Abstract
Two proteins that differ at the N terminus (l-KlCpo and s-KlCpo) are derived from KlHEM13, a single-copy-number gene in the haploid genome of Kluyveromyces lactis. Two transcriptional start site (tss) pools are detectable using primer extension, and their selection is heme dependent. One of these tss pools is located 5′ of the first translation initiation codon (TIC) in the open reading frame of KlHEM13, while the other is located between the first and second TICs. In terms of functional significance, only s-KlCpo complements the heme deficiency caused by the Δhem13 deletion in K. lactis. Data obtained from immune detection in subcellular fractions, directed mutagenesis, chromatin immunoprecipitation (ChIP) assays, and the functional relevance of ΔKlhem13 deletion for KlHEM13 promoter activity suggest that l-KlCpo regulates KlHEM13 transcription. A hypothetical model of the evolutionary origins and coexistence of these two proteins in K. lactis is discussed.
INTRODUCTION
Cell complexity depends on regulatory mechanisms that control gene expression and adapt levels of specific proteins to internal and environmental changes. One gene can produce one protein or several proteins through mechanisms controlling transcription, mRNA processing, translation, and posttranslational events. In yeast, changes in gene transcription and the regulation of mRNA translation play critical roles in the response to oxygen availability.
In the yeast Kluyveromyces lactis, the HEM13 gene (KlHEM13) encodes coproporphyrinogen oxidase (Cpo), which catalyzes the sixth step of heme biosynthesis. Cpo promotes oxidative decarboxylation of the 2- and 4-propionyl groups of coproporphyrinogen III to form the vinyl groups of protoporphyrinogen IX. Northern blot analyses have revealed that KlHEM13 is a hypoxic gene and that its transcription is regulated by oxygen and heme (10). In Saccharomyces cerevisiae, the gene homologous to KlHEM13 is essential in the absence of a heme precursor under aerobiosis (normoxia, with approximately 20% O2) and hypoxic conditions (less than 0.05% O2). KlHEM13 complements a Δhem13 mutation in S. cerevisiae and restores its capacity to grow in the absence of hemin (protoporphyrin IX) (2). Sequence alignment of the protein derived from the KlHEM13 open reading frame (ORF) with the S. cerevisiae homolog demonstrates the presence of 52 additional amino acids at the N terminus of KlCpo. This extension includes the amino acids between the first and second in-frame ATG codons in the KlHEM13 ORF. Therefore, computer-aided translation from the second in-frame ATG (the second translation initiation codon [TIC-2]) produces a Saccharomyces-like Cpo, while translation from the first in-frame ATG (TIC-1) gives rise to an N-terminally extended Cpo (Fig. 1A).
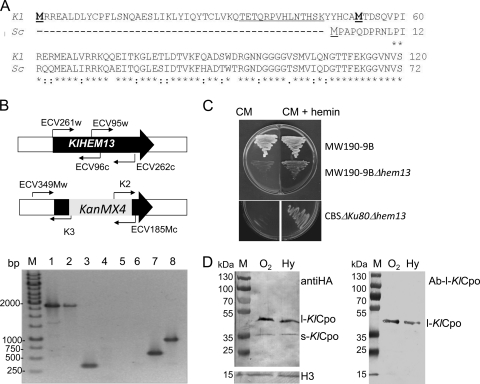
Fig. 1.
One gene and two proteins. (A) Alignment of the N-terminal regions of the ORFs translated from the KlHEM13 (Kl) and ScHEM13 (Sc) genes. The first and second in-frame Mets from the K. lactis protein are boldface and underlined. (B) Verification of the null strains. (Top) Schemes showing the primers used. (Bottom) PCR amplifications from MW190-9B (lanes 1, 3, 5, and 6) or MW190-9B-Δhem13 (lanes 2, 4, 7, and 8) DNA with primers ECV261w and ECV262c (lanes 1 and 2), ECV95w and ECV96c (lanes 3 and 4), ECV349Mw and K3 (lanes 5 and 7), or K2 and ECV185Mc (lanes 6 and 8). Lane M, 1-kbp GeneRuler (Fermentas). (C) Growth of wild-type and ΔKlhem13 strains in complete medium (CM) alone or supplemented with hemin. (D) Western blot of proteins expressed in MW190-9B from the pSK1-KlHEM13-3HA construct C-terminally tagged with HA and hybridized with anti-HA (left) or Ab-l-KlCpo, which is directed against the peptide underlined at the N terminus in panel A (right). Hybridization with ab21054, an HRP-conjugated anti-histone H3 antibody from Abcam (H3), was used as a loading control. l-KlCpo, protein translated from the first TIC; s-KlCpo, protein translated from the second TIC. The strains were grown under aerobiosis (O2) or hypoxia (Hy). The molecular sizes of the proteins used as molecular markers (PageRuler prestained protein ladder; Fermentas) are given to the left of each panel.
This study demonstrates that in K. lactis, KlHEM13, a single-copy-number gene, produces two proteins that differ in the N-terminal region (l-KlCpo and s-KlCpo). Diverse evidence supports a mechanism in which the use of alternative transcription start sites (tss) allows two different polypeptides to be synthesized. Complementation with s-KlCpo, but not with l-KlCpo, restores the growth of K. lactis Δhem13 mutants in media without hemin supplementation. Differential use of tss pools during hypoxia and other functional data indicate that l-KlCpo plays a role in the transcriptional regulation of KlHEM13 expression. A hypothetical model of the origin and coexistence of these two proteins that have differing functions is proposed.
MATERIALS AND METHODS
Yeast strains and growth conditions.
The K. lactis strains used in this study were NRRL-Y1140 (CBS2359), MW190-9B (MATα lac4-8 uraA Rag+), and CBSΔKU80 (CBS2359 ΔKlku80) (16). The S. cerevisiae strain BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0), used for efficient recombination, was obtained from EUROSCARF (http://web.uni-frankfurt.de/fb15/mikro/euroscarf/yeast.html). Yeast cells were grown at 30°C in YPD (2% glucose, 2% Bacto peptone, 1% yeast extract) or synthetic complete medium (CM) (29). Hypoxic conditions were generated in anaerobic jars with the AnaeroGen gas pack system (Oxoid Ltd.). For hypoxic growth, media were supplemented with Tween and ergosterol as described previously (2). When required, media were supplemented with hemin at a final concentration of 50 μg/ml.
Nomenclature used for gene regions, primers, and plasmids.
In the numbering of sequences, the A in the first ATG of the KlHEM13 ORF was considered position +1; negative positions were counted 5′-wards and positive positions 3′-wards. Using this nomenclature for the KlHEM13 ORF, TIC-1 was at position +1, TIC-2 at position +157, and the TGA stop codon at position +1126. In the primer sequences, capital letters represent the genomic sequences and lowercase letters represent additional sequences added for design convenience. Restriction sites are shown in italics. The letters “w” and “c” at the end of primer names indicate the forward and reverse strands, respectively.
