Abstract
We developed and validated a treatment to inactivate Escherichia coli O157:H7 on radish seeds without decreasing seed viability. Treatments with aqueous ClO2 followed by drying and dry-heat treatments were evaluated for efficacy to inactivate the pathogen. Conditions to dry radish seeds after treatment with water (control) or ClO2 were established. When treated seeds with high water activity (aw) (>0.99) were stored at 45°C and 23% relative humidity (RH), the aw decreased to <0.30 within 24 h. Drying high-aw seeds before exposing them to dry-heat treatment (≥60°C) was essential to preserve seed viability. The germination rate of radish seeds which had been immersed in water for 5 min, dried at 45°C and 23% RH for 24 h, and heated at 70°C for 48 h or at 80°C for 24 h was not significantly decreased (P ≤ 0.05) compared to that of untreated radish seeds. Sequential treatments with ClO2 (500 μg/ml, 5 min), drying (45°C, 23% RH, 24 h), and dry heating (70°C, 23% RH, 48 h) eliminated E. coli O157:H7 (5.9 log CFU/g) on radish seeds and, consequently, sprouts produced from them without decreasing the germination rate. These sequential treatments are recommended for application to radish seeds intended for sprout production.
INTRODUCTION
In the Republic of Korea and some other Asian countries, consumption of vegetable seed sprouts has increased in recent decades. Worldwide, the number and frequency of sprout-associated outbreaks of disease have increased during this period. There were at least 40 outbreaks implicating vegetable sprouts reported between 1973 and 2006 (30). The majority of these outbreaks were linked to alfalfa, mung bean, clover, radish, mustard, and cress sprouts (20, 23, 29). Pathogens most frequently involved in causing outbreaks were Salmonella and Escherichia coli O157:H7 (20, 22, 29). The largest outbreak was associated with radish sprouts in Japan (11). This outbreak involved more than 6,000 culture-confirmed cases of E. coli O157:H7 infections.
The source of pathogenic bacteria on sprouts is thought to originate largely from seeds rather than the contamination of sprouts during or after production (20). Thus, the National Advisory Committee on Microbiological Criteria for Foods (NACMCF) has recommended applying treatments to achieve a 5-log CFU/g reduction of pathogens on seeds. Even a 5-log CFU/g reduction, however, may not guarantee the absence of pathogens in sprouts. Pathogens that remain on seeds after treatment, even if present in very low numbers, can multiply rapidly to high levels during the sprouting process (1, 12, 19, 20). The goal of decontamination treatments should be to eliminate food-borne pathogens on seeds intended for sprout production.
Many studies have evaluated the effectiveness of various sanitizers, such as hypochlorites, organic acids, ozonated water, ethanol, and hydrogen peroxide, for reducing and eliminating Salmonella and E. coli O157:H7 on seeds (5, 9, 10, 17, 18, 24, 25, 27, 28). However, most treatments of seeds with a single chemical solution have not consistently reduced populations of pathogens by more than 3 log CFU/g (29). Noted exceptions are mung bean decontamination by treatment with acetic acid vapor at 45°C for 12 h (7) and elimination of E. coli O157:H7 and Salmonella in mung beans, soybeans, alfalfa seeds, and cress seeds without decreasing germination yields by treating with an oxychloro-based sanitizer (16). To achieve greater reductions in numbers of food-borne pathogens, sequential or simultaneous treatments with chlorine-based sanitizers, organic acids, heat, high pressure, and irradiation have been evaluated (6, 13, 17, 21, 23, 31). Although most studies using multiple treatments have been shown to result in greater reductions in pathogens than those using a single treatment, with some exceptions (3, 4), elimination of pathogens without decreasing the germination rate of seeds has been difficult. Bari et al. (4) reported that E. coli O157:H7 was eliminated from alfalfa, mung bean, and radish seeds without decreasing the germination rate and yield by applying dry heat (50°C for 1 h) and irradiation (2.5 kGy).
