Abstract
We determined the enzymatic characteristics of an industrially important biocatalyst, α-ketoglutarate-dependent l-isoleucine dioxygenase (IDO), which was found to be the enzyme responsible for the generation of (2S,3R,4S)-4-hydroxyisoleucine in Bacillus thuringiensis 2e2. Depending on the amino acid used as the substrate, IDO catalyzed three different types of oxidation reactions: hydroxylation, dehydrogenation, and sulfoxidation. IDO stereoselectively hydroxylated several hydrophobic aliphatic l-amino acids, as well as l-isoleucine, and produced (S)-3-hydroxy-l-allo-isoleucine, 4-hydroxy-l-leucine, (S)-4-hydroxy-l-norvaline, 4-hydroxy-l-norleucine, and 5-hydroxy-l-norleucine. The IDO reaction product of l-isoleucine, (2S,3R,4S)-4-hydroxyisoleucine, was again reacted with IDO and dehydrogenated into (2S,3R)-2-amino-3-methyl-4-ketopentanoate, which is also a metabolite found in B. thuringiensis 2e2. Interestingly, IDO catalyzed the sulfoxidation of some sulfur-containing l-amino acids and generated l-methionine sulfoxide and l-ethionine sulfoxide. Consequently, the effective production of various modified amino acids would be possible using IDO as the biocatalyst.
INTRODUCTION
Hydroxy amino acids are unusual hydroxylated amino acids and are ubiquitous in nature. They exist as secondary metabolites and components of peptides and proteins. Free amino acids are mostly found in higher plants (3, 29), and also, free threo-3-hydroxy-l-asparagine has been found in human urine (25) and free 3-hydroxy-l-valine has been isolated from the mushroom Pleurocybella porrigens (1). Alternatively, many hydroxy amino acids are components of glycopeptide antibiotics, such as vancomycin (30), bleomycin (35), and ramoplanin (7), and of cyclodepsipeptides, such as polytheonamides (14) and papuamides (10), and also of collagen (13). These peptides, which contain hydroxy amino acid residues, are known to have antifungal, antibacterial, antiviral, and anticancer activities. Some hydroxy amino acids have several chiral carbons and may be important as precursors and chiral auxiliaries in the chemical synthesis of other compounds (4, 26).
In particular, (2S,3R,4S)-4-hydroxy-l-isoleucine (HIL), which was first found in fenugreek seeds (12), is a promising compound for drugs and functional foods because of its antidiabetes activity (5, 6) and antiobesity activity (15). In our previous research, it was found that Bacillus thuringiensis 2e2 possessed a novel l-isoleucine (l-Ile) metabolic pathway involving the hydroxylation of l-Ile to (2S,3R,4S)-HIL and further oxidation to (2S,3R)-2-amino-3-methyl-4-ketopentanoate (AMKP), which is known as a vitamin B12 antimetabolite (27). The former reaction of the pathway was catalyzed by an α-ketoglutarate (αKG)-dependent l-Ile dioxygenase (IDO) (18) and the latter by a NAD+-dependent HIL dehydrogenase (23). In addition, we developed an efficient production system for HIL using Escherichia coli cells heterologously expressing IDO (31).
The enzymes belonging to the αKG-dependent dioxygenase superfamily primarily catalyze the hydroxylation of a wide range of small molecules accompanied by the oxidative decarboxylation of αKG into succinate (16). Several αKG-dependent dioxygenases are known to hydroxylate free amino acids. Among them, l-proline 4-hydroxylase (19), l-proline 3-hydroxylase (22), l-asparagine 3-hydroxylase (AsnO) (34), and l-arginine 3-hydroxylase (VioC) (37) were all found in Streptomyces species and have been well characterized. IDO is the first enzyme possessing C-4 hydroxylation activity toward a free aliphatic amino acid, and it is therefore important to investigate the ability of this novel dioxygenase to lead to various applications, such as pharmaceutical intermediates, functional peptides, and control molecules of eukaryotic gene expression.
In this study, we investigated IDO to determine its kinetic constants and substrate specificity toward amino acids and their derivatives. Interestingly, IDO catalyzed three types of oxidation reactions: stereoselective hydroxylation of various hydrophobic aliphatic l-amino acids, dehydrogenation of (2S,3R,4S)-HIL into (2S,3R)-AMKP, and sulfoxidation of some sulfur-containing l-amino acids; these results may be useful for the industrial production of optically active amino acids, as well as (2S,3R,4S)-HIL.