Construction of the K. lactis Δhem13 strains.
The KlHEM13 gene was amplified from MW190-9B genomic DNA using PCR with primers ECV184Mw (ggggggtaccTTTTTTGGCATGGAAGGAAGG, at position −1025) and ECV185Mc (ggggggtaccTAGGTGGAGAAGGTGCATGGG, at position +1751) and was cloned into the KpnI site of the YIplac204 vector (9). This construct was used as a template for inverse PCR carried out with the divergent primers ECV250Mc (gggggcggccgcGGCTAGCTAGTTTCCACCG, at position −129) and ECV251Mw (gggggcggccgcGGCCGTCGACAATACTTTT, at position +1253). Thus, after religation, deletion of the ORF was obtained. A NotI site included in the primers allowed the kanMX4 cassette for Geneticin (G418) selection to be introduced (28). This construct, YIplac204-KlΔhem13::kanMX4, was used as a PCR template to obtain a linear fragment containing the marker for G418 resistance and two flanking regions of homology to the K. lactis genome using primers ECV261Mw (GGAAGGAACGCGATCTTCAGC, annealing at position −1014) and ECV262Mc (CAGCAGCACAATGTCCGAACC, annealing at position +1715). K. lactis CBSΔKU80 and MW190-9B cells were transformed using this cassette by following the procedure of Ito et al. (13). Two rounds of selection in YPD-G418 (40 μg/ml) were carried out to eliminate false positives. Correct integration into the K. lactis genome was verified using PCR as described previously (26). Genomic DNA samples isolated from parental strains and their respective K. lactis Δhem13 mutants were used as templates for amplification with sets of primers designed to test the genotype. Internal primers K2 (CGCCTTAATTAACCCGGGGAT) and K3 (GCCTCGACATCATCTGCCCAG) were designed for annealing inside the kanMX4 cassette. In order to verify correct homologous integration, external primers ECV185Mc (described above) and ECV349Mw (gggggagctcACGACGAAGTTTGTTATTATTTTTTTT, at position −1046) were designed flanking KlHEM13, but external to the regions of homology used for the recombination event. Amplification with each pair of external/internal primers produced PCR products of the expected sizes. The expected size of the fragment obtained with ECV261w and ECV262c (described above) and the absence of amplification of an internal fragment of the ORF were tested with primers ECV95w (GGACGTTGCCTTGTACCCAAA, at position +687) and ECV96c (GACACGGGATCCTGGAGTTC, at position +1008). Further verifications of null phenotypes were accomplished using Cpo enzymatic assays and phenotypic analyses of the growth defect in CM in the absence of hemin.
Cell fractionation.
K. lactis cells (Δhem13 and wild type) were grown in heme-supplemented medium as described above. Cells were harvested at an optical density at 600 nm (OD600) of 2, washed (to remove adsorbed heme), and broken with glass beads (diameter, 0.45 mm) in 0.1 M potassium phosphate buffer, pH 7.2. The cell extract was centrifuged for 5 min at 3,000 × g to remove intact cells. The supernatant is referred to as the homogenate (H). An aliquot of the homogenate was centrifuged for 30 min at 45,000 × g to sediment membranous fractions containing nuclei (MF). The supernatant was referred to as the “soluble fraction” (SF). The pellet (the MF) was resuspended in potassium phosphate buffer and was solubilized by sonication.
Cpo activity.
Cpo activities were measured as described previously (18) on a Photon Technology International (PTI) spectrofluorometer in 1-ml cuvettes at 30°C. Tween 80, EDTA, dithiothreitol (DTT), and freshly prepared coproporphyrinogen solutions were added as described previously (18). The coupling enzyme was highly purified S. cerevisiae protoporphyrinogen oxidase (100 U/ assay). The excitation wavelength was 410 nm, and the emission wavelength was 632 nm. One unit of Cpo activity is defined as the amount of enzyme that catalyzes the formation of 1 nmol of protoporphyrinogen per hour at 30°C under standard conditions.
C-terminal tagging of KlCpo.
KlHEM13 was modified using the pFA6a-3HA-kanMX6 cassette (20). The 3HA-kanMX6 cassette was amplified using PCR with two primers, ECV436Mw (CAAGTATTGAAGAACCCTGTTGAATGGGTTcggatccccgggttaattaa) and ECV437Mc (ATACATGAGACGTTACATAAATAAAGGCATgaattcgagctcgtttaaac). These primers were designed to fuse the KlHEM13 ORF in frame to a DNA sequence encoding three copies of the hemagglutinin (HA) epitope. They had regions of homology to the pFA6a-3HA-kanMX6 cassette (lowercase letters in sequences) and to KlHEM13 (capital letters), near a BsmI restriction site at position +1127 that overlapped with the TGA stop codon of KlHEM13. Plasmid pSK1-KlHEM13 (2) was linearized by partial digestion with BsmI and was used together with the 3HA-kanMX6 PCR product to cotransform the S. cerevisiae strain BY4741. The transformed cells were selected in CM without uracil (CM-Ura) and in CM-Ura plates with 40 μg/ml G418. Growth in CM-Ura selected cells carrying plasmid pSK1-KlHEM13, and growth in media with G418 selected recombinant plasmids carrying the tagged protein. The recombinant plasmid was isolated from the transformed BY4741 cells and was amplified in DH10B bacteria by using LB medium supplemented with kanamycin (20 μg/ml) for selection. After verification by sequencing, plasmid pSK1-KlHEM13-3HA was used to transform the K. lactis strain MW190-9B.
Protein extracts.
Protein extracts for Western blotting and chromatin immunoprecipitation (ChIP) assays were obtained in phosphate-buffered saline (PBS) (0.8% NaCl, 0.02% KCl, 0.144% Na2HPO4, 0.024% KH2PO4 [pH 7.4]), and those for the measurement of β-galactosidase activity were obtained in Z-Buffer as described previously (2). Protein concentrations were determined using the method of Bradford (3).
Western blotting.
