Abstract
Experiments were conducted to examine the effects of cocultivating the important bioenergy crop switchgrass with the ectomycorrhizal fungus Sebacina vermifera under severe drought conditions. Plants cocultivated with the fungus produced significantly higher biomass and had a higher macronutrient content than uninoculated control plants under both adequately watered and drought conditions.
TEXT
Drought is a predominant factor limiting plant growth and yield in both dry land and irrigated agriculture (6, 27). Lack of soil water has a wide range of effects on morphological and biochemical processes in plants, including nutrient uptake from the soil, negatively impacting crop productivity (2). Unfortunately, the influence of drought on agriculture is expected to worsen in the future due to climate change (8) and increasing demands for water for municipal and residential consumption (9). Thus, various strategies are being developed to maximize water use efficiency and minimize the effects of drought on agriculture (3, 12, 14, 18, 23, 26, 29). However, utilization of naturally occurring symbiotic microbes to enhance drought tolerance of agricultural crops has remained largely unexplored.
Most plant species in natural ecosystems are in symbiotic relationships with mycorrhizal and/or endophytic fungi (21). Members of the newly defined basidiomycete order Sebacinales naturally form a wide spectrum of mycorrhizal types of relationships (31) with the roots of various mono- and dicotyledonous plants (4, 11, 15, 28, 30). Two species in particular, Sebacina vermifera [Serendipita vermifera (Oberw.) P. Roberts, comb. nov] and its close relative Piriformospora indica, have stimulated considerable attention over the past several years, because they form endophytic and mycorrhiza-like associations with most plant species studied to date (30, 32). This is of great interest, because both species are axenically cultivable, possess plant growth-promoting characteristics, and contribute several other benefits to their host plants (4, 11, 15, 28, 30). Two previous studies have shown that colonization of roots by P. indica confers drought tolerance in Arabidopsis thaliana and Chinese cabbage (24, 25). However, no similar studies have been performed to evaluate the potential of S. vermifera to impart drought tolerance to host plants. Our objective here was to investigate the effect of S. vermifera in mitigating biomass losses in switchgrass due to drought, with the ultimate goal of maximizing the utility of this important bioenergy crop and the range of lands upon which it can be grown.
An in vitro study was performed using 175-ml plant containers (65 mm in diameter by 65 mm in height) with lids. The containers were filled with 25 ml of modified PNM culture medium (24) and overlaid with a nylon disk (mesh pore size, 50 μm). Two strains of S. vermifera, MAFF-305828 and MAFF-305830, were used in the study. One 5-mm-diameter plug of fungal hyphae from a colony actively growing on malt extract agar (MEA) was placed at the center of the nylon disk and allowed to grow for 2 weeks, and a plug of similar size from an uninoculated MEA plate was used as a control. Subsequently, four germinated Alamo seeds with no visible contamination by fungi or bacteria were placed on each nylon disk at a distance of 15 mm from the plug. Containers were incubated at 24°C with a light-dark cycle of 16 h and 8 h (light illumination of 165 μ mol m−2 s−1) for 6 weeks. The lids were then replaced with air-pore filters to allow evaporative and transpiration water loss at ambient temperature. On the 7th day of drought exposure, leaf color, plant stature, and total fresh weight were recorded. The fibrous roots were stained (16) and examined under a microscope for fungal colonization. Each treatment consisted of 10 to 13 experimental units (containers), and the experiment was performed twice.
In a subsequent greenhouse experiment, 6-week-old rooted explants of switchgrass genotype VS16 were grown in 3.8-liter pots filled with a 4:1 (vol/vol) mixture of sterile Metromix-350 medium (Scotts-Sierra Horticultural Products, Marysville, OH) and S. vermifera (strain MAFF-305828)-colonized sorghum grains (Fig. 1). Control seedlings were grown in a mixture prepared with sterile sorghum grains. Plants were maintained at 24°C and 18°C in a light-dark cycle of 16 h and 8 h (light illumination of 165 μ mol m−2 s−1) for 5 weeks. Individual seedlings from both treatments were subsequently planted in 1-liter pots filled with a 3:1 (vol/vol) mixture of Metromix-350 medium and sand and grown in the greenhouse with an average temperature and average relative humidity of 20.2°C and 39.3%, respectively.
Fig. 1.
(a) Sorghum grains colonized by Sebacina vermifera. (b) Uninoculated control.
