Abstract
It is known that the stomach is colonized by indigenous lactobacilli in mice. The aim of this study was to examine the role of such lactobacilli in the development of the stomach. For a DNA microarray analysis, germ-free BALB/c mice were orally inoculated with 109 CFU lactobacilli, and their stomachs were excised after 10 days to extract RNA. As a result, lactobacillus-associated gnotobiotic mice showed dramatically decreased expression of the gastrin gene in comparison to germ-free mice. The mean of the log2 fold change in the gastrin gene was −4.3. Immunohistochemistry also demonstrated the number of gastrin-positive (gastrin+) cells to be significantly lower in the lactobacillus-associated gnotobiotic mice than in the germ-free mice. However, there was no significant difference in the number of somatostatin+ cells in these groups of mice. Consequently, gastric acid secretion also decreased in the mice colonized by lactobacilli. In addition, an increase in the expression of the genes related to muscle system development, such as nebulin and troponin genes, was observed in lactobacillus-associated mice. Moreover, infection of germ-free mice with Helicobacter pylori also showed the down- and upregulation of gastrin and muscle genes, respectively, in the stomach. These results thus suggested that indigenous lactobacilli in the stomach significantly affect the regulation of gastrin-mediated gastric acid secretion without affecting somatostatin secretion in mice, while H. pylori also exerts such an effect on the stomach.
INTRODUCTION
The human stomach contains only a few species of bacteria if it is free from infection with Helicobacter pylori (3). During fasting, gastric juice contains only small numbers of bacteria, approximately 102 to 103/ml, which include Streptococcus, Lactobacillus, and Veillonella. However, these bacteria are considered nonresidents that are only in transit from the oral cavity and throat (13, 19). The scarcity of such bacteria in the human stomach appears to be due to the high acidity of the luminal medium.
While H. pylori is a well-known pathogenic bacterium that causes peptic ulcers and cancer in the human stomach, the bacterium has also been proposed to belong to the indigenous gastric microbiota of humans from the earliest times (2). That hypothesis is supported by the fact that H. pylori is acquired in early childhood and thereafter remains stably colonizing the stomach for decades in substantial numbers. This raises questions about the role of H. pylori as the indigenous bacteria of the stomach in the physiological development and function of the organ. However, it is difficult to clarify the answers to this question by an infection study using H. pylori, because various pathogenic factors of H. pylori, such as CagA, vacuolating toxins, and urease and its metabolites, induce chronic pathological inflammation in the gastric tissue, which obscures the physiological role of H. pylori as an indigenous bacterium.
In a previous study (12), we found an indigenous microbiota that predominantly consists of lactobacilli, including Lactobacillus acidophilus, Lactobacillus delbrueckii, and Lactobacillus fermentum, in the stomachs of specific-pathogen-free (SPF) mice. The colonization of the murine stomach by several Lactobacillus species was also revealed by PCR amplification using Lactobacillus species-specific primers (25). The lower acidity in the stomachs of mice was thought to enable the lactobacilli to colonize the stomach. Moreover, no evident inflammatory changes were observed in the stomachs of those mice by histological analysis (12). On the other hand, Zavros et al. (27) reported that hypochlorhydria induced in the mice predisposed their stomachs to develop bacterial overgrowth, resulting in mucosal inflammation, although the species in the expanded bacterial population remained unclear. These contradictory reports necessitated a study to elucidate the role of indigenous lactobacilli in the stomach. In the present study, a microarray analysis was performed to investigate how innate lactobacilli affect the stomach using germ-free (GF) and lactobacillus-associated gnotobiotic mice.
MATERIALS AND METHODS
Bacterial strains.
Lactobacillus gasseri OLL2716 (LG21) and Lactobacillus johnsonii no. 1088 (LJ88) were provided by Meiji Dairies Corporation, Tokyo, Japan, and Snowden Co., Tokyo, Japan, respectively. For inoculation into mice, lactobacilli were grown in Difco MRS broth (Becton Dickinson, Sparks, MD) for 1 day. For heat treatment, a lactobacillus suspension was incubated at 100°C for 10 min. The identity of an H. pylori strain named WT was verified by the presence of cagA, cagE, and cagG (24), and bacteria were incubated in Difco brucella broth containing 5% fetal bovine serum (FBS) at 37°C for 3 days.
Mice.
Animal treatment and care were carried out in accordance with the institutional guidelines of Tokai University. Male SPF and GF BALB/c mice were obtained from Nippon Clea Inc. (Tokyo, Japan). The reason why SPF mice were used in the present study was that they are considered to be free from pathogenic microbes but nevertheless still retain indigenous microbiota in the body. GF male littermate mice were maintained in Trexler-type flexible-film plastic isolators with sterile food and water. To construct the gnotobiotic mice with lactobacilli, 109 CFU of live lactobacilli suspended in 0.5 ml of phosphate-buffered saline (PBS) were intragastrically inoculated using a stomach tube once into 8-week-old mice, except where otherwise indicated. The mice were also intragastrically inoculated with 1010 CFU heat-killed lactobacilli suspended in 0.5 ml PBS every day for 10 days. For omeprazole treatment, 8-week-old SPF mice were injected subcutaneously with 0.4 mg omeprazole sodium (AstraZeneca, London, England) in 0.2 ml PBS every 2 days for 20 days. Control SPF mice were treated in the same manner with the vehicle alone. For infection with H. pylori, GF mice were intragastrically inoculated using a stomach tube on three consecutive days with 109 CFU of H. pylori bacteria that were freshly prepared and resuspended in 0.5 ml PBS. In the control mice, 0.5 ml PBS was orally administered in the same way. As reported in a previous study (12), this protocol enabled H. pylori to easily colonize the stomachs of the GF mice. After the treatment, the stomach was excised for analysis. The number of H. pylori in the homogenate of the stomach was around 108 CFU/g tissue in all of the mice. The experimental designs for the animal study in the present study are summarized in Table 1.
