Abstract
The gastrointestinal tracts of neonates are colonized by bacteria immediately after birth. It has been discussed that the intestinal microbiota of neonates includes strains transferred from the mothers. Although some studies have indicated possible bacterial transfer from the mother to the newborn, this is the first report confirming the transfer of bifidobacteria at the strain level. Here, we investigated the mother-to-infant transmission of Bifidobacterium longum subsp. longum by genotyping bacterial isolates from the feces of mothers before delivery and of their infants after delivery. Two hundred seven isolates from 8 pairs of mothers and infants were discriminated by multilocus sequencing typing (MLST) and amplified fragment length polymorphism (AFLP) analysis. By both methods, 11 strains of B. longum subsp. longum were found to be monophyletic for the feces of the mother and her infant. This finding confirms that these strains were transferred from the intestine of the mother to that of the infant. These strains were found in the first feces (meconium) of the infant and in the feces at days 3, 7, 30, and 90 after birth, indicating that they stably colonize the infant's intestine immediately after birth. The strains isolated from each family did not belong to clusters derived from any of the other families, suggesting that each mother-infant pair might have unique family-specific strains.
INTRODUCTION
The intestinal microbiota plays an important role in the maintenance of human health. Microbial colonization during infancy is critical for a lifetime of good health (11); suboptimal colonization can predispose the individual to diseases later in life (19). Establishment of an optimal microbial community immediately after birth and the maintenance of a balanced intestinal microbiota are important in the development of the immune system (11).
The gastrointestinal tract is suggested to be sterile at birth. Microbial colonization starts with facultative bacteria, such as Enterobacteriaceae, Enterococcus, and Streptococcus, which are followed by anaerobic bacteria such as Bifidobacterium, Bacteroides, Clostridium, and Eubacterium (10). The origin of these intestinal microbes has attracted continuous attention. It has been hypothesized that they are acquired during transit through the birth canal or immediately after birth from the surroundings (13). Recent studies have also discussed that breast milk can be another source of intestinal microbes (20, 22, 28, 31). Several studies employing molecular biology techniques have suggested the possibility of vertical mother-to-infant transmission of intestinal microbes (1, 25, 26, 32) and the presence of these microorganisms in breast milk (12, 21, 28). However, the molecular methods used in those studies, such as real-time quantitative PCR (qPCR), while effective in identifying microorganisms at the species level, do not allow comparisons at the strain level. Furthermore, randomly amplified polymorphic DNA (RAPD) analysis is questioned as a highly sensitive strain-level typing technique due to the failure of discrimination of related clones (7, 14, 18). Therefore, further analyses are required to confirm how bacteria are transferred from the mother or any other environmental sources to the infant. A more comprehensive analysis at the strain level is necessary.
Molecular techniques such as multilocus sequencing typing (MLST) and amplified fragment length polymorphism (AFLP) analysis are precise and powerful tools for characterizing and classifying bacterial strains. MLST is based on use of the sequence polymorphism of a set of several genes in the genome to generate data that can be used to differentiate between bacterial strains (5, 17, 36). AFLP analysis is based on the detection of genomic restriction fragments by PCR amplification (16, 37). These methods have the capacity for high resolution and provide reproducible data, therefore being suitable for both species identification and strain typing (3, 6, 8).
Bifidobacterium is an important genus in the microbiota of infants. In general, bifidobacteria become the dominant microorganisms in the intestine within a week after birth and remain so throughout until weaning (29, 35). Moreover, bifidobacteria are thought to play a crucial role in the protection of the gut mucosa against pathogenic bacteria, and they also contribute to the development of the infant's mucosal immune system (29, 35). In this study, we have focused on B. longum subsp. longum, one of the predominant bifidobacterial species in the intestines of mothers and infants (24). Our objective was to verify the mother-to-infant transmission of B. longum subsp. longum by isolating strains from fecal samples and breast milk of mother-infant pairs and analyzing them by both MLST and AFLP methods.
MATERIALS AND METHODS
Samples.
Samples of fresh breast milk and feces from mother and infant were aseptically collected from 8 mothers and their respective infants who had been delivered by the vaginal route. The mothers' fecal samples were taken twice before delivery (see Table S1 in the supplemental material), and the infants' fecal samples were taken at 0 (meconium), 3, 7, 30, and 90 days of age. Breast milk samples were taken at 7 and 30 days after delivery. All the mothers were healthy, had a full-term pregnancy, and breast fed their infants for 6 months. These subjects belong to a larger observational study conducted in Belgium (ISRCTN66704989).
