Abstract
The ARC (amidoxime reducing component) proteins are molybdenum cofactor (Moco) enzymes named hmARC1 and hmARC2 (human ARCs [hmARCs]) in humans and YcbX in Escherichia coli. They catalyze the reduction of a broad range of N-hydroxylated compounds (NHC) using reducing power supplied by other proteins. Some NHC are prodrugs or toxic compounds. YcbX contains a ferredoxin (Fd) domain and requires the NADPH flavin reductase CysJ to reduce NHC. In contrast, hmARCs lack the Fd domain and require a human cytochrome b5 (hCyt b5) and a human NADH Cyt b5 reductase (hCyt b5-R) to reduce NHC. The ARC proteins in the plant kingdom are uncharacterized. We demonstrate that Chlamydomonas reinhardtii mutants defective in Moco biosynthesis genes are sensitive to the NHC N6-hydroxylaminopurine (HAP). The Chlamydomonas reinhardtii ARC protein crARC has been purified and characterized. The six Chlamydomonas Fds were isolated, but none of them are required by crARC to reduce HAP. We have also purified and characterized five C. reinhardtii Cyt b5 (crCyt b5) and two flavin reductases, one that is NADPH dependent (crCysJ) and one that is NADH dependent (crCyt b5-R). The data show that crARC uses crCyt b5-1 and crCyt b5-R to reduce HAP. The crARC has a Zn-dependent activity, and the presence of Zn increases its Vmax more than 14-fold. In addition, all five cysteines of crARC were substituted by alanine, and we demonstrate that the fully conserved cysteine 252 is essential for both Moco binding and catalysis. Therefore, it is proposed that crARC belongs to the sulfite oxidase family of Moco enzymes.
INTRODUCTION
All eukaryotic molybdenum (Mo)-containing enzymes that have been studied have Mo chelated with an organic motif (molybdopterin [MPT]) forming the so-called Mo cofactor (Moco) (Fig. 1A). Moco is widespread in all kingdoms and synthesized by a conserved pathway, divided in several steps according to the biosynthesis of its intermediates from a guanosine derivative (probably GTP): cyclic pyranopterin monophosphate (cPMP), MPT, and MPT-AMP (adenylated molybdopterin). In Chlamydomonas reinhardtii, the CNX2 and CNX3 enzymes catalyze the conversion of GTP into cPMP, CNX5, CNX6, and CNX7 from cPMP into MPT, CNX1G from MPT into MPT-AMP, and CNX1E from MPT-AMP into Moco (21). Two families of Moco-containing enzymes are present in eukaryotes, the sulfite oxidase (SO) family and the xanthine oxidase (XO) family. In Moco proteins, Mo is chelated via two thiol groups of MPT and also with two oxo groups (Fig. 1A). In the SO family, the fifth Mo ligand is a protein-derived cysteine, and in the XO family, this is an inorganic sulfur (28).
Fig. 1.
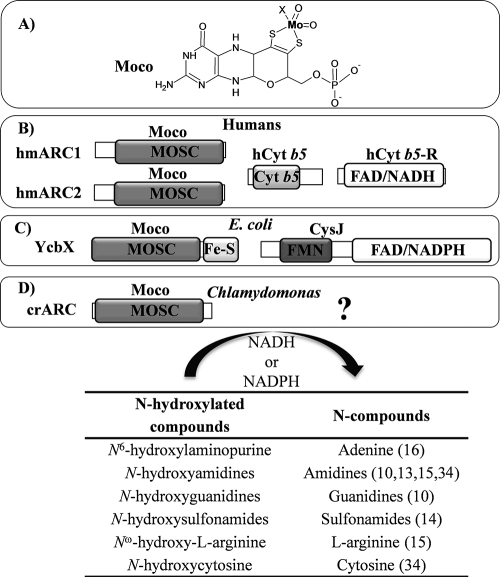
Schematic structure of Moco, ARC proteins, its partners, and the reaction catalyzed. (A) Structure of the Moco molecule with the organic motif (MPT) and the Mo atom shown in bold type. The X indicates that the fifth Mo ligand in the ARC proteins is unknown. (B to D) Schematic representations of the ARC system in humans (B), E. coli (C), and Chlamydomonas reinhardtii (D). Each of the protein domains found in the ARC protein system, MOSC (molybdenum cofactor sulfurase C-terminal domain), Cyt b5 (Cyt b5 domain), FAD/NADH (FAD- and NADH-binding domain), FAD/NADPH (FAD- and NADPH-binding domain), Fe-S (2Fe-2S-binding domain), and FMN (FMN-binding domain), is shown in boxes (most boxes with a gray background). The MOSC domain is able to bind Moco, which is indicated by Moco above the domain, but it is not known to which amino acids Moco binds. The proteins involved with crARC in the HNC reduction are unknown (question mark). In the table at the bottom of the figure, some reactions carried out by these proteins are shown with some examples of HNC substrates studied and references in the parentheses. The electron donors are NADH in the human system and NADPH in the E. coli system.
A newly identified enzyme called ARC (amidoxime reducing component) is involved in the reduction of a broad range of N-hydroxylated compounds (NHC), present in eukaryotic and prokaryotic organisms (12). An important group of base analogues are for example the NHC of adenine, N6-hydroxylaminopurine (HAP), a very powerful mutagen in phages, bacteria, and eukaryotic cells (24). In bacteria, the defect in any enzyme involved in the Moco biosynthesis pathway gives a HAP-hypersensitive phenotype (17), the first evidence of a Moco-dependent enzyme involved in the detoxification of HAP. However, the deletion of known molybdoenzymes in Escherichia coli failed to reveal any HAP sensitivity (18), suggesting that a novel type of Moco-dependent activity was involved in HAP detoxification. Nevertheless, a novel Moco-dependent enzymatic activity involved in the reduction of NHC was discovered in humans, hmARCs (human mARCs), operating in conjunction with a human Cyt b5 (hCyt b5) and a human NADH Cyt b5 reductase (hCyt b5-R), capable of converting the prodrug, benzamidoxime, to its active form, benzamidine (12). This conversion is analogous to the reduction of HAP to adenine, as it entails the reduction of an NHC (benzamidoxime) to the corresponding amino form (benzamidine) (Fig. 1). Two highly homologous hmARC proteins are present in humans, hmARC1 and hmARC2 (Fig. 1B). The subcellular localization of ARC proteins is not well defined. The hmARCs were localized in the outer mitochondrial membrane (9), mouse ARC proteins were localized in the inner mitochondrial membrane (5), and rat ARC proteins were localized in peroxisomal membranes (14).
In bacteria, the enzyme involved in the HAP detoxification was identified by analyzing an E. coli mutant defective in the YcbX protein. YcbX was capable of avoiding the HAP toxicity by its reduction to adenine (16).
YcbX and hmARCs are hypothetical members of the MOSC protein superfamily. These proteins contain a domain homologous to the C-terminal domain (MOSC) of the eukaryotic Moco sulfurases (MOS). The MOS enzymes are involved in the transfer of a sulfide ligand, yielding sulfurated Moco, that is essential for the activity of the XO family of Moco enzymes. However, except for MOS, all other members of the MOSC superfamily are proteins without any confirmed function (28). MOSC-containing proteins are widely distributed in prokaryotes and eukaryotes and contain a fully conserved cysteine (1).
YcbX contains, in contrast to hmARCs, a ferredoxin (Fd) [Fe2-S2] domain in the C terminus, essential for its activity (16) (Fig. 1C). Recently, the CysJ component of the sulfite reductase complex (8CysJ4CysI) has been identified as one additional component of this system (19) (Fig. 1C). The role of CysJ in HAP reduction is unique and independent of CysI and sulfite reductase. CysJ provides via its NADPH flavin reductase activity the reducing equivalents needed by YcbX to reduce HAP. Therefore, the role of bacterial CysJ seems to be analogous to hCyt b5-R, that is, to provide the reducing power needed for the reduction of the NHC. In bacteria, the electrons would be funneled from Fd to the MOSC domain of YcbX, while in humans, the electrons would go from the hCyt b5 to the MOSC domain of hmARC proteins. Thus, it appears that the ARC proteins are a widely distributed class of molybdoenzymes existing in organisms from bacteria to mammalian cells with similar biochemical properties.
Although there have been substantial advances in the role of ARC proteins in bacteria and human cells, nothing is known about ARC proteins in the plant kingdom. We have studied this protein in the green alga Chlamydomonas reinhardtii, which has a single gene encoding a protein with similarity to ARC that we call crARC (for Chlamydomonas reinhardtii ARC). In this work, we have shown that crARC is a molybdoenzyme critical for detoxifying HAP, and similar to its human homologue, it requires the NADH-dependent Cyt b5 flavin reductase and Cyt b5-1 but none of the six Chlamydomonas ferredoxins. The enzymatic reduction reaction of HAP by crARC has also been studied in vitro and found to have a Zn-dependent activity. Finally, crARC mutants with each of its cysteine residues substituted were obtained, demonstrating that cysteine 252 is essential for Moco binding and catalysis; therefore, it is proposed that crARC belongs to the SO family of Moco enzymes.
MATERIALS AND METHODS
Chemicals.
N6-Hydroxylaminopurine (HAP) was purchased from ICN Biochemicals. The other chemicals were purchased from Sigma-Aldrich.
Chlamydomonas reinhardtii strains and culture conditions.
The Chlamydomonas reinhardtii strains used in this work have been described in references 7 and 21. Cells were cultured under continuous light at 23°C in liquid and solid media containing 8 mM ammonium chloride or bubbled (for liquid cultures) with 5% (vol/vol) CO2-enriched air in minimal medium (11).
