Abstract
Human plasmin(ogen) is regarded as a component of the molecular cross talk between the probiotic species Bifidobacterium animalis subsp. lactis and the human host. However, up to now, only in vitro studies have been reported. Here, we demonstrate that the probiotic strain B. animalis subsp. lactis BI07 is capable of recruiting plasmin(ogen) present at physiological concentrations in crude extracts from human feces. Our results provide evidence that supports the significance of the B. lactis-plasmin(ogen) interaction in the human gastrointestinal tract.
TEXT
Bifidobacterium animalis subsp. lactis is a Gram-positive lactic acid bacterium belonging to the Actinobacteria phylum. Originally isolated from fermented milk (13, 14, 22), B. animalis subsp. lactis inhabits the gut of healthy adults and infants (20, 24). Merging health-promoting activities with technological properties, B. animalis subsp. lactis is one of the most common probiotic bifidobacterial species utilized in commercial dairy products in North America and Europe (3). However, despite its broad usage as a probiotic, few molecules that mediate the B. animalis subsp. lactis-host interaction have been described. We previously reported the capability of this probiotic microorganism to bind human plasmin(ogen) (5, 6, 7). According to our data, the probiotic strain B. animalis subsp. lactis BI07 (21) possesses a dose-dependent binding activity to human plasminogen (Plg) in concentrations ranging from 5 to 100 ng/μl (7). Plg (92 kDa) is the zymogen of the serine protease plasmin which is involved in fibrinolysis, homeostasis, and degradation of basement membranes and the extracellular matrix (17). Although it is produced mainly by hepatocytes, other tissue sources of Plg have been identified, including the intestine (25). Plg activation in human tissues is a process tightly regulated by the balance between plasminogen activators (PA), such as the urokinase-type activator (uPA) and the tissue-type activator (tPA), and their specific inhibitors, PAI-1 and PAI-2.
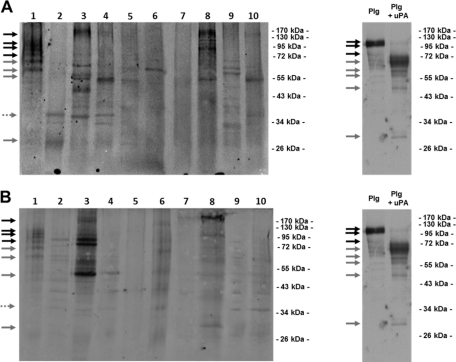
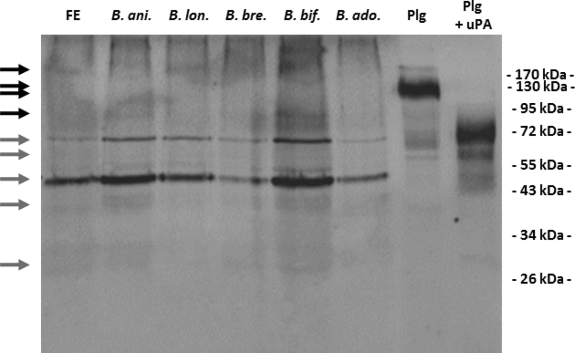
Plasmin captured on the bacterial cell surface has been reported to favor the bacterial dissemination in host tissues, and the capacity to intervene with the host Plg system has been traditionally regarded as a paradigm of bacterial pathogenicity (4, 12). However, Plg binding to commensal species of Lactobacillus, Bifidobacterium, and streptococci has been recently reported, raising a debate on the role of Plg binding in bacterial commensalism (2, 7, 11). In a previous in vitro study, we demonstrated that in the presence of Plg and host PA, B. animalis subsp. lactis acquires a surface-bound Plg-derived proteolytic activity effective in the degradation of physiological substrates (6). Thus, we hypothesized that the interaction with components of the host Plg system in the luminal compartment of the human gastrointestinal tract (GIT) would endow B. animalis subsp. lactis with a surface-associated plasmin activity useful to gain access to the mucus layer overlying enterocytes, the ideal habitat for commensal microorganisms (10, 19). However, even if the intervention with the host plasmin(ogen) system has been recently regarded as a component in the B. animalis subsp. lactis-host molecular cross talk (8, 18), plasmin(ogen) binding to B. animalis subsp. lactis has been studied only in phosphate-buffered saline (PBS) suspensions, and its relevance in the complex human gastrointestinal ecosystem remains an unanswered question. With the intent to shed light on this last issue, we evaluated the capability of the probiotic strain B. animalis subsp. lactis BI07 to recruit plasmin(ogen) present at physiological concentrations in human crude fecal extracts. Reflecting the entire content of the full length of the colon/rectum (1), fecal extracts represent the only possibility for a noninvasive sampling of the human GIT