MATERIALS AND METHODS
Preparation of purified recombinant IDO.
The 6×His-tagged IDO-expressing strain E. coli Rosetta2(DE3) carrying pET-IDO (2e2) was constructed by a method similar to that in our previous report (23), except for the primer set used: CATATGAAAATGAGTGGCTTTAGCATAGAA and CTCGAGTTTTGTCTCCTTATAAGAAAATGT. The recombinant IDO was heterologously expressed in the strain and purified in the same manner as in the previous report (23). After checking the homogeneity by SDS-PAGE (see Fig. S1 in the supplemental material), the purified IDO was used for further characterization.
Reaction conditions for IDO.
For catalytic characterization of l-Ile hydroxylation by IDO, the reaction mixture was composed of 10 mM l-Ile, 10 mM αKG, 0.5 mM FeSO4·7H2O, 10 mM ascorbic acid, 50 mM Bis-Tris (pH 6.0), and 0.2 mg/ml purified recombinant IDO, and the reaction was allowed to proceed at 25°C for 10 min. In order to determine Km values, l-Ile and αKG were used at concentrations of 0.02 to 5 mM and 0.01 to 1 mM, respectively. Enzymatic activity was measured by amino acid analysis. For substrate specificity analysis of IDO, the reaction mixture was composed of 2 mM substrate, 10 mM αKG, 0.5 mM FeSO4·7H2O, 10 mM ascorbic acid, 50 mM Bis-Tris (pH 6.0), and 0.2 mg/ml purified recombinant IDO, and the reaction was allowed to proceed at 25°C for 10 min. Enzymatic activity was determined as the production of succinic acid measured spectrometrically with an F-kit (Roche Diagnostics, Basel, Switzerland).
Amino acid analysis conditions with HPLC.
Amino acids were derivatized using the AccQ-Tag method (Waters, Milford, MA). The amino acid derivatives were analyzed using an Alliance 2695 high-performance liquid chromatography (HPLC) System (Waters) equipped with a fluorescence detector. The XBridge C18 column (5 μm; 2.1 by 150 mm; Waters) was used for separation at 40°C. The mobile phases were 10 mM ammonium acetate at pH 5.0 (eluent A) and methanol (eluent B), and the flow rate of the eluent was 0.3 ml/min. The eluent gradients were 0 to 1% (vol/vol) B for 0 to 0.5 min, 1 to 5% B for 0.5 to 18 min, 5 to 9% B for 18 to 19 min, 9 to 17% B for 19 to 29.5 min, 17 to 60% B for 29.5 to 40 min, and 60% B for 40 to 43 min.
Liquid chromatography/electrospray ionization-mass spectrometry (LC/ESI-MS) analysis conditions.
The reaction products of IDO were analyzed using an LCMS-2010A (Shimadzu, Kyoto, Japan). A TSKgel Amide-80 HR column (4.6 mm by 250 mm; Tosoh, Tokyo, Japan) was used for separation at 40°C. The mobile phase was 1.5 mM ammonium acetate (pH 5.0) in 85% acetonitrile, and the flow rate was 1 ml/min. MS conditions were as follows: block temperature, 200°C; curved desolvation line (CDL) temperature, 250°C; detector voltage, 1.5 kV; nebulizing gas flow, 1.5 liters/min.
Identification of amino acid products in the IDO reaction.
Preparative isolation of amino acids produced in the IDO reaction mixture was performed with the Alliance 2695 HPLC System (Waters) equipped with a UV detector. A TSKgel Amide-80 column (7.8 mm by 300 mm; Tosoh) was used for separation at 40°C. The mobile phase was 1.5 mM ammonium acetate (pH 5.0) in 85% acetonitrile, and the flow rate was 2 ml/min. Amino acids were detected using UV absorbance at 210 nm. Each eluate containing isolated amino acid was freeze-dried and dissolved in D2O. Proton nuclear magnetic resonance (1H-NMR) spectra were recorded on an Avance 500 (Bruker, Billerica, MA).
RESULTS
Catalytic properties of recombinant IDO.
The catalytic parameters for the hydroxylation of l-Ile were measured using purified recombinant IDO. The optimal reaction conditions for the reaction by recombinant IDO were pH 6.0 and 25°C. Although the optimal reaction pH of recombinant IDO was the same as that of the native form of the IDO purified from B. thuringiensis 2e2 cells, its optimal reaction temperature was 5°C lower than that of the native form (18). Addition of a 6×His tag to the C terminus of IDO may therefore influence its thermostability. Normal hyperbolic kinetics was observed with l-Ile in the reaction by recombinant IDO. Lineweaver-Burk treatment of the data yielded apparent Km values for l-Ile of 0.27 mM and for αKG of 0.23 mM, and the Vmax was 1.13 μmol·min−1·mg−1.