Bacterial strains and culture conditions.
The Escherichia coli strains were grown on LB medium or minimal Vogel-Bonner medium (VB) (33) containing 0.2% glucose as the carbon source. The E. coli strain ycbX mutant JW5126-1 CGSC strain 11193 and the cysJ mutant JW2734-1 CGSC strain 10150 came from the E. coli Genetic Stock Center (3). The E. coli strain TP1000 (mobA mutant) (31) was used for expression of ARC recombinant proteins because it accumulates eukaryotic molybdenum cofactor (Moco). The E. coli strain BL21(DE3) was used for expression of the remaining recombinant proteins (8).
Tests for inhibition by HAP of E. coli growth.
A freshly transformed single E. coli colony of each strain to be tested was inoculated into 1 ml of liquid LB and grown for 2 h at 37°C. Then, it was diluted 50-fold in 0.9% NaCl and 2.5 μl was transferred to minimal VB plates. After the spots had dried, 100 μg of HAP was spotted onto the center of the plate. The plates were incubated for 24 h at 37°C and inspected for growth inhibition zones.
Tests for inhibition by HAP of Chlamydomonas growth.
A 5-μl drop from liquid culture of ammonium-grown cells containing about 1,000 cells was laid, in three replicate samples, on 2 ml liquid ammonium media with the indicated amounts of HAP. The cells were then grown under continuous light for 5 days, and the chlorophyll content was measured (2).
Cloning of proteins.
The cloning vector used was pSPARK-I (Canvax Biotech, Córdoba, Spain). Two expression vectors were used, pQE80, which allows the expression of fusion proteins tagged with six histidines at the N-terminal region of the protein (Qiagen, Hilden, Germany) in E. coli and pASK-IBA5C (IBA GmbH, Göttingen, Germany), which allows tagging of the protein in the N terminus with a streptactin affinity tag, but in all the constructions used, this tag was removed by using specific primer sets. The two expression vectors were used when two proteins had to be expressed in the same E. coli strain, since pASK-IBA5C confers chloramphenicol resistance and pQE80 confers ampicillin resistance. All the Chlamydomonas proteins were identified by analyzing the Chlamydomonas genome (22) in the JGI database (http://genome.jgi-psf.org/Chlre4/Chlre4.home.html). All the Chlamydomonas cDNAs were amplified from total cDNA. Table 1 summarizes the specific primer sets used.
Table 1.
Primers used in this study
| Primer set | Primer | Primer sequencea (5′-3′) | DNA clonedb | Vector |
|---|---|---|---|---|
| 1 | crARC5BamHI | GGATCCATGCTCACAATCGGCGGTCT | crARC | pQE80 |
| crARC3HindIII | AAGCTTTCACGGCGCTGGCACCAGGTCCG | |||
| 2 | crARC5NheI | GCTAGCATGCTCACAATCGGCGGTCT | crARC | pASK-IBA5C |
| crARC3SalI | GTCGACTCACGGCGCTGGCACCAGGT | |||
| 3 | crARC5-C15 | ATCAAGTCAGCTCGCGGTGTG | crARC-C15A | pQE80 |
| crARC3-C15 | CACACCGCGAGCTGACTTGAT | |||
| 4 | crARC5-C137 | GGGACTGCCCGCTCGCCTGGTGCGC | crARC-C137A | pQE80 |
| crARC3-C137 | CGCACCAGGCGAGCGGGCAGTCCCA | |||
| 5 | crARC5-C243 | GATGTGGCCGCCGGCGCCGACGG | crARC-C234A | pQE80 |
| crARC3-C243 | CCGTCGGCGCCGGCGGCCACATC | |||
| 6 | crARC5-C249 | TCAAGCCCGCCTCCCGCTGCAAGGTGAC | crARC-C249A | pQE80 |
| crARC3-C249 | GTCACCTTGCAGCGGGAGGCGGGCTTGA | |||
| 7 | crARC5-C252 | TCAAGCCCTGCTCCCGCGCCAAGGTGAC | crARC-C252A | pQE80 |
| crARC3-C252 | GTCACCTTGGCGCGGGAGCAGGGCTTGA | |||
| 8 | crARC5-Cdm | TCAAGCCCGCCTCCCGCGCCAAGGTGAC | crARC-C249/252A | pQE80 |
| crARC3-Cdm | GTCACCTTGGCGCGGGAGGCGGGCTTGA | |||
| 9 | FDX15NheI | GCTAGCTACAAGGTCACCCTGAAGACCCCTTC | crFDX1 | pASK-IBA5C |
| FDX13PstI | CTGCAGTTAGTACAGGGCCTCCTCCTGGTGGGT | |||
| 10 | FDX25NheI | GCTAGCTTTAAGGTCACGTTTAAGACCCCCAA | crFDX2 | pASK-IBA5C |
| FDX23PstI | CTGCAGTTAGAGCTTGGACTCCTGGTCGGTCA | |||
| 11 | FDX35NheI | GCTAGCTACAAGGTCACCTTCGTCGGTGCCGA | crFDX3 | pASK-IBA5C |
| FDX33XhoI | CTCGAGCTACTTCTGCAGCTCCGCCCAACCCT | |||
| 12 | FDX45NheI | GCTAGCTACAAGATCAGCCTGACGCATGAAGG | crFDX4 | pASK-IBA5C |
| FDX43PstI | CTGCAGTTACTGGCTGGTCATCAGCTGCATG | |||
| 13 | FDX55NheI | GCTAGCTTTCAGGTGACGCTGCGCATGC | crFDX5 | pASK-IBA5C |
| FDX53PstI | CTGCAGTTACTGGTGCTTGCCGTACTCGCAGG | |||
| 14 | FDX65NheI | GCTAGCCCGGTGCACAAGATCAAGATCTTTGACC | crFDX6 | pASK-IBA5C |
| FDX63XhoI | CTCGAGTCACTCGTCCATGTTGGCAATGGACA | |||
| 15 | Citb515SacI | GAGCTCATGGCCCCCTCAGGGAAAACATA | crCyt b5-1 | pQE80 |
| Citb513SalI | GTCGACTCATCGCGTCGCCGCACTCTTA | |||
| 16 | Citb525SacI | GAGCTCATGGCGAACACGGCCCC | crCyt b5-2 | pQE80 |
| Citb523SalI | GTCGACCTATAAGCTGAACAAACGCTTGAAGATG | |||
| 17 | Citb535SacI | GAGCTCATGAGCGCGGACGACCTCGGT | crCyt b5-3 | pQE80 |
| Citb533PstI | CTGCAGCTATGACGAGGCCGGCTTGGC | |||
| 18 | Cytb545SacI | GAGCTCGTCGCGCAGCTCGACCCTAAGAA | crCyt b5-4 | pQE80 |
| Cytb543SalI | GTCGACTCACTCCTCGCCCGCCGCAA | |||
| 19 | Cytb555SacI | GAGCTCAGCAACACGTTCACGCAGGAGGA | crCyt b5-5 | pQE80 |
| Cytb535SalI | GTCGACTCACGCCGTCAAAGCCGTAGCCG | |||
| 20 | Citb5R5KpnI | GGTACCCGGAAGAAGACCAAGAAGCCGTTCC | crCyt b5-R | pQE80 |
| Citb5R3PstI | CTGCAGTCAGAACTGGAACTGCTTGTCCTCGG | |||
| 21 | CrCysJ5SacI | GAGCTCGAGCCGGTTGGCTCTACTGGCA | crCysJ | pQE80 |
| CrCysJ3SalI | GTCGACTCAGTACCAGACATCGCGCTGGT | |||
| 22 | Ycbx5KpnI | GGTACCGCGACATTAATCCGGCTTTTTATTCATC | YcbX | pQE80 |
| Ycbx3MoscPstI | CTGCAGCTATACATTTGCGTCCGGTTGTTGCGT | |||
| 23 | Ycbx5MoscKpnI | GGTACCGCGACATTAATCCGGCTTTTTATTCATC | ecMOSC | pQE80 |
| Ycbx3MoscPstI | CTGCAGCTATACATTTGCGTCCGGTTGTTGCGT | |||
| 24 | Ycbx5FerKpnI | GGTACCGAGGTGGAAATTCTGGCAACGGCTC | ecFDX | pQE80 |
| Ycbx3FerPstI | CTGCAGCTAACGCGCCAACTTAAGTGCAGTCTTC | |||
| 25 | Ycbx5FerKpnI | GGTACCGGAGGTGGAAATTCTGGCAACGGCTC | ecFDX | pASK-IBA5C |
| Ycbx3FerPstI | CTGCAGCTAACGCGCCAACTTAAGTGCAGTCTTC | |||
| 26 | mARC1SacI | GAGCTCTGGCCCACGCGGCGCCGGC | hmARC1 | pQE80 |
| mARC KpnI | GGTACCTTACTGGCCCAGCAGGTACA | |||
| 27 | mARC2SacI | GAGCTCTGGCCCAGGCGGCGCCGGC | hmARC2 | pQE80 |
| mARC2KpnI | GGTACCCTACACCATCCGATACACA |
The restriction site and mutation introduced are indicated by underlined and italic sequences, respectively. The identity of the restriction enzyme is shown at the end of the primer name.
crARC-C15A, crARC with the C15A mutation; crARC-C249/252A, crARC with the C249A C252A double mutation.
The crARC was identified by a BLAST search using ARC sequences as queries that gave as a result one sequence with GenPept accession no. XP_001694549. The crARC cDNA was amplified, and the resulting cDNA encoding a 330-amino-acid protein was cloned in pQE80 (Table 1, primer set 1) and pASK-IBA5C (Table 1, primer set 2). The generation of the cysteine-to-alanine variants of crARC (C15A, C137A, C234A, C249A, C252A, and double mutant C249A C252A) were made by PCR mutagenesis (Table 1, primer sets 3 to 8), and the resulting cDNAs were cloned in pQE80.
