation was performed in a Gene Pulser Xcell Electroporation System (Bio-Rad) with two pulses of 0.85 kV/25 μF with a resistance-capacitance (RC) time of 1 ms, separated by a 5-s interval. For Vm measurements, 20 μl of cell suspension was immediately taken out of the electroporation chamber and placed in a petri dish in DB and measured 10 min later.
Statistics.
Pearson's correlation coefficient and a chi-square test were performed when pertinent. All data points were presented as means ± standard errors of the means (SEM) averaged from at least three measurements.
RESULTS
Extracellular pH and K+ concentration regulated Vm in Dictyostelium cells.
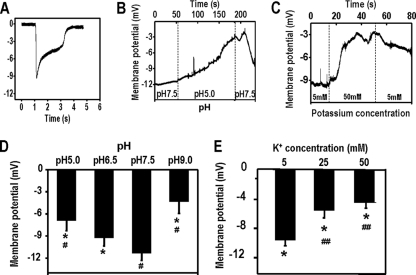
A typical negative peak potential was transiently observed upon impalement of a Dictyostelium cell with a microelectrode. Vm transiently reached a peak value within several milliseconds of impalement; the potential quickly decreased due to leakage (Fig. 1A). The initial peak value was used to reflect the Vm, as in previous studies (24, 25). The values were verified with the continuous measurement. We achieved continuous recording using microelectrodes with an extremely fine tip (resistance up to 30 MΩ) that penetrates a cell so the Vm can be reliably monitored continuously for a much longer time, usually several minutes in contrast to milliseconds in the first method (Fig. 1B and C).
Fig. 1.
Extracellular pH and K+ concentration regulate the membrane potential (Vm) of Dictyostelium cells. Developed Dictyostelium cells were bathed in DB with different pH values and K+ concentrations. Vm was measured by microelectrode impalement. (A) Typical peak-shaped potential transient that was recorded upon microelectrode penetration of a D. discoideum cell bathed in DB with different pH values or K+ concentrations. (B and C) Continuous recording of Vm with an extra-fine electrode showed stable stationary Vm of D. discoideum. The dotted vertical line indicates the time when the bathing solution was replaced with a buffer of a different pH value or different K+ concentration. (D) Averaged Vms from 21 cells (pH 5.0), 16 cells (pH 6.5), 16 cells (pH 7.5), and 16 cells (pH 9.0). *, P < 0.001 compared to that in pH 7.5; #, P < 0.001 compared to that in pH 6.5. (E) Averaged Vms from 13 cells (5 mM K+), 16 cells (25 mM K+), and 24 cells (50 mM K+). *, P < 0.001 compared to that in buffer with K+ concentration of 0.5 mM K+; #, P < 0.001 compared to that in K+ concentration of 5 mM K+.
We first quantified the effect of extracellular pH and [K+] on Vm. Being bathed in solutions of different pH values and K+ concentrations resulted in significant and consistent changes in Vm (Fig. 1). An example of continuous recording of the dynamic change in Vm, in which extracellular pH dropped from 7.5 to 5.0 and then went back up to 7.5, showed that the Vm remained stable and was responsive to pH changes over several minutes (Fig. 1B). The membrane significantly depolarized when the pH either dropped to 5.5 or increased to 9.0 (Fig. 1D). The maximal Vms were recorded at pH 7.5 and 5 mM K+.
Chemotaxis and electrotaxis in bathing solutions with different pH values.
To test the effects of depolarizing Vm on chemotaxis, we used a needle chemotaxis assay and quantitatively analyzed the directional cell migration. Without cAMP gradient, cells bathed in different solutions showed similar patterns of migration in random directions. In buffer solutions of pH 5.0, pH 6.5, and pH 9.0, no significant differences in cell morphology, behavior, or trajectory speed were observed among cells in response to the cAMP gradient (Fig. 2A and D; Table 1). This is consistent with a previous report (25).