Fecal samples were collected with a sterile plastic spatula and transferred into a sterile glass tube containing 6 ml of anaerobic transport medium [containing, in 1 liter, 0.225 g KH2PO4, 0.225 g K2HPO4, 0.45 g NaCl, 0.225 g (NH4)2SO4, 0.0225 g CaCl2, 0.0225 g MgSO4, 0.5 g l-cysteine hydrochloride, 0.001 g resazurin, 0.5 g agar, 10 g Lab Lemco powder, 100 ml glycerol, and 2.1 ml 8% Na2CO3] (15). Breast milk samples were collected in a sterile tube using sterile gloves. The nipples and mammary areola were cleaned with medical gauze wet with sterile saline, and then breast milk was collected by using a manual breast pump (Philips AVENT; Philips, Surrey, United Kingdom). All the samples were kept at 4°C to 7°C until delivery to the laboratory, which occurred within 72 h after collection.
This study was approved by the ethics committee of the hospital network of Antwerp (Ziekenhuisnetwerk Antwerpen), and informed written consent was obtained from the mothers.
Isolation of bifidobacteria.
The fecal samples in the anaerobic transport medium were homogenized with a Vortex mixer to make fecal suspensions. The fecal suspensions or breast milk samples were serially diluted with phosphate-buffered saline (PBS) (pH 7.2). Serial dilutions of samples were inoculated onto TOS propionate agar (Yakult Pharmaceutical Industry Co., Ltd., Tokyo, Japan) containing 50 μg/ml mupirocin (TOS-M agar) and incubated anaerobically at 37°C for 72 h. Two to six colonies per sample, showing different morphologies on the medium, were isolated for subsequent analyses.
DNA extraction.
DNA from all samples was extracted as described previously (39) with some modifications. Briefly, all the isolated strains were cultured anaerobically in TOS-M broth at 37°C for 48 h. Cell pellets were collected from 1.0 ml of the culture by centrifugation (20,000 × g, 5 min, 4°C). Pellets were resuspended in 250 μl extraction buffer (100 mM Tris-HCl, 40 mM EDTA, pH 9.0). Glass beads (diameter, 0.1 mm; 700 mg), 500 μl of phenol, and 50 μl of 10% sodium dodecyl sulfate were added to the suspension, and the mixture was vortexed vigorously for 30 s in a FastPrep-24 (M.P. Biomedicals, Irvine, CA) at a setting of 6.5 ms−1. Then, 150 μl of 3 M sodium acetate was added, and the mixture was cooled on ice for 5 min. After centrifugation (20,000 × g, 8 min, 4°C), the supernatant was collected and DNA was precipitated with isopropanol. Finally, the DNA was diluted in 100 μl TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0).
Identification of the bacterial isolates.
Identification of the strains at the species level was carried out by PCR sequencing of the 16S rRNA gene. The universal primers 8F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-ACGGCTACCTTGTTACGACTT-3′) (34) were employed for amplification. PCR was carried out in 25-μl final volumes containing 10 mM Tris-HCl (pH 8.3), 50 mM KCl, 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate (dNTP), 0.5 U Taq DNA polymerase (TaKaRa, Shiga, Japan), 0.4 μM each respective primer, and 10 ng DNA template. The PCR amplification program consisted of an initial heating step at 94°C for 2 min, 32 cycles of 94°C for 20 s, 55°C for 20 s, and 72°C for 20 s, and a final extension step at 72°C for 3 min. All amplifications were performed with the DNA Engine Peltier thermal cycler (Bio-Rad, Hercules, CA). The amplicons were purified using ExoSAP-IT (USB, Cleveland, OH) and were sequenced using the primers 8F and 520R (5′-ACCGCGGCTGCTGGC-3′) (27) and BigDye Terminator v1.1 chemistry (Applied Biosystems, Foster City, CA) on ABI PRISM 3130xl Genetic Analyzers (Applied Biosystems). The resulting sequences were used to search sequences deposited in the EMBL database by using the BLAST algorithm, and the identities of the isolates were determined on the basis of the highest scores. Subspecies of the isolated strains that belonged to B. longum were identified by PCR using primers BiINF-1 (5′-TTCCAGTTGATCGCATGGTC-3′) and BiINF-2 (5′-GGAAACCCCATCTCTGGGAT-3′) for B. longum subsp. infantis and BiLON-1 (5′-TTCCAGTTGATCGCATGGTC-3′) and BiLON-2 (5′-GGGAAGCCGTATCTCTACGA-3′) for B. longum subsp. longum (23). The PCR amplification program consisted of an initial heating step at 94°C for 4.5 min, 35 cycles of 94°C for 30 s, 55°C for 30 s, and 72°C for 1 min, and a final extension step at 72°C for 10 min. The PCR products were separated by electrophoresis on a 1% agarose gel using 1× Tris-acetate-EDTA buffer, stained with SYBR green (Lonza, Rockland, ME), and visualized under UV light.