Table 1.
Experimental design using mice
| Assay | Type of mice | Inoculation of bacteria |
Sampling time (age of mice) | ||
|---|---|---|---|---|---|
| Strain | No. (CFU) | Time (age of mice) | |||
| Count of lactobacilli | SPF | (-)a | 2, 4, 8 wk | ||
| Gnotobiotic | LG21 | 107 | At birth | 2, 4, 8 wk | |
| Microarray | GF | (-) | 8 wk + 10 days | ||
| Gnotobiotic | LG21 | 108 | 8 wk | 8 wk + 10 days | |
| Gnotobiotic | H. pylori | 3 × 109 | 8 wk | 8 wk + 10 days | |
| Immunohistochemistry | GF | (-) | |||
| Gnotobiotic | LG21 | 108 | 8 wk | 8 wk + 10 days | |
| Gnotobiotic | LJ88 | 108 | 8 wk | 8 wk + 10 days | |
| Gnotobiotic | H. pylori | 3 × 109 | 8 wk | 8 wk + 10 days | |
| SPF | (-) | Treated with PBS | 8 wk + 20 days | ||
| SPF | (-) | Treated with omeprazole | 8 wk + 20 days | ||
| GF | (-) | Treated with PBS | 8 wk + 10 days | ||
| GF | Heat-killed LG21 | 10 × 1010 | 8 wk | 8 wk + 10 days | |
| GF | Heat-killed LJ88 | 10 × 1010 | 8 wk | 8 wk + 10 days | |
| RT-PCR | GF | (-) | 8 wk + 10 days | ||
| Gnotobiotic | LG21 | 108 | 8 wk | 8 wk + 10 days | |
| Gastric acid secretion | GF | (-) | 8 wk + 10 days | ||
| Gnotobiotic | LG21 | 108 | 8 wk | 8 wk + 10 days | |
| PF | (-) | 8 wk + 10 days | |||
| Gnotobiotic | H. pylori | 3 × 109 | 8 wk | 8 wk + 10 days | |
| Histology | GF | (-) | 8 wk | ||
| Gnotobiotic | LG21 | 107 | At birth | 8 wk | |
(-), inoculation of bacteria was not done.
Gastric acid production.
Under light ether anesthesia, an epigastric laparotomy was performed. After exposing the stomach, the pylorus was ligated and the abdominal incision was sutured. Gastric juice was collected for 2 h after the pylorus ligation. The gastric juice was centrifuged at 3,000 × g for 5 min, and then the volume, pH, and total acidity were measured. The pH and total-acidity values were measured using a pH meter (M-7; Horiba, Tokyo, Japan) and a titration method, respectively.
Count of lactobacilli in the stomach by real-time PCR.
Bacterial DNA from the samples was analyzed by real-time PCR to count the lactobacilli according to the method reported by Mikami et al. (16). The Lactobacillus genus-specific paired primers, LactoF (5′-TGGAAACAGRTGCTAATACCG-3′) and LactoR (5′-GTCCATTGTGGAAGATTCCC-3′), used in this study have been described previously by Byun et al. (5). The composition of Lactobacillus species in the stomachs of SPF mice was examined using 11 different species-specific primers reported by Walter et al. (26). The extraction of bacterial DNA from the stomach and PCR amplification were done according to a method described elsewhere (16).
DNA microarray analysis.
Total RNA was prepared individually from the stomachs of mice using a Qiagen (Hilden, Germany) RNeasy Mini Kit. The time point of sampling shown in Table 1 was determined based on the study of Hooper et al. (11). The quality and quantity of RNA in the samples were examined by measuring the optical densities at 260 nm and 280 nm and by observing the gel electrophoresis pattern. Ten micrograms of pooled RNA samples from 3 mice in each group was subjected to the One-Cycle cDNA Synthesis Kit (Affymetrix, Santa Clara, CA) to generate cDNA. The cDNA was then purified and served as a template in the subsequent in vitro transcription reaction for cRNA amplification and biotin labeling using a GeneChip IVT labeling kit (Affymetrix). The biotinylated cRNAs were then cleaned up, fragmented into 50 to 200 bases by heating them at 94°C for 35 min, and hybridized to GeneChip Test3 Arrays (Affymetrix) in order to assess the target quality and labeling efficiency prior to utilizing genome array products. The bound RNAs were stained and amplified by treatment with streptavidin-phycoerythrin and biotinylated anti-streptavidin antibody (Ab), respectively. Thereafter, a 10-μg aliquot was used for hybridization to GeneChip Mouse Genome 430 2.0 arrays according to the manufacturer's instructions. Fluorescence was measured by a GeneChip Scanner 3000 (Affymetrix). The scanning data were transformed into numerical data and then scaled by using the GeneChip Operating Software program (Affymetrix). This application was used to compare the expression arrays from gnotobiotic and germ-free stomach samples and generated a list of genes with significantly affected expression. Genes with a “change call” of “increase” or “decrease” were selected based on the degree of the change.
RT-PCR analysis.
A quantitative real-time RT-PCR analysis was performed according to a method described elsewhere (6). The target gene expression was normalized by calculating the difference between the threshold cycle (CT) of the target gene and the CT of the control (β-actin). The ΔCT value was calculated as follows: ΔCT = CT (target gene) − CT (control gene). The relative threshold cycles of transcripts were exhibited as 2−ΔCT.
