Abstract
Enteral tube feeding is widely used to maintain nutrition for elderly adults with eating difficulties, but its long-term use alters the environment of the oral ecosystem. This study characterized the tongue microbiota of tube-fed elderly adults by analyzing the 16S rRNA gene. The terminal restriction fragment length polymorphism (T-RFLP) profiles of 44 tube-fed subjects were compared with those of 54 subjects fed orally (average age, 86.4 ± 6.9 years). Bar-coded pyrosequencing data were also obtained for a subset of the subjects from each group (15 tube-fed subjects and 16 subjects fed orally). The T-RFLP profiles demonstrated that the microbiota of the tube-fed subjects was distinct from that of the subjects fed orally (permutational multivariate analysis of variance [perMANOVA], P < 0.001). The pyrosequencing data revealed that 22 bacterial genera, including Corynebacterium, Peptostreptococcus, and Fusobacterium, were significantly more predominant in tube-fed subjects, whereas the dominant genera in the subjects fed orally, such as Streptococcus and Veillonella, were present in much lower proportions. Opportunistic pathogens rarely detected in the normal oral microbiota, such as Corynebacterium striatum and Streptococcus agalactiae, were often found in high proportions in tube-fed subjects. The oral indigenous microbiota is disrupted by the use of enteral feeding, allowing health-threatening bacteria to thrive.
INTRODUCTION
Enteral tube feeding is widely used to maintain nutrition in patients with a functional gastrointestinal tract but inadequate oral intake. It is frequently used to address eating problems in frail older adults, especially those with dementia. In the United States, approximately one-third of nursing home residents with advanced dementia are tube-fed (22). Nevertheless, tube feeding in the demented elderly remains controversial. Several studies have shown that tube feeding is associated with poor survival (1, 16, 23) and an increased risk of developing pneumonia (12, 15, 28).
The oral indigenous microbiota exists in a state of balance with the host (7), but the long-term use of tube feeding alters the intraoral conditions. The absence of food passage results in an absence of mechanical clearance within the mouth and reduces saliva secretion. The mucosal surfaces often dry out, and dried sputum adheres to the palate. These ecological changes should affect the bacterial population of the indigenous microbiota. Aspiration pneumonia is a major cause of death in tube-fed elderly patients, and it mainly involves oral contents (21). Unexpected bacteria may thrive in the disused oral cavity and threaten the lives of these patients.
Some well-known respiratory pathogenic bacteria, such as Pseudomonas aeruginosa, are isolated more frequently from the oropharynges of tube-fed older adults than from adults fed orally (17, 18, 30). However, the overall composition of the oral microbiota remains poorly understood due to its complexity. In addition, opportunistic bacteria could be critical etiologic agents in compromised elderly adults. Recently, we found that the global composition of the tongue microbiota is associated with the risk of pneumonia-related health problems in older adults by using terminal restriction fragment length polymorphism (T-RFLP) analysis of the 16S rRNA gene, which is a culture-independent community-fingerprinting approach (31). The current study examined the rough composition of the tongue microbiota of bedridden elderly adults by using T-RFLP and in a subset of the subjects in more detail by using bar-coded pyrosequencing. In this study, the microbiota of subjects fed enterally was compared comprehensively with that of subjects fed orally to characterize the oral indigenous microbiota of tube-fed elderly adults.
MATERIALS AND METHODS
Study population.
The subjects were a subgroup of the population analyzed in our previous study (31). Of the 11 hospitals or nursing homes in the previous study, we selected two hospitals and one nursing home that had sufficient numbers of both tube-fed and orally fed bedridden patients; this study enrolled 98 bedridden elderly residents aged 65 and over (12 men and 86 women; mean age, 86.4 ± 6.9 years). Forty-four subjects were fed with enteral tubes (31 with percutaneous endoscopic gastrostomy [PEG] tubes and 13 with nasogastric [NG] tubes), and 54 were fed orally. The ethics committee of the Kyushu University Faculty of Dental Science approved this study and the procedure for obtaining informed consent. The clinical condition of each subject was evaluated using previously described criteria (31).
Sample preparation.
Sample collection and DNA extraction from each sample were performed in our previous study (31). Tongue-coating samples were collected by scraping from the base to the tip of the tongue dorsum with a sterile plastic spatula (Muddler; Nihon Dixie, Yokohama, Japan), and DNA was extracted from each sample.
T-RFLP analysis.