The Chlamydomonas reinhardtii genome encodes six plant type Fds previously identified and characterized (32) (GenPept accession numbers given in parentheses after the Fd): PETF or crFDX1 (XP_001692808), crFDX2 (XP_001697912), crFDX3 (XP_001691381), crFDX4 (XP_001700106), crFDX5 (XP_001691603), and crFDX6 (XP_001702961). The six Fd cDNAs were amplified using specific primers that remove the coding sequence for the putative N-terminal chloroplastic targeting sequence. The resulting cDNAs encoding 94 (crFDX1), 93 (crFDX2), 129 (crFDX3), 101 (crFDX4), 103 (crFDX5), and 132 (crFDX6) amino acids were cloned in pASK-IBA5C (Table 1, primer sets 9 to 14).
A BLAST search in the Chlamydomonas JGI database using the hCyt b5 sequence (GenPept accession no. NP_085056) as a query resulted in 5 sequences with GenPept accession numbers (shown in parentheses): crCyt b5-1 (XP_001697920), crCyt b5-2 (XP_001697853), crCyt b5-3 (XP_001693518), crCyt b5-4 (XP_001693863), and crCyt b5-5 (XP_001697852). These five crCyt b5 cDNAs were amplified using specific primers, and the resulting cDNAs encoding 111 (crCyt b5-1), 113 (crCyt b5-2), 182 (crCyt b5-3), 100 (crCyt b5-4), and 108 (crCyt b5-5) amino acids were cloned in pQE80 (Table 1, primer sets 15 to 19).
A BLAST search in the Chlamydomonas JGI database using the human hCyt b5-R sequence (GenPept accession no. NP_015565) as a query resulted in one sequence with a GenPept accession no., crCyt b5-R (XP_001695724). This cDNA was amplified using specific primers, and the resulting cDNA encoding 250 amino acids was cloned in pQE80 (Table 1, primer set 20).
A BLAST search in the Chlamydomonas JGI database using the E. coli CysJ sequence (GenPept accession no. NP_417244) as a query resulted in one sequence with GenPept accession no. XP_001703247 (crCysJ). This cDNA was amplified using specific primers, and the resulting cDNA encoding 668 amino acids was cloned in pQE80 (Table 1, primer set 21).
The full-length ycbX open reading frame was amplified from total genomic E. coli DNA according to GenPept accession no. NP_415467. The resulting cDNA encoding 368 amino acids was cloned in pQE80 (Table 1, primer set 22). The cDNA encoding the N-terminal MOSC domain of YcbX (ecMOSC [E. coli MOSC]) resulting in 290 amino acids was cloned in pQE80 (Table 1, primer set 23), and the cDNA encoding the C-terminal Fd domain (ecFDX) resulting in 110 amino acids was cloned in both pQE80 and pASK-IBA5C (Table 1, primer sets 24 and 25).
Full-length cDNA clones of hmARC1 and hmARC2 were obtained from Source BioScience LifeSciences genomic services (Source BioScience LifeSciences, Nottingham, United Kingdom) IMAGE ID 3872779 for hmARC1 and IMAGE ID 3849257 for hmARC2 according to GenPept accession nos. NP_073583 and NP_060368, respectively. The cDNAs were amplified using specific primers, and the resulting cDNAs encoding 294 (hmARC1) and 292 (hmARC2) amino acids were cloned in pQE80 (Table 1, primer sets 26 and 27).
The accuracy of all cDNA sequences was confirmed by DNA sequencing.
Expression and purification of recombinant proteins.
Standard expression of the crARC, crARC cysteine-to-alanine variants, hmARC-1, hmARC-2, and YcbX proteins was performed in freshly transformed E. coli TP1000 (mobA mutant) cells (31). The expression of the other proteins was performed in E. coli BL21(DE3). The cells were grown aerobically in LB medium to an A550 of 0.1 before induction. TP1000 cells were induced with 10 μM isopropyl-β-d-thiogalactopyranoside (IPTG) and additionally supplemented with 0.1 mM sodium molybdate to initiate recombinant expression. E. coli BL21 cells were induced with 100 μM IPTG to start expression. Cells expressing proteins with a heme group were supplemented with 1 mM aminolevulinic acid to support heme synthesis. After induction, the cells were grown for an additional 36 h at 22°C. Purification of recombinant proteins expressed was performed by Ni-nitrilotriacetic acid (Ni-NTA) matrix, as recommended by the supplier (Qiagen), under native conditions at 4°C, using minimal volumes of washing buffers to reduce dissociation of bound Mo and Zn from the proteins. The protein fractions were analyzed by SDS-PAGE, and only the pure fractions were taken and immediately desalted on a PD10 gel filtration column previously equilibrated with 100 mM Tris-HCl, pH 7.2. The protein concentration was determined by UV absorption measurements using the calculated extinction coefficient (23) of the analyzed polypeptides.
DNA sequencing and sequence analysis.
DNA sequencing was performed at the Servicio Central de Apoyo a la Investigación (SCAI) (University of Córdoba, Spain). Sequences were analyzed using the DNAstar software v.4.05 (Lasergene Navigator), the Bioedit Sequence Alignment Editor v. 7.0.9 (Department of Microbiology, North Carolina State University), the NCBI BLAST server (http://www.ncbi.nlm.nih.gov/BLAST/), and the Chlamydomonas JGI server (http://www.chlamy.org).
Enzyme assays. (i) The in vitro HAP reduction by crARC.
The HAP reduction by crARC was determined as described previously for hmARCs (9) with minor modifications. Incubations were carried out under aerobic conditions at 37°C in a shaking water bath. Unless stated otherwise, standard incubation mixtures of the reconstituted system contained 100 pmol crARC, 10 pmol crCyt b5-R (or crCysJ), 100 pmol crCyt b5-1 (or b5-2, b5-3, b5-4, or b5-5), 0.5 mM HAP, and 1.0 mM NADH or NADPH in a total volume of 150 μl of 100 mM potassium phosphate buffer, pH 6.5. After preincubation for 3 min at 37°C, the reaction was started by the addition of NADH or NADPH and terminated after 15 min by adding 150 μl of methanol. The precipitated proteins were sedimented by centrifugation, and the supernatant was analyzed by high-performance liquid chromatography (HPLC). One unit of crARC activity is defined as the amount of enzyme causing the production of 1 μmol of adenine per minute under the described conditions.
The apparent kinetic parameters Km and Vmax were estimated using nonlinear regression analysis.
(ii) Determination of the Cyt b5 heme content.
For the determination of heme binding to Cyt b5, the absorption at 413 nm was monitored, and the heme/protein ratio was calculated using an extinction coefficient of 117 mM−1 cm−1 (29).
(iii) Determination of the flavin reductase FAD content.
Binding of FAD to the different reductases used in this work was determined at 450 nm, and the FAD/protein ratio was calculated using an extinction coefficient of 11.3 mM−1 cm−1 (35).
(iv) Determination of the NAD(P)H flavin reductase activity.
The rate of NAD(P)H flavin reductase activity was measured by two methods, the reduction of potassium ferricyanide which acts as an artificial electron acceptor or the reduction of crCyt b5.
The activity of crCyt b5-R and crCysJ with potassium ferricyanide was assayed as previously described (30). The reaction mixture contained 0.1 M potassium phosphate buffer (pH 7.5), 0.112 mM NADH or NADPH, 0.2 mM potassium ferricyanide, and the appropriate concentration of enzyme in a final volume of 1 ml. The reaction was started by the addition of NADH or NADPH, and ferricyanide reduction was followed by recording the absorbance decrease at 420 nm. The enzyme activity was calculated using the extinction coefficient of 1.02 mM−1 cm−1 (26). One unit of reductase activity is defined as the amount of enzyme causing reduction of 1 μmol of potassium ferricyanide per minute.
The activity of crCyt b5-R and crCysJ in the presence of crCyt b5 was assayed by the method of Strittmatter and Velick (29). The reaction mixture contained 0.1 M potassium phosphate buffer (pH 6.8), 0.112 mM NADH or NADPH, 2 nmol of purified crCyt b5, and the appropriate amount of crCyt b5-R or crCysJ in a final volume of 1 ml at 25°C. The reaction was initiated by the addition of NADH or NADPH. The reaction was followed by the increase in absorbance at 423 nm. The molar extinction coefficient increase between the reduced and oxidized forms of crCyt b5 was taken as 100 mM−1 cm−1. One unit of reductase is defined as the amount of enzyme catalyzing the reduction of 1 μmol of crCyt b5 per minute.
HPLC method for HAP and adenine quantification.
HAP and adenine were separated and quantified by HPLC. The HPLC analysis was performed on an Agilent series 1200 from Agilent Technologies. The separation was carried out by isocratic elution with 3 mM 1-octanesulfonic acid sodium salt (pH 4) and 15% methanol (vol/vol). The flow rate was kept at 0.5 ml/min. The detection wavelength was set at 260 nm. The separation was carried out with a symmetric C18 column (Zorbax Eclipse XDB-C18 column) (4.6 mm by 150-mm inner diameter [ID]; 5 μm). The characteristic retention times were 4.2 ± 0.02 min for HAP and 5.3 ± 0.02 min for adenine. For the determination of the recovery rate, reaction mixtures with defined concentrations of synthetic reference substance (1 to 1,000 μM) were incubated and worked up under the same conditions as those used for the experimental samples. The standard curves were linear over this range with correlation coefficients of 0.999 (n = 12).