In a recent study, we observed a synergistic lethal effect of ClO2 treatment (50 or 200 μg/ml, 5 min) and subsequent air drying (25°C, 40% relative humidity [RH], 24 h) in killing E. coli O157:H7 on radish seeds (14). In a follow-up study, a combination of ClO2 (500 μg/ml, 5 min), air drying (25°C, 40% RH, 2 h), and dry-heat (55°C, 23% RH, 36 h) treatments reduced the total aerobic bacteria (TAB) count by >5 log CFU/g and E. coli O157:H7 by >4.8 log CFU/g without significantly decreasing seed viability (1). In the research reported here, we extended the latter study with the aim of eliminating E. coli O157:H7 on radish seeds without substantially decreasing the germination rate. We demonstrated the importance of the drying procedure between ClO2 and dry-heat treatments in preserving seed viability. Conditions for dry-heat treatment to eliminate E. coli O157:H7 without substantially reducing the viability of seeds were established.
MATERIALS AND METHODS
Bacterial strains and preparation of inoculum.
Five strains of E. coli O157:H7 were used: ATCC 43895 (isolated from hamburger), E0018 (isolated from bovine feces), F4546 (isolated from a patient in an alfalfa sprout-associated outbreak), H1730 (isolated from a lettuce-associated outbreak), and 932 (isolated from a patient with hemorrhagic colitis). We prepared the inoculum as described in earlier studies (1, 14) with some modifications. In brief, E. coli O157:H7 strains were adapted to grow in tryptic soy broth (TSB; Difco, BD Diagnostics, Sparks, MD) containing 50 μg/ml of nalidixic acid (TSBN) at 37°C. After three consecutive transfers at 24-h intervals, 150 ml of a five-strain cocktail was prepared by combining 30 ml of culture of each strain. The cocktail (150 ml) was centrifuged at 2,000 × g for 15 min at 25°C. The supernatant was decanted, and cells in the pellet were resuspended in sterile distilled water (1,500 ml) to give a population of ca. 8 log CFU/ml.
Inoculation of E. coli O157:H7 on radish seeds.
Radish seeds (350 g) purchased from Saessakmart (Seoul, Republic of Korea) were immersed in 1,050 ml of E. coli O157:H7 suspension (ca. 8 log CFU/ml) with gentle swirling for 5 min at 25 ± 2°C. Seeds were then placed on a sterile sieve (203-mm diameter by 41-mm depth; 600-μm pore size) and held for 2 h at 25 ± 2°C in a laminar flow biosafety hood before using in experiments.
Establishment of a 23% RH environment.
To create an atmosphere with 23% RH, 250 ml of saturated, filter-sterilized (bottle-top filter, 0.2-μm pore size; Corning Costar, Lowell, MA) potassium acetate (Sigma-Aldrich Inc., Milwaukee, WI) solution was deposited in a propylene container (2.1 liters; 25 cm long by 18 cm wide by 8 cm high; Lock & Lock, Seoul, Republic of Korea). The container was sealed with polyethylene film (Seven Wrap; Cleanson, Seoul, Republic of Korea) and incubated at 45, 60, 70, or 80°C for at least 48 h before using in experiments.
Preparation of ClO2 solution.
An aqueous solution of ClO2 was prepared by combining 450 ml of sodium chlorite solution (10,000 μg/ml) with 21 ml of hydrochloric acid (1 N) and incubating the mixture at 25 ± 2°C for 1 h. The solution was diluted in sterile distilled water to give a ClO2 concentration of 500 μg/ml. The pH of the ClO2 solution was 8.5 ± 0.4. The concentration of ClO2 was measured immediately before experiments using a chlorine colorimeter (model Dr/820; Hach, Loveland, CO).
Determination of germination rate.
Radish seeds (n = 100) were placed on sterile cheesecloth in a commercial sprout cultivator (225 by 325 by 150 mm; Shinhan Innovation & Creative, Suwon, Republic of Korea) containing sterile distilled water. The seeds were incubated at 25°C for 5 days, and the number of seeds that germinated and grew normally was counted. The germination percentage was calculated.