Histology and immunohistochemistry.
For histology of the stomach, one-half of each resected stomach along the great curvature was fixed in 4% buffered formalin, embedded in paraffin wax, and cut into 2-μm sections. The sections were then stained with hematoxylin and eosin following standard procedures. The thickness of the muscle layer in the stomach was measured with a microscope (AX80; Olympus, Tokyo, Japan) along its perpendicular axis using an analysis software package (Olympus BP2-BSW). A total of 10 different points were measured to obtain the mean thickness in each region of a specimen. The number of nuclei in a rectangle (150 by 200 μm) was also determined in the muscle layer of each stomach region. For immunohistochemistry, the tissue sections were deparaffinized, boiled in a microwave oven for 15 min at 98°C in 10 mM citrate buffer (pH 6.0), and blocked with 5% normal goat serum-PBS for 15 min at room temperature. The sections were incubated with primary Abs overnight at 4°C and then with secondary Abs labeled with a fluorescent marker for 2 h at room temperature. Finally, the samples were treated with DAPI (4,6-diamidino-2-phenylindole) and DABCO [1,4-diazobicyclo(2,2,2)octane]. The slides were visualized with a microscope configured for fluorescence imaging (BZ-9000; Keyence, Woodcliff Lake, NJ). Morphometric analysis using AxioVision release 4.8 (Zeiss, Jena, Germany) was performed on sections stained for gastrin-positive (gastrin+) and somatostatin+ cells in random fields 1 mm in length along the corpus-antrum axis from the antra of mice. Anti-gastrin Ab diluted to 1:300 (rabbit polyclonal; Dako, Glostrup, Denmark), mouse anti-somatostatin monoclonal Ab diluted to 1:100 (GeneTex, Irvine, CA), control rabbit IgG (Dako), and control mouse IgG (Dako) were used as the primary Abs. Goat anti-rabbit IgG-Alexa 488 (Molecular Probe, Eugene, OR) and goat anti-mouse IgG-Alexa 594 (Molecular Probe) were used as the secondary Abs.
Statistical analyses.
The results were statistically evaluated by either Student's t test or a 1-way analysis of variance (ANOVA), as appropriate. The SSPS (SSPS, Chicago, IL) version 16.0 or GraphPad Prism 5 (GraphPad Software, San Diego, CL) software program was used for data analysis.
RESULTS
Number of lactobacilli colonizing the stomach.
The lactobacillus count indicated by the real-time PCR method revealed 108 CFU/g tissue of indigenous lactobacilli in the whole stomach of SPF BALB/c mice at the age of 2 weeks (Table 2). The numbers of LG21 bacteria in the whole stomachs of gnotobiotic mice inoculated with 107 CFU of LG21 at birth gradually increased and reached more than 108 CFU by the age of 8 weeks. The number of LG21 bacteria attached to the glandular epithelium (corpus and antrum) was almost 10 times more than that on the squamous (pregastric) epithelium in gnotobiotic mice at the age of 8 weeks. It was therefore demonstrated that the LG21 strain could establish stable colonization in the stomachs of mice, and thus, it is considered to be an appropriate Lactobacillus strain to examine the role of lactobacilli innately colonizing the stomach. An examination of the composition of Lactobacillus species colonizing the stomach by PCR detected L. acidophilus, L. gasseri, and L. johnsonii in SPF mice (data not shown).
Table 2.
Numbers of lactobacilli in the stomach
| Mouse age (wk) | No. of lactobacilli in the stomach (log10 CFU/g tissue)c |
|||
|---|---|---|---|---|
| SPF mice (wholea) | GF mice inoculated with 107 CFU of LG21 at birth |
|||
| Whole | Pregastricb | Corpus + antrumb | ||
| 2 | 8.0 ± 0.24 | 5.6 ± 0.23 | NDd | ND |
| 4 | 8.3 ± 0.82 | 7.8 ± 0.39 | ND | ND |
| 8 | 8.9 ± 0.38 | 8.4 ± 0.60 | 6.6 ± 0.94 | 7.7 ± 0.88 |
The stomach with its contents was homogenized before counting the lactobacilli (n = 7).
The stomach was excised along the great curvature and rinsed with PBS to remove the contents. Then, the pregastric region or corpus and antrum were excised in order to count the colonizing lactobacilli (n = 15).
Mean ± standard deviation (SD).
ND, not done.
Changes in the gene expression in the stomach after lactobacillus or H. pylori colonization.
To perform a comprehensive analysis of the genome-wide expression in the stomach colonized by lactobacilli, the transcription level of each gene in the organ was compared between LG21-associated gnotobiotic (LG21AG) and GF mice using a microarray analysis (Table 3). In the comparative analysis, 45,000 genes in total were examined, and 259 and 104 genes were increased (>2) and decreased (<0.5), respectively, by colonization with the LG21 strain. Table 3 shows the down- and upregulated genes in order of their degree of change. Among the genes whose expression markedly changed, genes related to gastric acid secretion, such as gastrin and potassium voltage channel genes, exhibited marked decreases in their expression. The reduction of gastrin mRNA was especially impressive, as the mean of the log2 fold change in two independent experiments was −4.3. On the other hand, genes related to muscular system development and function, such as nebulin, troponin, and myosin heavy chain genes, showed the greatest increases in expression.
Table 3.