Substrate specificity of IDO.
Recombinant IDO protein was purified and reacted with various kinds of amino acids: 20 proteinogenic l-amino acids, nonproteinogenic l-amino acids, and d-form amino acids and amino acid derivatives, such as N-substituted amino acids, amino acid esters, and so on. When some l-form amino acids, including l-leucine, l-norleucine, l-norvaline, l-allo-Ile, (2S,3R,4S)-HIL, l-methionine, l-ethionine, l-cysteine, and dl-homocysteine, were used as substrates, IDO showed succinate-generating activity, as well as l-Ile. The initial reaction rates for these substrates are listed in Table 1. On the other hand, no succinate generation was observed in the reaction mixture of IDO using any d-form amino acids and amino acid derivatives, such as N-substituted amino acids and amino acid esters, as the substrate. Particularly referring to compounds structurally related to l-Ile, IDO did not react with d-Ile, d-allo-Ile, tert-butoxycarbonyl (Boc)-l-Ile, 9-fluorenylmethoxy carbonyl (Fmoc)-l-Ile, N-carbamoyl-l-Ile, l-Ile methyl ester, and N-acetyl-l-Ile methyl ester, but it did react with l-allo-Ile.
Table 1.
Substrate specificity of IDO
| Substrate | Initial reaction rate (μmol·min−1·mg−1) | ESI-MS (m/z) [M+H] |
|
|---|---|---|---|
| Substrate | Product | ||
| l-Ile | 0.71 ± 0.29 | 132 | 148 |
| l-Norleucine | 0.39 ± 0.05 | 132 | 148, 148 |
| l-Leucine | 0.31 ± 0.04 | 132 | 148 |
| l-Norvaline | 0.27 ± 0.10 | 118 | 134 |
| dl-Homocysteine | 0.27 ± 0.17 | 136 | NDa |
| l-Methionine | 0.26 ± 0.05 | 150 | 166 |
| l-Ethionine | 0.19 ± 0.06 | 164 | 180 |
| l-Cysteine | 0.18 ± 0.05 | 122 | ND |
| (2S,3R,4S)-HIL | 0.18 ± 0.07 | 148 | 146 |
| l-allo-Ile | 0.13 ± 0.03 | 132 | 148 |
| 4-Hydroxy-l-norleucine | 0.12 ± 0.06 | 148 | ND |
| (S)-4-Hydroxy-l-norvaline | 0.09 ± 0.02 | 134 | ND |
ND, not detected.
Analysis of IDO reaction products.
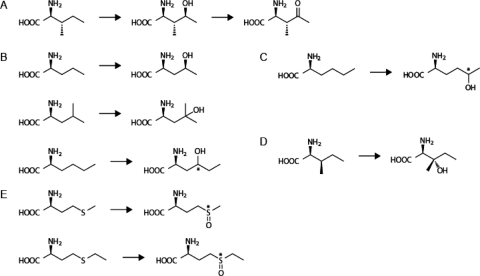
Amino acid analysis was performed on the reaction mixtures in which succinate was generated by IDO from αKG. A single peak of newly produced amino acid was observed in each reaction mixture using l-leucine, l-norvaline, l-allo-Ile, (2S,3R,4S)-HIL, l-methionine, or l-ethionine as a substrate. Only when l-norleucine was used in the IDO reaction were two novel peaks of amino acids produced. Despite significant succinate generation observed in the reaction mixture, including l-cysteine or dl-homocysteine, no novel amino acid peak was detected by amino acid analysis, even though a substantial amount of succinate was produced. IDO reaction products from these amino acids might be undetectable by the analysis method used in this study. The molecular weights of newly generated amino acids were directly analyzed by LC/ESI-MS and compared with those of the substrates used (Table 1). The products from l-leucine, l-norvaline, l-allo-Ile, l-methionine, and l-ethionine were all heavier by 16 than their original substrates, indicating that monooxygenation of these substrates was catalyzed by IDO, as well as hydroxylation of l-Ile. In the reaction mixture containing l-norleucine, the molecular weights of the two products were both 148, so they were also monooxygenated products of l-norleucine. Surprisingly, the retention time in amino acid analysis and the molecular weight of the reaction product arising from (2S,3R,4S)-HIL corresponded to that of (2S,3R)-AMKP, which was the C-4 keto form of l-Ile previously found in B. thuringiensis 2e2. This result indicates that IDO dehydrogenated the C-4 hydroxyl group of HIL and formed a C-4 keto group, coupled with oxidative decarboxylation of αKG (Fig. 1A).