Optimization of temperature and time for drying seeds with high aw.
Radish seeds (40 g) were immersed in sterile distilled water (120 ml) with intermittent swirling for 5 min, spread on the surface of a sterile sieve (88.9-mm diameter by 41-mm depth; 600-μm pore size), placed above the surface of saturated potassium acetate solution (140 ml) in a propylene container (1.2 liters; 16 cm long by 16 cm wide by 9 cm high; Lock & Lock), and incubated at 25°C or 45°C for up to 48 h. After drying for 4, 8, 12, 24, 36, and 48 h, the water activity (aw) of seeds (3 g) was measured using a water activity meter (AquaLab Series 3TE; Decagon Devices, Inc., Pullman, WA).
Optimization of temperature and time for dry-heat treatments.
Radish seeds (40 g) were immersed in sterile distilled water (120 ml) for 5 min with gentle swirling. Seeds were then placed on a sterile sieve (88.9-mm diameter by 41-mm depth; 600-μm pore size), dried at 45°C in air containing 23% RH for 24 h, and heated at 60, 70, or 80°C and 23% RH for up to 48 h. Radish seeds (40 g) were also treated with water (120 ml) for 5 min and, without drying at 45°C, heated at 60, 70, or 80°C and 23% RH for up to 48 h. Germination percentages were determined after dry-heat treatment for 24 and 48 h.
Inactivation of E. coli O157:H7 on seeds by sequential treatments with ClO2, drying, and dry heat.
Immersion-inoculated seeds held for 2 h at 25 ± 2°C contained E. coli O157:H7 at a population of 5.9 log CFU/g. Seeds (220 g) were immersed in 660 ml of sterile distilled water or ClO2 solution (500 μg/ml) in a sterile glass bottle for 5 min, with intermittent swirling, and rinsed twice in sterile distilled water (660 ml) for 1 min. Treated seeds (40 g) were placed on a sterile sieve (88.9-mm diameter by 41-mm depth; 600-μm pore size) and positioned on a rack above 250 ml of saturated potassium acetate in a propylene container. The container was sealed with plastic wrap and held at 45°C at an internal RH of 23% for 24 h. After drying, seeds were incubated at 70°C and 23% RH for 24 and 48 h or at 80°C and 23% RH for 6, 12, 24, and 48 h.
Microbiological analyses of seeds.
Populations of total aerobic bacteria (TAB), E. coli O157:H7, and molds and yeasts (MY) on radish seeds were determined before treatment with water (control) or ClO2 (0 h), after treatment with water or ClO2 for 5 min, after drying (45°C, 23% RH, 24 h), and after dry-heat treatment (70°C, 23% RH, 24 and 48 h; 80°C, 23% RH, 6, 12, 24, and 48 h). At each sampling time, seeds (5 g) were deposited in TSB (45 ml) in a polyolefin stomacher bag (400 ml; Interscience, St. Nom La Breteche, France) and pummeled for 1 min. The TSB in the TSB-seed mixtures was serially diluted in 0.1% peptone water (or not diluted) and surface plated on tryptic soy agar (TSA) for enumerating TAB, MacConkey sorbitol (Difco, BD Diagnostics) agar supplemented with nalidixic acid (50 μg/ml) (MSAN) for enumerating E. coli O157:H7 cells, and dichloran rose bengal chloramphenicol (DRBC; Difco, BD Diagnostics) agar for enumerating MY. TSA and MSAN plates were incubated at 37°C for at least 24 h, and DRBC plates were incubated at 25°C for 5 days before colonies were counted. For seeds heated at 70°C for 24 or 48 h or at 80°C for 6, 12, 24, or 48 h, mixtures of seeds and TSB were incubated at 37°C for 48 h to enrich for E. coli O157:H7. The enriched suspension was streaked on TSA and MSAN and incubated at 37°C for 24 h. Colonies presumptive for E. coli O157:H7 that formed on TSA and MSAN were randomly selected and tested using an E. coli O157:H7 latex agglutination test (Oxoid, Basingstoke, United Kingdom). The detection limit by direct plating was 9 CFU/g of seeds (0.95 log CFU/g); the limit by enrichment was 1 CFU/5 g of seeds (−0.70 log CFU/g). Germination percentages were determined after dry-heat treatment, as described above.