All 98 samples were examined using T-RFLP analysis. From each sample, the internal regions of 16S rRNA genes were amplified using the universal forward primer 8F (5′-AGA GTT TGA TYM TGG CTC AG-3′) labeled at the 5′ end with 6-carboxyfluorescein (6-FAM) and the universal reverse primer 806R (5′-GGA CTA CCR GGG TAT CTA A-3′) labeled at the 5′ end with hexachlorofluorescein (HEX). PCR was performed using the KOD DNA polymerase (Toyobo, Osaka, Japan) and cycling conditions of 98°C for 2 min followed by 30 cycles of 98°C for 15 s, 60°C for 20 s, and 75°C for 30 s. The 16S rRNA gene amplicons were gel purified using a Wizard SV gel and PCR purification kit (Promega, Madison, WI). Digestion with the restriction enzyme HaeIII and electrophoresis were performed as described previously (31). The 98 T-RFLP profiles containing the electropherogram data were aligned using two different fluorescent dyes (6-FAM and HEX) per subject. The aligned T-RFLP profiles, excluding those terminal restriction fragments (TRFs) detected in fewer than 10% of the subjects, were subjected to principal-component analysis (PCA) and displayed as a PCA diagram. PCA was performed using R 2.10.0 (26). Candidate bacterial species corresponding to important TRFs were selected based on their sizes from 755 oral bacterial 16S rRNA gene sequences (HOMD 16S rRNA RefSeq version 10.1) deposited in the Human Oral Microbiome Database (9). The matching window was set to a molecular weight (MW) of ±660.
Bar-coded pyrosequencing analysis.
Pyrosequencing of the 16S rRNA gene was performed for 15 subjects fed by tube and 16 subjects fed orally; subjects were selected randomly from the 25 PEG tube-fed subjects and 42 subjects fed orally who had not used antibiotics in the preceding month. For each extracted DNA sample, we reamplified the 16S rRNA gene using 806R with the 454 Life Sciences adaptor B sequence (5′-CCT ATC CCC TGT GTG CCT TGG CAG TCT CAG-3′) and 8F with the 454 Life Sciences adaptor A and subject-specific 6-base bar code sequences (5′-CCA TCT CAT CCC TGC GTG TCT CCG ACT CAG NNN NNN-3′). The PCR amplification was performed under the same conditions as for the T-RFLP analysis. The proper size of amplicons was confirmed by agarose gel electrophoresis, and amplicons were gel purified using a Wizard SV Gel and PCR clean-up system (Promega) according to the manufacturer's instructions. The DNA concentration and quality were assessed using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE), and equal amounts of DNA from 31 subjects were pooled together. One microliter of the mixture was electrophoresed on an agarose gel to reconfirm the proper size of the amplicons. Pyrosequencing was carried out on a 454 Life Sciences genome sequencer FLX instrument (Roche, Basel, Switzerland) at the Dragon Genomics Center of Takara Bio (Yokkaichi, Japan) and was used to determine the 16S rRNA gene sequences containing the hypervariable regions V1 and V2.
Informatics analysis.
The pyrosequencing reads were processed according to the procedure described by Costello et al. (6), with some modification. Sequences were excluded from the analysis using a script written in PHP if they were shorter than 240 bases or had an average quality score of <25 and were subsequently removed using a script written in R if they did not include the correct primer sequence, had a homopolymer run of >6 nucleotides (nt), or contained three or more ambiguous characters. The remaining sequences were assigned to each subject by examining the six-base bar code sequence. Similar sequences were clustered into operational taxonomic units (OTUs) using the complete-linkage clustering tool of the RDP pyrosequencing pipeline (5) at a distance cutoff of 0.03, and representative sequences of each cluster were selected using the Dereplicate request function. The representative sequences from each OTU were aligned using PyNAST (4) and the Greengenes database (8) using a minimum percent identity of 75%. Chimeras were removed from the representative set on the basis of identification as chimeric via Chimera Slayer (13) and verification that the putative chimera appeared in only one sample. After chimera elimination, a relaxed neighbor-joining tree was built using FastTree (25). To determine the dissimilarity between any pair of bacterial communities, we used the UniFrac metric (20) calculated by Fast UniFrac (14). UniFrac distances are based on the fraction of branch length shared between two communities within a phylogenetic tree constructed from all communities being compared. The similarity relationship assessed using the unweighted UniFrac metric was represented in a principal-coordinate analysis (PCoA) plot drawn by R. The taxonomy of representative sequences was determined using the RDP classifier with a minimum support threshold of 60% and the RDP taxonomic nomenclature (down to the genus level). To detect the OTUs characteristically detected in the tube-fed subjects, we considered only OTUs containing at least 100 sequences. For each representative sequence of the OTUs detected in significantly higher proportions in the tube-fed group than in the orally fed group, the nearest-neighbor species with over 98% identity were first searched using BLAST against 755 oral bacterial 16S rRNA gene sequences (HOMD 16S rRNA RefSeq Version 10.1) deposited in the Human Oral Microbiome Database (9). Sequences with no hits were further compared against a local database comprising 81,679 nonchimeric 16S rRNA gene sequences of “ncbi_tax_string” not deposited as “environmental samples” in the Greengenes database (8).
