Abstract
In humans, uropathogenic Escherichia coli (UPEC) is the most common etiological agent of uncomplicated urinary tract infections (UTIs). Cranberry extracts have been linked to the prevention of UTIs for over a century; however, a mechanistic understanding of the way in which cranberry derivatives prevent bacterial infection is still lacking. In this study, we used a fliC-lux reporter as well as quantitative reverse transcription-PCR to demonstrate that when UPEC strain CFT073 was grown or exposed to dehydrated, crushed cranberries or to purified cranberry-derived proanthocyanidins (cPACs), expression of the flagellin gene (fliC) was inhibited. In agreement with these results, transmission electron microscopy imaging of bacteria grown in the presence of cranberry materials revealed fewer flagella than those in bacteria grown under control conditions. Furthermore, we showed that swimming and swarming motilities were hindered when bacteria were grown in the presence of the cranberry compounds. Because flagellum-mediated motility has been suggested to enable UPEC to disseminate to the upper urinary tract, we propose that inhibition of flagellum-mediated motility might be a key mechanism by which cPACs prevent UTIs. This is the first report to show that cranberry compounds inhibit UPEC motility via downregulation of the fliC gene. Further studies are required to establish whether these inhibitors play a role in vivo.
INTRODUCTION
There are more than 150 million cases of urinary tract infection (UTI) in the world every year, with correspondingly significant morbidity and health care costs (12). Uncomplicated UTIs, i.e., infections that occur in healthy, immunocompetent individuals, are caused over 80% of the time by uropathogenic Escherichia coli (UPEC) (44). Among UPEC strains, E. coli CFT073, a strain isolated from the blood and urine of a woman diagnosed with acute pyelonephritis (34), is one of the most prevalent clonal lines (21, 22). The majority of UTIs develop in an ascending manner (1, 44) that commences when bacteria inoculate the periurethral area and then the bladder (20, 35, 46). The bacteria may then ascend to the upper urinary tract and kidneys and establish a secondary infection (44). Once in the kidneys, the bacteria can access the bloodstream, causing bacteremia and (potentially) death (44).
The bacterial flagellum is a molecular machine driven by a motor which rotates a long, curved filament (2). This filament extends from the basal body outward and is a polymer of flagellin subunits encoded by the fliC gene (6, 31). Mutations in fliC result in loss of flagellation and motility (31). Flagellum-mediated motility has been suggested to contribute to virulence by enabling UPEC to disseminate to the urinary tract, to escape host immune responses, and to disperse to new sites within the urinary tract (24). Even though this hypothesis remains to be demonstrated, several groups have shown that fliC mutants are outcompeted by motile, wild-type strains during experimental cochallenge of mice (24, 40, 45), thereby demonstrating that flagella provide a fitness advantage in the colonization of the urinary tract. Furthermore, flagellar motility has been shown to be essential for the pathogenesis of other bacteria, including Proteus mirabilis (5, 33), Salmonella species (39, 47), Helicobacter pylori (42), and enteropathogenic E. coli (8).
The standard management approach for uncomplicated UTIs is empirical therapy with antibiotics (17, 32); however, current rising antimicrobial resistance (9, 10) has resulted in increasing clinical failure rates (3, 26), emphasizing the need to develop alternate options for infection prevention and treatment. North American cranberries (Vaccinium macrocarpon) have long been considered to have protective properties against UTIs. Some of the first scientific research conducted on this topic concluded that acidification of the urine following the consumption of cranberries was responsible for the preclusion of UTIs (4); however, subsequent studies showed that the pH of urine is not altered significantly by the intake of cranberries (11, 16). Several studies have also demonstrated that cranberry products are nonbacteriostatic (15, 36). Current research supports the hypothesis that cranberries, and specifically the condensed tannins that they contain, known as proanthocyanidins (PACs), hinder bacterial attachment to abiotic surfaces (7, 28) and to uroepithelial and kidney cells (16, 29, 37, 43), therefore preventing infection. However, a mechanistic understanding of the way in which cranberries influence bacterial behavior is still lacking.