Fig. 1.
Oxidative reactions catalyzed by IDO. (A) C-4 hydroxylation of l-Ile into (2S,3R,4S)-HIL and further dehydrogenation of (2S,3R,4S)-HIL into (2S,3R)-AMKP. (B) C-4 hydroxylation of l-norvaline, l-leucine, and l-norleucine, as well as l-Ile. (C) C-5 hydroxylation of l-norleucine. (D) C-3 hydroxylation of l-allo-Ile. (E) Sulfoxidation of l-methionine and l-ethionine.
Amino acid hydroxylation by IDO.
The IDO reaction products of l-norvaline, l-leucine, l-norleucine, and l-allo-Ile were isolated by preparative liquid chromatography and subjected to structural analysis using NMR spectrometry (Table 2). The product from l-norvaline was determined to be 4-hydroxy-l-norvaline and corresponded to the previously reported NMR spectrum (2) of (S)-4-hydroxy-l-norvaline (Fig. 1B). Similar to l-Ile, IDO introduced a hydroxyl group onto the methylene carbon at the 4 position of l-norvaline, and the product of l-leucine oxidation was confirmed to be 4-hydroxy-l-leucine (Fig. 1B). IDO also hydroxylated the methine carbon at the 4 position of l-leucine and generated an achiral tertiary alcohol group. One of the two products formed from l-norleucine was determined to be 4-hydroxy-l-norleucine, formed in the same manner as the hydroxylation of l-Ile (Fig. 1B). On the other hand, the other product of l-norleucine was found to be 5-hydroxy-l-norleucine (Fig. 1C). The production ratio of 4-hydroxy-l-norleucine to 5-hydroxy-l-norleucine was approximately 11:1 in HPLC analysis. Thus, IDO simultaneously catalyzed the hydroxylation of C-4 and C-5 methylene carbons of l-norleucine and generated two monohydroxylated l-norleucines, but a dihydroxylated product was not detected in the reaction mixture. Based on the results of amino acid analysis, one of two possible diastereomers of 4-hydroxy-l-norleucine or 5-hydroxy-l-norleucine was also stereospecifically produced. Moreover, the product from l-allo-Ile was determined to be 3-hydroxy-l-allo-Ile and corresponded to the previously reported NMR spectrum of (S)-3-hydroxy-l-allo-Ile (4). Interestingly, IDO hydroxylated only methine carbon at the 3-position of l-allo-Ile, which differed from the C-4 methylene hydroxylation of l-Ile into HIL (Fig. 1D). Also, IDO activity was measured for (S)-4-hydroxy-l-norvaline and 4-hydroxy-l-norleucine, as well as HIL (Table 1). Although these hydroxy amino acids were weakly reacted with IDO, the reaction products were not identified as dehydrogenated amino acids because of their small amounts (Table 1).
Table 2.