All plants were watered to saturation at planting. Thereafter, half of the plants from the cocultivation and control treatments were subjected to drought whereas the remaining plants were watered normally. Experimental pots were checked individually for soil volumetric water content (VWC) on alternate days using a Field Scout TDR 100 soil moisture probe (Spectrum Technologies, Plainfield, IL). Plants receiving drought treatments were allowed to grow until the VWC dropped to ≤1%, at which point subsets of the plants subjected to drought and of the adequately watered plants were harvested. Plants with normal watering were maintained at a constant 10 to 20% VWC but were harvested at the same time points as the plants subjected to drought. These water levels were derived empirically in a previous experiment in which switchgrass seedlings at 10 to 20% VWC grew normally, seedlings at 5% VWC showed initial wilt symptoms, and plants at ≤1% VWC wilted permanently when grown for 2 days.
Experimental plants were arranged in a factorial randomized-block design. Each treatment was applied to 48 individually potted plants. One third of the experimental plants were harvested after the first drought stress (i.e., when the average VWC in the corresponding drought treatment pots reached 1%). The remaining plants were subjected to a second drought stress treatment, and half of those were subjected to a subsequent third drought stress treatment. All experimental plants were rewatered to saturation prior to subsequent drought stress treatments. Data on tiller number, shoot length, root length, shoot dry matter (DM), and root DM were recorded in each harvest. Leaf chlorophyll content was measured using a SPAD-502 Plus chlorophyll meter (Konica Minolta Sensing Americas Inc., Ramsey, NJ). The shoot and root tissues were analyzed for nitrogen (N), phosphorous (P), potassium (K) calcium (Ca), magnesium (Mg), and sulfur (S) content at Ward Laboratories, Inc. (Kearney, NE). Data on biomass and biomass-related parameters were analyzed using PROC GLM in SAS statistical software package version 9.1.3 (22), and least-significant-difference (LSD) tests were performed to compare treatments at ≥95% confidence levels.
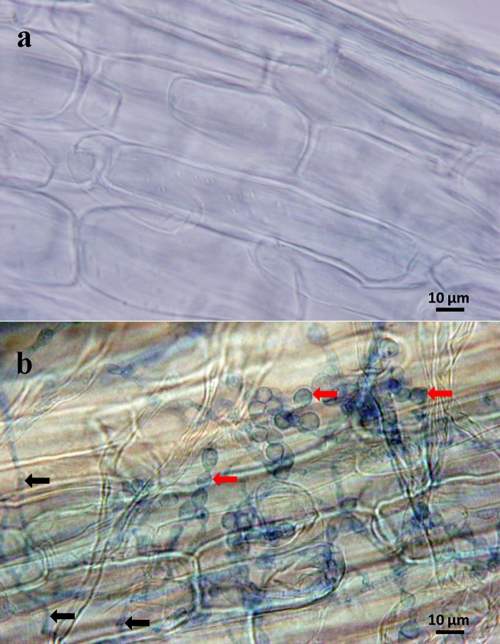
Roots of cocultivated plants from the in vitro study were effectively colonized by S. vermifera (Fig. 2). After a weeklong exposure to ambient temperature, the appearance of cocultivated plants was barely affected whereas control seedlings were pale green and withered (Fig. 3). Plants cocultivated with MAFF-305828 and MAFF-305830 produced 71% and 53% higher levels of fresh biomass, respectively, than control plants (Table 1; P < 0.01). The absence of fungus-mediated water and nutrient uptake might be the reason for the poor performance of control seedlings. Similar effects on the drought tolerance of Arabidopsis thaliana seedlings inoculated with P. indica were observed previously, with up to a 300% increase in fresh biomass production (24).
Fig. 2.
Switchgrass roots. (a) Uninoculated control. (b) Roots of cocultivated switchgrass seedling. Aniline blue-stained mycelia on the root surface are indicated with black arrows. Sebacina vermifera chlamydospores in the epidermal cells are indicated with red arrows.
Fig. 3.
Switchgrass seedlings after exposure to mild drought stress. Cocultivated seedlings with Sebacina vermifera strain MAFF 305830 are shown on the left, with S. vermifera strain MAFF 305828 shown in the middle and controls shown on the right.
Table 1.