With the purpose of elucidating how bacterial gene expression is affected by cranberry PACs (cPACs), our laboratory previously examined the transcriptional profiles of E. coli CFT073 cultures grown in Luria-Bertani (LB) broth and in the presence or absence of cPACs (15). Among the genes that were found to be downregulated in the presence of cPACs was the flagellin gene (fliC) (15). Additional research has shown that in Pseudomonas aeruginosa, swarming motility is blocked by cPACs and other tannins (36). The main goal of this study was to further investigate if exposure to cranberry materials would result in downregulation of the fliC gene in E. coli CFT073 and whether this would impact this bacterium's motility.
MATERIALS AND METHODS
Bacterial strains and media.
The strains and plasmids used in this study are detailed in Table 1. E. coli strain CFT073 (ATCC 700928) was used as the test bacterium in this study. Cultures were grown in LB medium (10 g/liter tryptone, 10 g/liter NaCl, and 5 g/liter yeast extract) or M9 minimal medium, as indicated. M9 medium contained the following (per liter): 12.8 g Na2HPO4·7H2O, 3 g KH2PO4, 0.5 g NaCl, 1.0 g NH4Cl, 0.24 g MgSO4, 0.01 g CaCl2, and 4 g of glucose as the sole carbon source. The pH of all M9 growth media used in this study, whether supplemented or not with cranberry powder (CP) or cPACs, was adjusted to 7.1 with NaOH as required, using an Accumet AR20 pH meter. Planktonic bacterial cultures were incubated at 37°C with rotary shaking at 150 rpm unless otherwise indicated. Ampicillin and kanamycin were added as needed, to final concentrations of 100 μg/ml and 50 μg/ml, respectively. Dehydrated, crushed cranberry powder (Canneberges Atoka Cranberries, Quebec, Canada) and dry cPAC extract purified by high-performance liquid chromatography (HPLC) (Marucci Center for Blueberry and Cranberry Research, Rutgers University) were solubilized in distilled, deionized water and sterilized by filtration.
Table 1.
E. coli strains and plasmids used in this study
| Strain, plasmid, or primer | Relevant characteristics or sequence (5′-3′) | Source or reference |
|---|---|---|
| Strains | ||
| CFT073 | Wild-type pyelonephritis isolate | Laboratory collection |
| CFT073 ΔfliC | CFT073 fliC::aphA Kanr | 24 |
| Plasmid | ||
| PfliC-lux | Flagellin transcription reporter vector; Ampr | 24 |
| Primers | ||
| fliC F | ACAGCCTCTCGCTGATCACTCAAA | 24 |
| fliC R | GCGCTGTTAATACGCAAGCCAGAA | 24 |
| gapA F | AAGTTGGTGTTGACGTTGTCGCTG | 24 |
| gapA R | ATAACCACTTTCTTCGCACCAGCGG | 24 |
Growth curves.
Cultures of E. coli CFT073 were grown in the presence or absence of cPACs at 0.1 mg/ml and of CP at 1, 5, 10, 15 and 20 mg/ml. An overnight culture of CFT073 grown at 37°C with shaking at 200 rpm was diluted 1,000-fold with M9 medium. This cell suspension, containing approximately 3 × 106 cells/ml, was aliquoted into sterile 96-well plates and incubated at 37°C until stationary phase was reached (∼12 h). The optical density at 600 nm (OD600) was recorded at intervals, using a Tecan Infinite M200 Pro instrument (Tecan Group Ltd., Männedorf, Switzerland). Each condition was set up in quadruplicate.
fliC downregulation.
An overnight culture of E. coli strain CFT073 PfliC-lux (24), grown as described above, was diluted 1,000-fold. Aliquots of the cell suspension (3 × 106 cells/ml) were mixed with CP at 0, 1, 5, 10, 15, and 20 mg/ml or with cPACs at 0.1 mg/ml, and the cultures were incubated at 37°C in a 96-well white polystyrene plate with a clear bottom. Luminescence and OD600 values were measured periodically, using a Tecan Infinite M200 Pro instrument (Tecan Group Ltd., Männedorf, Switzerland), for 12 h. Expression of the flagellar gene, fliC, was quantified by measuring the luminescence and normalizing it to cell concentration, which was calculated by subtracting the initial OD600 reading from the OD600 at each time point [expression of fliC = luminescence/(OD600 − initial OD600)]. This was done to account for the variability in cell numbers as well as for the changes in OD600 generated by the addition of CP or cPACs.