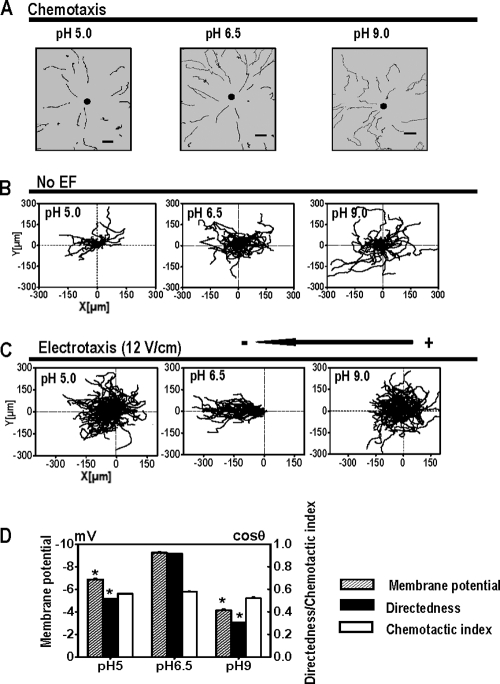
Fig. 2.
Extracellular pH plays different roles in chemotaxis and electrotaxis in Dictyostelium cells. (A) cAMP gradients were formed from the tip of a micropipette filled with 10 μM cAMP. Trajectories of cell migration toward cAMP in DB with different pH values are indicated. Dark spots represent the position of the micropipette. Scale bar, 20 μm. (B) Cell migration in random directions under control conditions without an EF although cells were bathed in DB with different pH values as indicated. (C) Cell migration trajectories in which the start point of each cell is set as the origin. Cells migrated cathodally in a pH of 6.5 in an EF. However, directed cell migration was significantly impaired under acidic (pH 5.0) or alkaline (pH 9.0) conditions. (D) The effects of extracellular pH on electrotaxis in Dictyostelium cells correlate with the effects on Vm. Dictyostelium cells bathed in pH 6.5 showed a greater Vm than that of the cells in pH 5.0 or pH 9.0 and significantly better electrotaxis. The changes in pH and corresponding changes in Vm did not significantly affect the chemotaxis (chemotactic index). *, P < 0.001 compared to that in pH 6.5.
Table 1.
Effects of extracellular pH and K+ on chemotaxisa
| Developing buffer | Trajectory speed (μm/min) | Displacement speed (μm/min) | Chemotactic index | Persistency |
|---|---|---|---|---|
| pH 5.0 | 4.69 ± 0.03 | 3.34 ± 0.03 | 0.56 ± 0.01 | 0.79 ± 0.01† |
| pH 6.5 | 5.01 ± 0.02 | 3.06 ± 0.02 | 0.58 ± 0.01 | 0.70 ± 0.01 |
| pH 9.0 | 3.89 ± 0.02‡ | 2.07 ± 0.02† | 0.52 ± 0.01 | 0.64 ± 0.01 |
| 5 mM K+ | 5.01 ± 0.02 | 3.06 ± 0.02 | 0.58 ± 0.01 | 0.70 ± 0.01 |
| 50 mM K+ | 4.26 ± 0.03 | 1.62 ± 0.03* | 0.30 ± 0.01* | 0.65 ± 0.01 |
The data represent means ± SEM. *, P < 0.001, compared to buffer using 5 mM K+; †, P < 0.01, compared to buffer at pH 6.5; ‡, P < 0.001, compared to buffer at pH 6.5.
The electrotaxis of Dictyostelium cells, however, is significantly altered by the pH of the bathing solution. The chemotactic indexes for cells bathed in DB of pH 5.0, pH 6.5, and pH 9.0 were 0.56 ± 0.01, 0.58 ± 0.01, and 0.52 ± 0.01, respectively. However, electrotaxis was significantly affected by bathing solution pH (Fig. 2C and D). Cells in solutions of pH 6.5 had more negative Vms and showed the best electrotaxis. Cells in pH 5.0 or pH 9.0 showed significantly reduced electrotaxis, with directedness values of 0.51 ± 0.01 or 0.30 ± 0.01, respectively (Fig. 2D).
Chemotaxis and electrotaxis of K+-induced depolarized Dictyostelium cells.
We then examined the effect of extracellular [K+] on chemotaxis and electrotaxis. Cells bathed in buffer of 5 mM K+ moved toward cAMP with a typically polarized morphology. In buffer of 50 mM K+, cells near the micropipette tip moved toward the cAMP source. Cells further away from the tip appeared to move less directionally toward the pipette. Nonetheless, directional migration was evident (Fig. 3A). Cells in 50 mM K+ migrated with a reduced chemotactic index of 0.30 ± 0.01, compared to that of 0.58 ± 0.01 for cells in 5 mM K+ (Fig. 3D).