Identification of amino acid products in IDO reactions
| Substrate | IDO reaction product |
||
|---|---|---|---|
| 1H-NMR spectrum | Identified compound | Reference | |
| l-Ile | See reference 18 | (2S,3R,4S)-HIL | |
| l-allo-Ile | δ = 0.92 (3H, t, J = 7.5), 1.35 (3H, s), 1.47-1.62 (2H, m), 3.63 (1H, s). | (S)-3-Hydroxy-l-allo-Ile | 4 |
| l-Leucine | δ = 1.31 (3H, s), 1.31 (3H, s), 1.90 (1H, dd, J = 15.4, 10.1 Hz), 2.08 (1H, dd, J = 15.2, 3.2 Hz), 3.92 (1H, dd, J = 10.2, 3.2 Hz). | 4-Hydroxy-l-leucine | 8 |
| l-Norleucine | δ = 0.90 (3H, t, J = 7.4 Hz), 1.42-1.58 (2H, m), 1.72 (1H, ddd, J = 15.0, 9.6, 9.6 Hz), 2.13 (1H, ddd, J = 15.0, 3.6, 3.6 Hz), 3.77-3.82 (1H, m), 3.86 (1H, dd, J = 9.0, 4.5 Hz). | 4-Hydroxy-l-norleucine | |
| δ = 1.17 (3H, d, J = 6.3 Hz), 1.45-1.61 (2H, m), 1.81-2.09 (3H, m), 3.70-3.80 (1H, m), 3.81-3.87 (1H, m). | 5-Hydroxy-l-norleucine | ||
| l-Norvaline | δ = 1.22 (3H, d, J = 6.3 Hz), 1.77 (1H, ddd, J = 15.0, 9.3, 9.3 Hz), 2.07 (1H, ddd, J = 14.9, 3.9, 3.9 Hz), 3.78 (1H, dd, J = 9.1, 4.5 Hz). 4.01-4.07 (1H, m). | (S)-4-Hydroxy-l-norvaline | 2 |
| l-Methionine | δ = 2.27-2.31 (2H, m), 2.72 (3H, s), 2.94-3.06 (2H, m), 3.86 (1H, t, J = 6.3 Hz). | l-Methionine sulfoxide | 17 |
| l-Ethionine | δ = 1.24 (3H, t, J = 7.5 Hz), 2.24-2.29 (2H, m), 2.80-2.96 (4H, m), 3.89 (1H, t, J = 6.3 Hz). | l-Ethionine sulfoxide | 17 |
| (2S,3R,4S)-HIL | See reference 23 | (2S,3R)-AMKP | |
Amino acid sulfoxidation by IDO.
The IDO reaction products of l-methionine and l-ethionine were isolated in a similar manner by preparative liquid chromatography and subjected to structural analysis using NMR spectrometry (Table 2). Unexpectedly, l-methionine and l-ethionine were oxidized by IDO into l-methionine sulfoxide and l-ethionine sulfoxide, respectively, instead of hydroxy amino acids (Fig. 1E). Based on the results of amino acid analysis, one of two possible diastereomers of these sulfoxides also was produced stereospecifically. This result indicates that IDO catalyzed the stereospecific sulfoxidation of some sulfur-containing amino acids, coupled with oxidative decarboxylation of αKG.
Amino acid conversion observed in B. thuringiensis 2e2 culture.
Bioconversion of amino acids by B. thuringiensis 2e2 was performed in the same way as previously reported (18), except for using l-allo-Ile, l-leucine, l-norleucine, l-norvaline, l-methionine, and l-ethionine instead of l-Ile in the IDO broth. All of the amino acid conversions observed in the reactions using recombinant IDO also proceeded in B. thuringiensis 2e2 cultures. These results indicate that native IDO in B. thuringiensis 2e2 cells catalyzed amino acid conversions with substrate specificity similar to that of the recombinant IDO. Thus, these converted amino acids most likely exist as trace secondary metabolites of B. thuringiensis 2e2 in the natural environment.
DISCUSSION
Based on detailed analysis of IDO substrate specificity and identification of reaction products, it was first demonstrated in this study that an αKG-dependent dioxygenase catalyzed three distinct types of reactions: hydroxylation, sulfoxidation, and dehydrogenation, depending on the reaction substrates.
Hydroxylation seems to be the primary reaction catalyzed by IDO, which is typical of most αKG-dependent dioxygenases. IDO catalyzed the strictly stereoselective hydroxylation of the C-3, C-4, or C-5 position in several l-form amino acids with aliphatic hydrophobic side chains. Substrate recognition by IDO required more than 5 carbons in the main chain of the amino acid, and rather than a methyl carbon, a methylene or methine carbon of the substrate was hydroxylated; hence, l-alanine, l-aminobutyrate, l-valine, and l-tert-leucine did not react at all with IDO. l-Norleucine was converted to 4-hydroxynorleucine and 5-hydroxynorleucine simultaneously by IDO. Because only l-norleucine had a C-5 methylene carbon among the amino acids tested in this study, the possibility remains that IDO hydroxylates C-5 methylene carbons, and possibly C-5 methine carbons, in other long aliphatic amino acids. Interestingly, the C-3 methine carbon of l-allo-Ile was hydroxylated by IDO instead of the C-4 methylene carbon, in contrast to results with l-Ile. It is possible that the steric constraint of the side chain methyl group in l-allo-Ile prevented C-4 hydroxylation to generate (2S,3S,4S)-4-hydroxyisoleucine.