Western blotting and control of loading were carried out as described previously (1). The HA probe (F-7) (sc-7392; Santa Cruz Biotechnology, Inc.), directed against the HA-tagged proteins (l-KlCpo and s-KlCpo) at their C termini, was used according to the manufacturer's instructions. A polyclonal probe specific for l-KlCpo, directed to its N terminus (Ab-l-KlCpo), was obtained by chemical synthesis of the TETQRPVHLNTHSK peptide (positions T34 to K47) and rabbit immunization (at the facilities of GenScript USA Inc.). Membranes were incubated with a 1:325 dilution of Ab-l-KlCpo in PBS with 0.25% nonfat dry milk and 0.1% Tween 20 at room temperature for 25 min. The secondary antibody, horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (sc-2004; Santa Cruz Biotechnology, Inc.), was diluted 1:200 in PBS with 0.1% Tween 20, and membranes were incubated at room temperature for 25 min. An HRP-conjugated antibody against human histone H3 (ab21054; Abcam) was used as a loading control, since it cross-reacts with the homologous protein from K. lactis. The immune complexes on the membrane were visualized using diaminobenzidine (Sigma-Aldrich Co.) and H2O2.
Protein identification by mass spectrometry.
Samples were analyzed using matrix-assisted laser desorption-tandem time of flight (MALDI-TOF/TOF) mass spectrometry by following procedures described previously (24). Peptides were identified using ProteinPilot software, version 4.0 (AB Sciex Pte. Ltd.).
RT-PCR.
RNA was isolated from MW190-9B cells grown in CM to an OD600 of 0.8 with the Aurum Total RNA Mini kit (Bio-Rad). To ensure that the RNA preparation was DNA free, it was treated with RQ1 RNase-free DNase (Promega) according to the manufacturer's instructions. After this treatment, the absence of DNA was verified by the failure to amplify a control region without the addition of reverse transcriptase (RT). The cDNA was obtained by using 1 ng of RNA, 2 pmol of primer oligo(dT)18, and the SuperScript II reverse transcriptase kit (Invitrogen) according to the manufacturer's instructions. After cDNA synthesis, primer ECV550Mc (ATTTTACTCCTGTTGCATCTG, annealing at position +475) and forward primers ECV551Mw (ACTGAAATGAGAAGAGAAGCA, annealing at position −6) and ECV548Mw (TGGTAAAGCAAACAGAAACT, at position +108) were used for PCR amplification of the corresponding fragments, including TIC-1.
Determination of tss using primer extension.
Total RNA (60 μg) was incubated at 70°C for 10 min with 0.5 μg of primer ECV300Mc (ATGTAAAGTTTTATCAATGATTCTGC, at position +74) or primer ECV405Mc (AGTATCCAAAGTTTCCAGAC, at position +252) in two independent reactions. The mixtures were cooled briefly on ice and were reverse transcribed with the SuperScript II reverse transcriptase kit according to the manufacturer's protocol (Invitrogen) by using [α-32P]dATP for labeling. After cDNA synthesis, RNA was hydrolyzed by alkali treatment. The cDNA was precipitated with an equal volume of isopropanol at −80°C for 2 h and was pelleted by centrifugation at 13,000 rpm for 30 min. The purified cDNA samples were dissolved in 10 μl water. Two sequencing reactions were carried out using the Sanger method on template DNA from the pSK1-KlHEM13 clone, and the same primer was used for the primer extension reaction. Reaction mixtures were loaded onto a 7% polyacrylamide gel and were exposed to screens for visualization in the PhosphorImager system (Pharmacia Biotech) after electrophoresis and vacuum drying.
Fusion of KlHEM13 promoter regions to the lacZ reporter gene and measurement of β-galactosidase activity.
The upstream region of KlHEM13 from −1025 to +14 was amplified with ECV163Mw (gggggatccTTTTTTGGCATGGAAGGAACG) and ECV154c (gtcgacGCTTCTCTTCTCATTTCAGTT), and after digestion with BamHI and SalI, the product was cloned into the pXW1 vector (5) upstream of, and in frame with, the lacZ reporter gene. The upstream KlHEM13 region including the sequence between TIC-1 and TIC-2 (to position +190) was cloned into the pXW1 polylinker in a similar manner, but using the reverse primer ECV394Mc (gggggaagcttTTCTCTCTCTAATTGGCACTTGTG), which contains a HindIII site for cloning. The upstream region of KlHEM13 from −494 to +190 was amplified with ECV396Mw (ggggggtcgaccattcatcgtaactgccaacc) and ECV394Mc. These constructs, named pTIC-1-lacZ, pTIC-2-lacZ, and pTIC-2494-lacZ, respectively, were verified by sequencing and were used to transform K. lactis strain MW190-9B and its Δhem13 derivative (this work) as required. β-Galactosidase activity was measured at 30°C using protein extracts (as described above) in four replicates. Average values ± standard deviations are presented. Values are presented as nmol of ortho-nitrophenyl-β-d-galactopyranoside hydrolyzed per min per mg protein.
KlHEM13 constructs in pCXJ18.
Constructs of KlHEM13 expressed from its own promoter and terminator regions, or containing only the desired ORF (starting from TIC-1 or TIC-2) but under the control of heterologous promoter and terminator regions, were prepared in the K. lactis centromeric (KlCEN2) vector pCXJ18 (4). The pCXJ18-KlHEM13 construct was obtained from an available KlHEM13 clone; KlHEM13, including its own promoter and terminator regions, was digested with HindIII and BamHI and was inserted into the pCXJ18 polylinker. The pCXJ18-l-KlCpo and pCXJ18-s-KlCpo constructs were obtained as follows. Two DNA sequences, including the ORF from the first (from +1 to +1126) or second (from +157 to +1126) in-frame ATG start codon, respectively, were amplified from genomic DNA obtained from the K. lactis strain MW190-9B using PCR. In both cases, the amplified DNA sequences contained BsmFI and NotI restriction sites (included in the design of the primers) at the 5′ and 3′ ends, respectively, and were used to insert the clones into the pKLAC1 vector (New England Biolabs Inc.). These constructs were amplified using PCR with primers ECV694AVc (gggggtcgacGAAGTCCATATTGTACACCCGG) and pKLAC7391w (ggggtgatcaATTAAGTTGGGTAACGCCAGG), containing HindIII and BamHI sites, respectively. The PCR products were digested with HindIII and BamHI and were cloned into the same sites of the pCXJ18 vector. In these constructs, transcription was under the control of the LAC4 promoter and the LAC4 terminator from the pKLAC1 vector. All the constructs were verified by sequencing.
Directed mutagenesis.
Plasmid pSK1-KlHEM13-3HA was subjected to directed mutagenesis in order to eliminate the codon for the second in-frame Met present in KlCpo. For directed mutagenesis, the Megaprimer PCR protocol was used (27). The mutagenic oligonucleotide AVV019w (ACATTCTAAATATTATCATTGTGCGgcGACAGATTCACAAGTGCCAATTAGA), for changing Met to Ala, and ECV120Mc (AGGCGAAGAATTTTACTCCTG) were used to obtain the megaprimer, and PCR mutagenesis was carried out by our laboratory protocol using Dream-Taq (Fermentas). After the PCRs, products were first treated with DpnI and then transformed into Escherichia coli ECOS Blue (Yeastern Biotech Co.), and the DNA was amplified with the GeneJET Plasmid Miniprep kit (Fermentas). After the transformation of MW190-9B yeast cells, the absence of s-KlCpo production from this construct was verified by Western blotting.