Effect of Sebacina vermifera and switchgrass cocultivation on switchgrass biomass production under drought stress in vitro conditionsa
| Treatment | No. of seedlings per container after: |
Fresh wt (mg/container) (mean ± LSD) | |
|---|---|---|---|
| Planting | Drought exposure (mean ± LSD) | ||
| Expt 1 | |||
| Cocultivation | |||
| Strain MAFF 305828 | 4 | 3.46 ± 0.26 A | 148.62 ± 13.45 A |
| Strain MAFF 305830 | 4 | 3.69 ± 0.26 A | 143.08 ± 13.45 A |
| Control | 4 | 3.54 ± 0.26 A | 97.46 ± 13.45 B |
| Significance for treatment results | n.s. | ** | |
| Expt 2 | |||
| Cocultivation | |||
| Strain MAFF 305828 | 4 | 3.80 ± 0.20 A | 122.59 ± 12.20 A |
| Strain MAFF 305830 | 4 | 3.60 ± 0.20 A | 96.48 ± 12.20 B |
| Control | 4 | 3.10 ± 0.20 B | 58.11 ± 12.20 C |
| Significance for treatment results | ** | ** | |
| Expt 1 and 2 | |||
| Cocultivation | |||
| Strain MAFF 305828 | 4 | 3.61 ± 0.17 A | 137.30 ± 10.65 A |
| Strain MAFF 305830 | 4 | 3.65 ± 0.17 A | 122.82 ± 10.65 A |
| Control | 4 | 3.35 ± 0.17 A | 80.35 ± 10.65 B |
| Significance for treatment results | n.s. | ** | |
**, P ≤ 0.01; n.s., not significant. Values with different letters within the drought exposure and fresh weight columns for a given experiment represent significantly different results at a ≥99% confidence level.
In the greenhouse study, treatment results differed significantly with respect to shoot length, shoot DM, root DM, and shoot-to-root DM ratio (Table 2). Shoot lengths increased by 109%, 59%, and 95% and shoot biomass increased by 337%, 215%, and 267%, respectively, at the first, second, and third harvests in response to inoculation under conditions of adequate watering. Intriguingly, cocultivated plants exposed to drought produced significantly taller plants with a higher shoot DM level than well-watered control plants. Under conditions of adequate watering, cocultivated plants consistently produced higher levels (290%, 270%, and 166%, respectively, at first, second, and third harvest) of root biomass than the controls. Further, when subjected to one, two, or three drought cycles, cocultivated plants produced 303%, 127%, and 112% higher root biomass levels, respectively, than the controls. Further, as was evident for shoot tissues, cocultivated plants exposed to one, two, or three drought stress treatments produced, respectively, 353%, 131%, and 148% higher root biomass levels than corresponding controls that had been adequately watered (Table 2; P ≤ 0.01). Thus, plants colonized by S. vermifera exhibited simultaneous increases in both shoot and root biomass levels, indicating that above-ground biomass gains were not simply a consequence of reallocated carbohydrate. Indeed, cocultivated plants consistently produced higher root biomass levels than control plants, suggesting a greater potential to sequester carbon and hold soils, both highly desired properties in a crop grown under low-input conditions.
Table 2.