Statistical analysis.
All statistical analyses were conducted with R. Fisher's exact test was conducted to look for differences by sex, institution, severity of dementia, coexisting conditions, denture use, amount of tongue coating, and tongue moisture. Student's t test was performed to compare age and the numbers of teeth and decayed teeth. Wilcoxon's signed-rank test was performed to compare the relative abundance of bacteria. Permutational multivariate analysis of variance (perMANOVA) with the function adonis in the vegan package was performed to test for differences in bacterial community structure among groups of samples. Statistical significance was set at a P value of <0.05.
RESULTS
Of the 98 bedridden elderly persons in this study, 44 were fed via enteral tubes (31 by PEG tubes and 13 by NG tubes), and 54 were fed orally. Table 1 summarizes the general and oral conditions of the subjects in each group. Although significantly more men and severely demented persons were included in the tube-fed group, no statistically significant differences were observed for the other general conditions. The amount of tongue coating was significantly greater in the tube-fed group than in the group fed orally. No denture users were included in the tube-fed group.
Table 1.
Baseline characteristics of bedridden elderly adults fed orally or by tube
| Characteristic | Valuea for: |
P value | |
|---|---|---|---|
| Tube-fed patients (n=44) | Orally fed patients (n=54) | ||
| General conditions | |||
| Age, yr | 85.1 ± 7.5 | 87.4 ± 6.3 | 0.10 |
| Female sex, no. (%) | 35 (79.5) | 51 (94.4) | 0.03 |
| Institution, no. (%) | 0.06 | ||
| Hospital A | 27 (61.3) | 40 (74.0) | |
| Hospital B | 14 (21.6) | 7 (12.9) | |
| Nursing home A | 3 (6.8) | 7 (12.9) | |
| Dementia, no. (%) | <0.001 | ||
| Mild | 5 (11.3) | 29 (53.7) | |
| Severe | 39 (88.6) | 25 (46.2) | |
| Coexisting conditions, no. (%) | |||
| Diabetes mellitus | 5 (11.3) | 5 (9.2) | 0.74 |
| Stroke | 38 (86.3) | 37 (68.5) | 0.05 |
| Cancer | 5 (11.3) | 9 (16.6) | 0.56 |
| Chronic gastroenteritis | 3 (6.8) | 4 (7.4) | 1.00 |
| Cardiovascular disease | 18 (40.9) | 24 (44.4) | 0.83 |
| Kidney disease | 2 (4.5) | 3 (5.5) | 1.00 |
| Liver disease | 6 (13.6) | 8 (14.8) | 1.00 |
| Oral conditions | |||
| No. of natural teeth | 8.4 ± 9.3 | 7.8 ± 7.6 | 0.70 |
| No. of decayed teeth | 1.7 ± 3.3 | 2.1 ± 3.6 | 0.53 |
| Denture use, no. (%) | 0 (0) | 16 (29.6) | <0.001 |
| Amt of tongue coating, no. (%) | 0.004 | ||
| No or slight | 15 (34.0) | 35 (64.8) | |
| Moderate or much | 29 (65.9) | 19 (35.1) | |
| Tongue moisture (mm) | 1.00 | ||
| ≥5.0 | 13 (29.5) | 13 (24.0) | |
| 1.0-4.9 | 14 (31.8) | 22 (40.7) | |
| <1.0 | 17 (38.6) | 19 (35.1) | |
Values with errors are means ± standard deviations.