content. In our study, 10 healthy Italian individuals ≥30 years of age were enrolled. None of the subjects had a history of gastrointestinal disorders at the time of sampling. With the exception of antibiotics, probiotics, and functional foods, no dietary restriction was prescribed for at least 4 weeks prior to sampling. Informed consent was obtained from each enrolled subject. Fecal extracts were obtained by following the procedure reported by Ivanov et al. (9) with slight modifications. Briefly, 6 g of feces was resuspended in 10 ml of PBS with complete protease inhibitor (Roche). After the addition of 3-mm glass beads (BioSpec Products), samples were treated with FastPrep (MP Biomedicals) at 5.5 ms for 1 min 3 times. Debris and glass beads were removed by centrifugation at 12,000 rpm for 10 min. The protein concentration in supernatant was determined by NanoDrop (NanoDrop Technologies). The presence of human plasmin(ogen) in the crude fecal extracts was investigated by Western blotting. For each subject, 10 μg of fecal proteins was resolved by SDS-PAGE and Western blotted with polyclonal anti-human Plg IgG antibodies (Kordia) as reported by Candela et al. (7). As positive controls, Plg (Sigma) and Plg activated to plasmin by uPA (Calbiochem) were loaded. While the fecal extracts from subjects 1, 3, and 8 also showed bands attributable to Plg isoforms, all the fecal extracts analyzed, with the only exception being that of subject 7, showed bands attributable to plasmin heavy chain A and light chain B isoforms (Fig. 1A). These data confirm the presence of Plg and its derivatives in the healthy human GIT and strengthen previous data reporting the occurrence of plasma proteins in fecal extracts (1, 16). The capability of B. animalis subsp. lactis BI07 to recruit plasmin(ogen) and derivatives present at physiological concentrations in crude fecal extracts was next investigated. To this aim, stationary-phase B. animalis subsp. lactis BI07 cells (7) were resuspended at a concentration of 109 CFU/ml in 10 ml of crude fecal extracts from subjects 1 to 10. After 1 h of incubation at 37°C, bacterial cells were recovered by centrifugation and washed in 50 mM Tris-HCl (pH 7.6). In order to detect the Plg captured on the B. animalis subsp. lactis BI07 cell surface, the bacterial cell wall protein fractions were purified as previously described (7), resolved by SDS-PAGE, and Western blotted with anti-human Plg IgG antibodies (Kordia) as reported above. As negative controls, stationary-phase B. animalis subsp. lactis BI07 cells were incubated with PBS, and fecal proteins were incubated without bacterial cells. Interestingly, the cell wall fractions from B. animalis subsp. lactis BI07 cells incubated with the fecal extracts from subjects 1, 3, and 8 exhibited a Plg- and plasmin-related band pattern attributable to the one detected in the respective crude fecal fractions (Fig. 1B). As expected, negative controls did not show any Plg- and plasmin-related bands (data not shown). These data demonstrate that B. animalis subsp. lactis BI07 is capable of recruiting Plg and plasmin isoforms present at physiological concentrations in crude extracts of human feces. As far as we know, this represents the first experimental evidence of a direct interaction between a probiotic bifidobacterial strain and a human protein in a human microenvironment. The capacity to interact with plasmin(ogen) present in crude extract from human feces was investigated in 4 human Bifidobacterium strains: Bifidobacterium longum ATCC 15707, Bifidobacterium breve ATCC 15700, Bifidobacterium bifidum DSM20456, and Bifidobacterium adolescentis ATCC 15703 (13) (Fig. 2). The interaction experiments were carried out as reported above. Interestingly, all the bifidobacterial strains studied were capable of recruiting plasmin(ogen) present in human feces. B. animalis subsp. lactis BI07 and B. bifidum DSM20456 showed the highest efficiencies in plasmin(ogen) recruitment.