The response of the fliC-lux reporter was also tested by growing the bacteria to mid-exponential phase (OD600 − initial OD600 ≈ 0.25) in M9 medium, harvesting the cells, and spiking them with cPACs or CP at the concentrations described above. Luminescence and optical density were recorded for the following 5 h, and fliC expression was quantified as described above.
RNA extraction, cDNA synthesis, and comparative qPCR.
A culture of E. coli CFT073 that was grown overnight was diluted 1,000-fold in M9 medium. Aliquots of this suspension (approximately 3 × 106 cells/ml) were mixed with CP at 0, 5, 10, and 20 mg/ml or with cPACs at 0.1 mg/ml and incubated at 37°C with agitation to mid-exponential growth phase (OD600 − initial OD600 ≈ 0.25). Total RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. After elution, nucleic acid concentrations and quality were determined by spectrophotometry using an Eppendorf BioPhotometer Plus instrument (Eppendorf, Hamburg, Germany). Expression of target genes (fliC and the housekeeping gene gapA) was quantified using two-step quantitative reverse transcription-PCR (qRT-PCR) analysis. Total RNA (200 ng) was mixed with 0.25 ng of random hexamers (Invitrogen, Carlsbad, CA) and reverse transcribed with 200 U of Superscript reverse transcriptase II (Invitrogen, Carlsbad, CA) and RNasin (Promega). The equivalent of 2 ng of total RNA was loaded into each well with TaqMan universal PCR master mix (Applied Biosystems), and qRT-PCR was performed with an ABI Prism 7900 HT thermal cycler (Applied Biosystems). Conditions for qRT-PCR were as follows: 50°C for 2 min, initial denaturation at 95°C for 10 min, and 45 cycles of 15 s at 95°C and 1 min at 60°C. Results were analyzed with SDS software, version 2.2 (Applied Biosystems). Data were normalized to the endogenous reference gene gapA and analyzed by the threshold cycle method (2−ΔΔCT) (30). Three independent isolated cDNA samples were analyzed. Sequences of the primers used to amplify gapA and fliC are detailed in Table 1.
Electron microscopy.
For transmission electron microscopy, bacteria from the outer motility rings of 0.5% agar LB plates supplemented with 0.5% glucose were cultured in LB, with and without CP at 10 mg/ml, in a rotary shaker set at 200 rpm and 30°C for 16 h. Next, 5 μl of bacterial suspension and 5 μl of 2.5% glutaraldehyde were placed on carbon-coated copper grids (SPI Supplies, West Chester, PA) for 2 min. Bacteria were stained with 1% uranyl acetate for 1 min. The grids were examined with an FEI Tecnai 12 transmission electron microscope at an operating voltage of 120 kV. Digital images were captured with a Gatan Bioscan model 792 charge-coupled device (CCD) camera.
Motility.
Swimming and swarming motilities were evaluated using soft-agar plates. For the swimming assays, 0.25% agar M9 plates with CP added at concentrations ranging from 1 to 20 mg/ml or cPACs added at 0.1 mg/ml were allowed to dry at room temperature overnight before use. Swimming plates were seeded with overnight cultures of E. coli CFT073 by use of a sterile inoculating needle. Control plates without CP or cPACs were also set up. Swarming motility was assessed in 0.5% Eiken agar (Eiken Chemical, Tokyo, Japan) LB plates supplemented with 0.5% glucose. CP or cPACs were also added at the concentrations described above. An overnight culture of E. coli CFT073 was diluted 1,000-fold and incubated until early stationary phase (OD600 − initial OD600 ≈ 0.5). At that point, 5 μl of the culture was spotted onto the surface of a plate. Swimming and swarming plates were incubated at 30°C and 37°C, respectively, and motility was recorded at various time points.
RESULTS AND DISCUSSION
A whole-transcriptome analysis of UPEC CFT073 grown in LB in the presence or absence of 0.1 mg/ml cPACs was conducted previously by our lab (15). The study revealed that in the culture grown with cPACs, the flagellin gene (fliC) was downregulated 2.4-fold (15). Given that several works have demonstrated that flagella play an important role during ascending UTIs (24, 40, 45), we decided to further investigate the downregulation of fliC during bacterial growth in the presence of cranberry materials.