ChIP assays.
Protein extracts were prepared from cells of strain MW190-9B transformed with plasmid pSK1-KlHEM13-3HA or with its derivative pSK1-KlHEM13-3HA-2TIC mutant, obtained by mutagenesis, and were grown in the selective medium CM-Ura under aerobic or hypoxic conditions. ChIP assays were carried as described previously (19) using magnetic Dynabeads (Invitrogen S.A.) according to the manufacturer's instructions and anti-HA (sc-7392; Santa Cruz Biotechnology, Inc.) for precipitation of the HA-tagged protein. Each assay was carried out in duplicate from two independent cultures.
The promoter region of KlHEM13, from −494 to −200 and from −434 to −200, was amplified with primers ECV396Mw (ggggggtcgacCATTCATCGTAACTGCCAACC) and ECV555Mc (ggggggTCGACAGTCCGAGCACCACAAGATGAATA) or primers ECV554Mw (gggggggatccCGATAGCCTTTTCTTGACCCAATT) and ECV555Mc, respectively. Negative controls for amplification from the HA immunoprecipitates were included. The primers used, AVV026 (GCCTTGTACCACAGGTTTCG-3′) and AVV027 (AGAGTTTCACACAATTTCCGC), were designed to amplify a region of the promoter of the unrelated KLLA0E17623g ORF.
RESULTS
Only one gene encodes Cpo in K. lactis.
A null allele of KlHEM13 was constructed in the K. lactis haploid strains CBSΔKU80 and MW190-9B. Correct integration of the disruption cassette was verified and is demonstrated for strain MW190-9B (Fig. 1B). Similar results were obtained with CBSΔKU80 (data not shown). Both ΔKlhem13 null strains were unable to grow in the absence of hemin (Fig. 1C). Cpo enzymatic activity was determined in CBSΔKU80 and its CBSΔKU80-ΔKlhem13 derivative using fluorometric assays (18). Cpo enzymatic activity was absent in the CBSΔKU80-ΔKlhem13 strain. Cpo activity in the strain with wild-type HEM13, CBSΔKU80, was detected only in the soluble fraction (40 ± 4 U/μg protein), not in the membranous fraction. This result is in agreement with the subcellular location of Cpo activity in the soluble cytosolic fraction of K. lactis cells, as reported for S. cerevisiae (17). Analysis of the complete sequence of the K. lactis genome with BLAST tools at Génolevures (http://www.genolevures.org) yielded no other K. lactis ORFs with homology to HEM13 genes. These data confirm that only one gene, KlHEM13 (KLLA0F18546g), encodes a functional Cpo enzyme in K. lactis.
Two KlCpo proteins are translated from KlHEM13.
In order to ascertain which of the two in-frame TICs present in KlHEM13 was used for translation, a recombinant K. lactis strain expressing KlCpo tagged at the C terminus with three copies of HA was constructed. After growth of this strain under aerobic or hypoxic conditions, protein extracts were obtained; 30 μg of total protein was fractionated using electrophoresis in 12% polyacrylamide gel electrophoresis (PAGE) gels, and Western blotting was performed with an HA-specific primary antibody. Two proteins with different Mrs were obtained (Fig. 1D). The Mr calculated for the slowly migrating band had the expected size for the KlCPO-3HA construct translated from TIC-1 (l-KlCpo). The Mr calculated for the rapidly migrating band was coincident with that expected for the protein translated from TIC-2 (s-KlCpo). In order to verify the nature of these two proteins, the corresponding bands were fractionated by PAGE, excised from the gel, and analyzed using MALDI-TOF/TOF. The sequences of the internal peptides obtained confirmed that the two proteins were KlCpo, but peptides from the N terminus and C terminus were not present in the pools detected, and therefore it was not possible to verify by these methods whether the proteins differed in their N-terminal regions. In order to test whether l-KlCpo contained the N-terminal region corresponding to translation from TIC-1 and whether s-KlCpo lacked this region, Western blotting was performed using a specific antibody (Ab-l-KlCpo) directed against a peptide encoded by the region between the first and second TICs (underlined in Fig. 1A). The results (Fig. 1D) revealed that this antibody interacts with l-KlCpo but not with s-KlCpo. Considering that (i) both proteins contained the C-terminal tag, (ii) only l-KlCpo interacted with Ab-l-KlCpo, (iii) experimentally calculated Mrs were as expected from TIC-1 and TIC-2 translation, and (iv) no introns could be identified when KlHEM13 was analyzed using the yeast intron program EXPLORA (14), it was concluded that the two KlCpo proteins detected were derived from translation initiation from TIC-1 (l-KlCPo) and TIC-2 (s-KlCpo). The production of different proteins by translation from different TICs has been described previously in eukaryotic systems, including yeasts (21, 22).
KlHEM13 is transcribed from two different tss pools, and selection of a specific tss depends on the availability of oxygen and heme.
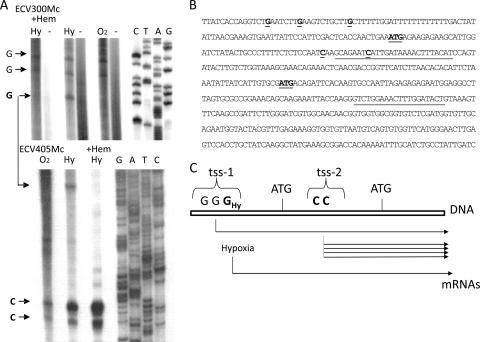
To better characterize the promoter regions in the 5′ upstream region of KlHEM13 and their relative positions to the TICs, the precise positions of tss on the KlHEM13 promoter were determined using primer extension. The data (Fig. 2) confirmed the presence of two tss pools. One pool (tss-1) was located 5′ of TIC-1 and included three weak starts at G positions −87, −80, and −69. A second tss pool (tss-2), between TIC-1 and TIC-2, included two strong starts at C positions +46 and +56 (positions −111 and −101 if the relative positions from TIC-2, which would be the only possible starting AUG codon for an mRNA transcribed from this tss-2 pool in this case, are considered). The positions of the determined tss relative to the TICs are represented on the KlHEM13 sequence (Fig. 2B). The tss pools were detected during aerobiosis or hypoxia. However, the tss at position −69 in the tss-1 pool is specific for hypoxia and disappeared when hemin was added (Fig. 2A). The long transcript could be produced to a minor extent from tss-1 under both aerobic and hypoxic conditions. The short transcript would be more abundant than the larger transcript under both conditions, but production of the long transcript would increase during hypoxia owing to heme-regulated use of the tss at the −69 position (Fig. 2C).