Effect of Sebacina vermifera colonization on switchgrass biomass production under conditions of adequate watering and drought in greenhousea
| Treatment | Shoot length (cm) (mean ± SE) | Root length (cm) (mean ± SE) | Shoot DM (mg/plant) (mean ± SE) | Root DM (mg/plant) (mean ± SE) | Shoot-to-root ratio (mean ± SE) | No. of tillers (mean ± SE) | Chlorophyll content (%) (mean ± SE) |
|---|---|---|---|---|---|---|---|
| First harvest (one drought cycle) | |||||||
| Cocultivation | |||||||
| Watered | 70.5 ± 1.1 | 24.1 ± 0.4 | 752 ± 29 | 316 ± 16 | 2.48 ± 0.06 | 2.38 ± 0.06 | 31.0 ± 0.3 |
| Dry | 56.9 ± 1.0 | 30.5 ± 0.9 | 500 ± 23 | 367 ± 16 | 1.40 ± 0.03 | 2.06 ± 0.03 | 30.9 ± 0.3 |
| Control | |||||||
| Watered | 33.8 ± 0.6 | 20.7 ± 0.5 | 172 ± 9 | 81 ± 4 | 2.16 ± 0.07 | 2.38 ± 0.06 | 29.7 ± 0.3 |
| Dry | 28.5 ± 0.7 | 20.3 ± 0.4 | 105 ± 6 | 91 ± 5 | 1.23 ± 0.07 | 1.88 ± 0.10 | 31.7 ± 0.3 |
| Significance tests | |||||||
| Cocultivation | ** | ** | ** | ** | * | n.s. | n.s. |
| Drought | ** | * | ** | n.s. | ** | ** | n.s. |
| Interaction | * | ** | * | n.s. | n.s. | n.s. | n.s. |
| Second harvest (two drought cycles) | |||||||
| Cocultivation | |||||||
| Watered | 73.6 ± 1.0 | 36.2 ± 0.6 | 1,600 ± 53 | 1,236 ± 80 | 1.46 ± 0.05 | 2.56 ± 0.06 | 28.6 ± 0.3 |
| Dry | 61.8 ± 1.0 | 36.7 ± 0.8 | 900 ± 20 | 771 ± 22 | 1.20 ± 0.03 | 2.69 ± 0.06 | 32.2 ± 0.3 |
| Control | |||||||
| Watered | 46.2 ± 0.8 | 37.9 ± 0.7 | 508 ± 17 | 334 ± 11 | 1.56 ± 0.05 | 2.63 ± 0.06 | 31.1 ± 0.3 |
| Dry | 36.5 ± 0.7 | 41.8 ± 0.6 | 388 ± 14 | 339 ± 11 | 1.18 ± 0.04 | 2.50 ± 0.07 | 34.2 ± 0.5 |
| Significance tests | |||||||
| Cocultivation | ** | * | ** | ** | n.s. | n.s. | ** |
| Drought | ** | n.s. | ** | ** | ** | n.s. | ** |
| Interaction | n.s. | n.s. | ** | ** | n.s. | n.s. | n.s. |
| Third harvest (three drought cycles) | |||||||
| Cocultivation | |||||||
| Watered | 72.9 ± 0.7 | 43.3 ± 0.9 | 1,598 ± 35 | 2,342 ± 41 | 0.68 ± 0.01 | 2.69 ± 0.08 | 16.7 ± 0.9 |
| Dry | 61.8 ± 1.0 | 41.1 ± 0.8 | 1,047 ± 25 | 2,189 ± 40 | 0.48 ± 0.01 | 2.38 ± 0.08 | 16.5 ± 1.2 |
| Control | |||||||
| Watered | 37.4 ± 1.1 | 43.8 ± 0.9 | 435 ± 20 | 882 ± 36 | 0.49 ± 0.01 | 2.25 ± 0.07 | 24.7 ± 0.3 |
| Dry | 34.0 ± 0.5 | 47.6 ± 1.5 | 406 ± 16 | 1,032 ± 43 | 0.41 ± 0.01 | 2.31 ± 0.06 | 28.4 ± 0.4 |
| Significance tests | |||||||
| Cocultivation | ** | n.s. | ** | ** | ** | n.s. | ** |
| Drought | ** | n.s. | ** | n.s. | ** | n.s. | n.s. |
| Interaction | * | n.s. | ** | n.s. | ** | n.s. | n.s. |
*, P ≤ 0.05; **, P ≤ 0.01; n.s., not significant.
Except for the second harvest, cocultivated plants had significantly higher shoot-to-root DM ratios than the corresponding control plants (Table 2). In all treatments, the shoot-to-root DM ratio was highest at the first harvest and declined in the subsequent harvests, falling between 66 and 77% from the first to the third harvest. These results suggest that nutrient availability determines how plants allocate their resources to root or shoot tissues. As experimental plants were grown in pots without supplemental nutrition, plants at the first harvest may have had access to relatively higher nutrient conditions than those of second and third harvests. Accordingly, development prior to subsequent harvests may have shifted in favor of root growth (thereby reducing shoot-to-root DM levels) to maximize nutrient acquisition potential. These observations are consistent with other studies that reported increased biomass allocation to roots under low-nutrient conditions (7, 17).