MLST analysis.
Seven housekeeping genes encoding proteins were chosen for analysis as previously described (2, 8, 30, 36). The selected genes encode the following proteins: class III stress response-related ATPase with chaperone activity (clpC), DNA primase (dnaG), chaperone protein DnaJ (dnaJ), GTP-binding protein chain elongation factor G (fusA), the β subunit of DNA gyrase (gyrB), amidophosphoribosyltransferase (purF), and the β subunit of RNA polymerase (rpoB) (Table 1). The DNA sequences of these candidate loci were selected based on the genome sequence data for strains Bifidobacterium longum DJO10A (NC_010816) and Bifidobacterium longum NCC2705 (NC_004307). Each 25-μl PCR mixture contained 1× PCR buffer, 200 μM deoxynucleoside triphosphates, 2 mM MgCl2, 0.4 μM each primer, 10 μl GC-RICH solution, 2 U Fast Taq polymerase (Roche, Basel, Switzerland), and 10 ng template DNA. The PCR amplification program consisted of an initial heating step at 95°C for 5 min, 30 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 1 min, and a final extension step at 72°C for 10 min. Sequencing was performed as described above.
Table 1.
Primers for MLST analysis
| Gene | Primer | Sequence (5′ → 3′) | Size (bp) |
|---|---|---|---|
| clpC | Bilon-clpC-F | CCTGAAGAAGGTGCTGAAGG | 563 |
| Bilon-clpC-R | TTCTCCTGCTTGTCGCGCAGT | ||
| dnaG | Bilon-dnaG-F | GTTGCCGTAGATTTGGGCTTGG | 449 |
| Bilon-dnaG-R | ATGACTTCGGTGTTCCGCAC | ||
| dnaJ | Bilon-dnaJ-F | GCTGAGCAAGAAGGAAGATCGC | 421 |
| Bilon-dnaJ-R | TGAACTTCTTGCCGTCCACGG | ||
| fusA | Bilon-fusA-F | CACCATCAAGGAGAAGCTGG | 536 |
| Bilon-fusA-R | ACGAGCTTGCCGTAGAACG | ||
| gyrB | Bilon-gyrB-if1 | AAGTGCGCCGTCAGGGCTT | 473 |
| Bilon-gyrB-R | GTGTTCGCGAAGGTGTGCAC | ||
| purF | Bilon-purF-if1 | ATGGCGGTTTCGCCTACC | 510 |
| Bilon-purF-ir1 | AGAGAGCTTCATACGCACAC | ||
| rpoB | Bilon-rpoB-F | AGACCGACAGCTTCGATTGG | 575 |
| Bilon-rpoB-R | AACACGATGGCGGACTGCTT |
AFLP analysis.
An AFLP analysis described previously (33) was used with the following modifications. B. longum subsp. longum DNA samples were digested with MspI and MesI, and the resulting fragments were ligated to MspI and MesI double-stranded adapters (Table 2). Primer sets for amplifying the restriction fragments were designed using the program In Silico AFLP-PCR Amplification (http://insilico.ehu.es/AFLP/) (4), as shown in Table 2.
Table 2.
Adapters and PCR primers for AFLP analysis
| Adapter or primer | Sequence (5′–3′) | Reference |
|---|---|---|
| Adapters | ||
| MspI | CTCGTAGACTGCGTACA | This study |
| CGTGTACGCAGTCTAC | This study | |
| MseI | GACGATGAGTCCTGA | 37 |
| TACTCAGGACTCAT | 37 | |
| Preselective primers | ||
| MspI | GACTGCGTACACGGA | This study |
| MseI | GATGAGTCCTGAGTAA | 37 |
| Selective primers | ||
| MspI-A | FAMa-GACTGCGTACACGGAA | This study |
| MseI-T | GATGAGTCCTGAGTAAT | 37 |
FAM, 6-carboxyfluorescein.