Fig. 1.
(A) Immunoblot analysis of crude fecal extracts from subjects 1 to 10 carried out by using polyclonal anti-Plg IgG antibodies. As positive controls, 5 μg (each) of Plg and Plg activated to plasmin by a preincubation with 0.06 kallikrein inhibitor units (KIU) uPA was loaded. Black arrows indicate protein bands attributable to plasminogen isoforms (∼90 to 100 kDa). Gray arrows indicate plasmin heavy chain A (∼65-kDa), plasmin heavy chain A short form (∼57-kDa), and plasmin light chain B (∼25-kDa) isoforms. Dotted arrow indicates angiostatin (∼38 kDa), which corresponds to the first four kringle domains of Plg isoforms. (B) Immunoblot analysis with polyclonal anti-Plg IgG antibodies of cell wall proteins purified from B. animalis subsp. lactis BI07 cells incubated with fecal extracts from subjects 1 to 10. Arrows indicate Plg and plasmin isoforms as reported above.
Fig. 2.
Ten to 9 CFU/ml (each) of B. animalis subsp. lactis BI07 (B. ani.), B. longum ATCC 15707 (B. lon.), B. breve ATCC 15700 (B. bre.), B. bifidum DSM20456 (B. bif.), and B. adolescentis ATCC 15703 (B. ado.) was incubated in parallel with crude extracts from human feces (FE). Bacterial cell wall proteins were purified, and plasmin(ogen) was detected by immunoblot analysis with polyclonal anti-Plg IgG antibodies. Black arrows indicate protein bands attributable to plasminogen isoforms (∼90 to 100 kDa). Gray arrows indicate plasmin heavy chain A (∼65-kDa), plasmin heavy chain A short form (∼57-kDa), and plasmin light chain B (∼25-kDa) isoforms.
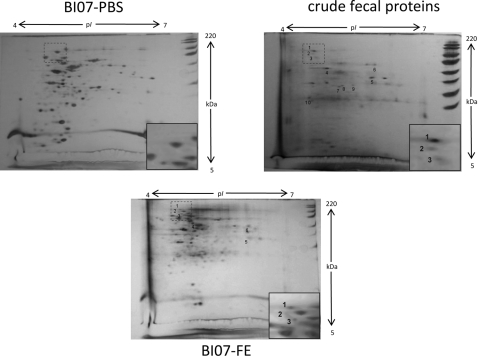
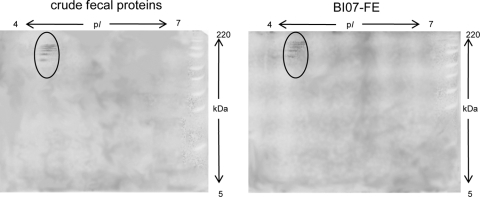
With the intent to investigate whether B. animalis subsp. lactis BI07 is capable of interacting with other human fecal proteins besides plasmin(ogen), stationary-phase B. animalis subsp. lactis BI07 cells were incubated with the fecal extract from subject 1 as previously described. Subsequently, the cell wall protein fractions from fecal extract-incubated B. animalis subsp. lactis BI07 cells (BI07-FE) and PBS-incubated B. animalis subsp. lactis BI07 cells (BI07-PBS) and the fecal proteins from subject 1 (FP) were analyzed by comparative proteomics as reported by Candela et al. (5) (Fig. 3). Six protein spots specific for the human fecal protein fraction were detected in the cell wall fraction from BI07-FE, demonstrating that B. animalis subsp. lactis BI07 is capable of recruiting different components of the human fecal proteome to its cell surface. A tentative matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based identification of spots 1 to 6 was performed as reported by Candela et al. (7) (Table 1). Confirming the data obtained by Western blotting, spots 1 and 3 were identified as human Plg and spot 2 as plasmin heavy chain A. These last data were also confirmed by two-dimensional Western blot analysis with anti-human Plg IgG antibodies (Kordia) (Fig. 4). Spot 4 was assigned to human keratin 8 and spot 6 to a hypothetical protein from Oryza sativa, whereas no identification was obtained for spot 5. Plant food-related proteins have already been identified as microbiota-associated proteins in the human fecal metaproteome (23). Our data provide the first experimental evidence of the B. animalis subsp. lactis plasmin(ogen) binding activity in the human GIT microenvironment. Allowing B. animalis subsp. lactis to acquire a host-derived surface-associated proteolytic activity, the interaction with components of the host Plg system can impact the dynamics of the B. lactis-host interaction process in the human GIT. Since gastrointestinal inflammatory processes involve imbalances of the host Plg system (15), it can be expected that the inflammation-dependent fluctuations of the components of the Plg system can modulate the biological outcome of the B. lactis-host interaction process. Studies in this direction should be carried out, leading to a more rational usage of this probiotic bifidobacterial species in inflammatory diseases.