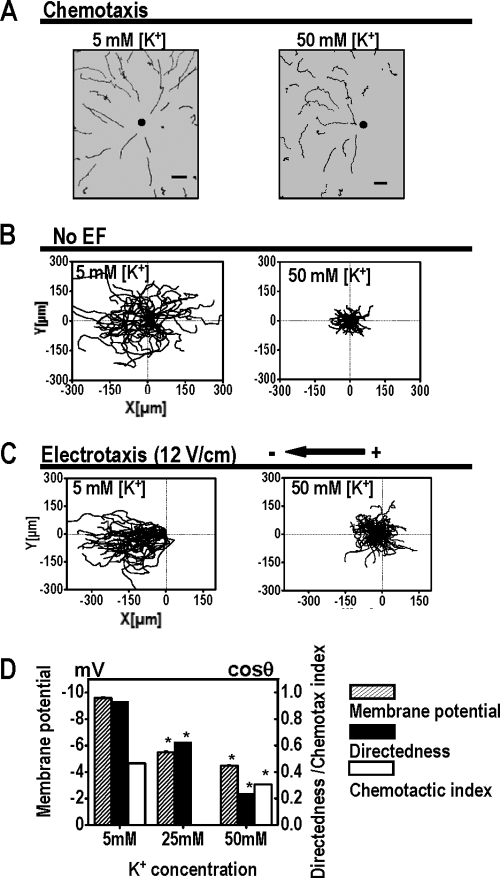
Fig. 3.
Extracellular K+ significantly affects electrotaxis but does not affect chemotaxis of Dictyostelium cells. (A) Chemotaxis of Dictyostelium cells in buffer with different extracellular K+ concentrations. Cells in buffer with 5 mM or 50 mM K+ were able to undergo chemotaxis. Trajectories show that cells migrate toward cAMP. Dark spots represent the positions of micropipette tips. Scale bar, 20 μm. (B) Cells migrate in random directions in buffer with 5 mM or 50 mM K+ without an applied EF. (C) Cells migrate cathodally in DB with 5 mM and 50 mM K+. When the K+ concentration increased Vm decreased (Fig. 1), and electrotaxis was significantly reduced. (D) The effects of extracellular K+ on electrotaxis in Dictyostelium cells correlated with the effects on Vm. Cells in buffer with 50 mM K+ had a significantly reduced Vm as well as significantly reduced electrotaxis. Slightly reduced chemotactic activity was seen with higher concentrations of K+ solution.
Extracellular [K+] significantly affected electrotaxis. Cells bathed in buffer with 5 mM K+ showed robust electrotaxis with a directedness value of 0.93 ± 0.01 (Fig. 3D). The directedness gradually decreased to 0.64 in 25 mM K+ and 0.24 in 50 mM K+ (Fig. 3D). Cells bathed in buffer with 50 mM K+ had a significantly lower directedness value, representing a decrease of 73%. Although increasing extracellular [K+] appeared to inhibit both chemotaxis and electrotaxis, it seemed to have a more significant effect on electrotaxis (Table 2).
Table 2.
Effects of pH and K+ on electrotaxisa
| Developing buffer | Trajectory speed (μm/min) | Displacement speed (μm/min) | Directedness | Persistency |
|---|---|---|---|---|
| pH 5.0 | 9.27 ± 0.02*† | 4.79 ± 0.02*† | 0.51 ± 0.01*† | 0.52 ± 0.01*† |
| pH 6.5 | 14.79 ± 0.08* | 10.75 ± 0.07* | 0.95 ± 0.01 | 0.73 ± 0.02 |
| pH 7.5 | 11.68 ± 0.06† | 9.20 ± 0.06† | 0.97 ± 0.01 | 0.78 ± 0.01 |
| pH 9.0 | 9.05 ± 0.02*† | 4.42 ± 0.02*† | 0.30 ± 0.01*† | 0.50 ± 0.01*† |
| 5 mM K+ | 13.09 ± 0.05 | 8.55 ± 0.04 | 0.93 ± 0.01 | 0.66 ± 0.01 |
| 25 mM K+ | 6.15 ± 0.03‡ | 3.57 ± 0.02‡ | 0.64 ± 0.01‡ | 0.59 ± 0.01 |
| 50 mM K+ | 6.46 ± 0.01‡ | 2.95 ± 0.01‡ | 0.24 ± 0.01‡ | 0.46 ± 0.00‡ |
The data represent means ± SEM. *, P < 0.001 compared to buffer at pH 7.5; †, P < 0.001 compared to buffer at pH 6.5; ‡, P < 0.001 compared to that in buffer with 5 mM K+.