The composition of the restriction reaction mixtures was as follows: 1× NE buffer 4, 1× bovine serum albumin (BSA), 5 U MspI (New England BioLabs, Ipswich, MA), 5 U MesI (New England BioLabs), and 2.9 μl of DNA in a total volume of 5 μl. The restriction reaction mixtures were incubated at 37°C for 2 h. For the next step, the ligation reaction mixture composition was as follows: 1× T4 DNA ligase buffer, 2 μM MspI adapter, 2 μM MesI adapter (Table 2), 1 U T4 DNA ligase (New England BioLabs), and 5 μl of digested DNA in a total volume of 10 μl. The ligation reaction mixtures were incubated at 20°C for 2 h. Following the addition of 90 μl of TE buffer, the digested and ligated DNA was used as the template for the preselective PCR template. For preselective PCR, the 10-μl reaction mixture contained 1× buffer, 1.5 mM MgCl2, 250 μM each dNTP, 0.025 U Taq DNA polymerase (TaKaRa), 2.5 μM each preselective primer (Table 2), and 1 μl of diluted digestion and ligation DNA. The PCR amplification conditions included an initial heating step at 94°C for 2 min, 28 cycles of 94°C for 20 s, 56°C for 30 s, and 72°C for 2 min, and a final extension step at 72°C for 5 min. Following the addition of 90 μl of TE buffer, the preselective PCR product was used as the template for the selective PCR. For the selective PCR, the 10-μl reaction mixture contained 1× buffer, 1.5 mM MgCl2, 100 μM each dNTP, 0.025 U Taq DNA polymerase (TaKaRa), 3 μM each selective primer (Table 2), and 1 μl of diluted preselective PCR template. The PCR amplification program consisted of an initial heating step at 94°C for 2 min and 13 cycles at 94°C for 20 s, 65°C for 30 s, and 72°C for 60 s, with a decrease in the annealing temperature of 0.7°C/cycle, followed by 24 cycles at 94°C for 20 s, 56°C for 30 s, and 72°C for 60 s, with a final extension at 72°C for 2 min.
One microliter of the selective PCR products was mixed with 9 μl of Hi-Di formamide (Applied Biosystems) and 1 μl of GeneScan 600 LIZ size standards (Applied Biosystems) and denatured at 95°C for 1 min. The samples were then analyzed with ABI PRISM 3130xl Genetic Analyzers (Applied Biosystems) with the following conditions: 15-s injection time, 1.6-kV injection voltage, 15-kV run voltage, and 30-min running time.
Phylogenetic analysis.
BioNumerics software version 6.0 (Applied Maths, Sint-Martens-Latem, Belgium) was used to perform all phylogenetic analyses. For MLST data, the sequences obtained for the 7 genes were aligned and compared. Each distinct gene sequence was assigned an allele number, and each unique combination of seven allele numbers was assigned a sequence type (ST). We created a dendrogram using the multiscale setting for comparisons and the unweighted pair group method with arithmetic mean (UPGMA) for clustering.
The AFLP data in FSA format were imported into BioNumerics 6.0. The AFLP analysis was performed for fragments ranging from 60 to 600 nucleotides in length, and a threshold of 1% was used for position tolerance. We created a dendrogram using the Pearson product-moment correlation coefficient for comparisons and UPGMA for clustering.
RESULTS
B. longum subsp. longum strains for molecular analysis.
Two hundred seven B. longum subsp. longum strains were isolated from 8 mother-infant pairs (see Table S2 in the supplemental material). One hundred ninety-five of them were obtained from the mothers' feces before delivery and from infants' feces from several different periods. The other 12 strains were isolated from breast milk samples from 4 mothers.
MLST genetic diversity at seven loci.
Seven pairs of primers were designed to partially amplify DNA regions of 421 to 575 bp of the 7 housekeeping genes (clpC, dnaG, dnaJ, fusA, gyrB, purF, and rpoB) of B. longum subsp. longum according to the genome sequences of B. longum strains DJO10A (NC_010816) and NCC2705 (NC_004307). The sequences of the 7 genes were determined for the 207 B. longum subsp. longum strains. All 7 genes were successfully amplified and sequenced for all B. longum subsp. longum isolates tested.
We analyzed a total of 3,527 bp (23.28% coverage of the seven complete coding sequences [CDSs]) (see Table S3 in the supplemental material). Nucleotide variation was observed in all genes, with polymorphic nucleotide sites ranging from 0.89% (clpC) to 45.37% (dnaJ) (11.62% of the 7 complete CDSs). The number of alleles ranged from 5 to 9. By combining the 7 gene loci, 26 distinct sequence types (STs) were identified in 207 strains. Strains that had the same STs and were isolated from the same source and sampling period were defined as the same strain; otherwise, they were identified as individual strains. Out of 207 B. longum subsp. longum strains, 69 individual strains were identified.
Comparison of B. longum subsp. longum strains isolated from feces and breast milk, based on MLST profiles.