Fig. 2.
Determination of transcriptional start sites in the KlHEM13 upstream region. (A) Primer extension analysis. Aerobic or hypoxic conditions are indicated as in Fig. 1. +Hem, medium supplemented with hemin, -, negative control. (B) Sequence of the upstream region of KlHEM13 and the ORF (partial). The positions of primers ECV300Mc and ECV405Mc are underlined. The ATGs of the first and second TICs and the experimentally determined tss are underlined. (C) Scheme showing the relative positions and use of tss pools and TICs.
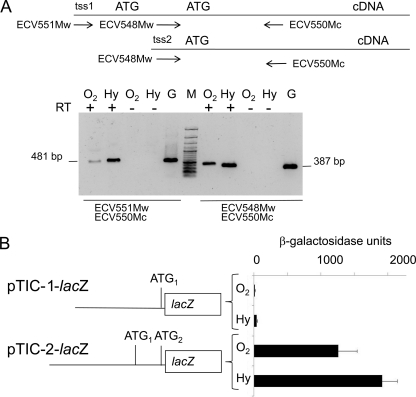
In order to confirm the functionality of these tss pools, total RNA was isolated from K. lactis cells grown under aerobiosis or hypoxia, and cDNA was obtained. This cDNA was used as a template in PCR amplifications using two pairs of primers, ECV551Mw/ECV550Mc and ECV548Mw/ECV550Mc. The long transcript, starting 5′ of TIC-1, is present in the total RNA isolated from K. lactis cells under aerobic and hypoxic conditions (Fig. 3A).
Fig. 3.
Analyses of the 5′ region of KlHEM13. (A) Amplification from cDNAs obtained with oligo(dT) from total mRNA isolated from MW190-9B using the primer pairs shown. Aerobic and hypoxic conditions are indicated as in Fig. 1. G, positive control for amplification, carried out with genomic DNA; RT+ and RT−, reactions with and without the addition of reverse transcriptase, respectively; M, molecular size marker (100-bp GeneRuler; Fermentas). The sizes of the PCR products are indicated. (B) Expression of the lacZ reporter gene driven by two KlHME13 regions, described in the text and schematized on the left.
The upstream regions of KlHEM13 from −1025 to TIC-1 (pTIC-1-lacZ) and to TIC-2 (pTIC-2-lacZ) were fused with the reporter gene lacZ, and β-galactosidase activity in protein extracts obtained from transformed K. lactis cells after aerobic and hypoxic growth was measured. The results presented in Fig. 3B indicate that the sequence located upstream of TIC-1 had poor activity in terms of promoting the expression of the reporter gene. When this sequence was extended 3′ of TIC-2, its promoter activity increased by 3 orders of magnitude. From these data it was concluded that the importance of the region between TIC-1 and TIC-2 in the control of KlHEM13 transcription, under aerobic and hypoxic conditions, is due to the presence of two major initiation sites in the tss-2 pool. However, minimal transcription is possible through the tss-1 pool in the region upstream of TIC-1. These results are compatible with the use of alternative tss to generate two KlHEM13 transcripts differing at their 5′ termini.
s-KlCpo but not l-KlCpo expression complements the heme deficiency of the ΔKlhem13 mutant strain.
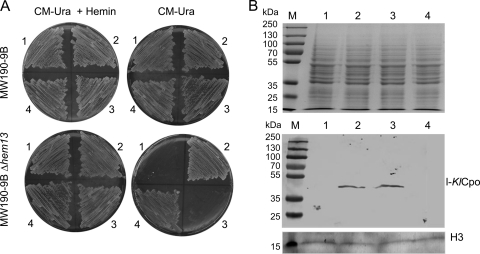
The results indicated that the two proteins, l-KlCpo and s-KlCpo, were obtained from the single gene KlHEM13. Therefore, the question of whether the two proteins were functionally equivalent was investigated. Constructs that expressed only one of the two proteins were obtained from a heterologous LAC4 promoter, avoiding the coexpression caused by the use of the native KlHEM13 promoter. The pCXJ18-l-KlCpo (expressing l-KlCpo) and pCXJ18-s-KlCpo (expressing s-KlCpo) constructs and the control pCXJ18-KlHEM13 construct (expressing both l-KlCpo and s-KlCpo) are described in Materials and Methods. These plasmids were used to transform the MW190-9B and MW190-9B-Δhem13 strains, and the selected transformed cells were plated on the selective synthetic medium CM-Ura with galactose as a carbon source in the absence or presence of hemin. Complementation of the heme deficiency of the Δhem13 mutant strain implies that the protein expressed from the plasmid construct has Cpo activity. This was evident with the pCXJ18-s-KlCpo construct and the control pCXJ18-KlHEM13 construct but not with pCXJ18-l-KlCpo. Therefore, only s-KlCpo complements the mutant phenotype (Fig. 4A). However, despite the lack of complementation, expression of l-KlCpo from the pCXJ18-KlHEM13 and pCXJ18-l-KlCpo constructs in the Δhem13 strain was confirmed using Western blotting with the l-KlCpo-specific antibody (Fig. 4B). In conclusion, l-KlCpo is expressed from these constructs but lacks Cpo activity.
Fig. 4.
Test of complementation of the heme deficiency of the MW190-9B-Δhem13 strain by l-KlCpo and s-KlCpo expression. (A) Growth of cells transformed with pCXJ18 (sector 1), pCXJ18-KlHEM13 (sector 2), pCXJ18-l-KlCpo (sector 3), or pCXJ18-s-KlCpo (sector 4) in selective medium. The addition of hemin to the medium is indicated (+ Hemin). (B) (Top) Protein-stained gel before blotting. (Bottom) Western blot demonstrating the expression of l-KlCpo from the pCXJ18-KlHEM13 and pCXJ18-l-KlCpo constructs. Hybridization with ab21054, an HRP-conjugated anti-histone H3 antibody from Abcam (H3), was used as a loading control. Lane numbers correspond to sector numbers in panel A.
Autoregulation of the KlHEM13 promoter.