Profile of the molecules that underwent the greatest change in the stomach for LG21-associated gnotobiotic mice vs GF mice as determined by a microarray analysis
| Gene | Log2 fold change |
Gene reference | ||
|---|---|---|---|---|
| 1st expt | 2nd expt | Avg | ||
| Downregulated genes | ||||
| Gastrin | −5.4 | −3.2 | −4.3 | NM010257.1 |
| Potassium voltage channel (Kcne 3) | −3.1 | −2.7 | −2.9 | NM020574.1 |
| Chloride channel calcium activated 3 | −2.6 | −2.9 | −2.8 | NM017474 |
| Secreted phosphoprotein 1 | −3.6 | −1.9 | −2.8 | NM009263.1 |
| Fatty acid binding protein 2 | −2.4 | −2.4 | −2.4 | NM007980.1 |
| Embryo whole-body cDNA | −2.8 | −1.9 | −2.4 | AK004474.1 |
| 6 BAC RP24-180C9 | −2.6 | −1.8 | −2.2 | |
| Adult male small intestine cDNA | −2.5 | −1.8 | −2.2 | AK008023.1 |
| DNA segment, chromasome 15 | −2.2 | −2.0 | −2.1 | BC010337.1 |
| Claudin 7 | −2.0 | −2.1 | −2.1 | BC008104.1 |
| Upregulated genes | ||||
| Nebulin | 5.1 | 2.4 | 3.8 | AY189120 |
| Mast cell protease 1 | 3.6 | 2.6 | 3.1 | NM008570.1 |
| Weakly similar to V-ATPase | 4.6 | 1.5 | 3.1 | AV204216 |
| Chromosome 1 | 1.0 | 4.3 | 2.7 | |
| Troponic C | 2.6 | 2.6 | 2.6 | NM009394.1 |
| Myosin heavy chain IIX | 2.5 | 2.4 | 2.5 | AJ293626.1 |
| Hydroxysteroid 17-β dehydrogenase | 1.1 | 3.6 | 2.4 | NM030611.1 |
| Myosin heavy chain 2X | 2.3 | 2.4 | 2.4 | AJ002522. |
| Adult male stomach cDNA | 2.0 | 2.5 | 2.3 | AK008863.1 |
| Myosin heavy polypeptide 8 | 2.4 | 2.1 | 2.3 | BB011213 |
We next performed another microarray analysis of the murine stomach inoculated with H. pylori in order to compare the expression profile with those of genes induced by lactobacilli. Table 4 shows the down- and upregulated genes in order of their fold changes in mice with H. pylori infection. The changes in gene expression found in this analysis were comparable to those found in the analysis of stomachs of mice colonized by lactobacilli, as indicated by the genes in boldface in Table 4. That is, genes related to gastric acid secretion, such as the gastrin gene (with the 2nd largest decrease), were significantly downregulated, and genes related to the muscle, such as the myosin heavy chain gene (with the greatest increase), were highly upregulated.
Table 4.
Profile of the molecules that underwent the greatest change in the stomach for H. pylori-infected gnotobiotic mice versus GF mice as determined by a microarray analysis
| Gene | Log2 fold change |
Gene reference | ||
|---|---|---|---|---|
| 1st expt | 2nd expt | Avg | ||
| Downregulated genes | ||||
| NK6 homeobox 3 | −1.6 | −3.3 | −2.5 | AK018683.1 |
| Gastrina | −1.1 | −2.7 | −1.9 | NM010257.1 |
| Secreted phosphoprotein 1 | −1.2 | −2.2 | −1.7 | NM009263.1 |
| Pancreatic homeobox gene 1 | −1.1 | −2.0 | −1.6 | AK020261.1 |
| 6 BAC RP24-180C9 | −0.9 | −2.0 | −1.5 | AV069898 |
| Gastrokine 3 | −0.9 | −1.9 | −1.4 | GU220566 |
| Potassium voltage channel (Kcne 3) | −1.1 | −1.5 | −1.3 | NM020574.1 |
| BAC clone PR23-413F14 | −1.5 | −0.8 | −1.2 | AC140322 |
| Adipsin | −1.5 | −0.7 | −1.1 | NM013459.1 |
| Urokinase-type plasminogen | −1.0 | −1.1 | −1.1 | X62701.1 |
| Upregulated genes | ||||
| Myosin heavy chain 2X | 8.5 | 7.2 | 7.9 | AJ002522.1 |
| Troponin C | 8.4 | 6.8 | 7.6 | NM009394.1 |
| Myosin heavy chain IIX | 7.9 | 6.8 | 7.4 | AJ293626.1 |
| Ornithine transcarbamylase | 5.3 | 8.2 | 6.8 | AI786408 |
| Myoglobin | 6.3 | 4.4 | 5.4 | BC025172.1 |
| Sarcolipin | 5.9 | 3.1 | 4.5 | AK008863.1 |
| Skeletal muscle α-actin | 5.0 | 3.9 | 4.5 | M12233.1 |
| Myosin, heavy polypeptide 8 | 5.0 | 3.8 | 4.4 | BB011213 |
| Nebulin | 4.9 | 3.9 | 4.4 | AI595938 |
| BAC clone PR23-138F21 | 5.0 | 3.5 | 4.3 | BB099116 |
Analysis of gastrin-positive cells in the stomach.