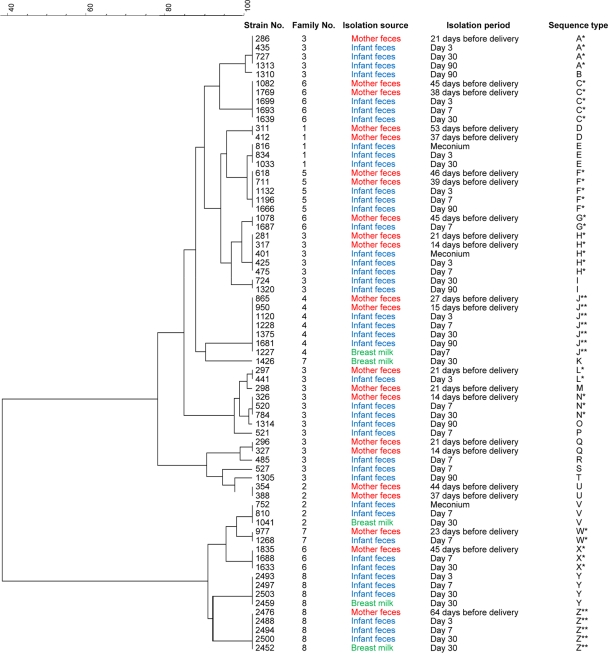
A UPGMA dendrogram was generated based on the MLST profiles for all 69 individual B. longum subsp. longum strains (Fig. 1). Out of the 69 sequenced strains, 11 strains were monophyletic between the mother's feces and her infant's feces, from 6 families out of 8 (STs A, C, F, G, H, J, L, N, W, X, and Z). Of these, 2 strains were also monophyletic with strains from breast milk (STs J and Z). Two other strains were monophyletic among the same infant's feces collected at different sampling periods (STs E and I), and 3 strains were monophyletic only among the mother's feces (STs D, Q, and U). There were 2 strains that were monophyletic between breast milk and the infant's feces (STs V and Y), although monophyletic strains were not isolated from the mother's feces.
Fig. 1.
Dendrogram derived from a comparison of MLST profiles of B. longum subsp. longum isolates from mothers' and infants' feces and breast milk for all 8 families. The dendrogram was generated with a multiscale setting for comparison and UPGMA for clustering. *, strains isolated from mothers' and infants' feces showing the same MLST profiles within a given cluster. **, strains isolated from breast milk and mothers' and infants' feces showing the same MLST profiles within a given cluster.
Isolates from each mother-infant pair formed their own cluster. Pairs of different mother-infant strains were not monophyletic. Strains isolated from family 3 were diverse, forming 13 individual clusters (STs A, B, H, I, L, M, N, O, P, Q, R, S, and T). Out of the 13 strains, four strains were monophyletic between the mother's and infant's feces (STs A, H, L, and N). For ST A, the following strains were monophyletic: strain 286, isolated from the mother's feces 21 days before delivery; strain 435, isolated from day 3 infant feces; strain 727, isolated from day 30 infant feces; and strain 1313, isolated from day 90 infant feces. Likewise, for STs H, L, and N, these monophyletic stains were isolated from the mother's feces before delivery at different times and also from the infant's feces at different times. Mother-infant monophyletic strains were found in 5 other families, for a total of 6 mother-infant pairs (no. 3, 4, 5, 6, 7, and 8). Monophyletic strains were not identified in 2 families (mother-infant pairs 1 and 2). Strains isolated from these 2 mother-infant pairs formed separate clusters for each source.
Comparison of B. longum subsp. longum strains isolated from mother and infant feces and breast milk, based on AFLP profiles.
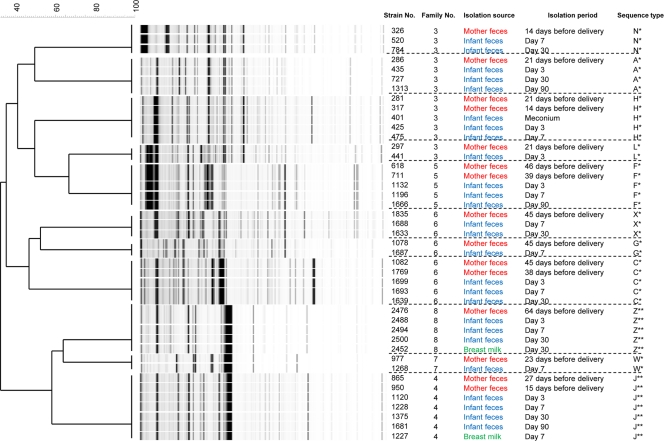
We performed AFLP analysis on 43 individual strains that were monophyletic for mothers' feces and infants' feces based on MLST data. An average of 206 (±23) fragments were detected from each strain. These 43 strains were distributed into 11 fragment patterns (Fig. 2). Isolates from family 3 were clustered into 4 groups. Likewise, we identified 1 cluster for family 4 isolates, 1 cluster for family 5 isolates, 3 clusters for family 6 isolates, 1 cluster for family 7 isolates, and 1 cluster for family 8 isolates.
Fig. 2.