Determination of the organic motif of Moco (MPT).
To measure the amounts of molybdopterin (MPT) bound to the proteins, the analysis of FormA was performed as reported previously (27).
ICP-OES.
To measure the amounts of Mo and Zn bound to the proteins, inductively coupled plasma optical emission spectrometry (ICP-OES) analysis was used. Determinations were carried out on a Yobin-Ivon Ultima 2 ICP-OES. The instrument response was optimized and calibrated with standards prepared from Merck multielement solution VI plus a solution of nitric acid 5% (vol/vol) as a blank. The accuracy of the system was evaluated by running control standards prepared at concentrations lower and higher than the concentration of the samples at the beginning and end of sample runs. Blanks were introduced in sequences before and after sample runs. Recovery was evaluated on samples. The instrument settings were as follows: power, 1,200 W; plasma gas, 12 liters/min; and carrier gas, 0.6 liters/min. Zn was analyzed at a wavelength (λ) of 206.200 nm. Mo was analyzed at a λ of 202.030 nm.
RESULTS
The Chlamydomonas reinhardtii Moco mutants are sensitive to HAP.
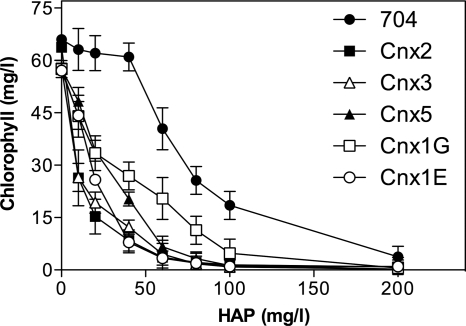
The E. coli mutants defective in molybdenum cofactor (Moco) biosynthesis are hypersensitive to the toxic effect of N6-hydroxylaminopurine (HAP) present in the growth media (18). In order to determine the relationship between N-hydroxylated compound (NHC) toxicity and Moco, we have investigated the phenotypes of different Chlamydomonas Moco mutants in the presence of HAP. Screening the Chlamydomonas mutant library generated in our lab by insertional mutagenesis (7) allowed us to identify five mutations in Moco biosynthesis genes (21). These strains are affected at genes Cnx2 and Cnx3 of the first step of Moco biosynthesis, Cnx5 of the second step, Cnx1G of the third step, and Cnx1E of the fourth step (see introduction). These mutants allowed us to study systematically the effect of inactivated genes in each of the defined Moco biosynthetic steps. As shown in Fig. 2, all the Chlamydomonas Moco mutants tested were hypersensitive to HAP compared to Chlamydomonas parental strain 704. This result indicates that the mutations blocking the synthesis of any Moco intermediate and thus of a functional Moco leads to a HAP hypersensitivity phenotype. Thus, our results suggest that Chlamydomonas reinhardtii has a Moco-dependent HAP detoxification system. The questions arising now are whether or not this system depends on ARC proteins and if so, whether this is similar to the system found in bacteria or to the one found in humans (Fig. 1). Thus, further studies were performed in order to identify the enzyme complex involved in HAP detoxification.
Fig. 2.
HAP toxicity in the Chlamydomonas reinhardtii Moco mutants. The strains were laid on liquid ammonium medium with the indicated amount (in milligrams per liter) of HAP. The cells were grown under continuous light for 5 days; after 5 days, the amount of growth was estimated by measuring the amount of chlorophyll of the culture.
Cyt b5, but not Fd, is involved in HAP resistance in Chlamydomonas.
The protection system against NHC toxicity in a photosynthetic organism like Chlamydomonas has been uncovered. The HAP toxicity found in every Chlamydomonas Moco mutant suggests that an ARC protein could be involved in HAP detoxification in this organism. A search in the Chlamydomonas genome resulted in one sequence that we call crARC. The main difference among YcbX, hmARCs, and crARC is the presence of a Fd domain [Fe2-S2] in the E. coli protein that is absent in the Chlamydomonas and humans proteins. However, the MOSC domain is present in the three proteins (Fig. 1). crARC shows 26.3, 26.1, and 21.3% sequence identity with hmARC1, hmARC2, and YcbX, respectively.
None of the mutants screened from our Chlamydomonas insertional mutant collection (6) were defective in the crARC gene, most probably because the selection medium was not appropriate for this purpose. As the E. coli ycbX mutant is hypersensitive to the toxic effect of HAP, the participation of the different proteins in HAP detoxification could be analyzed from their ability to revert the HAP toxicity found in this mutant (we refer to these experiments as in vivo experiments). Therefore, to resolve whether crARC is involved in the detoxification of HAP, this protein was expressed heterologously in the E. coli ycbX mutant.
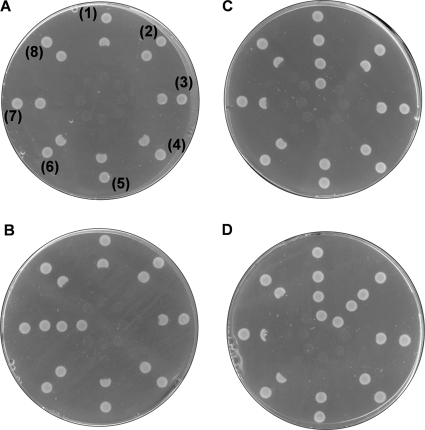
Figure 3A shows that the HAP toxicity was not reverted in any E. coli ycbX mutant transformed with crARC. The question that now arises is whether crARC needs one ferredoxin (Fd) similar to YcbX or one Cyt b5 similar to hmARCs to be fully functional. We cloned the six ferredoxins present in the Chlamydomonas genome (32). For a control, we also cloned the Fd domain of YcbX (ecFDX [E. coli FDX]). The percentages of identity between the E. coli Fd and Chlamydomonas Fd are 17.3 (crFDX1), 15.4 (crFDX2), 17.1 (crFDX3), 14.5 (crFDX4), 15.7 (crFDX5), and 14.4 (crFDX6). Fig. 3A, spots 1 to 6, shows that the HAP toxicity was not reverted in the E. coli ycbX mutant cotransformed with crARC plus each of the six crFDX or the ecFDX. This result suggests that none of the Chlamydomonas Fd participate with crARC in the detoxification of HAP. For a control, we also cloned the entire YcbX and its MOSC domain (ecMOSC). The entire YcbX (data not shown) and the cotransformant with ecMOSC plus ecFDX domains were able to revert the HAP toxicity (Fig. 3B, spots 7). This result shows that our strategy of analyzing whether or not there are in vivo protein interactions by coexpressing proteins or their domains in two expression vectors functions; at least between previous predicted interactions, what motivated us to find by this way the partners of crARC. None of the six Chlamydomonas Fds were able to revert the HAP toxicity when coexpressed with ecMOSC (Fig. 3B, spots 1 to 6). These results suggest that the interaction between ecMOSC and ecFDX domains is very specific and that the crFDXs cannot replace the function of the ecFDX domain.
Fig. 3.
HAP toxicity of E. coli strains expressing proteins. (A) Effect of Fd on HAP toxicity. The E. coli ycbX mutant was cotransformed with crARC cloned in pQE80 plus crFDX1 (1), crFDX2 (2), crFDX3 (3), crFDX4 (4), crFDX5 (5), crFDX6 (6), ecFDX (7), or none (8) cloned in pASK-IBA5C. (spots 8). (B) As in panel A but changing the crARC by the N-terminal MOSC domain of YcbX (ecMOSC). (C) Effect of Chlamydomonas Cyt b5 on HAP toxicity. The E. coli ycbX mutant was cotransformed with crARC cloned in pASK-IBA5C plus crCyt b5-1 (1), crCyt b5-2 (2), crCyt b5-3 (3), crCyt b5-4 (4), crCyt b5-5 (5), or none (6, 7, and 8) cloned in pQE80. (D) As in panel C but adding 100 μM molybdate to the growth medium. Suspensions of each different strain were applied in a series of spots radiating out from the center. The spot numbers are shown in the first plate in panel A, but the others follow the same code. The center of all the plates contained 100 μg HAP. The plates were incubated for 24 h at 37°C and inspected for a zone of growth inhibition around the center.
As the crFDXs have failed as crARC partners, we analyzed the Chlamydomonas Cyt b5, since the partner of hmARCs is a hCyt b5. A search in the Chlamydomonas genome using the hCyt b5 sequence as a query resulted in 5 sequences (crCyt b5-1 to crCyt b5-5) that were cloned. The percentages of identity between the hCyt b5 and each crCyt b5 are 28.8 (crCyt b5-1), 26.4 (crCyt b5-2), 15.7 (crCyt b5-3), 28.8 (crCyt b5-4), and 16.3 (crCyt b5-5) (Fig. 4). Interestingly, as shown in Fig. 3C, spots 1, only crCyt b5-1 was able to revert the HAP toxicity when cotransformed with crARC. However, when 100 μM molybdate was also included in the medium, crCyt b5-2 was also able to revert the HAP toxicity (Fig. 3D, spots 2). These results show clearly that crCyt b5 but not crFDX participates with crARC in the HAP detoxification, at least in vivo. These data allowed us to hypothesize that the proteins crARC and crCyt b5-1 or crCyt b5-2 are forming a complex in vivo in the bacterial cell that functions analogously as YcbX. This means that in vivo one or more E. coli proteins should be able to donate electrons to this predicted complex.
Fig. 4.