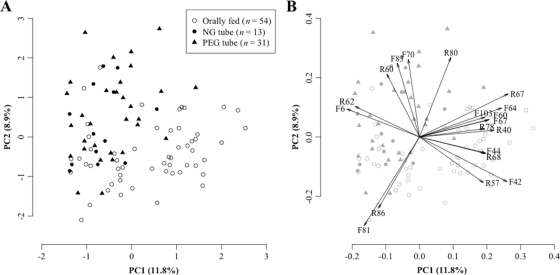
The tongue microbiota compositions of all 98 subjects were compared based on the T-RFLP profiles of the 16S rRNA gene. The overall profiles contained 235 distinct peaks (TRFs), 121 TRFs (F1 to F121) in the 6-FAM profiles and 114 TRFs (R1 to R114) in the HEX profiles. To visualize the similarity of T-RFLP profiles, they were plotted in a PCA diagram of the first principal component (PC1) and the second principal component (PC2) using different dots to represent each feeding mode (Fig. 1 A). These two components explained only 20.7% of the total variation. This low value represents the large diversity in the microbiota structures of bedridden elderly subjects. Their diverse T-RFLP patterns containing various uncommon TRFs might not be well explained by using only two virtual factors. The performance of two-factorial PCA to explain overall microbial community variability is limited. Nevertheless, the diagram of these two primary principal components showed that the T-RFLP profiles of both PEG tube-fed subjects and NG tube-fed subjects differed greatly from those of subjects fed orally. The differences between the two groups were confirmed statistically using perMANOVA (P < 0.001). No significant difference was observed between PEG and NG tube feeding.
Fig. 1.
(A) Principal-component analysis (PCA) diagram showing the similarity relationships among the 98 T-RFLP profiles. The T-RFLP profile of each subject is plotted according to feeding mode: orally (○), by percutaneous endoscopy gastrostomy (PEG) tube (▴), and by nasogastric (NG) tube (•). These two components explain 20.7% of the variance. (B) Loading plot of the first two principal components. Only 19 TRFs with large factor loading (>0.5 in absolute value) on the first or second principal component were selected, and these are indicated by arrows.
The loading plot of the first two principal components gave us some phylogenetic information on the microbiota of tube-fed subjects (Fig. 1B). TRFs with a large (>0.5 in absolute value) factor loading in the negative direction of PC1 were F6 and R62. Based on the fragment size, Corynebacterium or Propionibacterium species were selected from the oral bacterial database as candidate bacterial species corresponding to these TRFs (see Table S1 in the supplemental material). Conversely, 11 TRFs (R67, F42, F64, R40, F60, F67, R68, F44, F103, R78, and R57) had a large positive loading on PC1; they corresponded to other bacterial species, including Prevotella and Veillonella. Two TRFs with high loading in the negative direction of PC2 (F81 and R86) corresponded to Streptococcus or Bacillus species, while bacteria of the genus Fusobacterium and family Peptostreptococcaceae were assigned to four TRFs (R80, F70, F83, and R60) with large positive loading on PC2. Tube-fed subjects were localized in the negative direction of PC1 and the positive direction of PC2 (upper left area in the diagram), suggesting that their microbiotas contain lower proportions of common oral bacteria such as Streptococcus, Veillonella, and Prevotella and higher proportions of other bacterial species, including Corynebacterium and Fusobacterium, than those of orally fed subjects.
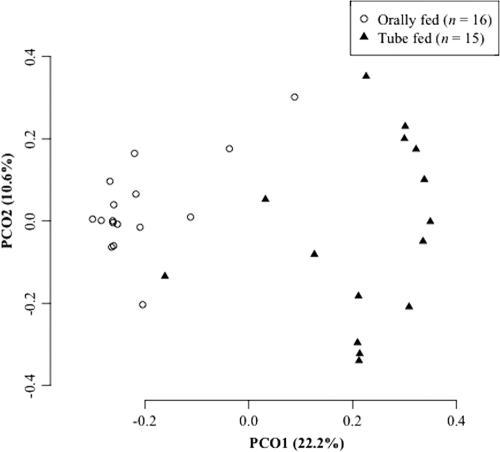
To obtain more-detailed phylogenetic information, bar-coded pyrosequencing analysis was performed for 15 PEG tube-fed and 16 orally fed subjects. We determined 131,888 sequences, and 103,391 bacterial 16S rRNA gene sequences with an average length of 358 ± 71 bases passed quality control (Table 2). The sequences were assigned to 3,118 species-level OTUs using a cutoff distance of 0.03. The PCoA plot based on UniFrac, which is a phylogeny-based metric (20), also revealed that the overall microbiota composition in the tube-fed subjects was distinct from that in those fed orally (Fig. 2).
Table 2.