Microbiological analysis of sprouts.
Radish seeds (20 g) treated with ClO2 solution (500 μg/ml) for 5 min, dried at 45°C and 23% RH for 24 h, and dry heated at 70°C and 23% RH for 24 h and 48 h or at 80°C and 23% RH for 6, 12, 24, and 48 h were soaked in sterile water at 35°C for 2 h, placed in a commercial sprout cultivator, and incubated at 25°C for 5 days. Sprouts (10 g) were aseptically collected, combined with 90 ml of TSB in a stomacher bag, and pummeled for 1 min. The homogenate was serially diluted in sterile 0.1% peptone, surface plated (0.1 ml in duplicate) on TSA, MSAN, and DRBC agar, and incubated at 37°C for 24 h (TSA, MSAN) or 25°C for 5 days (DRBC agar). To enrich for E. coli O157:H7 on sprouts, the remaining sprout and TSB mixture was incubated at 37°C for 48 h, streaked on TSA and MSAN, and incubated at 37°C for 48 h. Several colonies that formed on TSA and MSAN were randomly selected and tested for E. coli O157:H7 using an E. coli O157:H7 latex agglutination test. The detection limit by direct plating was 10 CFU/g sprout (1.0 log CFU/g); the limit by enrichment was 1 CFU/10 g sprout (−1.0 log CFU/g).
Statistical analysis.
All experiments were replicated at least three times. Data were analyzed using the general linear model of the Statistical Analysis Systems procedure (SAS; SAS Institute, Cary, NC). Analysis to determine the effects of drying, heating temperature, and heating time on germination rate and the effect of sequential ClO2, drying, and dry-heat treatments on TAB, E. coli O157:H7, and MY populations recovered from radish seeds and sprouts was done using Fisher's least significant difference (LSD) test. Significant differences are presented at a 95% confidence level (P ≤ 0.05).
RESULTS
Optimization of temperature and time for drying seeds with high aw.
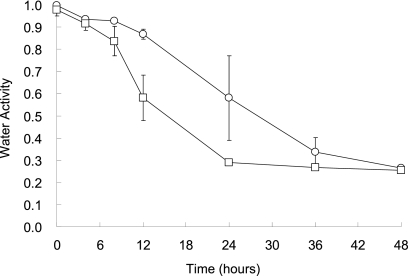
To minimize the adverse effect of wet heat on seed viability, radish seeds with high aw were dried before being exposed to dry-heat treatment. Figure 1 shows the aw of radish seeds which were immersed in water for 5 min and incubated at 25°C or 45°C and 23% RH for 24 or 48 h. The aw of seeds was >0.99 after treatment with water for 5 min. When these seeds were stored at 25°C and 23% RH, the aw decreased to <0.30 within 48 h. When stored at 45°C, the aw was <0.30 within 24 h and remained constant for an additional 24 h.
Fig. 1.
Water activity of radish seeds dried at 25°C (○) or 45°C (□). Radish seeds were immersed in water for 5 min and incubated at 25 or 45°C for up to 48 h at 23% relative humidity (RH).
Optimization of temperature and time for dry-heat treatments.