Multiple-sequence alignment of Chlamydomonas reinhardtii and human Cyt b5 proteins. The consensus sequences have been calculated with a threshold of 75% with the BioEdit v.7.0.9 program. The sequences and GenPept accession numbers (shown in parentheses) are crCyt b5-1 (XP_001697920), crCyt b5-2 (XP_001697853), crCyt b5-3 (XP_001693518), crCyt b5-4 (XP_001693863), and crCyt b5-5 (XP_001697852) (crCyt b5-1) (the crCyt b5-1 to crCyt b5-5 are from Chlamydomonas reinhardtii), and hCyt b5 (NP_085056) from humans. Highly conserved amino acids are shown on a black background, and moderately conserved amino acids are shown on a gray background. The numbers in the sequence alignment (43, 15, and 13) represent the lengths of poorly conserved inserts that have not been shown in the alignment. The conserved histidines that bind the heme group are indicated by white letters on a black background. The coding sequences for the putative membrane-binding domains are underlined. Gaps introduced to maximize the alignment are indicated by dashes.
In E. coli, the cysJ mutation also causes a HAP hypersensitivity phenotype (19). CysJ provides via its NADPH flavin reductase activity the reducing equivalents needed for the reduction of HAP by YcbX (19). The E. coli cysJ mutant was also cotransformed with crARC plus each of the five crCyt b5. However, none of them were able to revert the HAP toxicity of the cysJ mutant, even in the presence of molybdate in the medium (data not shown). This means that the CysJ protein is able to transfer, at least in vivo, the reducing equivalents to the predicted complex of crARC with crCyt b5-1 or crCyt b5-2.
Purification and characterization of recombinant proteins.
To analyze in vitro which Chlamydomonas reductase is involved in the reduction of HAP, we first purify and characterize the potential proteins involved in this reduction.
In contrast to hmARC proteins, crARC does not contain any predicted targeting sequences to the mitochondria or to any other cell compartment. Therefore, we cloned the full-length cDNA, and the recombinant crARC was expressed in a soluble form of 35 kDa. The hmARC1 and hmARC2 proteins were cloned without their predicted signal sequences for mitochondrial export. The hmARC1 and hmARC2 proteins were obtained in a soluble form of 33.2 and 33.6 kDa, respectively. YcbX after the recombinant expression was obtained as a soluble protein of 40.6 kDa with a dark red color, corresponding to the bound Fd domain.
Purified crARC, hmARC1, hmARC2, and YcbX were subjected to inductively coupled plasma optical emission spectrometry (ICP-OES) analysis to measure the Mo content which reflects the amount of Moco. As shown in Table 2, these four proteins present similar and almost fully saturated (1:1) Mo/protein ratios.
Table 2.
Cofactor content of recombinant proteins
| Protein | Cofactor contenta (mol/mol of protein) |
|---|---|
| crARC | 0.83 ± 0.06 Moco |
| crCyt b5-1 | 0.17 ± 0.01 heme |
| crCyt b5-2 | 0.20 ± 0.03 heme |
| crCyt b5-3 | 0.35 ± 0.02 heme |
| crCyt b5-4 | 0.19 ± 0.01 heme |
| crCyt b5-5 | 0.10 ± 0.01 heme |
| crCyt b5-R | 0.34 ± 0.02 FAD |
| crCysJ | 0.55 ± 0.06 FAD |
| YcbX | 0.81 ± 0.07 Moco |
| hmARC1 | 0.85 ± 0.11 Moco |
| hmARC2 | 0.90 ± 0.09 Moco |
Moco content was determined by quantifying Mo by ICP-OES (n = 3). Heme and FAD contents were determined via extinction coefficient (n = 3).
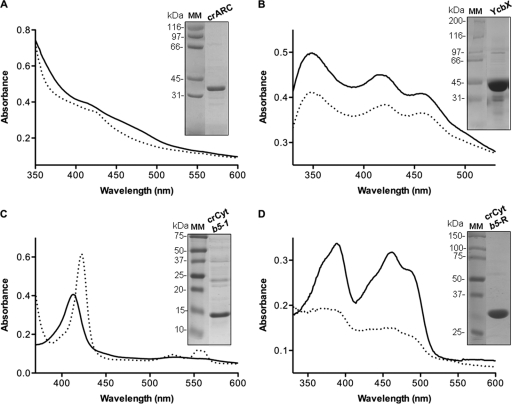
The visible absorption spectra of crARC in the oxidized state showed an absorption shoulder around 410 nm and a broad shoulder at 465 nm. When crARC was reduced with dithionite, the 410-nm shoulder shifted to 425 nm and the broad shoulder at 465 nm disappeared (Fig. 5A), in agreement with the spectra of hmARCs (34).
Fig. 5.
UV-visible absorption spectra and purity of recombinant crARC, YcbX, crCyt b5-1, and crCyt b5-R. Absorption spectra of 60 μM crARC (A), 50 μM YcbX (B), 6 μM crCyt b5-1 (C), and 20 μM crCyt b5-R (D) recorded in 100 mM Tris-HCl (pH 7.2). The absorption spectra of oxidized (solid black line) and reduced (dashed line) crARC, YcbX, crCyt b5-1, and crCyt b5-R are shown. Proteins were reduced with 2 mM dithionite. Recombinantly expressed proteins (10 μg) were separated on SDS-polyacrylamide gels and stained with Coomassie brilliant blue. An image of the gel with the positions of molecular mass markers (MM) (in kilodaltons) is shown for each spectrum.
The UV-visible absorption spectrum of YcbX showed maxima at 350 nm, 420 nm, and 455 nm typical of a Fd domain (Fig. 5B). After dithionite reduction, all these absorption peaks decreased their intensity.
Cyt b5 corresponds to an amphipathic protein consisting of two domains, a water-soluble heme-binding domain and a hydrophobic membrane-anchoring domain. The crCyt b5-1 and crCyt b5-4 proteins had a hydrophobic membrane-anchoring domain at the C terminus and N terminus, respectively (Fig. 4). In order to improve the solubility and thus expression and purity of crCyt b5-1 and crCyt b5-4, these proteins were partially truncated to remove the hydrophobic membrane-anchoring domain. The other crCyt b5 proteins lack any predicted anchoring domain. After purification, all the crCyt b5 were obtained in a soluble form between 12 and 19 kDa and characterized by their high purity and a dark red color, which indicated the binding of the heme group. The typical absorption spectrum signature of Cyt b5 proteins (29) was obtained for all five recombinant crCyt b5 proteins with a pronounced Soret peak at 413 nm and faint absorbance between 520 and 570 nm in the oxidized state (Fig. 5C). After dithionite reduction, the Soret peak shifted to 423 nm and two minor peaks at 526 and 556 nm were observed (Fig. 5C). The determination of crCyt b5-bound heme (29) revealed that these proteins were saturated with heme at an average of 10 to 35% (Table 2).
We have shown above that crARC together with crCyt b5-1 or crCyt b5-2 needs in vivo the presence of CysJ to detoxify HAP. Therefore, a CysJ homologous protein was searched for in the Chlamydomonas genome. A Chlamydomonas homologue (crCysJ) was found with 29% identity to CysJ. This protein belongs to the NADPH diflavin reductase family and like CysJ contains a N-terminal FMN domain and a C-terminal FAD/NADPH domain. However, as the crARC partner is a Cyt b5, a protein homologous to hCyt b5-R was also searched for in the Chlamydomonas genome. We identified a close homologue to hCyt b5-R with a 38% identity that we called crCyt b5-R. Both reductases belong to the NADH Cyt b5 reductase family that are flavoproteins with one FAD attached as a prosthetic group catalyzing the electron transfer from NADH to Cyt b5 (4).
The crCyt b5-R and crCysJ proteins contain in their N-terminal region a predicted hydrophobic membrane-anchoring domain and a peptide signal for chloroplast export, respectively. These sequences were removed in order to increase the expression and purification of the protein. After purification, crCyt b5-R and crCysJ were obtained as soluble proteins of 28.1 kDa and 71.2 kDa, respectively. These proteins were characterized by a light yellow color indicative of bound FAD. When crCyt b5-R and crCysJ were subjected to UV-visible absorption spectroscopy in the oxidized state, they showed two distinct absorption peaks at 390 and 460 nm accompanied by a shoulder at around 480 nm (Fig. 5D; data not shown for crCysJ). After dithionite reduction, all these absorption peaks were replaced by broad absorptions between 315 and 500 nm. These spectra are in agreement with the typical signature of flavin reductases. The determination of bound FAD (35) to crCyt b5-R and crCysJ revealed that these proteins were saturated at an average of 34% and 55%, respectively (Table 2). In summary, the spectral properties of the five crCyt b5, crCyt b5-R, and crCysJ indicate that these recombinant proteins meet the demands of electron carrier proteins.
The flavin reductase activity of crCyt b5-R and crCysJ was evaluated by measuring the reduction rate with the artificial electron acceptor ferricyanide and with each of the five crCyt b5. As shown in Table 3, crCyt b5-R was able to efficiently reduce ferricyanide with NADH but not with NADPH (data not shown). However, crCysJ was able to reduce efficiently ferricyanide with NADPH but not with NADH (data not shown). The crCyt b5-R protein was more efficient than crCysJ in the reduction of ferricyanide. These results indicated that crCyt b5-R and crCysJ are NADH and NADPH-dependent reductases, respectively. The crCyt b5-R protein was able to efficiently reduce crCyt b5-1, crCyt b5-2, and crCyt b5-4 with NADH but failed to reduce crCyt b5-3 and crCyt b5-5 (Table 3). However, crCysJ was able to reduce all the crCyt b5 with NADPH, except for crCyt b5-5. crCyt b5-R was more efficient in reducing crCyt b5-1 than crCysJ. These data show that the reductase activity of these proteins is fully active.
Table 3.