Electroporation depolarized Vm and abolished electrotaxis.
To further verify the role of Vm in electrotaxis, we used electroporation to depolarize Dictyostelium cells. Electroporation with high-voltage pulses permeabilizes the cell plasma membrane, thus significantly depolarizing Vm by causing a large increase in non-ion-selective membrane permeability. Measurements in electroporated cells showed that the membrane is significantly depolarized following electroporation. In the data shown in Fig. 4, the starting time (0 min) was set as the time of electroporation. All cells measured at 10 min following electroporation showed a significantly reduced Vm, which recovered gradually. The recovery took roughly 30 to 40 min following electroporation (Fig. 4A).
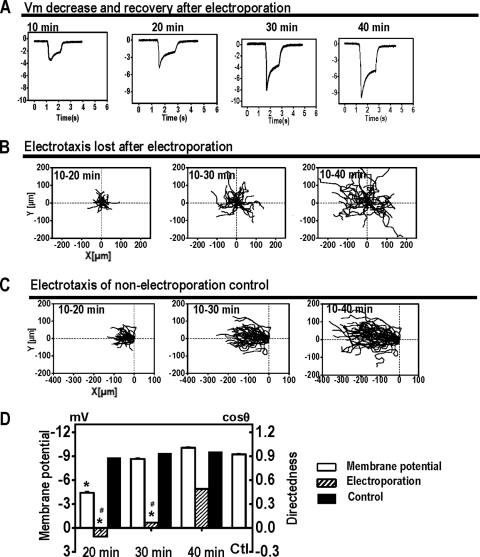
Fig. 4.
Electroporation depolarizes membrane potential (Vm) and abolishes electrotaxis. (A) Representative Vm measured at pH 6.5 after electroporation. The moment of electroporation was set as time zero. (B) Electroporated cells lost the electrotactic response, which showed some degree of recovery 30 min later (see video S6 in the supplemental material). (C) Control cells showed robust electrotaxis. (D) Loss of electrotaxis correlated well with Vm. When Vm recovered, electrotaxis recovered significantly. Control, Vm in control cells not electroporated. The EF was 12 V/cm. *, P < 0.001 compared to that of cells not being electroporated; #, P < 0.001 compared to that of cells after 40 min of recovery following electroporation.
Electrotaxis in electroporation-induced depolarized cells was lost. Batch-matched control cells had good directional migration with a directedness value of 0.96 ± 0.01 within 10 min in an EF, which was maintained through 20 to 30 min (Fig. 4C). Following electroporation, electrotaxis was completely inhibited (Fig. 4B). In the first 20 min following electroporation, the directedness of cells in an EF was almost zero. At 30 to 40 min, some directedness reappeared as Vm recovered to a certain degree (Fig. 4D).
We analyzed the relationship between these parameters and Vm. We found that Vm correlates significantly well with electrotaxis, with a correlation coefficient of −0.77 (Fig. 5A), whereas chemotaxis did not appear to correlate with the changes in Vm (Fig. 5B).
Fig. 5.