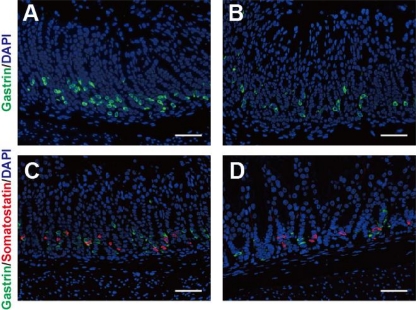
As the message level of gastrin dramatically decreased in the stomachs of LG21AG mice, according to a microarray analysis, the protein level of the molecule was quantitatively examined by immunohistochemical staining of gastrin-positive cells (green fluorescence) in the stomach (Fig. 1). The cells expressing somatostatin (red fluorescence), the main inhibitor of gastrin production, were also examined as a control in this analysis. A morphometric analysis (Table 5) demonstrated the number of gastrin+ cells in the LG21AG mice to be significantly lower than in GF mice (51.5 versus 84.3; P < 0.001). A similar decrease in the number of gastrin+ cells by lactobacillus colonization was also found in LJ88AG mice, whose stomachs were colonized by the L. johnsonii strain (37.1 versus 84.3; P < 0.001). H. pylori infection also decreased the number of gastrin+ cells in the GF mice (68.6 versus 84.3; P < 0.001).
Fig. 1.
Immunohistochemistry of the stomach for gastrin. The stomachs of GF (A and C) and LJ88AG (B and D) mice were excised 10 days after inoculation, and the antrum regions were stained for gastrin (green fluorescence) (A and B) or somatostatin (red fluorescence) together with gastrin (C and D). Nuclei (blue) were stained with DAPI. Bars, 50 μm.
Table 5.
Numbers of gastrin/somatostatin-positive cells in the antra of stomachs
| Host mice | Treatment | No. of hosts | No. of gastrin+ cells/mma,b | No. of somatostatin+ cells/mmb |
|---|---|---|---|---|
| GF | 7 | 84.3 ± 1.7 | 28.1 ± 0.9 | |
| LG21 AGc | 7 | 51.5 ± 2.8g | 26.4 ± 1.8 | |
| LJ88 AGc | 5 | 37.1 ± 2.8g | 27.9 ± 1.3 | |
| H. pylori AGd | 4 | 68.6 ± 2.0g | 27.1 ± 1.6 | |
| SPF | PBS | 6 | 45.7 ± 1.7 | 21.6 ± 1.3h |
| SPF | Omeprazolee | 6 | 69.8 ± 3.1g | 29.2 ± 2.2 |
| GF | PBS | 3 | 80.8 ± 4.1 | ND |
| GF | Heat-killed LG21f | 4 | 47.9 ± 2.5g | ND |
| GF | Heat-killed LJ88f | 4 | 48.0 ± 2.0g | ND |
Number of positive cells in a 1-mm width along the horizontal axis of the specimen.
Mean ± standard error (SE).
GF host mice were intragastrically inoculated with 108 CFU lactobacilli at 8 weeks of age and assayed 10 days later.
GF host mice were intragastrically inoculated with 109 CFU H. pylori on three consecutive days at 8weeks of age and assayed 10 days later.
Omeprazole (0.4 mg in 0.2 ml PBS) was injected subcutaneously into SPF mice every 2 days for 20 days.
Host mice at 8 weeks of age were intragastrically inoculated with 1010 CFU heat-killed lactobacilli every day for 10 days before the assay.
P < 0.001 in comparison to the GF, SPF/PBS, or GF/PBS group by Tukey's t test.
P < 0.01 in comparison to the GF, SPF/PBS, or GF/PBS group by Tukey's t test.
In SPF mice whose stomachs were colonized by indigenous lactobacilli, the number of gastrin+ cells was lower than in GF mice (45.7 versus 84.3). The SPF mice showed a significant increase in the number of gastrin+ cells after treatment with omeprazole (45.7 versus 69.8; P < 0.001), a proton pump inhibitor used as a positive control to increase gastrin+ cells. The oral administration of heat-killed Lactobacillus strains, including both LG21 and LJ88, in GF mice could also significantly reduce the number of gastrin+ cell to a degree comparable to that induced by viable lactobacilli (80.8 to 47.9 and 80.8 to 48.0, respectively), while a 10-times-larger number of bacteria was administered every day for 10 days. In the count of cells expressing somatostatin (Table 5), no significant differences were found among the GF (28.1), LG21AG (26.4), and LJ88AG (27.9) groups. Also, no significant change in the expression level of somatostatin was found in a microarray analysis in which the mean log2 fold change was 0.3 in LG21AG mice compared with GF mice (data not shown). Taken together, these analyses demonstrated that lactobacilli, including killed lactobacilli, significantly suppress the production of gastrin in the stomach without affecting somatostatin secretion.
Examination of G cell differentiation.
The RNA levels of Nkx6.3, Pdx1, and Ngn3, which are transcriptional regulators for G cell differentiation, in the stomach were measured by quantitative real-time reverse transcription (RT)-PCR in order to examine whether a decrease in the number of gastrin+ cells was caused by suppression of either the differentiation of progenitor G cells or the expression of the gastrin gene in mature G cells (Table 6). The RNA level of gastrin was also significantly lower in LG21AG than in GF mice in this analysis. Both the levels of Nkx6.3 and Ngn3 were significantly lower in LG21AG than in GF mice. Pdx1 tended to be expressed at a lower level in LG21AG mice. These results suggested that the differentiation of the G cell lineage was also suppressed in the stomachs of LG21AG mice.
Table 6.
RNA expression of transcription factors for G cell differentiation
| RNA | Relative threshold cycle (2−ΔCT)c |
||
|---|---|---|---|
| GF | LG21AGa | P valueb | |
| Gastrin | 0.57 ± 0.31 | 0.17 ± 0.14 | 0.015 |
| Nkx 6.3 | 0.05 ± 0.04 | 0.009 ± 0.006 | 0.025 |
| Pdx 1 | 0.009 ± 0.001 | 0.001 ± 0.001 | 0.31 |
| Ngn 3 | (5.4 ± 3.5) × 10−4 | (7.9 ± 5.8) × 10−5 | 0.015 |
GF mice at 8 weeks of age were orally inoculated with 108 CFU LG21, and their stomachs were removed for RNA extraction 10 days later.