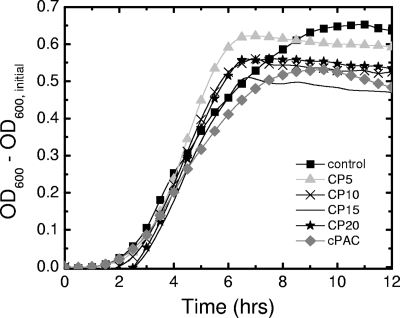
Our prior work showed that concentrations of cPACs in LB as high as 1.6 mg/ml did not inhibit the growth of CFT073 (15). However, LB is an undefined medium that is rich in essential elements (38). Thus, to test whether cPACs or CP added to M9 medium, a defined, minimal medium with no added trace metals, would hinder bacterial growth, CFT073 was grown in this medium with cPACs or CP at various concentrations. As shown in Fig. 1, bacterial growth rates and yields were not altered significantly by the introduction of either cPACs or CP at any of the working concentrations. This is an important result, because conditions that are adverse to growth, such as high temperature, high concentrations of salts or certain alcohols, or the presence of gyrase inhibitors, have been reported to inhibit motility (27, 41). Based on these results, we concluded that the differences in gene expression and phenotype that were observed in the bacterial cultures exposed to cranberry compounds did not result from toxic effects of these compounds.
Fig. 1.
Growth curve for E. coli CFT073 grown in the presence of cranberry compounds. The graph shows the OD600 − initial OD600 versus time for cultures of E. coli CFT073 grown in M9 medium (▪) or in M9 medium supplemented with cPACs at 0.1 mg/ml (♦) or with CP at 5 (▴), 10 (×), 15 (solid line), or 20 (★) mg/ml. Results are representative of at least three independent experiments. Abbreviations: CPx, cranberry powder at x mg/ml (e.g., CP5 indicates cranberry powder at 5 mg/ml); cPAC, cranberry proanthocyanidins at 0.1 mg/ml.
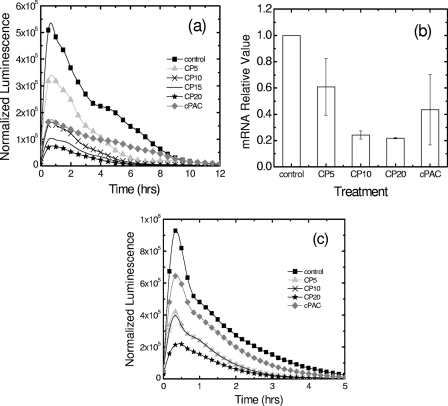
The effect of cPACs or CP on the regulation of fliC transcription was first assayed using a luminescent fliC reporter (24). A culture of CFT073 harboring the PfliC-lux plasmid was grown in M9 medium supplemented with cPACs at 0.1 mg/ml or with CP at various concentrations (Fig. 2a). Our results show that the normalized luminescence decreased with increasing CP concentrations. Also, the normalized luminescence was lower when the bacteria were grown with cPACs than when they were grown in M9 medium alone. Because a decrease in the transcription of fliC from the fliC-lux reporter correlates with a decrease in the luminescent signal, our data support the hypothesis that growth in the presence of CP or purified cPACs results in a reduction of fliC expression. These results were further validated by conducting RT-PCR to determine the relative quantities of fliC transcripts in the mRNAs extracted from UPEC cultures grown in the presence and absence of cPACs or CP (Fig. 2b). Relative mRNA expression of fliC was normalized to that of a stable housekeeping gene, gapA. The RT-PCR confirmed that in the presence of 0.1 mg/ml of cPACs, fliC was downregulated. Also, the flagellin gene was observed to be downregulated significantly when the bacteria were grown in M9 medium supplemented with CP at the concentrations tested (5, 10, and 20 mg/ml).
Fig. 2.