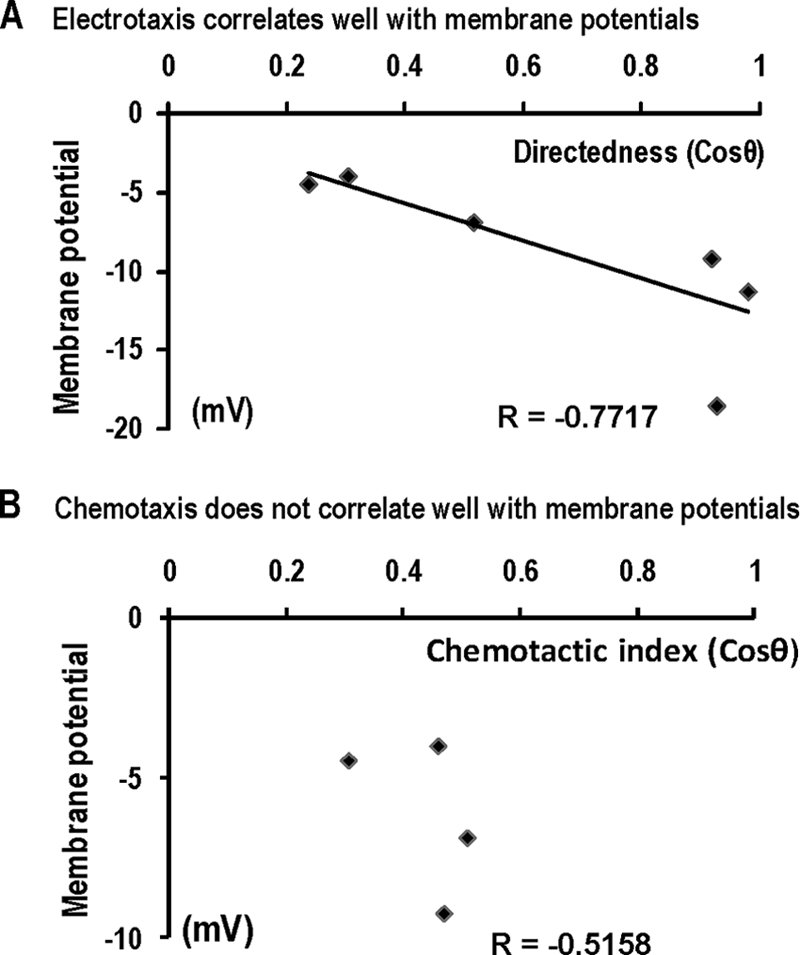
Significant correlation is between Vm and electrotaxis but not chemotaxis. (A) Scatter plot shows electrotactic directedness and resting membrane potential (r = −0.77). The best-fit line is shown. (B) Scatter plot shows chemotactic index and membrane potential (r = −0.51).
DISCUSSION
We tested the effects of extracellular pH and [K+] on electrotaxis using Dictyostelium cells, which have the unique property of tolerating changes in extracellular pH, [K+], and even electroporation, while maintaining good motility. We found that (i) changes in extracellular pH and [K+] and electroporation significantly affected Vm and that (ii) reduced Vm in response to these three factors significantly inhibited electrotaxis. The inhibitory effect on electrotaxis correlated well with the reduced Vm, but chemotactic effects did not.
In developed Dictyostelium cells, cAMP binds G protein-coupled receptors, activates Gα2βγ, small GTPase, and class 1 phosphatidylinositol-3 kinases (PI3K), thereby phosphorylating phosphatidylinositol-3,4-bisphosphate [PI(3,4)P2] into phosphatidylinositol-3,4,5-trisphosphate [PI(3,4,5)P3], and finally induces F-actin polymerization, resulting in pseudopod development. Several other pathways may also contribute to chemotaxis (9, 22). We demonstrated that Dictyostelium cells also show robust electrotaxis and are a good model for dissecting the molecular/genetic basis of electrotaxis (19, 29).
Extracellular pH, [K+], and electroporation significantly affected Vm and correspondingly reduced or abolished electrotaxis. When Vm recovered, electrotaxis was restored. Vm in Dictyostelium cells is mainly generated by electrogenic proton pumps (24, 25). By varying extracellular pH, we controlled the Vm with good reproducibility. The Vm values were smaller than those reported previously (24, 25). We used two different recording methods to confirm the measurements. The difference in Vm values may be due to other modifications: (i) the AX3 strain was used here whereas NC4 was used before; (ii) we used DB buffer while Van Duijn and coworkers used a Na+-saline (40 mM NaCl, 5 mM KCl, 1 mM CaCl2 and 5 mM HEPES-NaOH, pH 7.0); (iii) we used different development protocols (24, 25). The concentration of extracellular K+ affects Vm (26). Different extracellular K+ concentrations regulated Vm: the higher the K+ concentration, the lower the Vm (Fig. 1).