Summary of pyrosequencing analysis
| Group (n) and subject | No. of reads |
|---|---|
| Tube fed (15) | |
| T1 | 2,446 |
| T2 | 4,376 |
| T3 | 898 |
| T4 | 6,139 |
| T5 | 3,098 |
| T6 | 2,454 |
| T7 | 4,839 |
| T8 | 1,898 |
| T9 | 7,061 |
| T10 | 1,707 |
| T11 | 1,717 |
| T12 | 3,689 |
| T13 | 1,579 |
| T14 | 954 |
| T15 | 4,987 |
| Avg ± SD | 3,189 ± 1,913 |
| Orally fed (16) | |
| O1 | 2,530 |
| O2 | 2,781 |
| O3 | 1,347 |
| O4 | 6,237 |
| O5 | 2,879 |
| O6 | 1,896 |
| O7 | 8,381 |
| O8 | 3,435 |
| O9 | 6,986 |
| O10 | 2,431 |
| O11 | 1,336 |
| O12 | 1,468 |
| O13 | 3,068 |
| O14 | 1,504 |
| O15 | 6,490 |
| O16 | 2,780 |
| Avg ± SD | 3,471 ± 2,252 |
Fig. 2.
Principal-coordinate analysis (PCoA) plot showing the similarity relations among the 31 tongue microbiota compositions. Plots were generated using unweighted UniFrac distances. These two components explain 32.8% of the variance.
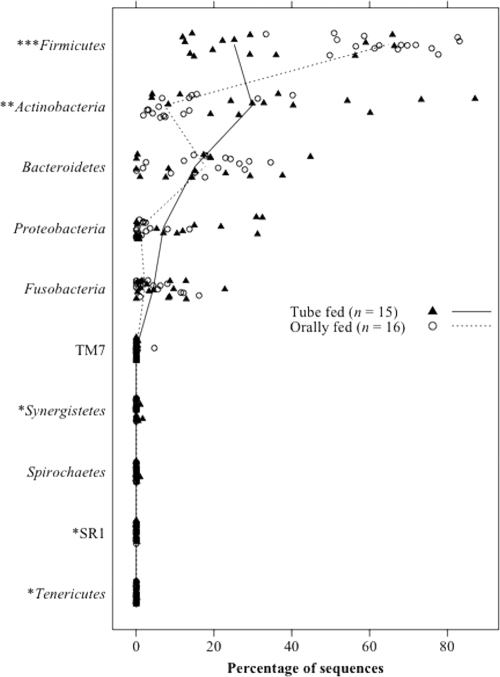
Although the microbiota diversity was confined largely to five phyla (Actinobacteria, Bacteroidetes, Fusobacteria, Firmicutes, and Proteobacteria) in both groups, the relative proportions of these phyla varied greatly between the two groups. The tube-fed subjects had a significantly higher proportion of Actinobacteria and a lower proportion of Firmicutes than those fed orally (Fig. 3). In addition, the relative abundances of three minor phyla, Synergistetes, Tenericutes, and SR1, were significantly greater in the tube-fed group.
Fig. 3.
Relative abundance of each phylum in the tongue microbiota of 31 subjects. The median percentages in the tube-fed and orally fed subjects are represented by solid and broken lines, respectively. P values were calculated using Wilcoxon's signed-rank test. *, P < 0.05; **, P < 0.01, ***, P < 0.001.
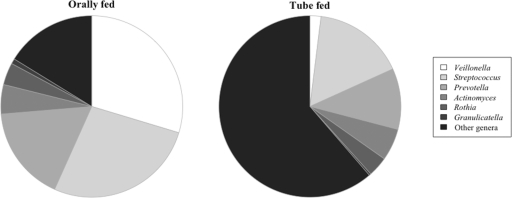
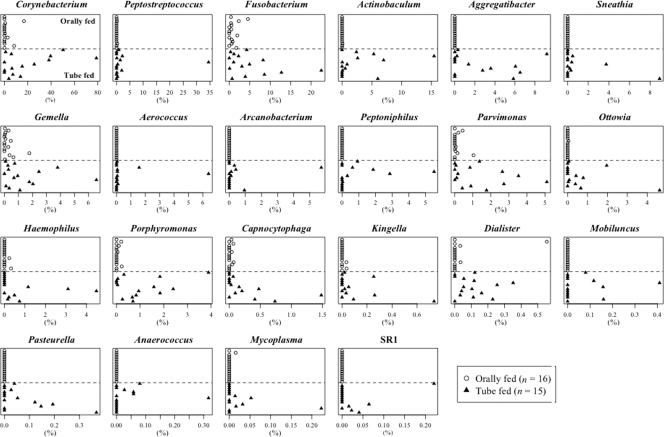
At the genus level, dominant bacterial genera commonly detected in the orally fed subjects, such as Veillonella and Streptococcus, were much less predominant in the tube-fed subjects (Fig. 4). Conversely, 22 minority bacterial genera in the usual oral cavity, including Corynebacterium, Peptostreptococcus, and Fusobacterium, accounted for markedly higher proportions in the microbiota of tube-fed subjects (Fig. 5). Seven bacteria unclassified at the genus level (family Flavobacteriaceae, family Neisseriaceae, family Pasteurellaceae, family Synergistaceae, family Incertae Sedis XI, order Bacteroidales, and phylum Bacteroidetes) were also more predominant in tube-fed subjects than in subjects fed orally. The genera Pseudomonas and Acinetobacter were detected only in the tube-fed group (one and three subjects, respectively). Staphylococcus was detected in four tube-fed subjects and one orally fed subject. Klebsiella was not detected in these subjects.