Cocultivated plants had lower concentrations of several macronutrients in shoot and root tissues compared to control plants (P ≤ 0.05) (see Table S1 and Table S2 in the supplemental material). However, the total acquisition of all macronutrients (except Ca in roots) was significantly higher in cocultivated plants than in the controls, likely reflective of their taller stature. As the plants were grown on the same amount of soil substrate, larger plants would have depleted this resource faster than smaller plants. Moreover, most plant species, especially perennials, effectively allocate resources to transport, growth, defense, and reproduction (10), with the remainder being committed to storage. The lower concentrations of these nutrients in Sebacina-infected plants observed in this study may reflect a depletion of cellular stores to fuel the demands for growth and maintenance in those substantially larger plants (19). Some of these macronutrients (e.g., N and Mg) are critical constituents of chlorophyll, and their lower concentrations in cocultivated plants might have affected the observed decline in leaf chlorophyll content (13).
This report confirms that cocultivation imparts extraordinary biomass gains to switchgrass such that the yield of such plants grown under our defined drought stress conditions significantly exceeded that of control plants grown under normal or restricted-water conditions. The cocultivated plants consistently produced higher levels of root biomass than control plants, suggesting a greater potential to sequester carbon and hold soils, both highly desired properties in a crop grown under low-input conditions. As with many warm-season perennial grasses, switchgrass can be difficult or slow to establish (1, 20), and this is a major impediment to its implementation as a primary bioenergy crop (5). Therefore, both the shoot- and root-growth-promoting effects of S. vermifera, particularly at early points in plant development, are likely to improve competitiveness of switchgrass seedlings, especially during the establishment process.
Supplementary Material
Acknowledgments
The BioEnergy Science Center is a U.S. Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science.
The fungal strains used in these studies were obtained from the National Institute of Agro-biological Sciences, Tsukuba, Ibaraki, Japan. We are thankful to Nikki Charlton, Jeremey Bell, and Myoung Chi for their help in greenhouse study and to Michael Udvardi and Twain Butler for reviewing the first draft of the manuscript.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print 12 August 2011.
REFERENCES
- 1. Aiken G. E., Springer T. L. 1995. Seed size distribution, germination and emergence of six switchgrass cultivars. J. Range Manage. 48:455–458 [Google Scholar]
- 2. Alam S. M. 1999. Nutrient uptake by plants under stress conditions, p. 285–313 In Pessarakli M.(ed.), Handbook of plant and crop stress, 2nd ed Marcel Dekker Inc., New York, NY [Google Scholar]
- 3. Anjum S. A., et al. 2010. Desertification in Pakistan: causes, impacts and management. J. Food Agric. Environ. 8:1203–1208 [Google Scholar]
- 4. Barazani O., Benderoth M., Groten K., Kuhlemeier C., Baldwin I. T. 2005. Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 146:234–243 [DOI] [PubMed] [Google Scholar]
- 5. Beckman J. J., Moser L. E., Kubik K., Waller S. S. 1993. Big bluestem and switchgrass establishment as influenced by seed priming. Agron. J. 85:199–202 [Google Scholar]
- 6. Begg J. E., Turner N. C. 1976. Crop water deficits. Adv. Agron. 28:161–217 [Google Scholar]
- 7. Boot R. G. A., Mensink M. 1990. Size and morphology of root systems of perennial grasses from contrasting habitats as affected by nitrogen supply. Plant Soil 129:291–299 [Google Scholar]
- 8. Burke E. J., Brown S. J., Christidis N. 2006. Modeling the recent evolution of global drought and projections for the twenty-first century with the Hadley Centre climate model. J. Hydrometeorol. 7:1113–1125 [Google Scholar]
- 9. CAST 2009. Water, people, and future: water availability for agriculture in the United States. Council for Agricultural Science and Technology, Ames, IA [Google Scholar]
- 10. Chapin F. S., Schulze E. D., Mooney H. A. 1990. The ecology and economics of storage in plants. Annu. Rev. Ecol. Syst. 21:423–447 [Google Scholar]