Mann-Whitney U test.
Mean ± SD; n = 6.
Gastric acid secretion.
As gastrin is the main stimulant of acid secretion in the stomach, gastric acid secretion was examined in LG21AG and SPF mice, in which the number of gastrin-producing cells was significantly decreased compared with GF mice (Table 7). There were no significant differences in the volumes of gastric juice accumulated in the stomach during the 2-h collection period among the GF, LG21AG, and SPF mice. However, the total acidity was significantly lower in LG21AG than in GF mice (5.8 versus 18.2 meq/ml; P < 0.05). Consistent with this finding, the pH value was significantly higher in LG21AG than in GF mice (6.5 versus 3.1; P < 0.01). A reduction in the total acidity accompanied by an elevation of the pH value was also found in SPF and H. pylori-associated gnotobiotic mice compared to GF mice, although to a lesser degree.
Table 7.
Secretion of gastric juice in the stomach
| Host mice | No. of hosts examined | Gastric juicea |
||
|---|---|---|---|---|
| Vol (ml) | Total acidity (meq/ml) | pH | ||
| GF | 6 | 0.58 ± 0.17 | 18.2 ± 5.5 | 3.1 ± 0.8 |
| LG21AG | 6 | 0.73 ± 0.12 | 5.8 ± 4.8b | 6.5 ± 0.7c |
| SPF | 7 | 0.69 ± 0.28 | 11.3 ± 5.6b | 4.8 ± 1.1c |
| H. pylori-associated gnotobiotic | 5 | 0.50 ± 0.37 | 10.6 ± 5.7b | 4.1 ± 1.6 |
Mean ± SD.
P < 0.05 in comparison to the germ-free group by the Kruskal-Wallis test.
P < 0.01 in comparison to the germ-free group by the Kruskal-Wallis test.
Histology of the stomach colonized by lactobacilli.
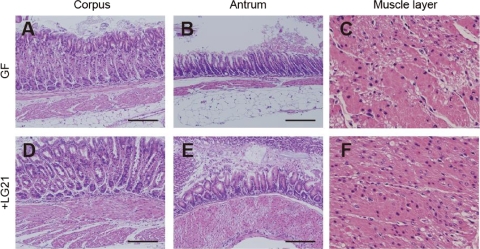
Finally, the stomach was examined by routine histology, because the microarray analysis in the present study demonstrated a significant increase in the expression of muscle genes in LG21AG mice (Fig. 2). A marked increase in the thickness of the muscle layer in the stomach was found in the gnotobiotic mice associated with LG21 at birth. The mean thickness of these muscle layers using four mice for each group revealed 1.7-fold (130 to 219 μm; P = 0.04) and 1.5-fold (185 to 284 μm; P = 0.03) increases in the corpus and antrum, respectively, in LG21AG mice compared with GF mice. No evident changes in the number of nuclei in the same area of the muscle layer were found between the GF and LG21AG groups (38 versus 40/150 by 200 μm; P = 0.25). There was no evidence of any infiltration of inflammatory cells in the mucosa of these mice.
Fig. 2.
Histology of the stomach. The stomachs of GF mice (A, B, and C) and gnotobiotic mice associated with LG21 at birth (+LG21; D, E, and F) were excised at 8 weeks of age and then stained with hematoxylin and eosin for histological analysis. Panels A and D, B and E, and C and F show the corpus, the antrum, and the muscle layer of the stomach, respectively. Bars, 200 μm. Magnification, ×400 (C and F).
DISCUSSION
While there are usually few or no bacteria present in the stomachs of humans due to the presence of strong gastric acid, commensal microbiota were found to colonize the stomachs of mice in significant numbers and predominantly consisted of lactobacilli (108.5 CFU/g stomach tissue) (12). It was thought that such successful colonization in the stomachs of mice was possibly due to the low acidity in the stomach. In fact, the pH value of the gastric lumen in SPF mice was more than pH 4 in the present study. On the other hand, there is another possibility that could explain the high number of lactobacilli in the stomach. Lactobacilli can easily colonize and grow in the stomach because they can attach to the pregastric region of mice, which is a squamous epithelium (25). However, the present study showed that the number of lactobacilli attached to the pregastric region was far less than that on the corpus and antrum region. LG21 could colonize in large numbers in the stomachs of GF mice as well as the indigenous lactobacilli.
Using LG21AG mice, comprehensive gastric transcriptional responses to colonization were examined by DNA microarrays. The most prominent finding was a marked downregulation in the mRNA expression of gastrin. The resulting decrease in the level of gastrin hormone in the stomach was confirmed by immunohistochemistry. Because the number of cells positive for somatostatin, the main hormone that inhibits gastrin production (21), was not found to increase by immunohistochemistry, lactobacilli were thought to directly decrease the number of gastrin-producing cells. Moreover, even heat-killed lactobacilli could reduce the number of gastrin+ cells. These findings suggest that relatively heat-stable bacterial components, not bioactive substances generated in situ by lactobacilli, may cause the suppression of gastrin secretion. When heat-killed lactobacilli were used in the present study, a far larger amount of bacteria was required to obtain a decrease in the number of gastrin+ cells that was comparable to the decrease obtained by live lactobacilli. Thus, a structural change in the bacterial components induced by heat treatment may cause a reduction in their binding affinity to the putative receptors on gastric mucosal cells, which results in reduced ability to cause a decrease in the number of gastrin+ cells.