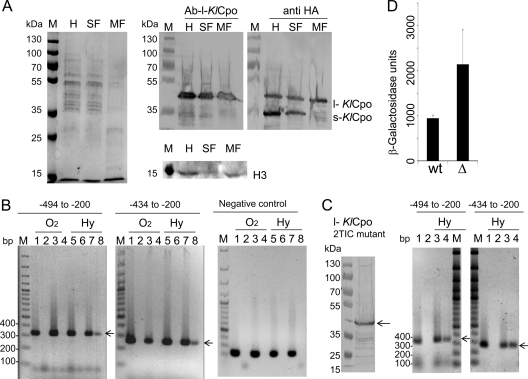
The l-KlCpo protein does not complement the heme deficiency of a ΔKlhem13 mutant strain (Fig. 4A). In addition, Cpo activity in the K. lactis wild-type strain was present only in the cytosolic soluble fraction (SF). These data excluded the possibility that s-KlCpo could be present in the membranous fraction that contains the nuclei (MF) but did not exclude the possibility that l-KlCpo could be present in this fraction, since it has no catalytic activity. Western blotting was carried out with protein extracts from both fractions, using anti-HA and the specific antibody Ab-l-KlCpo. The data presented in Fig. 5A demonstrate that l-KlCpo is found in both the soluble and membranous fractions, while s-KlCpo is present only in the soluble fraction. As expected, histone H3 was detected in the total extract (H) and the MF but not in the SF.
Fig. 5.
Nuclear regulatory function of l-KlCpo. (A) (Left) Protein-stained gel before blotting. (Center and right) Western blots showing the expression of l-KlCpo, s-KlCpo, and histone H3 in homogenates from whole-protein extracts (H), from the soluble fraction (SF), and from the membranous fraction containing nuclei (MF), obtained as described in Materials and Methods. The molecular sizes of the proteins used as molecular markers (lanes M) are given to the left. (B) ChIP assays demonstrating in vivo binding of the KlCpo protein to different regions of the KlHEM13 promoter (−494 to −200 and −434 to −200). The negative control shows the absence of binding to the KlHIS3 promoter. Lanes M, 100-bp GeneRuler (Fermentas). MW190-9B cells that were either left untransformed (lanes 1, 2, 5, and 6) or transformed with the pKS1-HEM13-3HA construct (lanes 3, 4, 7, and 8) were grown under aerobic (O2) or hypoxic (Hy) conditions. Odd-numbered lanes, DNA amplification from total input DNA; even-numbered lanes, DNA amplification from specific immunoprecipitates obtained with anti-HA. The primers used for amplification of the two promoter regions are described in the text. (C) (Left) Western blot showing the expression of the l-KlCpo protein in the 2TIC mutant. (Center and right) ChIP assays demonstrating in vivo binding of the l-KlCpo protein to regions of the KlHEM13 promoter during hypoxia. MW190-9B cells were either left untransformed (lanes 1 and 2) or transformed with the pKS1-HEM13-3HA-2TIC mutant construct (lanes 3 and 4). Odd- and even-numbered lanes show DNA amplification as described for panel B. (D) Expression of the lacZ reporter gene in MW190-9B (wt) and MW190-9B-Δhem13 (Δ) cells transformed with the pTIC-2494-lacZ construct described in the text.
In order to explore whether l-KlCpo could have a nuclear function and regulate KlHEM13 transcription, the interaction between the protein and the promoter was investigated in vivo using ChIP. The K. lactis strain MW190-9B was transformed with pSK1-KlHEM13-HA; protein extracts were obtained after aerobic or hypoxic growth; and ChIP assays were performed. The results revealed that under hypoxic conditions, an HA-tagged immune-precipitated protein was complexed with DNA in a region that extended from −494 to −200 in the KlHEM13 promoter and with a smaller subregion from −434 to −200 in the same promoter (Fig. 5B, lanes 8). To prove unambiguously that l-KlCpo, and not s-KlCpo, was binding to the KlHEM13 promoter, a mutant KlHEM13 gene without the ATG codon for the second in-frame Met was obtained (pSK1-KlHEM13-3HA-2TIC mutant). The K. lactis strain MW190-9B was transformed with this mutant construct, and ChIP assays were performed after growth during hypoxic conditions. The results obtained confirm that l-KlCpo binds to the KlHEM13 promoter (Fig, 5C).
A functional role for this region of the KlHEM13 promoter, which is coimmunoprecipitated with the protein in ChIP assays, was evident in terms of autorepression during hypoxia. The promoter region of KlHEM13 from −494 to +190 was fused in frame to the lacZ reporter in the pXW1 plasmid to make the construct pTIC-2494-lacZ. Cells from the K. lactis strain MW190-9B and its ΔKlhem13 derivative were transformed with this construct, and β-galactosidase activity was determined in protein extracts obtained from cells grown under hypoxic conditions. Comparative analysis of reporter gene activity demonstrated that the expression directed by the KlHEM13 promoter was lower in strain MW190-9B than in its ΔKlhem13 derivative (Fig. 5D). These data are compatible with a regulatory feedback control on KlHEM13 expression by l-KlCpo.
DISCUSSION
Sequence alignment of the protein derived from KlHEM13 with S. cerevisiae Cpo shows the presence of 52 additional amino acids at the N terminus of KlCpo. This extension includes the amino acids between two in-frame TICs in the KlHEM13 ORF. Other proteins from ascomycetes with homology to KlCpo accessed through Génolevures (http://www.genolevures.org/) do not have similar N-terminal extensions. This N-terminal extension does not contain a known sorting signal for either nuclear localization or other cellular localization as assayed with PSORT (http://psort.hgc.jp/).
KlHEM13 is a single-copy-number gene in K. lactis, and Cpo activity depends on its expression (Fig. 1B and C). Two tss pools (tss-1 and tss-2) have been mapped to the 5′ region of KlHEM13 (Fig. 2). The relative positions of the tss pools with reference to the TICs indicate that initiation of transcription from tss-1 would produce mRNA molecules containing TIC-1 and TIC-2, while initiation from tss-2 would produce mRNA molecules containing only TIC-2. Two proteins with different functions, l-KlCpo and s-KlCpo, are produced from this single gene. The s-KlCpo protein has Cpo activity and complements the Δhem13 deletion, unlike l-KlCpo (Fig. 4). Some evidence suggests that l-KlCpo (the only form present in the membranous fraction containing the nuclei) has a regulatory role in terms of transcriptional regulation of KlHEM13 during hypoxia (Fig. 5). The utilization of multiple tss, frequently very close to one another and forming tss pools, is a frequent event in yeasts, but its functional significance is not fully understood (11). The alternative use of tss has been related to the production of protein isoforms with the same function but different cellular locations. Genes containing more than one in-frame TIC in their 5′ regions may give rise to isoforms with different subcellular localizations due to heterogeneous N-terminal ends, depending on which of the AUG codons is used to initiate translation. Often, such genes have alternative transcription start sites, leading to the synthesis of two different mRNAs. For example, the genes coding for the yeast aminoacyl-tRNA synthetases (21) or glutathione reductase (22) use this mechanism. Generally, however, this has not been related to the utilization of alternative promoters or to the production of proteins with various functions, as reported here for KlHEM13. To our knowledge, this is the first report of the use of alternative tss as a mechanism for producing functionally different proteins in budding yeasts. However, this phenomenon has been described in the fission yeast Schizosaccharomyces pombe, although in that system one of the two transcripts is poorly translated, and its function remains to be elucidated (25).