Determination of flavin reductase activity of crCyt b5-R and crCysJ
| Protein | Flavin reductase activitya measured by: |
|||||
|---|---|---|---|---|---|---|
| Reduction of crCyt b5 proteinb |
Reduction of ferricyanidec | |||||
| crCyt b5-1 | crCyt b5-2 | crCyt b5-3 | crCyt b5-4 | crCyt b5-5 | ||
| crCyt b5-R | 563 ± 8.6 | 107 ± 13 | 0 | 253 ± 33 | 0 | 678 ± 27 |
| crCysJ | 57 ± 11 | 556 ± 125 | 162 ± 19 | 297 ± 36 | 0 | 44.8 ± 5.6 |
The flavin reductase activity was measured by two methods; the reduction of ferricyanide and the reduction of each of the five crCyt b5 proteins (described in detail in Materials and Methods). NADH was the electron donor for crCyt b5-R, and NADPH was the electron donor for crCysJ. Data are means ± standard deviations of 3 independent experiments.
Measured in milliunits per milligram of protein.
Measured in units per milligrams of protein.
The in vitro HAP reduction by crARC depends on crCyt b5-1 and crCyt b5-R.
The in vivo experiments had shown that the HAP detoxification in E. coli by crARC plus crCyt b5-1 or crCyt b5-2 is CysJ dependent (Fig. 3). To ascertain whether any of the two purified Chlamydomonas flavin reductases are involved in the reduction of HAP, we performed the following in vitro experiments. Since both reductases crCyt b5-R and crCysJ are functional, we tested their capacity to donate electrons through crCyt b5 to crARC for HAP reduction. As shown in Table 4, the in vitro HAP reduction by crARC was assayed using each of the two Chlamydomonas reductases plus each crCyt b5. Interestingly, the only combination that was able to promote a significant HAP reduction was crCyt b5-R with crCyt b5-1 plus crARC. The other crCyt b5 failed to do so even in the presence of 1 mM molybdate in the reaction mix (data not shown). Combining crCysJ with crCyt b5-1 was able to promote HAP reduction but only a minor amount, about 4% of the activity found with crCyt b5-R. The activity with crCysJ, although small, was significant and reproducible. This result explains the lack of reversion of HAP toxicity in the E. coli cysJ mutant after in vivo expression of crARC and crCyt b5. As CysJ is homologous to crCysJ, it should be able to donate electrons to crARC plus crCyt b5-1, at least in vivo, efficiently enough to revert the HAP toxicity.
Table 4.
HAP reduction by the crARC systema
| Protein | Reduction of HAPb by: |
||||
|---|---|---|---|---|---|
| crCyt b5-1 | crCyt b5-2 | crCyt b5-3 | crCyt b5-4 | crCyt b5-5 | |
| crCyt b5-R | 140 ± 12.5 | ND | ND | ND | ND |
| crCysJ | 3.5 ± 1.8 | ND | ND | ND | ND |
The in vitro reduction of HAP was performed under the standard conditions: 100 pmol of crARC, 100 pmol of each crCyt b5 and 10 pmol of crCyt b5-R or crCysJ, 0.5 mM HAP, 1 mM NADH (with crCyt b5-R) or 1 mM NADPH (with crCysJ) and 15 min of reaction time.
Measured in milliunits per milligram of crARC. Data are means ± standard deviations of 3 independent experiments. ND, not detected.
These results indicate that in Chlamydomonas, the HAP reduction occurs by a three-component system consisting of crCyt b5-R, crCyt b5-1, and crARC. The crCyt b5-2 was able in vivo to revert HAP toxicity in media with high molybdate, meanwhile in vitro, even with high molybdate (data not shown), crCyt b5-2 was unable to promote HAP reduction. These results are interesting because they suggest that alternative reductases from crCysJ and crCyt b5-R could be involved in the reduction of HAP using crCyt b5-2 and crARC.
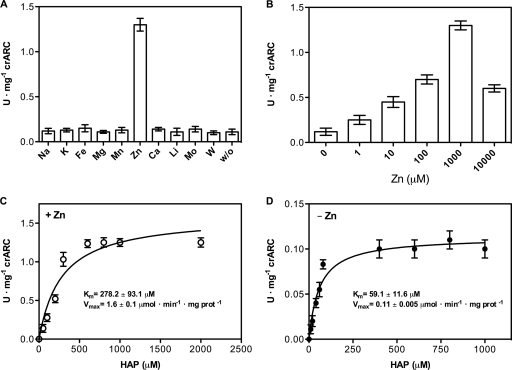
The crARC system has a Zn-dependent activity.
After determining that crCyt b5-R is the main reductase that with crCyt b5-1 and crARC can reduce HAP, the effects of different metals in the reaction mixture were also studied. The standard reduction of HAP was performed but in the presence of 1 mM concentrations of the different metals listed in Fig. 6A. The results were surprising; Zn caused 10-fold increases in activity in contrast to all other metals tested. The optimal Zn concentration was 1 mM, but even 10 μM increased the reaction rate 3 times; higher Zn concentrations were worse than the optimal but they still caused a positive effect (Fig. 6B).
Fig. 6.
The crARC system has a Zn-dependent activity. (A) HAP reduction by crARC, crCyt b5-1, and crCyt b5-R under standard conditions with 1 mM NaCl (Na), KCl (K), FeCl3 (Fe), MnCl2 (Mn), MgCl2 (Mg), ZnCl2 (Zn), CaCl2 (Ca), LiCl2 (Li), Na2MoO4 (Mo), and Na2WO4 (W) or with no metals (without [w/o]). (B) Same as panel A but with the indicated amounts of Zn. (C and D) Reaction rates of HAP reduction with 1 mM Zn (C) and without Zn (D). The curves in panels C and D were calculated from the Michaelis-Menten equation fitted to the data points (prot, protein). Error bars were derived from triplicate measurements.
The kinetic parameters of HAP reduction with Zn or without Zn were determined. The HAP reduction followed a Michaelis-Menten kinetics independent of the presence of Zn (Fig. 6C and D). The kinetic parameters for the reduction of HAP with and without Zn were as follows: with Zn, Km of 278 μM and Vmax of 1.60 μmol·min−1·mg crARC−1; without Zn, Km of 59 μM and Vmax of 0.11 μmol·min−1·mg crARC−1. Regarding the Km values, the substrate specificity for HAP was higher without Zn than with Zn. However, the Vmax was 14.4 times higher with Zn than without Zn, so it seems that Zn increases the crARC capacity to reduce HAP.
We measured the Zn content of crARC, hmARCs, and YcbX by ICP-OES, but we did not detect any Zn joined to these proteins (data not shown). This indicates that these recombinant proteins are expressed and purified without Zn, which might explain the need to add Zn to increase the crARC HAP reduction rate over 14 times.
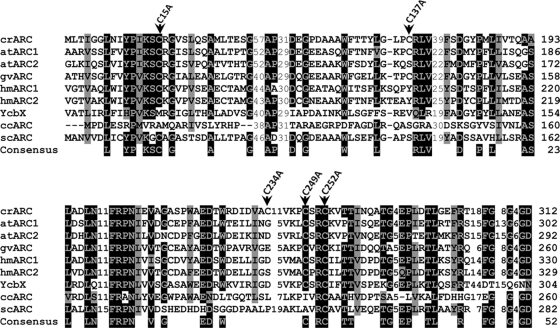
The fully conserved cysteine is essential for crARC activity.
Of all cysteines in ARC orthologous proteins, only one is fully conserved (1), that corresponding to cysteine 252 (C252) in crARC (Fig. 7). The 5 cysteines found in crARC were mutated to alanine. To find out whether the overall tertiary structure of the variants had changed in respect to the wild type, we performed a fluorescence spectroscopy study. As crARC has 10 tryptophan residues distributed along its sequence, if any mutation affected the folding of the protein, its fluorescence spectrum would be altered. We did not find any significant change in the fluorescence spectra in the mutants (data not shown). Therefore, it is unlikely that these cysteine-to-alanine changes significantly affected the folding of the proteins.
Fig. 7.
Multiple-sequence alignment of ARC proteins. The sequences and accession numbers (shown in parentheses) (GenPept accession numbers begin with XP and NP; others are proteins deducted from GenBank sequences) are as follows: crARC, Chlamydomonas reinhardtii ARC (XP_001694549); atARC1, Arabidopsis thaliana ARC1 (NP_174376); atARC2, Arabidopsis thaliana ARC2 (NP_199285); hmARC1, human ARC1 (Homo sapiens) (NP_073583); hmARC2, human ARC2 (NP_060368); YcbX, Escherichia coli YcbX (NP_415467); ccARC, Caulobacter crescentus ARC (AAK22857); scARC, Streptomyces coelicolor A3 ARC (CAC04053); and gvARC, Gloeobacter violaceus PCC 7421 ARC (NP_926027). The positions of the mutated cysteine to alanine in crARC are indicated by the black arrowheads. For other details, see the legend to Fig. 4.
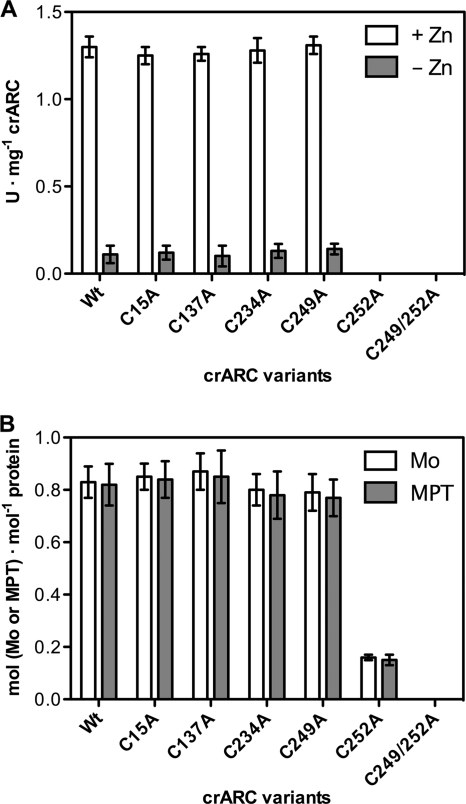
The HAP reduction activity was determined for each cysteine mutant with Zn and without Zn. None of them showed a HAP reduction rate that was different from that of the wild type, with the exceptions of the C252A mutant and the C249A C252A double mutant that totally abolished HAP reduction activity with and without Zn (Fig. 8A).