Tests were done to determine dry-heat conditions that minimize decreases in the germination rate of radish seeds. The influence of drying seeds between immersing in water and dry-heat treatment on the germination rate was also evaluated. Table 1 shows the germination rate of radish seeds as affected by drying, dry-heat temperature, and dry-heat treatment time. Seeds immersed in water for 5 min and, without drying, heated at 60°C and 23% RH for 24 or 48 h had germination rates of 57.3 and 63.7%, respectively. When radish seeds were immersed in water for 5 min, dried at 45°C and 23% RH for 24 h, and heated at 60°C and 23% RH for 24 or 48 h, germination rates were 87.7 or 84.5%, respectively. When water-treated radish seeds were heated without drying at 70°C and 23% RH for 24 or 48 h, germination rates were only 35.0 or 31.7%, respectively. However, when seeds were dried between water treatment and dry-heat treatment, at 70°C for 24 or 48 h, the germination rate was 84.3%. When the dry-heat temperature was increased to 80°C, the germination rate of seeds was 89.0% after dry-heat treatment for 24 h but decreased significantly to 69.5% after treatment for 48 h.
Table 1.
Effects of drying, dry-heat temperature, and heating time on the germination rate of radish seedsa
| Dry-heat temp (oC) | Dry-heat time (h) | Germination rate ± SD (%)b |
|
|---|---|---|---|
| Without drying | With drying | ||
| 60 | 24 | a 57.3 ± 9.0 B | a 87.7 ± 2.3 A |
| 48 | a 63.7 ± 4.7 B | a 84.5 ± 5.1 A | |
| 70 | 24 | a 35.0 ± 15.7 B | a 84.3 ± 7.6 A |
| 48 | a 31.7 ± 11.5 B | a 84.3 ± 4.6 A | |
| 80 | 24 | a 14.5 ± 12.0 B | a 89.0 ± 2.8 A |
| 48 | a 0.0 ± 0.0 B | b 69.5 ± 4.9 A | |
Seeds were immersed in water for 5 min, dried at 45°C and 23% RH for 24 h (or not dried), and incubated at 60, 70, or 80°C and 23% RH for 24 or 48 h before the germination rates were determined. The germination rate of untreated radish seeds (control) was 89.3 ± 6.7%.
Values in the same row that are not followed by the same uppercase letter are significantly different (P ≤ 0.05). Within the same temperature, values in the same column that are not preceded by the same lowercase letter are significantly different (P ≤ 0.05).
Inactivation of E. coli O157:H7 on seeds by sequential treatments with ClO2, drying, and dry heat.
The effects of the sequential treatments (ClO2, drying, and dry heat at 70°C or 80°C) on populations of microorganisms on radish seeds and germination rates were determined.
Table 2 shows the TAB, E. coli O157:H7, and MY populations on seeds treated with water or ClO2 (500 μg/ml) for 5 min, dried at 45°C and 23% RH for 24 h, and dry heated at 70°C and 23% RH for 24 or 48 h. The initial populations of TAB, E. coli O157:H7, and MY on seeds (6.1, 5.9, and 3.5 log CFU/g, respectively) were not significantly reduced by treatment with water for 5 min. Drying seeds at 45°C did not significantly reduce TAB and E. coli O157:H7 populations, but the number of MY was significantly lower (1.0 log CFU/g) than the number recovered from seeds treated with water. Subsequent dry-heat treatment at 70°C and 23% RH for 48 h decreased the populations of TAB and E. coli O157:H7 to 4.1 and 3.4 log CFU/g, respectively, but the population of MY did not significantly change.
Table 2.