AFLP profiles of the 11 B. longum subsp. longum strains found by MLST analysis to be monophyletic between feces from mothers and their infants. Dendrograms were generated with a multiscale setting for comparison and UPGMA for clustering.
DISCUSSION
The aim of our study was to investigate whether mothers' intestinal bacteria are transferred to their infants' intestines and, if so, whether these bacteria stably colonize the infants' intestines over time. To test this hypothesis, we isolated B. longum subsp. longum from the mothers' and infants' feces at different times and performed a strain-level analysis using MLST and AFLP methods for a total of 207 strains.
We identified 11 B. longum subsp. longum strains that were monophyletic for the mother's and infant's feces, from 6 families out of 8, by both the MLST and AFLP methods. For several strains, the positions within the evolutionary tree (dendrogram) were different by MLST and AFLP (Fig. 1 and 2). However, the focus of this study was to determine whether these strains were monophyletic and not to clarify the evolutionary relationship. Both methods identified the same monophyletic strains. All strains that had the same STs also had the same AFLP profiles (Fig. 1 and 2), confirming that these 11 strains were transferred from mother to infant. Monophyletic strains from family 5 (ST F) were detected in the mother's feces at 46 days and 39 days before delivery and in the infant's feces at day 3, day 7, and day 90 after birth (Fig. 1). This pattern, i.e., mother-infant monophyletic strains being continuously detected over time in the infant feces, was found among other families. These results suggest that predominant strains in the pregnant mother's intestine transfer to the infant's intestine, expand in numbers soon after birth, and subsequently colonize. Taken together, our results confirm, for the first time, that the first bacteria to colonize the intestine of an infant are transmitted from the mother. These findings confirm the data from previous studies that first indicated the importance of mother-to-infant transmission of bacteria in the colonization of the gastrointestinal tracts of neonates (20, 25, 32).
We also detected two monophyletic strains that were transferred from mother to infant in breast milk (Fig. 1, STs J and Z). Most breast milk isolates were found in the breast milk a month after delivery. Several studies have discussed the possibility of transfer of intestinal bacteria by breastfeeding (1, 12, 28). Although several authors have reported the presence of bifidobacterial DNA in breast milk samples (12, 22), its isolation seems to be more difficult. Martin et al. (22) were able to isolate bifidobacteria from only 8 out of 22 breast milk samples in which DNA was detected, suggesting fastidious growth requirements of the bacteria present in this type of samples. Further investigations are under way to clarify the importance of bacterial transfer by breastfeeding.
Among the 69 individual strains, isolates from each mother-infant pair formed their own clusters, suggesting that each mother-infant pair has unique family type strains.
We designed primer sets specifically for MLST and AFLP analysis of B. longum subsp. longum strains. The presence of nucleotide polymorphisms is important for MLST analysis, because this method is based on the characteristics of housekeeping genes. For the 7 housekeeping gene loci that we selected for MLST analysis, the number of polymorphic nucleotide sites ranged from 5 (clpC) to 191 (dnaJ) (see Table S3 in the supplemental material). The dnaG and dnaJ gene loci showed high levels of nucleotide polymorphisms (29.2% and 45.3%). The percentage of nucleotide polymorphisms was 11.62% of the complete CDSs of the 7 gene loci. This level of nucleotide polymorphisms can provide high discriminatory power for strain typing and is consistent with that reported in other studies using MLST analysis (9, 38). For AFLP analysis, the number of DNA fragments analyzed depends on the choice of restriction enzymes and the choice of PCR primers. The restriction enzyme pair EcoRI and MseI is usually used for AFLP analysis. However, this is the first time that an AFLP analysis targeting bifidobacteria has been performed. To target bifidobacteria, we chose the enzyme pair MspI and MseI because they offer two key advantages over EcoRI and MseI. First, MspI and MseI can be used at the same reaction temperature (37°C). Also, the number of fragments detected for B. longum subsp. longum was 206 on average, which is about 4 times higher than that generated with the conventionally used EcoRI-MseI pair (data not shown). Our results show that the developed MLST and AFLP are efficient methods for identifying B. longum subsp. longum strains.
In summary, we have shown that B. longum subsp. longum strains are transmitted from mothers' intestines to their infants immediately after birth and that these strains subsequently colonize the infants' intestines. We have shown that each strain is unique to a particular mother-infant pair and belongs to its own cluster. Bifidobacteria are the predominant bacterial group in the infants' intestines (29), and our data suggest that the mothers' intestinal bifidobacteria during pregnancy are an important component of their infants' intestinal microbiota.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by the Yakult Honsha European Research Center for Microbiology and Danone Research (Centre for Specialised Nutrition, Wageningen, The Netherlands, and Centre Daniel Carasso, Palaiseau, France).