Nkx6.3 transcripts localize in the epithelium of the distal stomach region (1). Nkx6.3−/− mice show markedly fewer G cells in the antrum of the stomach, indicating a crucial role of Nkx6.3 in the selective regulation of G cell lineage differentiation (6). Ngn3 has also been reported to operate in parallel with Nkx6.3 (14). RNA expression of both Nkx6.3 and Ngn3 was significantly lower in the stomachs of LG21AG mice than in those of GF mice in the current study. These results suggested that the differentiation of progenitor G cells into gastrin-expressing mature cells was also suppressed in the LG21AG mice.
Gastrin is predominantly produced in the G cells of the gastric antrum and stimulates the acid-secreting parietal cells directly. Moreover, it also stimulates enterochromaffin-like (ECL) cells, which leads to the secretion of histamine (21). Histamine then stimulates the parietal cells to secrete gastric acid. In accordance with the significant decrease in the gastrin level, gastric acid secretion was significantly lower in lactobacillus-associated gnotobiotic mice than in GF mice. The downregulation of both the potassium voltage and chloride channels revealed by microarray assay may also affect the reduction of gastric acid in the present study because the extrusion of K+ and Cl− by these apical channels is coupled with proton secretion via H+K+-ATPase by the parietal cells (10). It is likely that the SPF mice also showed a reduction in acid production, because their stomachs were colonized by indigenous lactobacilli. Acute infection with H. pylori has been reported to be associated with hypochlorhydria (9, 17). The present study found that gnotobiotic mice inoculated with H. pylori 10 days prior to the analysis also showed a significant reduction in gastric acid secretion in the same manner as in LG21AG mice via downregulation in the expression of gastrin.
In the present microarray analysis using H. pylori-infected mice, the pattern of changes in gene expression was similar to that found in lactobacillus-associated mice, that is, downregulation of genes involved in gastric acid secretion and upregulation of the genes related to muscle cell development. It therefore seems contradictory that both Gram-positive lactobacilli and Gram-negative H. pylori exert such similar effects on the gastric mucosa. However, it is noteworthy that lipopolysaccharide (LPS), a strong bioactive component of Gram-negative bacteria, has been reported to be unique in H. pylori (22). In general, Toll-like receptor 2 (TLR2) and TLR4 recognize peptidoglycan of Gram-positive species and LPS from most Gram-negative species, respectively. However, TLR2, but not TLR4, is required for the H. pylori-induced stimulation of gastric epithelial cells (22). These results strongly suggest that H. pylori is not only a pathogenic but also a commensal organism that plays a physiological role in the functional development of the human stomach similar to that of lactobacilli in the stomachs of mice.
Investigations of nonhuman primates have shown that essentially all of them possess an indigenous gastric microbiota that closely resembles H. pylori species (4, 7). Studies of humans who have had little contact with modern civilization indicate that virtually all carry H. pylori, although the prevalence of H. pylori infection is now much lower in developed countries (18). These observations suggested that Helicobacter species might be considered part of the indigenous gastric microbiota of humans and our prehuman ancestors from the earliest times (2). Considering that the prevalence of H. pylori infection is now very low in developed countries, large numbers of people in these countries might be considered to have “germ-free” stomachs with hyperacidity and a poor muscle layer, although they are free from the pathogenicity of H. pylori. Probiotic Lactobacillus strains, such as LG21, might be able to replicate the beneficial effects of H. pylori, although precisely how the presence of lactobacilli in an infant's stomach is influenced by environmental factors, such as the mode of delivery and method of feeding, still needs to be elucidated.
Finally, in the present study, histological surveys have substantiated the upregulation of the genes related to muscle cells that were observed by microarray analysis. That is, a marked increase in the thickness of the gastric muscle layer was demonstrated in LG21AG mice. Such an increase in thickness was considered to be due to enlargement in the mass of muscle cells rather than hypertrophy of individual muscle cells, because the density of nuclei did not change in the layer. The muscle layer is responsible for gastric motility (8). Weak or abnormal motility of the stomach due to poor development of the muscle layer leads to impaired gastric emptying, as develops during functional dyspepsia and peptic ulcer diseases (23). Saito et al. (20) reported that the muscle layer of the stomach was significantly thickened in SPF mice infected with H. pylori. In our microarray analysis using the stomachs of H. pylori-associated gnotobiotic mice, the genes of the muscle cell component were also highly upregulated. The stomachs of H. pylori-infected mice exhibit inflammation, as well as disturbance in gastric emptying (15). Therefore, such abnormally thick muscle layers accompanied by inflammation may cause a failure in gastric emptying. On the other hand, the stomach colonized by commensal lactobacilli exhibited no pathological inflammation (24). The absence of inflammation in the gastric mucosa was also identified by histological analysis of LG21AG mice in the present study. Therefore, indigenous or probiotic lactobacilli in the stomach are expected to contribute to the physiological development of the muscle layer in order to achieve normal gastric emptying.
ACKNOWLEDGMENTS
This study was financially supported by the Research Fund of Tokai University School of Medicine. The funding source had no role in study design, data collection, or the writing of the report.
The authors report no conflicts of interest.
Footnotes
Published ahead of print on 29 July 2011.