This is the first report demonstrating that sulfoxidation and dehydrogenation reactions are catalyzed by an enzyme of the αKG-dependent dioxygenase superfamily. Although several enzymes belonging to the cytochrome P450 monooxygenase superfamily were reported to catalyze hydroxylation, sulfoxidation, and dehydrogenation (20), it was shown for the first time in this paper that an αKG-dependent dioxygenase also differentially catalyzed these reactions, depending on the substrate. Sulfoxidation of two sulfur-containing amino acids was not mediated by a reactive oxygen species generated in an uncoupling reaction of IDO but was catalyzed enzymatically by IDO directly, because stereoselectivity of sulfoxidation was strictly controlled in the reaction. The absolute stereostructures of l-methionine sulfoxide and l-ethionine sulfoxide are still being investigated. l-Methionine sulfoxidation is known to be catalyzed by a flavin-containing monooxygenase (FMO), which is found in mammalian microsomes (9). However, this reaction might not occur in vivo, because the apparent Km of FMO toward methionine was above the physiological concentration. Also, detailed kinetic analysis of sulfoxidation by IDO would be needed to determine whether this reaction has physiological significance in B. thuringiensis 2e2 cells. On the other hand, IDO dehydrogenated (2S,3R,4S)-HIL into (2S,3R)-AMKP, but this activity has no physiological significance, because the specific activity of IDO was very low toward HIL and this conversion was strongly carried out by a NAD+-dependent HIL dehydrogenase in B. thuringiensis 2e2 (23).
In our previous work, together with the aldolase-transaminase coupling reaction for HIL production from acetaldehyde, α-ketobutyrate, and l-glutamate (24, 32), a highly efficient biotransformation system converting l-Ile into (2S,3R,4S)-HIL using IDO was developed (31). A genetically manipulated E. coli strain that accumulated αKG and was able to efficiently transport the branched-chain amino acid was used as the host strain in order to accelerate the hydroxylation reaction by heterologously expressed IDO. This system would be easy to apply to the production of other IDO-producible hydroxy amino acids found in this study. These hydroxy amino acids are sufficiently valuable to produce on a large scale. For instance, (S)-3-hydroxy-l-allo-Ile is a kind of β-hydroxy α-amino acid, which are important substances due to their biological activities and their ability to serve as intermediates for various compounds, such as β-lactams (4). 4-Hydroxy-l-leucine is a component of phalloin, which is one of the toxic bicyclic peptides produced by the mushroom Amantia phalloides (36). Fluorescently labeled phalloin is a useful reagent to stain actin filaments for light microscopy in cell biology (33). (S)-4-Hydroxy-l-norvaline solely stimulated the insulin release of isolated rat islets, as well as (2S,3R,4S)-HIL (6). Also, this hydroxy amino acid was isolated from the seeds of Lathyrus odoratus (11), and Crotalaria juncea seeds contain 5-hydroxy-l-norleucine (28). Interestingly, the organisms that accumulate large amounts of hydroxy hydrophobic amino acids in their seeds, including L. odoratus, C. juncea, and fenugreek, all belong to the Leguminosae. Although it is still unknown what role these amino acids play in the seeds, they are expected to have a beneficial function, such as some physiological activity. Lactones of 4-hydroxy-l-norvaline and 4-hydroxy-l-norleucine are analogs of acyl-homoserine lactones, which are signaling molecules involved in bacterial quorum sensing. These analogs can be used as control molecules for gene expression in eukaryotic cells (21). Large-scale preparation of these useful hydroxy amino acids has now become possible by using IDO as a biocatalyst, and also, it would allow further discovery of their useful features.
The hydroxy amino acids and amino acid sulfoxides found as the reaction products of IDO could also be naturally occurring secondary metabolites of B. thuringiensis 2e2, as well as (2S,3R,4S)-HIL and (2S,3R)-AMKP. These metabolites may have some role, such as that of (2S,3R)-AMKP (27), in natural complex biological communities.
Supplementary Material
ACKNOWLEDGMENTS
This work was partially supported by a Grant-in-Aid for Scientific Research (no. 21780070 to M.H.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Project for Development of a Technological Infrastructure for Industrial Bioprocesses on R&D of New Industrial Science and Technology Frontiers (to S.S. and J.O.) from the New Energy and Industrial Technology Development Organization (NEDO) of Japan.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 5 August 2011.