Downregulation of fliC expression by cranberry materials. (a) Cultures of CFT073 PfliC-lux were grown in the presence (♦) or absence (▪) of 0.1 mg/ml cPACs or with CP at 5 (▴), 10 (×), 15 (solid line), or 20 (★) mg/ml. Expression of the fliC gene was calculated as follows: normalized luminescence = [luminescence/(OD600 − initial OD600)]. Three independent experiments were performed, and the results of a typical experiment are presented. (b) Expression of the fliC gene assessed on the mRNA level by quantitative reverse transcription-PCR. Relative mRNA expression of fliC was first normalized to that of a housekeeping gene, gapA, and then related to the normalized expression level of the same gene in the control treatment. Results represent mean values ± standard deviations (SD) for three independent experiments. (c) fliC expression versus time for CFT073 PfliC-lux cultures harvested at mid-log phase and spiked with sterile water (▪), 0.1 mg/ml cPACs (♦), or CP at 5 (▴), 10 (×), 15 (solid line), or 20 (★) mg/ml. Similar results were obtained in at least three other experiments.
To further assess the conditions under which cranberry compounds may reduce fliC transcription, CFT073 PfliC-lux bacteria were grown in M9 medium, harvested, and then spiked with CP or cPACs. Luminescence and OD600 values were measured as described above, and the normalized luminescence was calculated (Fig. 2c). The results obtained show that relative to the control value, a spike with cPACs (0.1 mg/ml) or with 5 mg/ml or more of CP reduce the normalized luminescence. Based on these data, we concluded that growth with CP or cPACs is not necessary to achieve a reduction in the level of expression of the flagellin gene.
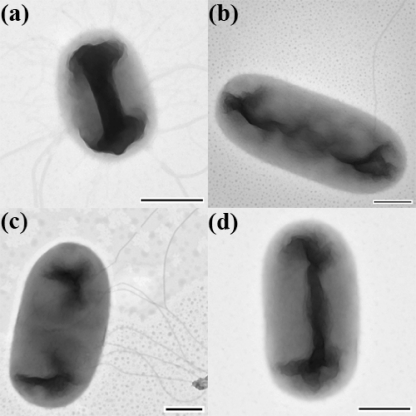
Additional evidence that growth of UPEC in media supplemented with cranberry compounds precludes flagellar synthesis was obtained by using electron microscopy to image bacteria cultivated under conditions that optimize motility, as described by Lane et al. (25). Abundant flagella were observed in bacteria that were grown in the control medium (Fig. 3a and c); however, for the samples obtained from bacteria grown with CP, few or no flagella were imaged (Fig. 3b and d). This result agrees with our expectations, because if exposure to cranberry materials results in a decrease in the transcription of the flagellin gene, fewer flagellar filaments should be synthesized by the bacteria in the presence of those materials.
Fig. 3.
Electron microscopic images of CFT073 cells. Bacteria were cultured in LB broth alone (a and c) or supplemented with 10 mg/ml CP (b and d). Magnification, ×43,700. Bars, 500 nm.
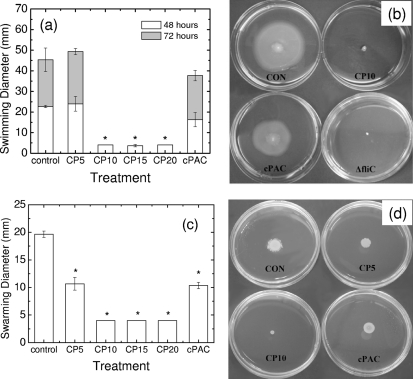
Next, we set out to evaluate whether the downregulation of fliC that results from growth or exposure to cranberry compounds impairs bacterial motility. Swimming motility is a type of bacterial movement that is powered by rotating flagella and that takes place as individual cells move in liquid environments (23). Swarming is also a type of motility that is powered by rotating helical flagella; however, it differs from swimming in that it requires an increase in the number of flagella per cell, as well as the secretion of surfactants to reduce surface tension and allow spreading, and in that the movement occurs in a coordinated manner across a surface (23). Because flagella are essential for both swimming and swarming, the effect of cranberry compounds on both of these motility phenotypes was tested. Figure 4a shows that concentrations of CP of >10 mg/ml completely blocked swimming motility. Moreover, the figure illustrates that relative to the control level, 0.1 mg/ml of cPACs also resulted in a decrease in motility. Figure 4b shows representative images of swimming motility plates containing CFT073 in the absence and presence of CP (10 mg/ml) or cPACs (0.1 mg/ml). An image of CFT073 ΔfliC grown under control conditions was included to illustrate that CP blocked the swimming motility in a manner similar to that seen with fliC gene deletion.