REFERENCES
- 1. Alanentalo T., et al. 2006. Cloning and analysis of Nkx6.3 during CNS and gastrointestinal development. Gene Expr. Patterns 6:162–170 [DOI] [PubMed] [Google Scholar]
- 2. Blaser M. J. 1998. Helicobacters are indigenous to the human stomach: duodenal ulceration is due to changes in gastric microecology in the modern era. Gut 43:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Blaser M. J., Falkow S. 2009. What are the consequences of the disappearing human microbiota? Nat. Rev. Microbiol. 7:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bronsdon M. A., Goodwin C. S., Sly L. I., Chilvers T., Schoenknecht F. D. 1991. Helicobacter nemestrinae sp. nov., a spiral bacterium found in the stomach of a pigtailed macaque (Macaca nemestrina). Int. J. Syst. Bacteriol. 41:148–153 [DOI] [PubMed] [Google Scholar]
- 5. Byun R., et al. 2004. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J. Clin. Microbiol. 42:3128–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Choi M. Y., et al. 2008. Requirement of the tissue-restricted homeodomain transcription factor Nkx6.3 in differentiation of gastrin-producing G cells in the stomach antrum. Mol. Cell. Biol. 28:3208–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubois A., et al. 1994. Natural gastric infection with Helicobacter pylori in monkeys. A model for human infection with spiral bacteria. Gastroenterology 106:1405–1417 [DOI] [PubMed] [Google Scholar]
- 8. Eisenberg M. M., Fondacaro P. F., Dunn D. H. 1995. Applied anatomy and anomalies of the stomach, p. 561–581In Haubrich W. S., Schaffner F., Berk J. E. (ed.), Bockus gastroenterology, 5th ed., vol. 1 W. B. Saunders, Philadelphia, PA [Google Scholar]
- 9. Harford W. V., et al. 2000. Acute gastritis with hypochlorhydria: report of 35 cases with long-term follow-up. Gut 47:467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heitzmann D., Warth R. 2007. No potassium, no acid: K+ channels and gastric acid secretion. Physiology 22:335–341 [DOI] [PubMed] [Google Scholar]
- 11. Hooper L. V., et al. 2001. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291:881–884 [DOI] [PubMed] [Google Scholar]
- 12. Kabir A. M. A., Aiba Y., Takagi A., Kamiya S., Koga Y. 1997. Prevention of Helicobacter pylori infection by lactobacilli in a gnotobiotic murine model. Gut 41:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lambert J., Hull R. 1996. Upper gastrointestinal tract disease and probiotics. Asia Pacific J. Clin. Nutr. 5:31–35 [PubMed] [Google Scholar]
- 14. Lee C. S., Perreault N., Brestelli J. E., Kaestner K. H. 2002. Neurogenin 3 is essential for the proper specification of gastric enteroendocrine cells and maintenance of gastric epithelial cell identity. Genes Dev. 16:1488–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mearin F., et al. 1995. Does Helicobacter pylori infection increase gastric sensitivity in functional dyspepsia? Gut 37:47–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mikami K., et al. 2009. Influence of maternal bifidobacteria on the establishment of bifidobacteria colonizing the gut. Pediatr. Res. 65:669–674 [DOI] [PubMed] [Google Scholar]
- 17. Morris A., Nicholson G. 1987. Ingestion of Campylobacter pyloridis causes gastritis and raised fasting gastric pH. Am. J. Gastroenterol. 82:192–199 [PubMed] [Google Scholar]
- 18. Pounder R., Ng D. 1995. The prevalence of Helicobacter pylori infection in different countries. Aliment. Pharmacol. Ther. 9:S33–S39 [PubMed] [Google Scholar]
- 19. Ruddel W. S. J., Axon A. T. R., Findlay J. M., Bartholomew B. A., Hill M. J. 1980. Effect of cimetidine on the gastric bacterial flora. Lancet i:672–674 [PubMed] [Google Scholar]
- 20. Saito Y., et al. 2011. Dysfunctional gastric emptying with downregulation of muscle-specific microRNAs in Helicobacter pylori-infected mice. Gastroenterology 140:189–198 [DOI] [PubMed] [Google Scholar]
- 21. Schubert M. L., Peura D. A. 2008. Control of gastric acid secretion in health and disease. Gastroenterology 134:1842–1860 [DOI] [PubMed] [Google Scholar]
- 22. Smith M. F., Jr., et al. 2003. Toll-like receptor (TLR) 2 and TLR5, but not TLR4, are required for Helicobacter pylori-induced NF-κB activation and chemokine expression by epithelial cells. J. Biol. Chem. 278:32552–32560 [DOI] [PubMed] [Google Scholar]
- 23. Stanghellini V., et al. 1996. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology 110:1036–1042 [DOI] [PubMed] [Google Scholar]
- 24. Tamura A., et al. 2006. Suppression of Helicobacter pylori-induced interleukin-8 production in vitro and within the gastric mucosa by a live Lactobacillus strain. J. Gastroenterol. Hepatol. 21:1399–1406 [DOI] [PubMed] [Google Scholar]
- 25. Tannock G. W., Archibald R. D. 1984. The derivation and use of mice which do not harbor lactobacilli in the gastrointestinal tract. Can. J. Microbiol. 30:849–853 [DOI] [PubMed] [Google Scholar]
- 26. Walter J., et al. 2000. Detection and identification of gastrointestinal Lactobacillus species by using denaturing gradient gel electrophoresis and species-specific PCR primers. Appl. Environ. Microbiol. 66:297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zavros Y., Reider G., Ferguson A., Samuelson L. C., Merchant J. L. 2002. Genetic or chemical hypochlorhydria is associated with inflammation that modulates parietal and G-cell populations in mice. Gastroenterology 122:119–133 [DOI] [PubMed] [Google Scholar]