We thank Elena Stefanelli (University of Verona, Italy) for helping with the MLST analysis.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 5 August 2011.
REFERENCES
- 1. Albesharat R., Ehrmann M. A., Korakli M., Yazaji S., Vogel R. F. 2011. Phenotypic and genotypic analyses of lactic acid bacteria in local fermented food, breast milk and faeces of mothers and their babies. Syst. Appl. Microbiol. 34:148–155 [DOI] [PubMed] [Google Scholar]
- 2. Alexandre A., Laranjo M., Young J. P. W., Oliveira S. 2008. dnaJ is a useful phylogenetic marker for alphaproteobacteria. Int. J. Syst. Evol. Microbiol. 58:2839–2849 [DOI] [PubMed] [Google Scholar]
- 3. Badali H., de Hoog G. S., Curfs-Breuker I., Klaassen C. H. W., Meis J. F. 2010. Use of amplified fragment length polymorphism to identify 42 Cladophialophora strains related to cerebral phaeohyphomycosis with in vitro antifungal susceptibility. J. Clin. Microbiol. 48:2350–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bikandi J., Millan R. S., Rementeria A., Garaizar J. 2004. In silico analysis of complete bacterial genomes: PCR, AFLP-PCR and endonuclease restriction. Bioinformatics 20:798–799 [DOI] [PubMed] [Google Scholar]
- 5. Bilhère E., Lucas P. M., Claisse O., Lonvaud-Funel A. 2009. Multilocus sequence typing of Oenococcus oeni: detection of two subpopulations shaped by intergenic recombination. Appl. Environ. Microbiol. 75:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cleenwerck I., De Wachter M., González ´A., De Vuyst L., De Vos P. 2009. Differentiation of species of the family Acetobacteraceae by AFLP DNA fingerprinting: Gluconacetobacter kombuchae is a later heterotypic synonym of Gluconacetobacter hansenii. Int. J. Syst. Evol. Microbiol. 59:1771–1786 [DOI] [PubMed] [Google Scholar]
- 7. Daxboeck F., et al. 2005. Characterization of clinically isolated Ralstonia mannitolilytica strains using random amplification of polymorphic DNA (RAPD) typing and antimicrobial sensitivity, and comparison of the classification efficacy of phenotypic and genotypic assays. J. Med. Microbiol. 54:55–61 [DOI] [PubMed] [Google Scholar]
- 8. Delétoile A., et al. 2010. Species delineation and clonal diversity in four Bifidobacterium species as revealed by multilocus sequencing. Res. Microbiol. 161:82–90 [DOI] [PubMed] [Google Scholar]
- 9. Diancourt L., et al. 2007. Multilocus sequence typing of Lactobacillus casei reveals a clonal population structure with low levels of homologous recombination. Appl. Environ. Microbiol. 73:6601–6611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Favier C. F., De Vos W. M., Akkermans A. D. 2003. Development of bacterial and bifidobacterial communities in feces of newborn babies. Anaerobe 9:219–229 [DOI] [PubMed] [Google Scholar]
- 11. Guarner F., Malagelada J. R. 2003. Gut flora in health and disease. Lancet 361:512–519 [DOI] [PubMed] [Google Scholar]
- 12. Gueimonde M., Laitinen K., Salminen S., Isolauri E. 2007. Breast milk: a source of bifidobacteria for infant gut development and maturation? Neonatology 92:64–66 [DOI] [PubMed] [Google Scholar]
- 13. Inoue R., Ushida K. 2003. Vertical and horizontal transmission of intestinal commensal bacteria in the rat model. FEMS Microbiol. Ecol. 46:213–219 [DOI] [PubMed] [Google Scholar]
- 14. Ipek M., Ipek A., Simon P. W. 2003. Comparison of AFLPs, RAPD markers, and isozymes for diversity assessment of garlic and detection of putative duplicates in germplasm collections. J. Am. Soc. Hort. Sci. 128:246–252 [Google Scholar]
- 15. Iwaya A., et al. 2006. Change in the bacterial flora of pouchitis. Hepatogastroenterology 53:55–59 [PubMed] [Google Scholar]
- 16. Janssen P., et al. 1996. Evaluation of the DNA fingerprinting method AFLP as a new tool in bacterial taxonomy. Microbiology 142:1881–1893 [DOI] [PubMed] [Google Scholar]
- 17. Jost B. H., Trinh H. T., Songer J. G. 2006. Clonal relationships among Clostridium perfringens of porcine origin as determined by multilocus sequence typing. Vet. Microbiol. 116:158–165 [DOI] [PubMed] [Google Scholar]
- 18. Kafkas S., et al. 2006. Detecting DNA polymorphism and genetic diversity in a wide Pistachio germplasm: comparison of AFLP, ISSR and RAPD markers. J. Am. Soc. Hort. Sci. 131:522–529 [Google Scholar]