Fig. 4.
Effects of cranberry materials on motility. (a) Characterization of the effects of CP or cPACs on swimming motility in soft agar plates. *, P < 0.001 by one-way analysis of variance for comparison with the control. (b) Representative images of swimming motility plates for the control (CON), CP at 10 mg/ml (CP10), and cPACs at 0.1 mg/ml (cPAC). An image of the result obtained with a fliC mutant is included. (c) Swarming motility of CFT073 cells. Error bars represent the SD for triplicate samples. (d) Representative images of swarming motility plates for the control, CP at 5 and 10 mg/ml, and cPACs at 0.1 mg/ml.
Evaluation of swarming motility was performed with LB plates instead of M9 plates because attempts at evaluating swarming motility in M9 medium were not successful (data not shown). This is not surprising given that swarming motility has been shown to require a glucose-supplemented, energy-rich medium (13, 18) and that swarming cell differentiation has been shown to be repressed in some standard laboratory media (18, 23). The swarming motility tests revealed that the swarming of UPEC CFT073 was impaired by cPACs and by CP, in a dose-dependent manner, as depicted in Fig. 4c. It is noteworthy that the inhibitory effects on swarming motility of both CP and cPACs were more pronounced than those on swimming motility, i.e., CP at 5 mg/ml was enough to significantly reduce swarming motility, but a higher concentration (10 mg/ml) was required for significant reduction of swimming motility. Swarmer cells are hyperflagellated, but one flagellum is sufficient for swimming motility (13, 14, 23). It is possible that the decrease in expression of fliC upon exposure to CP still allows for the synthesis of enough flagellar filaments to enable bacteria to swim but makes swarming prohibitive.
All of our experiments were conducted with HPLC-purified cranberry PACs but also with dried, crushed cranberries. The rationale for using both sources of cPACs was to test whether highly purified cPACs would elicit the same response as whole cranberries. As our data show, similar results were achieved with regard to fliC expression and motility regardless of whether cPACs or CP was utilized. These results highlight the role that consumption of whole, unpurified cranberries might have in the prevention and treatment of UTIs.
Cranberry ingestion has long been associated with the prevention of UTIs; however, a mechanistic understanding of the way in which the consumption of cranberries results in UTI prevention is achieved remains elusive. Recent research has focused on the inhibitory effect that PACs from cranberries, blueberries, and other Vaccinium species have on the adherence of E. coli to epithelial cells and surfaces (7, 29, 37, 43). The anti-adhesive properties of cranberries have been attributed to inhibition of p-fimbria expression (19, 28, 37). However, a relationship between cranberry compounds and the downregulation of the flagellin gene has not been reported.
In this study, we demonstrate that growth with or exposure to cranberry PACs or powder results in the downregulation of the flagellin gene (fliC). Moreover, we show that the decrease in the level of fliC expression precludes the synthesis of flagella, which then results in hindered swimming and swarming motility. The cause of the downregulation of fliC transcription and the accompanying decrease in motility as a result of the exposure to cranberry compounds remains to be determined. There is compelling evidence that UPEC strains express flagellin and utilize flagellum-mediated motility to ascend to the upper urinary tract during UTI (24, 40, 45). Therefore, we hypothesize that the prevention of UTIs that can result from the consumption of cranberries is linked with a decreased expression of fliC. Before this hypothesis is tested, additional research needs to be conducted to determine whether fliC is downregulated by cranberry compounds in vivo.
ACKNOWLEDGMENTS
We acknowledge the financial support of the Natural Sciences and Engineering Research Council of Canada, the Canada Research Chairs Program, the Wisconsin Cranberry Board, and the Cranberry Institute.
We are grateful to T. Quinn, D. Rosenzweig, and J. Mui (McGill University) for technical assistance. We thank A. Howell (Rutgers University) for providing cranberry PACs, Canneberges Atoka Cranberries for providing cranberry powder, and H. Mobley (University of Michigan) for the PfliC-lux plasmid and the ΔfliC strain.
Footnotes
Published ahead of print on 5 August 2011.