Fig. 8.
HAP reduction and Moco contents of crARC and their variants. (A) HAP reduction by crARC and their mutant variants with crCyt b5-1 and crCyt b5-R under standard conditions with Zn (+ Zn) or without Zn (− Zn). (B) Mo and MPT contents of crARC and their variants measured by ICP-OES and FormA, respectively. Wt, wild-type crARC; C249/252A, C249A C252A double mutant.
The crARC and its variants were subjected to FormA analysis, which allows the quantification of the organic motif of Moco (MPT) bound to the protein and to ICP-OES to measure the Mo content. In all of the crARC variants, the Mo and MPT contents were similar to those of the wild type except in the C252A mutant and C249A C252A double mutant that had lost 80% and 100% bound Mo and MPT, respectively (Fig. 8B).
These data indicate that the C252 is essential for the binding of Moco to crARC and that the HAP reduction activity depends on the presence of Moco. Interestingly, the neighboring C249 should be also mutated together with the C252 to fully abolish the Moco binding. These data can be explained by assuming that the close residue C249 is able to replace partially the function of the C252 at least with respect to Moco binding. However, since HAP reduction activity of the C252A mutants is zero, this residual Moco binding (20%) is inappropriate to promote a productive HAP reduction. This is the first evidence showing that the fully conserved cysteine in an ARC protein is essential for both Moco binding and catalysis.
DISCUSSION
The ARC (amidoxime reducing component) protein was discovered in 2006 as a new molybdenum cofactor (Moco)-containing enzyme involved in the reduction of N-hydroxylated compounds (NHC) (12) (Fig. 1). Proteins of this family are distributed throughout the three kingdoms of living organisms and occur either as stand-alone forms or fused to other domains (1). However, the study of this protein in the plant kingdom has not been addressed, and this is the first study of this protein in a plant-like organism, the green alga Chlamydomonas reinhardtii.
Chlamydomonas reinhardtii mutants defective in several Moco biosynthesis genes and sensitive to N6-hydroxylaminopurine (HAP) (Fig. 2) gave the first clue on the existence of a dependent NHC detoxification system. To investigate whether Chlamydomonas has a HAP reduction system related or not with the ARC proteins (Fig. 1D), we have cloned, purified, and studied the Chlamydomonas reinhardtii ARC protein, crARC, the six Chlamydomonas ferredoxins (Fds), five Chlamydomonas Cyt b5, and two Chlamydomonas reinhardtii flavin reductases (crCytb5-R and crCysJ), using the human ARCs (hmARCs) and YcbX proteins as controls for comparative experiments.
We have been able to demonstrate by heterologous expression in E. coli that in vivo the detoxification of HAP by crARC is dependent on crCyt b5-1, crCyt b5-2, and CysJ, but independent of Fd. This signifies that in Chlamydomonas reinhardtii, the HAP reduction system is more related to the three-component Cyt b5-dependent human system than to the two-component Fd-dependent bacterial system. The nature of this interaction indicates that CysJ has a broad spectrum of proteins with which it might interact and donate reducing equivalents; apart from its function in the sulfite reductase (10) and YcbX (19), it can even donate reducing equivalents to crCyt b5-1 and crCyt b5-2 proteins at least when they are heterologously expressed in E. coli. These facts raise the possibility of discovering other examples of redox carrier proteins functioning with multiple acceptor proteins.
Interestingly, crCysJ, which is homologous to CysJ, also promotes the reduction of HAP with crARC and crCyt b5-1 but in a minor way (Table 4). This explains why in vivo CysJ is needed by crARC plus crCyt b5-1 to detoxify HAP, because CysJ replaces the function of its homologous crCysJ.
In vivo crCyt b5-2 also reverts the toxicity of HAP if high molybdate is added to the growth medium (Fig. 3D), but this was not confirmed by the in vitro HAP reduction (Table 4). However, this result connects the HAP detoxification with the requirement of Moco cofactor for crARC activity. This means that there may be other reductases apart from crCyt b5-R and crCysJ that are able to interact with crCyt b5-2 and crARC in the reduction of HAP, or alternatively, the high molybdate concentration can alter the structure of one of these proteins, causing a conformational change needed for interactions. Future experiments are required to verify these hypotheses. The results obtained might explain the meaning of a three-component system, so that some of their members can be replaced by other proteins depending on demands of the cell.
The subcellular localization of ARC proteins is not well defined. Mammalian ARC proteins have been localized in the outer (9) and inner (5) mitochondrial membranes but also in the peroxisomal membranes (14). We have studied crARC and its partners with different prediction programs to determine whether they have any peptide signals for subcellular compartmentalization. No predicted signals for organellar compartmentalization could be found in crARC. However, their two partners crCyt b5-1 and crCyt b5-R contain a hydrophobic membrane-anchoring domain in their C-terminal and N-terminal sequences, respectively. These data suggest that crARC probably exerts its actions in a subcellular compartment. However, additional experiments will be needed to solve its subcellular localization.
Genomes of almost all eukaryotes that use molybdenum have two ARC proteins, with both showing strong similarities at amino acid and nucleotide levels. As there are unsequenced regions in the Chlamydomonas genome (22), the possibility of a second crARC protein cannot be excluded. In Arabidopsis thaliana, there are two homologous ARC proteins, Arabidopsis thaliana ARC1 (atARC1) and atARC2 (Fig. 7) (20). The atARC1 protein has a clear signal for chloroplast export that is absent in the atARC2, so it seems that in Arabidopsis, the ARC proteins may exert its functions in different subcellular compartments. Interestingly, we have also found ARC ortholog proteins in cyanobacterial genomes (Fig. 7), and as in Chlamydomonas, they lack the ferredoxin domain and there is only one ARC sequence per genome.
We have found that crARC has a Zn-dependent activity that with crCyt b5-1 and crCyt b5-R efficiently reduces HAP. The kinetic parameters for the reduction of HAP by the crARC system showed that Zn increases the crARC capacity to reduce HAP though it also increases its Km. However, the Michaelis-Menten equation fit much better the data points in the absence of Zn than in the presence of Zn. As it is a three-component system, a high number of protein interactions are required to complete the catalytic cycle, therefore a biphasic cooperative behavior cannot be discarded. The Km of crARC for HAP was similar to those reported for other NHC substrates with heterologously expressed hmARC proteins, but the Vmax of crARC with Zn was between 10 and 100 times higher (15, 34). As Zn is not essential for crARC activity but drastically increases its Vmax, we propose that a potential role of Zn could be related to increasing the capacity to eliminate the toxic NHC in the cell.
It is unknown which proteins of the crARC system requires Zn, although it could be related to increasing the proper binding between them to promote a correct electron transfer reaction.
The Vmax for benzamidoxime when the hmARC proteins were purified from human mitochondria was 12.2 μmol·min−1·mg protein−1 (12). This Vmax is more than 350 and 40 times higher than those obtained for heterologously expressed hmARC1 and hmARC2, respectively, but only 7 times greater than crARC with Zn (Vmax of 1.6 μmol·min−1·mg crARC−1). These facts suggest that hmARCs purified from living mitochondria could have retained Zn bound. Therefore, the Zn-dependent activity found with crARC could be a general feature for the other ARC proteins, though this should be confirmed by future experiments.
All ARC proteins contain a fully conserved cysteine that could be considered part of their signature (1) (Fig. 7). We have generated amino acid substitutions of each crARC cysteine to alanine. These substitutions had no effect on the Zn-dependent activity apart from the absolutely conserved cysteine 252 (Fig. 8A) that strongly affected Moco binding (Fig. 8B). Interestingly, to fully abolish Moco binding to crARC, the neighboring C249 also has to be mutated. This means that the C249 in the absence of a functional C252 is able to bind Moco to some extent, but this binding is not appropriate for productive enzyme catalysis. This is the first demonstration in an ARC protein that the fully conserved cysteine is involved in Moco chelation to the protein.
Until now, the Mo coordination spheres of the ARC proteins were unknown (Fig. 1A). In Moco proteins, Mo is chelated to two thiol groups of molybdopterin (MPT) and also with two oxo groups. The fifth Mo ligand is the sulfur of a cysteine in the sulfite oxidase (SO) family or an inorganic sulfur in the xanthine oxidase (XO) family (28), and the exchange of this conserved cysteine to serine or to alanine completely abolishes the enzyme function, as in the case of chicken sulfite oxidase (25). We propose that the fully conserved ARC cysteine is indeed the putative fifth Mo ligand of the ARC proteins (Fig. 9) and therefore, that the ARC proteins belong to the SO family of Moco proteins. Recently, Wahl et al. characterized the hmARCs and found that they do not belong to the XO family, since cyanide treatment neither released sulfur nor significantly affected the activities of hmARCs (34). In addition, by mutating each cysteine to serine, no difference in the activity of the hmARCs was found. It is possible that changing cysteine to serine, another polar amino acid and with a similar electronegative atom (S versus O), could have retained Mo chelation capacity and activity.