Alternative selection of tss as a mechanism for producing different proteins has been described in plants and mammals (6, 15). Examples of regulatory mechanisms based on tss selection have been reported in higher eukaryotes. Start site selection within many murine core promoters differs among tissues, and dynamic usage of tss in these promoters is associated with CpG islands, promoter structures, and imprinting (15). In the KlHEM13 promoter, transcription from the tss-2 pool results in s-KlCpo production under aerobic and hypoxic conditions (Fig. 2A and 3A). This is in accordance with the finding that only s-KlCpo is able to complement the heme deficiency caused by hem13 deletion in K. lactis (Fig. 4A). Cpo activity is necessary for cell viability during aerobiosis and hypoxia, and transcription from tss-2 would ensure cell survival under these conditions. The tss at position −69 in the tss-1 pool is specific for hypoxia and disappears in the presence of hemin (Fig. 2A). This feature argues in favor of l-KlCpo having a function that is more relevant during hypoxia. This hypothesis is supported by the finding that during hypoxia, a specific complex is formed that contains the tagged protein and a promoter region of KlHEM13 (delimited from −434 to −200 in our experiments [Fig. 5B and C]), which is also implied in the control of KlHEM13 expression under this condition (Fig. 5D). However, analysis of the l-KlCpo sequence does not reveal any known DNA-binding domains. It is possible that in vivo, the interaction of l-KlCpo with DNA may be anchored by another, as yet unknown regulatory factor that binds directly to the KlHEM13 promoter during hypoxia. However, the data presented here do not preclude a direct interaction.
A functional explanation for the repression exerted by l-KlCpo on the KlHEM13 promoter could be that this regulatory mechanism is directed to avoiding toxicity caused by an excess of Cpo activity that could eventually cause accumulation of the product, protoporphyrinogen IX, and consequently a bottleneck in subsequent steps of the heme biosynthetic pathway. Indeed, this compound accumulates when protoporphyrinogen oxidase, the next enzyme in the biosynthesis of heme, has decreased activity, and this metabolic failure produces variegate porphyria in humans (7).
An intriguing question concerns why the l-KlCpo form lacks catalytic activity. There are precedents, proteins in which a terminal extension blocks the catalytic site. For instance, proteases lack enzymatic activity until they are processed. Of course, processing is not the only mechanism suitable for opening the catalytic site, and conformational changes produced by interaction with other protein partners or regulatory molecules have to be considered as well. The crystal structure of S. cerevisiae Cpo has been resolved (23), and on the basis of sequence homology, a similar structure is predicted for s-KlCpo (data not shown). However, homology modeling of the N-terminal extension present in l-KlCpo is not possible, because there are no homologous models for this region. Currently, research is under way to purify and crystallize the two KlCpo forms, l-KlCpo and s-KlCpo, in order to determine their structures and investigate correlations between structure and function.
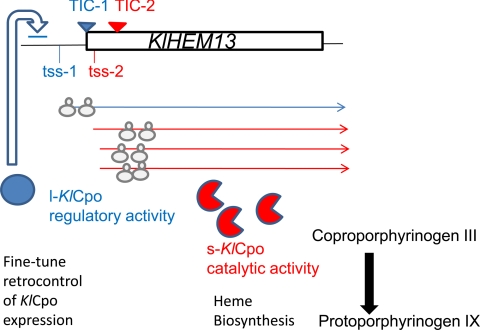
With regard to the evolutionary origin and selective pressure for conservation of the various functions identified in the l-KlCpo and s-KlCpo proteins, it can be speculated that the “first” function positively selected in K. lactis could be the high catalytic Cpo activity, essential for life and carried by s-KlCpo. During hypoxia, KlHEM13 transcription is significantly increased, and accumulation of protoporphyrinogen IX could become toxic. In this context, a new compatible function that decreased the expression of the catalytically active form would be favorable for selection, and a new function for l-KlCpo could be established (Fig. 6). It is interesting to speculate about the mechanism by which this new function could arise. It is likely that initially the gene was transcribed predominantly from the tss-2 pool, where the major transcription initiation sites still remain. Therefore, the short transcript that was translated to the catalytically active s-KlCpo would be actively produced. The new heme-regulated use of the transcriptional start site at position −69 in the tss-1 pool could increase the expression of the long transcript during hypoxia. To understand evolutionary positive selection for the coexistence of these two functions, l-KlCpo expression can be considered to have three advantages. First, it does not exclude s-KlCpo translation. Second, it lacks catalytic activity. Third, it is able to fine-tune hypoxic KlHEM13 gene expression, preventing the protoporphyrinogen IX excess that could be associated with overproduction of s-KlCpo.
Fig. 6.
Hypothetical model of the origins and functions of l-KlCpo and s-KlCpo.
Also of interest is the relationship between these two protein variants in K. lactis, l-KlCpo and s-KlCpo, and the phenomenon of moonlighting. Applying the moonlighting criteria (8, 12) to the coexistence of l-KlCpo and s-KlCpo in K. lactis, we find two coincidences: (i) they derive from a unique gene (without gene fusion or alternative splicing) but perform two different functions, and (ii) the two functions are independent in the sense that inactivation of one of the functions, as happens with the catalytic activity in l-KlCpo, does not affect the regulatory function. Still, we cannot strictly apply the term “moonlighting” to l-KlCpo and s-KlCpo, because we are able to fractionate them by PAGE, but we may consider them “moonlighting-like.”
In conclusion, two proteins with different N termini and different functions are derived from the single-copy-number KlHEM13 gene by a mechanism based on tss selection and the use of two TICs.
ACKNOWLEDGMENTS
We thank Jean Michel Camadro (Institut Jacques Monod, Paris, France) for collaboration with the Cpo assays carried out with the K. lactis strains CBSΔKU80 and CBSΔKU80-Δhem13 in his laboratory. We thank M. Wésolowski-Louvel (Université Claude Bernard, Villeurbanne, France) for strain MW190-9B and Yde Steensma (Delft University of Technology, Delft, The Netherlands) for strain CBSΔKU80. MALDI-TOF/TOF analyses were performed at the “Unidad de Proteomica-Nodo asociado a ProteoRed, INIBIC-Complejo Hospitalario Universitario de A Coruña,” A Coruña, Spain. We are grateful to Cristina Ruíz-Romero and Jesús Mateos Martín for valuable assistance with these techniques. We thank BioMedES for English language editing.