At 50 mM K+, electrotaxis was significantly inhibited (Fig. 3). Depolarization of cells following electroporation abolished the electrotactic response while recovery of Vm restored the electrotactic response (Fig. 4). Chemotaxis of the cells with an altered Vm, modulated by changes in extracellular pH or [K+], was largely unaffected. This is consistent with a previous report (25). Collectively, these results support the theory that the inhibition of electrotaxis by changes in extracellular pH, [K+], and electroporation appears to be a specific effect caused by changes to Vm. The genome of Dictyostelium cells shows at least two possible transient receptor potential (TRP) channel genes, a Ca2+ channel gene, and several K+ channel genes (14). Several signal transduction pathways related to electrotaxis could depend on Vm caused by the interactions between ion channels and other signaling proteins such as integrins (2, 5, 6, 12, 13, 16, 23). It may involve different membrane proteins, such as ion channels, transporters, receptors, and the actin cytoskeleton, and may also involve Ca2+ signaling (20). The reduced Vm might inhibit Ca2+ signaling and thereby affect electrotaxis. Another possibility is that Vm may control the sensors that detect the EFs. We are currently using a high-throughput strategy to screen for such sensing molecules in electrotaxis.
In conclusion, changes in extracellular pH, [K+], and electroporation all had significant effects on electrotaxis. When the Vm was depolarized, electrotaxis was significantly inhibited. Extracellular pH, [K+], and electroporation all had significant effects on electrotaxis, which appeared to be mediated by the changes in Vm. The initial directional sensing mechanisms for electrotaxis therefore differ from those in chemotaxis and may be mediated by changes in Vm.
ACKNOWLEDGMENTS
This work was supported by a grant from the NSF (MCB-0951199 to M.Z. and P.N.D.). M.Z. is also supported by NIH grant 1R01EY019101, California Institute of Regenerative Medicine grant RB1-01417, and the University of California at Davis (UC Davis) Dermatology Developmental Fund. This study was supported in part by an unrestricted grant from Research to Prevent Blindness, UC Davis Ophthalmology, and Yunnan Province Talented Recruiting Program (2009CI127). We thank the Wellcome Trust for continuous support (WT082887MA to M.Z.).
We thank Lin Cao and Kristen Swaney for sharing technical expertise and other members of the Zhao and Devreotes laboratories for their help. We thank Tsung-Yu Chen for kindly providing the electrophysiological equipment and Liping Nie for kindly helping us to use her electroporation device.
M.Z., P.N.D., A.M., X.-D.Z., and R.C.-G. designed the research; R.C.-G., X.-D.Z., Y.-H.S., and Y.K. performed research; M.Z., R.C.-G., X.-D.Z., and Y.-H.S. analyzed data; R.C.-G., X.-D.Z., and M.Z. wrote the paper with help from Y.-H.S.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print on 8 July 2011.
REFERENCES
- 1. Barreiro O., Martin P., Gonzalez-Amaro R., Sanchez-Madrid F. 2010. Molecular cues guiding inflammatory responses. Cardiovasc. Res. 86:174–182 [DOI] [PubMed] [Google Scholar]
- 2. Becchetti A., Pillozzi S., Morini R., Nesti E., Arcangeli A. 2010. New insights into the regulation of ion channels by integrins. Int. Rev. Cell Mol. Biol. 279:135–190 [DOI] [PubMed] [Google Scholar]
- 3. Berzat A., Hall A. 2010. Cellular responses to extracellular guidance cues. EMBO J. 29:2734–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cai H., et al. 2010. Ras-mediated activation of the TORC2-PKB pathway is critical for chemotaxis. J. Cell Biol. 190:233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dai S., Hall D. D., Hell J. W. 2009. Supramolecular assemblies and localized regulation of voltage-gated ion channels. Physiol. Rev. 89:411–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis M. J., et al. 2002. Regulation of ion channels by integrins. Cell Biochem. Biophys. 36:41–66 [DOI] [PubMed] [Google Scholar]
- 7. Gaudet P., Pilcher K. E., Fey P., Chisholm R. L. 2007. Transformation of Dictyostelium discoideum with plasmid DNA. Nat. Protoc. 2:1317–1324 [DOI] [PubMed] [Google Scholar]