Populations of TAB, E. coli O157:H7, and MY on radish seeds treated with water or ClO2 (500 μg/ml) for 5 min, dried at 45°C and 23% RH for 24 h, and dry heated at 70°C and 23% RH for 24 or 48 h
| Microorganism | Water or ClO2 treatment | Population ± SD (log CFU/g)a |
||||
|---|---|---|---|---|---|---|
| Treatment time |
Dried at 45°C for 24 h | Dry-heat treatment at 70°C |
||||
| 0 h | 5 min | 24 h | 48 h | |||
| TAB | Water | a 6.1 ± 0.2 A | a 5.7 ± 0.1 A | a 5.8 ± 0.6 A | a 4.8 ± 0.5 B | a 4.1 ± 0.2 C |
| ClO2 | a 6.1 ± 0.2 A | b 5.3 ± 0.1 B | b <1.3 ± 0.3 C | b <1.0 ± 0.0 C | b <1.4 ± 0.4 C | |
| E. coli O157:H7 | Water | a 5.9 ± 0.2 A | a 5.5 ± 0.1 AB | a 5.0 ± 0.8 B | a 4.2 ± 0.4 C | a 3.4 ± 0.2 D |
| ClO2 | a 5.9 ± 0.2 A | b 5.0 ± 0.1 B | b <1.0 (3/3)b C | b <1.0 (3/3) C | b <1.0 (0/3) C | |
| MY | Water | a 3.5 ± 0.5 A | a 3.3 ± 0.3 A | a 2.3 ± 0.1 B | a 2.0 ± 0.6 B | a 1.7 ± 0.4 B |
| ClO2 | a 3.5 ± 0.5 A | b 1.7 ± 0.3 B | b 1.1 ± 0.2 B | a <1.6 ± 0.7 B | a <1.2 ± 0.2 B | |
Values in the same row that are not followed by the same uppercase letter are significantly different (P ≤ 0.05). Within the same microorganism, values in the same column that are not preceded by the same lowercase letter are significantly different (P ≤ 0.05).
None detected by direct plating. Values in parentheses represent the number of samples out of three analyzed in three replicate trials that were positive for E. coli O157:H7 as determined by enrichment. Detection limit by direct plating was 9 CFU/g of seeds; detection limit by enrichment was 1 CFU/5 g of seeds.
When inoculated radish seeds were treated with ClO2 (500 μg/ml) for 5 min, populations of TAB, E. coli O157:H7, and MY were significantly decreased from 6.1, 5.9, and 3.5 log CFU/g to 5.3, 5.0, and 1.7 log CFU/g, respectively (Table 2). When the ClO2-treated seeds were dried at 45°C and 23% RH for 24 h, populations of TAB and E. coli O157:H7 significantly decreased by >4.0 log CFU/g. The E. coli O157:H7 population on seeds was decreased to an undetectable level (<0.95 log CFU/g) by direct plating but was detected by enrichment (≥1 CFU/5 g). The number of MY was not significantly decreased by drying seeds at 45°C. Dry-heat treatment of seeds at 70°C and 23% RH for 48 h caused TAB and MY to decrease to levels approaching the detection limit by direct plating. E. coli O157:H7 was not detected by enrichment in seeds exposed to dry heat for 48 h.
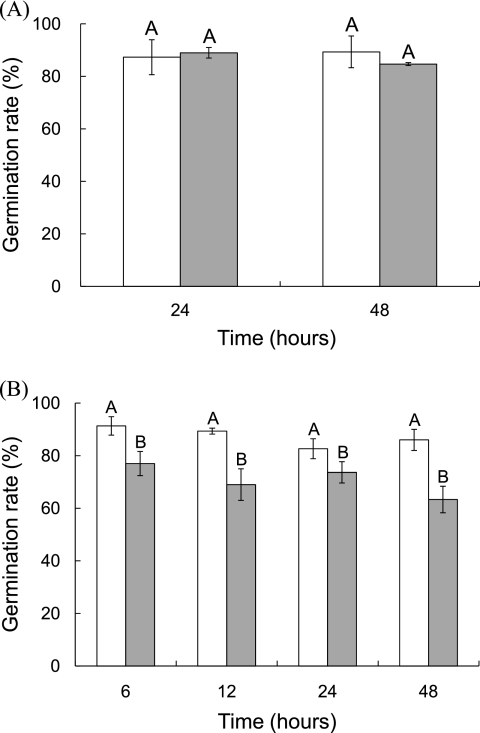
Figure 2A shows the germination rates of radish seeds initially containing E. coli O157:H7 (5.9 log CFU/g) after sequential treatments with water or ClO2 (500 μg/ml, 5 min), drying (45°C, 23% RH, 24 h), and dry heat (70°C, 23% RH, 24 or 48 h). The germination rate of seeds exposed to the harshest conditions (ClO2 [500 μg/ml, 5 min], drying [45°C, 23% RH, 24 h], and dry heating [70°C, 23% RH, 48 h]) was not significantly different than that of untreated radish seeds.