Fig. 3.
Comparative proteomics of the cell wall fractions from BI07-PBS and BI07-FE and the crude fecal protein extract from subject 1. Numbered spots were analyzed by MALDI-TOF MS: protein spots 1 to 6 were specific for the crude fecal protein fraction and detected in the cell wall fraction from BI07-FE; protein spots 7 to 10 were detected only in the fecal protein fraction. The two-dimensional gel regions where plasminogen spots were identified are magnified in the insets.
Table 1.
Protein spot identification by MALDI-TOF MS
| Spot IDa | Swiss-Prot accession no. | Protein name/description | Source | Theoretical Mr/pIb | Experimental Mr/pIb | % sequence coverage |
|---|---|---|---|---|---|---|
| 1 | B2R7F8 | cDNA, FLJ93426, highly similar to Homo sapiens plasminogen (PLG), mRNA | Homo sapiens | 91/7.0 | 100/4.7 | 26 |
| 2 | P00747 | Plasmin heavy chain A | Homo sapiens | 63/6.8 | 90/4.7 | 25 |
| 3 | P00747 | Plasminogen | Homo sapiens | 88/7.1 | 80/4.7 | 25 |
| 4 | P05787 | Keratin, type II cytoskeletal 8 | Homo sapiens | 54/5.5 | 59/4.9 | 30 |
| 5 | Unidentified | 40/5.9 | ||||
| 6 | Q943E1 | Putative uncharacterized protein | Oryza sativa | 73/6.2 | 70/6.1 | 27 |
| 7 | Unidentified | 36/5.2 | ||||
| 8 | Unidentified | 37/5.3 | ||||
| 9 | Unidentified | 37/5.5 | ||||
| 10 | A1L302 | LOC283685 protein | Homo sapiens | 41/4.8 | 30/4.5 | 30 |
Spots 1 to 6 were specific for the human crude fecal protein fraction and detected in the cell wall fraction from BI07-FP; spots 7 to 10 were detected only in the fecal protein fraction.
Mr, molecular weight, in thousands.
Fig. 4.
Two-dimensional Western blot analysis of the crude fecal protein extract from subject 1 and the purified cell wall fraction from BI07-FE carried out with polyclonal anti-Plg IgG antibodies. Protein bands attributable to Plg and plasmin isoforms are circled.
Footnotes
Published ahead of print on 5 August 2011.
REFERENCES
- 1. Ang C. S., Rothacker J., Patsiouras H., Burgess A. W., Nice E. C. 2010. Murine fecal proteomics: a model system for the detection of potential biomarkers for colorectal cancer. J. Chromatogr. A 1217:3330–3340 [DOI] [PubMed] [Google Scholar]
- 2. Antikainen J., Kuparinen V., Lähteenmäki K., Korhonen T. K. 2007. Enolases from Gram-positive bacterial pathogens and commensal lactobacilli share functional similarity in virulence-associated traits. FEMS Immunol. Med. Microbiol. 51:526–534 [DOI] [PubMed] [Google Scholar]
- 3. Barrangou R., et al. 2009. Comparison of the complete genome sequences of Bifidobacterium animalis subsp. lactis DSM 10140 and Bl-04. J. Bacteriol. 191:4144–4151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bergmann S., Hammerschmidt S. 2007. Fibrinolysis and host response in bacterial infections. Thromb. Haemost. 98:512–520 [PubMed] [Google Scholar]
- 5. Candela M., et al. 2010. DnaK from Bifidobacterium animalis subsp. lactis is a surface-exposed human plasminogen receptor upregulated in response to bile salts. Microbiology 156:1609–1618 [DOI] [PubMed] [Google Scholar]
- 6. Candela M., et al. 2008. Plasminogen-dependent proteolytic activity in Bifidobacterium lactis. Microbiology 154:2457–2462 [DOI] [PubMed] [Google Scholar]