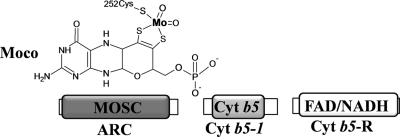
Fig. 9.
Scheme of the Chlamydomonas ARC system. The figure shows the structure of the Moco molecule. The Mo atom is shown in bold type, illustrating that the fifth Mo ligand in crARC is cysteine 252. The protein domains in the crARC system are shown in boxes (for more details, see Fig. 1 and Discussion).
Concerning the physiological functions of ARC proteins, it appears likely that one function could be to prevent the accumulation of mutagenic base analogues in the cell as HAP (16) and N-hydroxycytosine (34). The hmARC system is able to reduce N-hydroxysulfonamides, such as N-hydroxy-valdecoxib, which have considerable potential to treat a variety of disorders (13). In addition, hmARC proteins have recently been suggested to act as regulators for the l-arginine-dependent biosynthesis of nitric oxide (NO) by catalyzing the controlled elimination of the NO precursor Nω-hydroxy-l-arginine (NOHA) (15). Whether ARC proteins are involved in physiological NOHA reduction and/or are capable of physiologically affecting NO levels has to be investigated in future experiments.
In conclusion, we have characterized for the first time the ARC system in a photosynthetic organism showing that in Chlamydomonas this system is more related to the human system than to the bacterial system. The crARC partners are crCyt b5-1 and crCyt b5-R. The enzyme has a Zn-dependent activity that does increase its Vmax more than 10 times. We propose that the fully conserved cysteine 252 of crARC is involved in Moco chelation (Fig. 9). Therefore, the ARC protein would belong to the SO family of Moco enzymes.
ACKNOWLEDGMENTS
This work was supported by Ministerio de Ciencia e Innovación (MICINN) (grant BFU 2008-01798), Program Ramon y Cajal, Spain, Junta de Andalucía, Spain (PAI, BIO-128, and P08-CVI-04157) and Universidad de Córdoba. Genome analysis was conducted by the U.S. Department of Energy Joint Genome Institute (contract DE-AC02-05CH11231).
We thank Brian McDonagh for critical reading of the manuscript, Rafael Carrasco Dominguez of TSLADI, Universidad de Huelva for ICP-OES, Emanuel Sanz Luque for editing the manuscript, and Teresa Pineda for help with the fluorescence spectroscopic analysis.
Footnotes
Published ahead of print on 29 July 2011.
REFERENCES
- 1. Anantharaman V., Aravind L. 2002. MOSC domains: ancient, predicted sulfur-carrier domains, present in diverse metal-sulfur cluster biosynthesis proteins including molybdenum cofactor sulfurases. FEMS Microbiol. Lett. 207:55–61 [DOI] [PubMed] [Google Scholar]
- 2. Arnon D. I. 1949. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 24:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baba T., et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bando S., et al. 2004. Structure of human erythrocyte NADH-cytochrome b5 reductase. Acta Crystallogr. D Biol. Crystallogr. 60:1929–1934 [DOI] [PubMed] [Google Scholar]
- 5. Da Cruz C. S., et al. 2003. Proteomic analysis of the mouse liver mitochondrial inner membrane. J. Biol. Chem. 278:41566–41571 [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez-Ballester D., de Montaigu A., Galvan A., Fernandez E. 2005. Restriction enzyme site-directed amplification PCR: a tool to identify regions flanking a marker DNA. Anal. Biochem. 340:330–335 [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez-Ballester D., de Montaigu A., Higuera J. J., Galvan A., Fernandez E. 2005. Functional genomics of the regulation of the nitrate assimilation pathway in Chlamydomonas. Plant Physiol. 137:522–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Grodberg J., Dunn J. J. 1988. ompT encodes the Escherichia coli outer membrane protease that cleaves T7 RNA polymerase during purification. J. Bacteriol. 170:1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gruenewald S., et al. 2008. The fourth molybdenum containing enzyme mARC: cloning and involvement in the activation of N-hydroxylated prodrugs. J. Med. Chem. 51:8173–8177 [DOI] [PubMed] [Google Scholar]
- 10. Gruez A., et al. 2000. Four crystal structures of the 60 kDa flavoprotein monomer of the sulfite reductase indicate a disordered flavodoxin-like module. J. Mol. Biol. 299:199–212 [DOI] [PubMed] [Google Scholar]
- 11. Harris E. H. 2001. Chlamydomonas as a model organism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52:363–406 [DOI] [PubMed] [Google Scholar]
- 12. Havemeyer A., et al. 2006. Identification of the missing component in the mitochondrial benzamidoxime prodrug-converting system as a novel molybdenum enzyme. J. Biol. Chem. 281:34796–34802 [DOI] [PubMed] [Google Scholar]
- 13. Havemeyer A., et al. 2010. Reduction of N-hydroxy-sulfonamides, including N-hydroxy-valdecoxib, by the molybdenum-containing enzyme mARC. Drug Metab. Dispos. 38:1917–1921 [DOI] [PubMed] [Google Scholar]
- 14. Islinger M., Luers G. H., Li K. W., Loos M., Volkl A. 2007. Rat liver peroxisomes after fibrate treatment. A survey using quantitative mass spectrometry. J. Biol. Chem. 282:23055–23069 [DOI] [PubMed] [Google Scholar]
- 15. Kotthaus J., et al. 2010. Reduction of Nomega-hydroxy-l-arginine by the mitochondrial amidoxime reducing component (mARC). Biochem. J. 433:383–391 [DOI] [PubMed] [Google Scholar]
- 16. Kozmin S. G., Leroy P., Pavlov Y. I., Schaaper R. M. 2008. ycbX and yiiM, two novel determinants for resistance of Escherichia coli to N-hydroxylated base analogues. Mol. Microbiol. 68:51–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kozmin S. G., Pavlov Y. I., Dunn R. L., Schaaper R. M. 2000. Hypersensitivity of Escherichia coli (ΔuvrB-bio) mutants to 6-hydroxylaminopurine and other base analogs is due to a defect in molybdenum cofactor biosynthesis. J. Bacteriol. 182:3361–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kozmin S. G., Schaaper R. M. 2007. Molybdenum cofactor-dependent resistance to N-hydroxylated base analogs in Escherichia coli is independent of MobA function. Mutat. Res. 619:9–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kozmin S. G., Wang J., Schaaper R. M. 2010. Role for CysJ flavin reductase in molybdenum cofactor-dependent resistance of Escherichia coli to 6-N-hydroxylaminopurine. J. Bacteriol. 192:2026–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Llamas A., Tejada-Jimenez M., Fernandez E., Galvan A. 2011. Molybdenum metabolism in the alga Chlamydomonas stands at the crossroad of those in Arabidopsis and humans. Metallomics 3:578–590 [DOI] [PubMed] [Google Scholar]
- 21. Llamas A., et al. 2007. Chlamydomonas reinhardtii CNX1E reconstitutes molybdenum cofactor biosynthesis in Escherichia coli mutants. Eukaryot. Cell 6:1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Merchant S. S., et al. 2007. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 318:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pace C. N., Vajdos F., Fee L., Grimsley G., Gray T. 1995. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4:2411–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pavlov Y. I., et al. 1991. The genetic activity of N6-hydroxyadenine and 2-amino-N6-hydroxyadenine in Escherichia coli, Salmonella typhimurium and Saccharomyces cerevisiae. Mutat. Res. 253:33–46 [DOI] [PubMed] [Google Scholar]
- 25. Qiu J. A., et al. 2010. The structures of the C185S and C185A mutants of sulfite oxidase reveal rearrangement of the active site. Biochemistry 49:3989–4000 [DOI] [PubMed] [Google Scholar]
- 26. Schellenberg K. A., Hellerman L. 1958. Oxidation of reduced diphosphopyridine nucleotide. J. Biol. Chem. 231:547–556 [PubMed] [Google Scholar]
- 27. Schwarz G., Boxer D. H., Mendel R. R. 1997. Molybdenum cofactor biosynthesis. The plant protein Cnx1 binds molybdopterin with high affinity. J. Biol. Chem. 272:26811–26814 [DOI] [PubMed] [Google Scholar]
- 28. Schwarz G., Mendel R. R., Ribbe M. W. 2009. Molybdenum cofactors, enzymes and pathways. Nature 460:839–847 [DOI] [PubMed] [Google Scholar]
- 29. Strittmatter P., Velick S. F. 1956. The isolation and properties of microsomal cytochrome. J. Biol. Chem. 221:253–264 [PubMed] [Google Scholar]
- 30. Strittmatter P., Velick S. F. 1957. The purification and properties of microsomal cytochrome reductase. J. Biol. Chem. 228:785–799 [PubMed] [Google Scholar]
- 31. Temple C. A., Graf T. N., Rajagopalan K. V. 2000. Optimization of expression of human sulfite oxidase and its molybdenum domain. Arch. Biochem. Biophys. 383:281–287 [DOI] [PubMed] [Google Scholar]
- 32. Terauchi A. M., et al. 2009. Pattern of expression and substrate specificity of chloroplast ferredoxins from Chlamydomonas reinhardtii. J. Biol. Chem. 284:25867–25878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vogel H. J., Bonner D. M. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97–106 [PubMed] [Google Scholar]
- 34. Wahl B., et al. 2010. Biochemical and spectroscopic characterization of the human mitochondrial amidoxime reducing components hmARC-1 and hmARC-2 suggests the existence of a new molybdenum enzyme family in eukaryotes. J. Biol. Chem. 285:37847–37859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Whitby L. G. 1953. A new method for preparing flavin-adenine dinucleotide. Biochem. J. 54:437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]