Fig. 4.
Mean genus abundances in orally fed and tube-fed subjects. Only six genera commonly detected in the orally fed group (14 of 15 orally fed subjects) are shown.
Fig. 5.
Relative abundances of 22 bacterial genera that were significantly more predominant in the tube-fed group than in the orally fed group (P < 0.05). Statistical differences were calculated using Wilcoxon's signed-rank test.
At the species level, defined as the 3% dissimilarity level, 54 OTUs in the tube-fed group were found in significantly higher proportions than in the orally fed group (see Table S2 in the supplemental material). Eight of these OTUs corresponded to bacterial species rarely detected in the oral cavity, such as Corynebacterium striatum, Streptococcus agalactiae, and Streptococcus dysgalactiae.
DISCUSSION
This study demonstrated that the oral microbiota of tube-fed older adults is distinct from that of those fed orally. Although the microbial composition varied among the subjects fed orally, the difference according to the feeding mode exceeded the interindividual differences (Fig. 1 and 2). Predominant indigenous members such as Streptococcus and Veillonella were detected in much lower proportions in the tube-fed subjects, whereas as many as 22 bacterial genera, including Corynebacterium, occurred in significantly higher proportions than in the orally fed subjects (Fig. 4 and 5). Bacterial species normally uncommon in the oral cavity, such as C. striatum, were also found in high proportions in their microbiota. This characteristic microbiota composition was observed in both the PEG and NG tube-fed subjects (Fig. 1), suggesting that it is not derived from biofilm formed on the feeding tube. We postulate that the normal balance of the microbiota is disrupted in the oral cavity when it is not used for eating. The long-term absence of food passage is an extremely abnormal situation for the oral indigenous microbiota. While fluid and carbohydrate supply is stopped, mechanical clearance by mastication drastically decreases. In addition to a reduction in the salivary flow, the biochemical composition of saliva in tube-fed subjects differs from that in orally fed ones (18). These ecological changes would be involved in the disruption of the indigenous microbiota.
No significant increase in typical respiratory pathogens, such as P. aeruginosa, was observed in this study, but the species that thrived in their microbiota could threaten the lives of frail elderly adults. The predominant Corynebacterium species, especially C. striatum, are potentially pathogenic bacteria with the ability to cause nosocomial outbreaks and respiratory colonization (24, 27). Anaerobic bacterial genera such as Fusobacterium, Peptostreptococcus, Parvimonas, and Porphyromonas are associated with pulmonary infections, such as pneumonia, lung abscesses, and empyema (2, 3, 11, 34). In contrast to the oral Streptococcus species, S. agalactiae accounted for high proportions in the microbiota in tube-fed patients, and these cause invasive disease in elderly adults (10). Although mealtime aspiration is averted by the use of a feeding tube, elderly adults fed by tube commonly aspirate contaminated oral secretions (12). Therefore, our results imply that tube-fed elderly adults continuously inhale unusual, more virulent bacteria into the lower respiratory tract and lungs. In addition, the disturbed balance of beneficial and detrimental bacteria in the indigenous microbiota, or dysbiosis, has recently attracted attention in regard to the development of mucosal inflammation, including Crohn's disease (19, 29, 32, 35). The oral dysbiosis that occurs with enteral tube feeding could be a health-threatening factor for frail elderly adults.
Although performing a randomized controlled trial would be difficult, the poor outcome of enteral feeding in elderly adults has been reported in several observational studies (1, 12, 15, 16, 23, 28). In addition, in our study, the incidence of pneumonia or fever and mortality in the following 6 months were significantly higher in the tube-fed subjects than in those fed orally, although this may have been due to differences in the baseline conditions of the two groups (data not shown). One should pay careful attention to the bacterial populations in the oral cavity with the use of feeding tubes. While the benefits of oral care in preventing pneumonia in elderly adults are well documented (33, 36), oral care is generally neglected in patients receiving tube feeding due to the erroneous impression that their oral cavities are not used. Rather, our results suggest that tube-fed patients need aggressive oral care to prevent the overgrowth of a disturbed microbiota, even if such care might be ineffective at restoring the normal microbiota.