- 7. Candela M., et al. 2007. Binding of human plasminogen to Bifidobacterium. J. Bacteriol. 189:5929–5936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilad O., Svensson B., Viborg A. H., Stuer-Lauridsen B., Jacobsen S. 2011. The extracellular proteome of Bifidobacterium animalis subsp. lactis BB-12 reveals proteins with putative roles in probiotic effects. Proteomics 11:2503–2514 [DOI] [PubMed] [Google Scholar]
- 9. Ivanov D., et al. 2006. A serpin from the gut bacterium Bifidobacterium longum inhibits eukaryotic elastase-like serine proteases. J. Biol. Chem. 281:17246–17252 [DOI] [PubMed] [Google Scholar]
- 10. Johansson M. E., et al. 2008. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U. S. A. 105:15064–15069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kinnby B., Booth N. A., Svensäter G. 2008. Plasminogen binding by oral streptococci from dental plaque and inflammatory lesions. Microbiology 154:924–931 [DOI] [PubMed] [Google Scholar]
- 12. Lähteenmäki K., Edelman S., Korhonen T. K. 2005. Bacterial metastasis: the host plasminogen system in bacterial invasion. Trends Microbiol. 13:79–85 [DOI] [PubMed] [Google Scholar]
- 13. Lee J. H., O'Sullivan D. J. 2010. Genomic insights into bifidobacteria. Microbiol. Mol. Biol. Rev. 74:378–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Meile L., et al. 1997. Bifidobacterium lactis sp. nov., a moderately oxygen tolerant species isolated from fermented milk. Syst. Appl. Microbiol. 20:57–64 [Google Scholar]
- 15. Mondino A., Blasi F. 2004. uPA and uPAR in fibrinolysis, immunity and pathology. Trends Immunol. 25:450–455 [DOI] [PubMed] [Google Scholar]
- 16. Oleksiewicz M. B., Kjeldal H. O., Klenø T. G. 2005. Identification of stool proteins in C57BL/6J mice by two-dimensional gel electrophoresis and MALDI-TOF mass spectrometry. Biomarkers 10:29–40 [DOI] [PubMed] [Google Scholar]
- 17. Saksela O., Rifkin D. B. 1988. Cell-associated plasminogen activation: regulation and physiological functions. Annu. Rev. Cell Biol. 4:93–126 [DOI] [PubMed] [Google Scholar]
- 18. Sánchez B., Bressollier P., Urdaci M. C. 2008. Exported proteins in probiotic bacteria: adhesion to intestinal surfaces, host immunomodulation and molecular cross-talking with the host. FEMS Immunol. Med. Microbiol. 54:1–17 [DOI] [PubMed] [Google Scholar]
- 19. Sansonetti P. J. 2011. To be or not to be a pathogen: that is the mucosally relevant question. Mucosal Immunol. 4:8–14 [DOI] [PubMed] [Google Scholar]
- 20. Turroni F., et al. 2009. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl. Environ. Microbiol. 75:1534–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Turroni S., et al. 2010. Oxalate-degrading activity in Bifidobacterium animalis subsp. lactis: impact of acidic conditions on the transcriptional levels of the oxalyl coenzyme A (CoA) decarboxylase and formyl-CoA transferase genes. Appl. Environ. Microbiol. 76:5609–5620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ventura M., et al. 2007. From bacterial genome to functionality; case bifidobacteria. Int. J. Food Microbiol. 30:2–12 [DOI] [PubMed] [Google Scholar]
- 23. Verberkmoes N. C., et al. 2009. Shotgun metaproteomics of the human distal gut microbiota. ISME J. 3:179–189 [DOI] [PubMed] [Google Scholar]
- 24. Wall R., et al. 2008. Presence of two Lactobacillus and Bifidobacterium probiotic strains in the neonatal ileum. ISME J. 2:83–91 [DOI] [PubMed] [Google Scholar]
- 25. Zhang L., et al. 2002. Plasminogen has a broad extrahepatic distribution. Thromb. Haemost. 87:493–501 [PubMed] [Google Scholar]