This research was supported by grants BFU2006-03961/BMC and BFU2009-08854 from MICINN (Spain), cofinanced by FEDER (CEE). General support of the laboratory from 2008 to 2011 was funded by Xunta de Galicia (Consolidación C.E.O.U.2008/008), cofinanced by FEDER. A.V.V.'s salary was funded by the “Lucas-Labrada program-2008” from Xunta de Galicia.
Footnotes
Published ahead of print on 5 August 2011.
REFERENCES
- 1. Becerra M., Cerdán E., González Siso M. I. 1997. Heterologous Kluyveromyces lactis beta-galactosidase production and release by Saccharomyces cerevisiae osmotic-remedial thermosensitive autolytic mutants. Biochim. Biophys. Acta 1335:235–241 [DOI] [PubMed] [Google Scholar]
- 2. Blanco M., Becerra M., González-Siso M. I., Cerdán M. E. 2005. Functional characterization of KlHEM13, a hypoxic gene of Kluyveromyces lactis. Can. J. Microbiol. 51:241–249 [DOI] [PubMed] [Google Scholar]
- 3. Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 [DOI] [PubMed] [Google Scholar]
- 4. Chen X. J. 1996. Low- and high-copy-number shuttle vectors for replication in the budding yeast Kluyveromyces lactis. Gene 172:131–136 [DOI] [PubMed] [Google Scholar]
- 5. Chen X. J., Wesolowski-Louvel M., Fukuhara H. 1992. Glucose transport in the yeast Kluyveromyces lactis. II. Transcriptional regulation of the glucose transporter gene RAG1. Mol. Gen. Genet. 233:97–105 [DOI] [PubMed] [Google Scholar]
- 6. Dinkins R. D., et al. 2008. Changing transcriptional initiation sites and alternative 5′- and 3′-splice site selection of the first intron deploys Arabidopsis protein isoaspartyl methyltransferase2 variants to different subcellular compartments. Plant J. 55:1–13 [DOI] [PubMed] [Google Scholar]
- 7. Frank J., Christiano A. M. 1998. Variegate porphyria: past, present and future. Skin Pharmacol. Appl. Skin Physiol. 11:310–320 [DOI] [PubMed] [Google Scholar]
- 8. Gancedo C., Flores C. L. 2008. Moonlighting proteins in yeasts. Microbiol. Mol. Biol. Rev. 72:197–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gietz R. D., Sugino A. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527–534 [DOI] [PubMed] [Google Scholar]
- 10. González-Domínguez M., Freire-Picos M. A., Ramil E., Guiard B., Cerdán M. E. 2000. Heme-mediated transcriptional control in Kluyveromyces lactis. Curr. Genet. 38:171–177 [DOI] [PubMed] [Google Scholar]
- 11. Healy A. M., Zitomer R. S. 1990. A sequence that directs transcriptional initiation in yeast. Curr. Genet. 18:105–109 [DOI] [PubMed] [Google Scholar]
- 12. Huberts D. H., van der Klei I. J. 2010. Moonlighting proteins: an intriguing mode of multitasking. Biochim. Biophys. Acta 1803:520–525 [DOI] [PubMed] [Google Scholar]
- 13. Ito H., Fukuda Y., Murata K., Kimura A. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalogeropoulos A. 1995. Automatic intron detection in nuclear DNA sequences of Saccharomyces cerevisiae. Yeast 11:555–565 [DOI] [PubMed] [Google Scholar]
- 15. Kawaji H., et al. 2006. Dynamic usage of transcription start sites within core promoters. Genome Biol. 7:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kooistra R., Hooykaas P. J., Steensma H. Y. 2004. Efficient gene targeting in Kluyveromyces lactis. Yeast 21:781–792 [DOI] [PubMed] [Google Scholar]
- 17. Labbe P. 1997. Purification and properties of coproporphyrinogen III oxidase from yeast. Methods Enzymol. 281:367–378 [DOI] [PubMed] [Google Scholar]
- 18. Labbe P., Camadro J. M., Chambon H. 1985. Fluorometric assays for coproporphyrinogen oxidase and protoporphyrinogen oxidase. Anal. Biochem. 149:248–260 [DOI] [PubMed] [Google Scholar]
- 19. Lee T. I., et al. 2002. Transcriptional regulatory networks in Saccharomyces cerevisiae. Science 298:799–804 [DOI] [PubMed] [Google Scholar]
- 20. Longtine M. S., et al. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953–961 [DOI] [PubMed] [Google Scholar]
- 21. Natsoulis G., Hilger F., Fink G. R. 1986. The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell 46:235–243 [DOI] [PubMed] [Google Scholar]
- 22. Outten C. E., Culotta V. C. 2004. Alternative start sites in the Saccharomyces cerevisiae GLR1 gene are responsible for mitochondrial and cytosolic isoforms of glutathione reductase. J. Biol. Chem. 279:7785–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Phillips J. D., et al. 2004. Crystal structure of the oxygen-dependent coproporphyrinogen oxidase (Hem13p) of Saccharomyces cerevisiae. J. Biol. Chem. 279:38960–38968 [DOI] [PubMed] [Google Scholar]
- 24. Ruíz-Romero C., et al. 2009. Mitochondrial dysregulation of osteoarthritic human articular chondrocytes analyzed by proteomics: a decrease in mitochondrial superoxide dismutase points to a redox imbalance. Mol. Cell. Proteomics 8:172–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sehgal A., Hughes B. T., Espenshade P. J. 2008. Oxygen-dependent, alternative promoter controls translation of tco1+ in fission yeast. Nucleic Acids Res. 36:2024–2031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tizón B., Rodríguez-Torres A. M., Cerdán M. E. 1999. Disruption of six novel Saccharomyces cerevisiae genes reveals that YGL129c is necessary for growth in non-fermentable carbon sources, YGL128c for growth at low or high temperatures and YGL125w is implicated in the biosynthesis of methionine. Yeast 15:145–154 [DOI] [PubMed] [Google Scholar]
- 27. Tseng W., Lin J., Wei T., Fang T. 2008. A novel megaprimed and ligase-free, PCR-based, site-directed mutagenesis method. Anal. Biochem. 375:376–378 [DOI] [PubMed] [Google Scholar]
- 28. Wach A., Brachat A., Pohlmann R., Philippsen P. 1994. New heterologous modules for classical or PCR-based gene disruptions in Saccharomyces cerevisiae. Yeast 10:1793–1808 [DOI] [PubMed] [Google Scholar]
- 29. Zitomer R. S., Hall B. D. 1976. Yeast cytochrome c messenger RNA. In vitro translation and specific immunoprecipitation of the CYC1 gene product J. Biol. Chem. 251:6320–6326 [PubMed] [Google Scholar]