REFERENCES
- 1. Aoyagi Y., Sugahara T. 1988. β-Hydroxy-L-valine from Pleurocybella porrigens. Phytochemistry 27:3306–3307 [Google Scholar]
- 2. Ariza J., Font J., Ortuno R. M. 1990. Enantioselective synthesis of hydroxy α-amino acids. (-)-erythro- and (-)-threo-γ-Hydroxynorvaline. Tetrahedron 46:1931–1942 [Google Scholar]
- 3. Bell E. A. 1980. The non-protein amino acids of higher plants. Endeavour 4:102–107 [Google Scholar]
- 4. Blaskovich M. A., et al. 1998. Stereoselective synthesis of threo and erythro β-hydroxy and β-disubstituted-β-hydroxy α-amino acids. J. Org. Chem. 63:3631–3646 [Google Scholar]
- 5. Broca C., et al. 1999. 4-Hydroxyisoleucine: experimental evidence of its insulinotropic and antidiabetic properties. Am. J. Physiol. Endocrinol. Metab. 277:40–44 [DOI] [PubMed] [Google Scholar]
- 6. Broca C., et al. 2000. 4-Hydroxyisoleucine: effects of synthetic and natural analogues on insulin secretion. Eur. J. Pharmacol. 390:339–345 [DOI] [PubMed] [Google Scholar]
- 7. Ciabatti R., et al. 1989. Ramoplanin (A-16686), a new glycolipodepsipeptide antibiotic. III. Structure elucidation. J. Antibiot. (Tokyo) 42:254–267 [DOI] [PubMed] [Google Scholar]
- 8. De Kimpe N., Sulmon P., Brunet P. 1990. Synthesis of 2,2-dialkyl-1-aminocyclopropanecarboxylic acids from α-chloro ketimines. J. Org. Chem. 55:5777–5784 [Google Scholar]
- 9. Duescher R. J., Lawton M. P., Philpot R., Elfarra A. A. 1994. Flavin-containing monooxygenase (FMO)-dependent metabolism of methionine and evidence for FMO3 being the major FMO involved in methionine sulfoxidation in rabbit liver and kidney microsomes. J. Biol. Chem. 269:17525–17530 [PubMed] [Google Scholar]
- 10. Ford P. W., et al. 1999. Papuamides A-D, HIV-inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc. 121:5899–5909 [Google Scholar]
- 11. Fowden L. 1966. Isolation of γ-hydroxynorvaline from Lathyrus odoratus seed. Nature 209:807–808 [Google Scholar]
- 12. Fowden L., Pratt H. M., Smith A. 1973. 4-Hydroxyisoleucine from seed of Trigonella foenum-graecum. Phytochemistry 12:1707–1711 [Google Scholar]
- 13. Gelse K., Poschl E., Aigner T. 2003. Collagens-structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 55:1531–1546 [DOI] [PubMed] [Google Scholar]
- 14. Hamada T., Matsunaga S., Yano G., Fusetani N. 2005. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge, Theonella swinhoei. J. Am. Chem. Soc. 127:110–118 [DOI] [PubMed] [Google Scholar]
- 15. Handa T., Yamaguchi K., Sono Y., Yazawa K. 2005. Effects of fenugreek seed extract in obese mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 69:1186–1188 [DOI] [PubMed] [Google Scholar]
- 16. Hausinger R. P. 2004. Fe(II)/α-ketoglutarate-dependent hydroxylases and related enzymes. Crit. Rev. Biochem. Mol. Biol. 39:21–68 [DOI] [PubMed] [Google Scholar]
- 17. Holland H. L., Andreana P. R., Brown F. M. 1999. Biocatalytic and chemical routes to all the stereoisomers of methionine and ethionine sulfoxides. Tetrahedron Asymmetry 10:2833–2843 [Google Scholar]
- 18. Kodera T., et al. 2009. A novel L-isoleucine hydroxylating enzyme, L-isoleucine dioxygenase from Bacillus thuringiensis, produces (2S,3R,4S)-4-hydroxyisoleucine. Biochem. Biophys. Res. Commun. 390:506–510 [DOI] [PubMed] [Google Scholar]
- 19. Lawrence C. C., Sobey W. J., Field R. A., Baldwin J. E., Schofield C. J. 1996. Purification and initial characterization of proline 4-hydroxylase from Streptomyces griseoviridus P8648: a 2-oxoacid, ferrous-dependent dioxygenase involved in etamycin biosynthesis. Biochem. J. 313:185–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mansuy D. 1998. The great diversity of reactions catalyzed by cytochromes P450. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 121:5–14 [DOI] [PubMed] [Google Scholar]