- 11. Deshmukh S., et al. 2006. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc. Natl. Acad. Sci. U. S. A. 103:18450–18457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Endale D. M., Schomberg H. H., Jenkins M. B., Franklin D. H., Fisher D. S. 2010. Management implications of conservation tillage and poultry litter use for Southern Piedmont U. S. A. cropping systems. Nutr. Cycl. Agroecosys. 88:299–313 [Google Scholar]
- 13. Ericsson T. 1995. Growth and shoot: root ratio of seedlings in relation to nutrient availability. Plant Soil 168-169:205–214 [Google Scholar]
- 14. Fukai S., Sittisuang P., Chanphengsay M. 1998. Increasing production of rainfed lowland rice in drought prone environments: a case study in Thailand and Laos. Plant Prod. Sci. 1:75–82 [Google Scholar]
- 15. Ghimire S. R., Charlton N. D., Craven K. D. 2009. The mycorrhizal fungus, Sebacina vermifera, enhances seed germination and biomass production in switchgrass (Panicum virgatum L.). Bioenerg. Res. 2:51–58 [Google Scholar]
- 16. Grace C., Stribley D. P. 1991. A safer procedure for routine staining of vesicular arbuscular mycorrhizal fungi. Mycol. Res. 95:1160–1162 [Google Scholar]
- 17. Levang-Brilz N., Biondini M. E. 2003. Growth rate, root development and nutrient uptake of 55 plant species from the Great Plains grasslands, U. S. A. Plant Ecol. 165:117–144 [Google Scholar]
- 18. Major D. J., Morrison R. J., Blackshaw R. E., Roth B. T. 1991. Agronomy of dry land corn production at the northern fringe of the Great Plains. J. Prod. Agric. 4:606–613 [Google Scholar]
- 19. Millard P. 1988. The accumulation and storage of nitrogen by herbaceous plants. Plant Cell Environ. 11:1–8 [Google Scholar]
- 20. Moser L. E., Vogel K. P. 1995. Switchgrass, big blue stem, and Indian grass, p. 409–420 In Barnes R. F., Miller D. A., Nelson C. J. B. (ed.), Forage, an introduction to grassland agriculture, vol. 1 Iowa State University, Ames, IA [Google Scholar]
- 21. Petrini O. 1986. Taxonomy of endophytic fungi of aerial plant tissues, p. 175–187 In Fokkema N. J., van den Huevel J.(ed.), Microbiology of the phyllosphere. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 22. SAS 2004. Proc GLM, 9.1.3 ed SAS Institute Inc., Cary, NC [Google Scholar]
- 23. Schittenhelm S. 2010. Effect of drought stress on yield and quality of maize/sunflower and maize/sorghum intercrops for biogas production. J. Agron. Crop Sci. 196:253–261 [Google Scholar]
- 24. Sherameti I., Tripathi S., Varma A., Oelmuller R. 2008. The root-colonizing endophyte Pirifomospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress-related genes in leaves. Mol. Plant-Microbe Inter. 21:799–807 [DOI] [PubMed] [Google Scholar]
- 25. Sun C. A., et al. 2010. Piriformospora indica confers drought tolerance in Chinese cabbage leaves by stimulating antioxidant enzymes, the expression of drought-related genes and the plastid-localized CAS protein. J. Plant Physiol. 167:1009–1017 [DOI] [PubMed] [Google Scholar]
- 26. Tabatabaee J., Han M. Y. 2010. Rainwater harvesting potentials for drought mitigation in Iran. Water Sci. Technol. 62:816–821 [DOI] [PubMed] [Google Scholar]
- 27. Tazaki T., Ishihara K., Ushijima T. 1980. Influence of water stress on the photosynthesis and productivity of plants in humid stress, p. 309–322 In Turner N. C., Kratner P. J.(ed.), Adaptation of plants for water and high temperature stress. Wiley, New York, NY [Google Scholar]
- 28. Varma A., Verma S., Sudha, Sahay N., Butehorn B., Franken P. 1999. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 65:2741–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Voloudakis A. E., et al. 2002. Expression of selected drought-related genes and physiological response of Greek cotton varieties. Funct. Plant Biol. 29:1237–1245 [DOI] [PubMed] [Google Scholar]
- 30. Waller F., et al. 2008. Systemic and local modulation of plant responses by Piriformospora indica and related Sebacinales species. J. Plant Physiol. 165:60–70 [DOI] [PubMed] [Google Scholar]
- 31. Weiss M., Selosse M. A., Rexer K. H., Urban A., Oberwinkler F. 2004. Sebacinales: a hitherto overlooked cosm of heterobasidiomycetes with a broad mycorrhizal potential. Mycol. Res. 108:1003–1010 [DOI] [PubMed] [Google Scholar]
- 32. Weiss M., et al. 2011. Sebacinales everywhere: previously overlooked ubiquitous fungal endophytes. PLoS One 6:e16793 doi: 10.1371/journal.pone.0016793 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.