REFERENCES
- 1. Bacheller C. D., Bernstein J. M. 1997. Urinary tract infections. Med. Clin. North Am. 81:719–730 [DOI] [PubMed] [Google Scholar]
- 2. Berg H. C. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19–54 [DOI] [PubMed] [Google Scholar]
- 3. Bjerrum L., Dessau R. B., Hallas J. 2002. Treatment failures after antibiotic therapy of uncomplicated urinary tract infections. A prescription database study. Scand. J. Prim. Health Care 20:97–101 [PubMed] [Google Scholar]
- 4. Blatherwick N. R. 1914. The specific role of foods in relation to the composition of the urine. Arch. Intern. Med. XIV:409–450 [Google Scholar]
- 5. Burall L. S., et al. 2004. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect. Immun. 72:2922–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chilcott G. S., Hughes K. T. 2000. Coupling of flagellar gene expression to flagellar assembly in Salmonella enterica serovar Typhimurium and Escherichia coli. Microbiol. Mol. Biol. Rev. 64:694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eydelnant I. A., Tufenkji N. 2008. Cranberry derived proanthocyanidins reduce bacterial adhesion to selected biomaterials. Langmuir 24:10273–10281 [DOI] [PubMed] [Google Scholar]
- 8. Giron J. A., Torres A. G., Freer E., Kaper J. B. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361–379 [DOI] [PubMed] [Google Scholar]
- 9. Gupta K. 2002. Addressing antibiotic resistance. Am. J. Med. 113:29–34 [DOI] [PubMed] [Google Scholar]
- 10. Gupta K., Scholes D., Stamm W. E. 1999. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA 281:736–738 [DOI] [PubMed] [Google Scholar]
- 11. Habash M. B., Van der Mei H. C., Busscher H. J., Reid G. 1999. The effect of water, ascorbic acid, and cranberry derived supplementation on human urine and uropathogen adhesion to silicone rubber. Can. J. Microbiol. 45:691–694 [DOI] [PubMed] [Google Scholar]
- 12. Harding G. K. M., Ronald A. R. 1994. The management of urinary infections; what have we learned in the past decade? Int. J. Antimicrob. Agents 4:83–88 [DOI] [PubMed] [Google Scholar]
- 13. Harshey R. M. 1994. Dimorphic transition in Escherichia coli and Salmonella typhimurium: surface-induced differentiation into hyperflagellate swarmer cells. Proc. Natl. Acad. Sci. 91:8631–8635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Henrichsen J. 1972. Bacterial surface translocation: a survey and a classification. Bacteriol. Rev. 36:478–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hidalgo G., et al. 2011. Induction of a state of iron limitation in uropathogenic Escherichia coli CFT073 by cranberry-derived proanthocyanidins as revealed by microarray analysis. Appl. Environ. Microbiol. 77:1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howell A. B., Vorsa N., Der Marderosian A., Foo L. Y. 1998. Inhibition of the adherence of P-fimbriated Escherichia coli to uroepithelial-cell surfaces by proanthocyanidin extracts from cranberries. N. Engl. J. Med. 339:1085–1086 [DOI] [PubMed] [Google Scholar]
- 17. Hummers-Pradier E., Kochen M. M. 2002. Urinary tract infections in adult general practice patients. Br. J. Gen. Pract. 52:752–761 [PMC free article] [PubMed] [Google Scholar]
- 18. Inoue T., et al. 2007. Genome-wide screening of genes required for swarming motility in Escherichia coli K-12. J. Bacteriol. 189:950–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson B. J., et al. 2008. Impact of cranberry on Escherichia coli cellular surface characteristics. Biochem. Biophys. Res. Commun. 377:992–994 [DOI] [PubMed] [Google Scholar]
- 20. Johnson D. E., et al. 1998. Comparison of Escherichia coli strains recovered from human cystitis and pyelonephritis infections in transurethrally challenged mice. Infect. Immun. 66:3059–3065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Johnson J. R., Stell A. L. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261–272 [DOI] [PubMed] [Google Scholar]
- 22. Johnson J. R., et al. 2001. Phylogenetic and pathotypic similarities between Escherichia coli isolates from urinary tract infections in dogs and extraintestinal infections in humans. J. Infect. Dis. 183:897–906 [DOI] [PubMed] [Google Scholar]