- 21. McBride K., et al. January 2001. Polynucleotide that incorporates elements, e.g. promoter comprising an AHL (acetylated homoserine lactone)-response element, of a bacterial quorum sensing system, useful for modulating gene expression in a wide variety of plants and animals. U.S. patent WO200102593-A2 [Google Scholar]
- 22. Mori H., Shibasaki T., Yano K., Ozaki A. 1997. Purification and cloning of a proline 3-hydroxylase, a novel enzyme which hydroxylates free l-proline to cis-3-hydroxy-l-proline. J. Bacteriol. 179:5677–5683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ogawa J., et al. 2011. A novel L-isoleucine metabolism in Bacillus thuringiensis generating (2S,3R,4S)-4-hydroxyisoleucine, a potential insulinotropic and anti-obesity amino acid. Appl. Microbiol. Biotechnol. 89:1929–1938 [DOI] [PubMed] [Google Scholar]
- 24. Ogawa J., et al. 2007. Synthesis of 4-hydroxyisoleucine by the aldolase-transaminase coupling reaction and basic characterization of the aldolase from Arthrobacter simplex AKU 626. Biosci. Biotechnol. Biochem. 71:1607–1615 [DOI] [PubMed] [Google Scholar]
- 25. Okai H., Izumiya N. 1969. Resolution of amino acids. IX. Studies on the preparation of β-hydroxyasparagines and configuration of natural hydroxyasparagine. Bull. Chem. Soc. Jpn. 42:3550–3555 [Google Scholar]
- 26. Palomo C., et al. 1990. Highly stereoselective synthesis of α-hydroxy β-amino acids through β-lactams: application to the synthesis of the taxol and bestatin side chains and related systems. Tetrahedron Lett. 31:6429–6432 [Google Scholar]
- 27. Perlman D., Perlman K. L., Bodanszky M. 1977. Microbial production of vitamin B12 antimetabolites. II. 2-Amino-4-keto-3-methylpentanoic acids from Bacillus cereus 439. Bioorg. Chem. 6:263–271 [Google Scholar]
- 28. Pilbeam D. J., Bell E. A. 1979. A reappraisal of the free amino acids in seeds of Crotalaria juncea (Leguminosae). Phytochemistry 18:320–321 [Google Scholar]
- 29. Rosenthal G. A. 1982. Plant nonprotein amino and imino acids. Biological, biochemical and toxicological properties. Academic Press, New York, NY [Google Scholar]
- 30. Sheldrick G. M., Jones P. G., Kennard O., Williams D. H., Smith G. A. 1978. Structure of vancomycin and its complex with acetyl-D-alanyl-D-alanine. Nature 271:223–225 [DOI] [PubMed] [Google Scholar]
- 31. Smirnov S. V., et al. 2010. Metabolic engineering of Escherichia coli to produce (2S,3R,4S)-4-hydroxyisoleucine. Appl. Microbiol. Biotechnol. 88:719–726 [DOI] [PubMed] [Google Scholar]
- 32. Smirnov S. V., et al. 2007. A novel strategy for enzymatic synthesis of 4-hydroxyisoleucine: identification of an enzyme possessing HMKP (4-hydroxy-3-methyl-2-keto-pentanoate) aldolase activity. FEMS Microbiol. Lett. 273:70–77 [DOI] [PubMed] [Google Scholar]
- 33. Stopford C. R., Wolberg G., Prus K. L., Reynolds-Vaughn R., Zimmerman T. P. 1985. 3-Deazaadenosine-induced disorganization of macrophage microfilaments. Proc. Natl. Acad. Sci. U. S. A. 82:4060–4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Strieker M., Kopp F., Mahlert C., Essen L. O., Marahiel M. A. 2007. Mechanistic and structural basis of stereospecific Cβ-hydroxylation in calcium-dependent antibiotic, a daptomycin-type lipopeptide. ACS Chem. Biol. 2:187–196 [DOI] [PubMed] [Google Scholar]
- 35. Takita T., et al. 1981. Total synthesis of deglyco-bleomycin A2. Tetrahedron Lett. 22:671–674 [Google Scholar]
- 36. Wieland T. 1968. Poisonous principles of mushrooms of the genus Amanita. Four-carbon amines acting on the central nervous system and cell-destroying cyclic peptides are produced. Science 159:946–952 [DOI] [PubMed] [Google Scholar]
- 37. Yin X., Zabriskie T. 2004. VioC is a non-heme iron, α-ketoglutarate-dependent oxygenase that catalyzes the formation of 3S-hydroxy-L-arginine during viomycin biosynthesis. Chembiochem. 5:1274–1277 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.