This study was cross-sectional and thus cannot unequivocally demonstrate that feeding tube placement results in oral dysbiosis. A follow-up study would clarify the environmental trigger of the dysbiosis associated with tube feeding and may lead to the development of a novel approach to prevent the oral dysbiosis in tube feeding.
In the present study, we used two different molecular approaches for microbiota comparison, T-RFLP and bar-coded pyrosequencing. Although T-RFLP is highly effective for rapid comparisons of bacterial communities, it is unsuitable for predicting microbial community structures containing unexpected bacteria. Indeed, the high proportion of C. striatum in tube-fed subjects was unable to be predicted by T-RFLP because C. striatum is an uncommon bacterium in the oral cavity and thus is not deposited in the database which we used (the TRF size of C. striatum corresponded to F6 and R62). In addition, overgrowth of S. agalactiae in tube-fed subjects was masked by a decrease in the dominant oral Streptococcus species, such as S. salivarius, which generates TRFs with the same size (F81 and R86). Nevertheless, the microbiota characteristics of tube-fed subjects predicted from the T-RFLP data were globally consistent with the results of pyrosequencing analysis. Although some limitations exist, T-RFLP is useful for comparisons of oral microbiota, especially in analyses using a large number of samples.
The oral indigenous microbiota is thought to serve as a defensive barrier against the establishment of more pathogenic bacteria (7). Our results clearly demonstrated that the oral indigenous microbiota is disrupted by the use of enteral feeding, allowing health-threatening bacteria to thrive. It is suggested that oral food intake plays an important role not only in nutrition but also in maintenance of a healthy oral indigenous microbiota that acts to prevent exogenous infection.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported in part by Grants-in Aid for Young Scientists 23792517 (T.T.) and 22792121 (M.T.), by Grants-in Aid for Scientific Research 21592652 (Y.N.), 22406034 (Y.S.), and 23659986 (Y.Y.), and by a Strategic Research Base Development Program for Private Universities grant (Y.Y.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan as well as by the Sato Fund from the Nihon University School of Dentistry (Y.N.) and by the Uehara Memorial Foundation (Y.Y.).
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 5 August 2011.
REFERENCES
- 1. Abuksis G., et al. 2000. Percutaneous endoscopic gastrostomy: high mortality rates in hospitalized patients. Am. J. Gastroenterol. 95:128–132 [DOI] [PubMed] [Google Scholar]
- 2. Bartlett J. G. 1987. Anaerobic bacterial infections of the lung. Chest 91:901–909 [DOI] [PubMed] [Google Scholar]
- 3. Bartlett J. G. 2005. The role of anaerobic bacteria in lung abscess. Clin. Infect. Dis. 40:923–925 [DOI] [PubMed] [Google Scholar]
- 4. Caporaso J. G., et al. 2010. PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cole J. R., et al. 2009. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 37:D141–D145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costello E. K., et al. 2009. Bacterial community variation in human body habitats across space and time. Science 326:1694–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dahlen G. 2009. Bacterial infections of the oral mucosa. Periodontol. 2000 49:13–38 [DOI] [PubMed] [Google Scholar]
- 8. DeSantis T. Z., et al. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 72:5069–5072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dewhirst F. E., et al. 2010. The human oral microbiome. J. Bacteriol. 192:5002–5017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Edwards M. S., Baker C. J. 2005. Group B streptococcal infections in elderly adults. Clin. Infect. Dis. 41:839–847 [DOI] [PubMed] [Google Scholar]
- 11. El-Solh A. A., et al. 2003. Microbiology of severe aspiration pneumonia in institutionalized elderly. Am. J. Respir. Crit. Care Med. 167:1650–1654 [DOI] [PubMed] [Google Scholar]
- 12. Finucane T. E., Bynum J. P. 1996. Use of tube feeding to prevent aspiration pneumonia. Lancet 348:1421–1424 [DOI] [PubMed] [Google Scholar]