- 19. Kalliomäki M., et al. 2001. Distinct patterns of neonatal gut microflora in infants in whom atopy was and was not developing. J. Allergy Clin. Immunol. 107:129–134 [DOI] [PubMed] [Google Scholar]
- 20. Martin R., et al. 2003. Human milk is a source of lactic acid bacteria for the infant gut. J. Pediatr. 143:754–758 [DOI] [PubMed] [Google Scholar]
- 21. Martin R., et al. 2004. The commensal microflora of human milk: new perspectives for food bacteriotherapy and probiotics. Trends Food Sci. Technol. 15:121–127 [Google Scholar]
- 22. Martin R., et al. 2009. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl. Environ. Microbiol. 75:965–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matsuki T., et al. 2004. Quantitative PCR with 16S rRNA-gene-targeted species-specific primers for analysis of human intestinal bifidobacteria. Appl. Environ. Microbiol. 70:167–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Matsuki T., Watanabe K., Tanaka R., Fukuda M., Oyaizu H. 1999. Distribution of bifidobacterial species in human intestinal microflora examined with 16S rRNA-gene-targeted species-specific primers. Appl. Environ. Microbiol. 65:4506–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsumiya Y., Kato N., Watanabe K., Kato H. 2002. Molecular epidemiological study of vertical transmission of vaginal Lactobacillus species from mothers to newborn infants in Japanese, by arbitrarily primed polymerase chain reaction. J. Infect. Chemother. 8:43–49 [DOI] [PubMed] [Google Scholar]
- 26. Mikami K., et al. 2009. Influence of maternal bifidobacteria on the establishment of bifidobacteria colonizing the gut in infants. Pediatr. Res. 65:669–674 [DOI] [PubMed] [Google Scholar]
- 27. Miyake T., Watanabe K., Watanabe T., Oyaizu H. 1998. Phylogenetic analysis of the genus Bifidobacterium and related genera based on 16S rDNA sequences. Microbiol. Immunol. 42:661–667 [DOI] [PubMed] [Google Scholar]
- 28. Perez P. F., et al. 2007. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics 119:724–732 [DOI] [PubMed] [Google Scholar]
- 29. Salminen S., Isolauri E. 2006. Intestinal colonization, microbiota, and probiotics. J. Pediatr. 149:115–120 [Google Scholar]
- 30. Santos S. R., Ochman H. 2004. Identification and phylogenetic sorting of bacterial lineages using universally conserved genes and proteins. Environ. Microbiol. 6:754–759 [DOI] [PubMed] [Google Scholar]
- 31. Solís G., Reyse-Gavilan C. G., Fernandez N., Margolles A., Gueimonde M. 2010. Establishment and development of lactic acid and bifidobacteria microbiota in breast-milk and infant gut. Anaerobe 16:307–310 [DOI] [PubMed] [Google Scholar]
- 32. Takahashi H., et al. 2010. Comparative analysis of the properties of bifidobacterial isolates from fecal samples of mother-infant pairs. J. Pediatr. Gastroenterol. Nutr. 51:653–660 [DOI] [PubMed] [Google Scholar]
- 33. Thompson F. L., Hoste B., Vandemeulebroecke K., Swings J. 2001. Genomic diversity amongst Vibrio isolates from different sources determined by fluorescent amplified fragment length polymorphism. Syst. Appl. Microbiol. 24:520–538 [DOI] [PubMed] [Google Scholar]
- 34. Turner S., Pryer K. M., Miao V. P. W., Palmer J. D. 1999. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J. Eukaryot. Microbiol. 46:327–338 [DOI] [PubMed] [Google Scholar]
- 35. Turroni F., Ribbera A., Foroni E., van Sinderen D., Ventura M. 2008. Human gut microbiota and bifidobacteria: from composition to functionality. Antonie Van Leeuwenhoek 94:35–50 [DOI] [PubMed] [Google Scholar]
- 36. Ventura M., et al. 2006. Analysis of bifidobacterial evolution using a multilocus approach. Int. J. Syst. Evol. Microbiol. 56:2783–2792 [DOI] [PubMed] [Google Scholar]
- 37. Vos P., et al. 1995. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Res. 23:4407–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang W., Jayarao B. M., Knabel S. J. 2004. Multi-virulence-locus sequence typing of Listeria monocytogenes. Appl. Environ. Microbiol. 70:913–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhu H., Qu F., Zhu L. H. 1993. Isolation of genomic DNAs from plants, fungi and bacteria using benzyl chloride. Nucleic Acids Res. 21:5279–5280 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.