- 23. Kearns D. B. 2010. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 8:634–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lane M. C., Alteri C. J., Smith S. N., Mobley H. L. T. 2007. Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract. Proc. Natl. Acad. Sci. U. S. A. 104:16669–16674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lane M., Simms A. N., Mobley H. L. T. 2007. Complex interplay between type 1 fimbrial expression and flagellum-mediated motility of uropathogenic Escherichia coli. J. Bacteriol. 189:5523–5533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lawrenson R. A., Logie J. W. 2001. Antibiotic failure in the treatment of urinary tract infections in young women. J. Antimicrob. Chemother. 48:895–901 [DOI] [PubMed] [Google Scholar]
- 27. Li C., Louise C. J., Shi W., Adler J. 1993. Adverse conditions which cause lack of flagella in Escherichia coli. J. Bacteriol. 175:2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y., Black M. A., Caron L., Camesano T. A. 2006. Role of cranberry juice on molecular-scale surface characteristics and adhesion behavior of Escherichia coli. Biotechnol. Bioeng. 93:297–305 [DOI] [PubMed] [Google Scholar]
- 29. Liu Y., et al. 2008. Cranberry changes the physicochemical surface properties of E. coli and adhesion with uroepithelial cells. Colloids Surf. B Biointerfaces 65:35–42 [DOI] [PubMed] [Google Scholar]
- 30. Livak K. J., Schmittgen T. D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31. Macnab R. M. 1992. Genetics and biogenesis of bacterial flagella. Annu. Rev. Genet. 26:131–158 [DOI] [PubMed] [Google Scholar]
- 32. Michael M., Hodson E., Craig J., Martin S., Moyer V. 2002. Short compared with standard duration of antibiotic treatment for urinary tract infection: a systematic review of randomised controlled trials. Arch. Dis. Child. 87:118–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mobley H. L., et al. 1996. Construction of a flagellum-negative mutant of Proteus mirabilis: effect on internalization by human renal epithelial cells and virulence in a mouse model of ascending urinary tract infection. Infect. Immun. 64:5332–5340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mobley H. L., et al. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mulvey M. A., et al. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 282:1494–1497 [DOI] [PubMed] [Google Scholar]
- 36. O'May C., Tufenkji N. 2011. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl. Environ. Microbiol. 77:3061–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinzón-Arango P. A., Liu Y., Camesano T. A. 2009. Role of cranberry on bacterial adhesion forces and implications for Escherichia coli-uroepithelial cell attachment. J. Med. Food 12:259–270 [DOI] [PubMed] [Google Scholar]
- 38. Salomón R. A., Farías R. N. 1994. Influence of iron on microcin 25 production. FEMS Microbiol. Lett. 121:275–279 [DOI] [PubMed] [Google Scholar]
- 39. Schmitt C. K., et al. 2001. Absence of all components of the flagellar export and synthesis machinery differentially alters virulence of Salmonella enterica serovar Typhimurium in models of typhoid fever, survival in macrophages, tissue culture invasiveness, and calf enterocolitis. Infect. Immun. 69:5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schwan W. R. 2008. Flagella allow uropathogenic Escherichia coli ascension into murine kidneys. Int. J. Med. Microbiol. 298:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shi W., Li C., Louise C. J., Adler J. 1993. Mechanism of adverse conditions causing lack of flagella in Escherichia coli. J. Bacteriol. 175:2236–2240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Terry K., Williams S. M., Connolly L., Ottemann K. M. 2005. Chemotaxis plays multiple roles during Helicobacter pylori animal infection. Infect. Immun. 73:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tufenkji N., Rifai O. J., Harmidy K., Eydelnant I. A. 2010. Cranberry derived proanthocyanidins can prevent pathogen invasion of kidney epithelial cells. Food Res. Int. 43:922–924 [Google Scholar]
- 44. Warren J. W. 1996. Clinical presentation and epidemiology of urinary tract infections, p. 3–27 In Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, DC [Google Scholar]
- 45. Wright K. J., Seed P. C., Hultgren S. J. 2005. Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization. Infect. Immun. 73:7657–7668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yamamoto S., et al. 1997. Genetic evidence supporting the fecal-perineal-urethral hypothesis in cystitis caused by Escherichia coli. J. Urol. 157:1127–1129 [PubMed] [Google Scholar]
- 47. Zeng H., et al. 2003. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J. Immunol. 171:3668–3674 [DOI] [PubMed] [Google Scholar]