- 13. Haas B. J., et al. 2011. Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res. 21:494–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hamady M., Lozupone C., Knight R. 2010. Fast UniFrac: facilitating high-throughput phylogenetic analyses of microbial communities including analysis of pyrosequencing and PhyloChip data. ISME J. 4:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janssens J. P., Krause K. H. 2004. Pneumonia in the very old. Lancet Infect. Dis. 4:112–124 [DOI] [PubMed] [Google Scholar]
- 16. Kuo S., Rhodes R. L., Mitchell S. L., Mor V., Teno J. M. 2009. Natural history of feeding-tube use in nursing home residents with advanced dementia. J. Am. Med. Dir. Assoc. 10:264–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leibovitz A., Plotnikov G., Habot B., Rosenberg M., Segal R. 2003. Pathogenic colonization of oral flora in frail elderly patients fed by nasogastric tube or percutaneous enterogastric tube. J. Gerontol. A Biol. Sci. Med. Sci. 58:52–55 [DOI] [PubMed] [Google Scholar]
- 18. Leibovitz A., et al. 2003. Saliva secretion and oral flora in prolonged nasogastric tube-fed elderly patients. Isr. Med. Assoc. J. 5:329–332 [PubMed] [Google Scholar]
- 19. Ling Z., et al. 2010. Molecular analysis of the diversity of vaginal microbiota associated with bacterial vaginosis. BMC Genomics 11:488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lozupone C., Knight R. 2005. UniFrac: a new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71:8228–8235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marik P. E., Kaplan D. 2003. Aspiration pneumonia and dysphagia in the elderly. Chest 124:328–336 [DOI] [PubMed] [Google Scholar]
- 22. Mitchell S. L., Teno J. M., Roy J., Kabumoto G., Mor V. 2003. Clinical and organizational factors associated with feeding tube use among nursing home residents with advanced cognitive impairment. JAMA 290:73–80 [DOI] [PubMed] [Google Scholar]
- 23. Murphy L. M., Lipman T. O. 2003. Percutaneous endoscopic gastrostomy does not prolong survival in patients with dementia. Arch. Intern. Med. 163:1351–1353 [DOI] [PubMed] [Google Scholar]
- 24. Otsuka Y., et al. 2006. Emergence of multidrug-resistant Corynebacterium striatum as a nosocomial pathogen in long-term hospitalized patients with underlying diseases. Diagn. Microbiol. Infect. Dis. 54:109–114 [DOI] [PubMed] [Google Scholar]
- 25. Price M. N., Dehal P. S., Arkin A. P. 2009. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Mol. Biol. Evol. 26:1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. R Development Core Team. 2007. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria [Google Scholar]
- 27. Renom F., et al. 2007. Nosocomial outbreak of Corynebacterium striatum infection in patients with chronic obstructive pulmonary disease. J. Clin. Microbiol. 45:2064–2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rothan-Tondeur M., et al. 2003. Risk factors for nosocomial pneumonia in a geriatric hospital: a control-case one-center study. J. Am. Geriatr. Soc. 51:997–1001 [DOI] [PubMed] [Google Scholar]
- 29. Sartor R. B. 2008. Therapeutic correction of bacterial dysbiosis discovered by molecular techniques. Proc. Natl. Acad. Sci. U. S. A. 105:16413–16414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tada A., Hanada N., Tanzawa H. 2002. The relation between tube feeding and Pseudomonas aeruginosa detection in the oral cavity. J. Gerontol. A Biol. Sci. Med. Sci. 57:M71–M72 [DOI] [PubMed] [Google Scholar]
- 31. Takeshita T., et al. 2010. Microfloral characterization of the tongue coating and associated risk for pneumonia-related health problems in institutionalized older adults. J. Am. Geriatr. Soc. 58:1050–1057 [DOI] [PubMed] [Google Scholar]
- 32. Tamboli C. P., Neut C., Desreumaux P., Colombel J. F. 2004. Dysbiosis in inflammatory bowel disease. Gut 53:1–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Terpenning M., Shay K. 2002. Oral health is cost-effective to maintain but costly to ignore. J. Am. Geriatr. Soc. 50:584–585 [DOI] [PubMed] [Google Scholar]
- 34. Tsai T. H., Jerng J. S., Chen K. Y., Yu C. J., Yang P. C. 2005. Community-acquired thoracic empyema in older people. J. Am. Geriatr. Soc. 53:1203–1209 [DOI] [PubMed] [Google Scholar]
- 35. van Vliet M. J., Harmsen H. J., de Bont E. S., Tissing W. J. 2010. The role of intestinal microbiota in the development and severity of chemotherapy-induced mucositis. PLoS Pathog. 6:e1000879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yoneyama T., et al. 2002. Oral care reduces pneumonia in older patients in nursing homes. J. Am. Geriatr. Soc. 50:430–433 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.