- 8. Goetz J. G. 2009. Bidirectional control of the inner dynamics of focal adhesions promotes cell migration. Cell Adh. Migr. 3:185–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Insall R. H. 2010. Understanding eukaryotic chemotaxis: a pseudopod-centred view. Nat. Rev. Mol. Cell Biol. 11:453–458 [DOI] [PubMed] [Google Scholar]
- 10. Kamimura Y., Tang M., Devreotes P. 2009. Assays for chemotaxis and chemoattractant-stimulated TorC2 activation and PKB substrate phosphorylation in Dictyostelium. Methods Mol. Biol. 571:255–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Knecht D., Pang K. M. 1995. Electroporation of Dictyostelium discoideum. Methods Mol. Biol. 47:321–330 [DOI] [PubMed] [Google Scholar]
- 12. Liang W., et al. 2010. Role of phosphoinositide 3-kinase α, protein kinase C, and L-type Ca2+ channels in mediating the complex actions of angiotensin II on mouse cardiac contractility. Hypertension 56:422–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liu B., et al. 2009. Enhancement of BK(Ca) channel activity induced by hydrogen peroxide: involvement of lipid phosphatase activity of PTEN. Biochim. Biophys. Acta 1788:2174–2182 [DOI] [PubMed] [Google Scholar]
- 14. Martinac B., Saimi Y., Kung C. 2008. Ion channels in microbes. Physiol. Rev. 88:1449–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCaig C. D., Rajnicek A. M., Song B., Zhao M. 2005. Controlling cell behavior electrically: current views and future potential. Physiol. Rev. 85:943–978 [DOI] [PubMed] [Google Scholar]
- 16. Morini R., Becchetti A. 2010. Integrin receptors and ligand-gated channels. Adv. Exp. Med. Biol. 674:95–105 [DOI] [PubMed] [Google Scholar]
- 17. Mycielska M. E., Djamgoz M. B. 2004. Cellular mechanisms of direct-current electric field effects: galvanotaxis and metastatic disease. J. Cell Sci. 117:1631–1639 [DOI] [PubMed] [Google Scholar]
- 18. Robinson K. R. 1985. The responses of cells to electrical fields: a review. J. Cell Biol. 101:2023–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sato M. J., et al. 2009. Switching direction in electric-signal-induced cell migration by cyclic GMP and phosphatidylinositol signaling. Proc. Natl. Acad. Sci. U. S. A. 106:6667–6672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shanley L. J., Walczysko P., Bain M., MacEwan D. J., Zhao M. 2006. Influx of extracellular Ca2+ is necessary for electrotaxis in Dictyostelium. J. Cell Sci. 119:4741–4748 [DOI] [PubMed] [Google Scholar]
- 21. Song B., et al. 2007. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat. Protoc. 2:1479–1489 [DOI] [PubMed] [Google Scholar]
- 22. Swaney K. F., Huang C. H., Devreotes P. N. 2010. Eukaryotic chemotaxis: a network of signaling pathways controls motility, directional sensing, and polarity. Annu. Rev. Biophys. 39:265–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Uysal-Onganer P., Djamgoz M. B. 2007. Epidermal growth factor potentiates in vitro metastatic behaviour of human prostate cancer PC-3M cells: involvement of voltage-gated sodium channel. Mol. Cancer 6:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Duijn B., Vogelzang S. A. 1989. The membrane potential of the cellular slime mold Dictyostelium discoideum is mainly generated by an electrogenic proton pump. Biochim. Biophys. Acta 983:186–192 [DOI] [PubMed] [Google Scholar]
- 25. Van Duijn B., Vogelzang S. A., Ypey D. L., Van der Molen L. G., Van Haastert P. J. 1990. Normal chemotaxis in Dictyostelium discoideum cells with a depolarized plasma membrane potential. J. Cell Sci. 95:177–183 [DOI] [PubMed] [Google Scholar]
- 26. Van Duijn B., Ypey D. L., Van der Molen L. G. 1988. Electrophysiological properties of Dictyostelium derived from membrane potential measurements with microelectrodes. J. Membr. Biol. 106:123–134 [DOI] [PubMed] [Google Scholar]
- 27. von der Mark K., Park J., Bauer S., Schmuki P. 2010. Nanoscale engineering of biomimetic surfaces: cues from the extracellular matrix. Cell Tissue Res. 339:131–153 [DOI] [PubMed] [Google Scholar]
- 28. Zhao M., Agius-Fernandez A., Forrester J. V., McCaig C. D. 1996. Directed migration of corneal epithelial sheets in physiological electric fields. Invest. Ophthalmol. Vis Sci. 37:2548–2558 [PubMed] [Google Scholar]
- 29. Zhao M., Jin T., McCaig C. D., Forrester J. V., Devreotes P. N. 2002. Genetic analysis of the role of G protein-coupled receptor signaling in electrotaxis. J. Cell Biol. 157:921–927 [DOI] [PMC free article] [PubMed] [Google Scholar]