Fig. 2.
Germination rate of radish seeds after sequential ClO2, drying, and heat treatments. Radish seeds were immersed in water (white bars) or 500 μg/ml of ClO2 (gray bars) for 5 min, dried at 45°C and 23% relative humidity (RH) for 24 h, and dry heated at 70°C (A) or 80°C (B) and 23% RH for 24 or 48 h. Bars indicate standard deviations. For the same dry-heat treatment time, bars not noted by the same letter are significantly different (P ≤ 0.05).
Table 3 shows the populations of TAB, E. coli O157:H7, and MY on radish sprouts cultivated at 25°C for 5 days using seeds which had been subjected to sequential treatments with ClO2 (500 μg/ml, 5 min) followed by drying (45°C, 23% RH, 24 h) and dry heat (70°C, 23% RH, 24 or 48 h). Populations of TAB on radish sprouts were 7.4 to 8.4 log CFU/g, regardless of treatment with water or ClO2 or heating time applied to seeds. Sprouts produced using seeds that had been exposed to ClO2, drying, and dry-heat treatments were negative for E. coli O157:H7 by enrichment. However, sprouts cultivated from seeds that had been treated with water rather than ClO2 and dry heated for 48 h contained 6.1 log CFU/g; sprouts produced from seeds treated with ClO2 but heated for only 24 h contain E. coli O157:H7 at 7.2 log CFU/g. These results showed that E. coli O157:H7 can rapidly increase to a high population during cultivation, even after significant reduction on treated seeds. Sprouts produced from radish seeds treated with water had MY counts of 7.8 to 8.2 log CFU/g. Sprouts produced from seeds treated with ClO2 and subsequently subjected to dry-heat treatments contained only 3.0 to 3.9 log CFU/g.
Table 3.
Populations of TAB, E. coli O157:H7, and MY on radish sproutsa
| Microorganism | Water or ClO2 treatment | Population ± SD (log10 CFU/g)b after dry-heat treatment |
|
|---|---|---|---|
| 24 h | 48 h | ||
| TAB | Water | a 8.4 ± 0.4 A | a 7.8 ± 0.5 A |
| ClO2 | a 7.6 ± 0.3 A | a 7.4 ± 1.0 A | |
| E. coli O157:H7 | Water | a 6.7 ± 0.2 A | a 6.1 ± 0.5 A |
| ClO2 | a 7.2 ± 0.6 A | b <1.0 (0/3)c B | |
| MY | Water | a 8.2 ± 0.5 A | a 7.8 ± 0.3 A |
| ClO2 | b 3.9 ± 0.6 A | b 3.0 ± 2.6 A | |
Radish seeds inoculated with E. coli O157:H7 (5.9 log CFU/g) were treated with water or ClO2 (500 μg/ml) for 5 min, dried at 45°C and 23% RH for 24 h, and dry heated at 70°C and 23% RH for 24 or 48 h. Treated radish seeds were cultivated at 25°C for 5 days.
Values in the same row that are not followed by the same uppercase letter are significantly different (P ≤ 0.05). Within the same microorganism, values in the same column that are not preceded by the same lowercase letter are significantly different (P ≤ 0.05).
None detected by direct plating. Values in parentheses represent the number of samples out of three analyzed in three replicate trials that were positive for E. coli O157:H7 as determined by enrichment. Detection limit by direct plating was 10 CFU/g of sprouts; detection limit by enrichment was 1 CFU/10 g of sprouts.
Shown in Table 4 are populations of TAB, E. coli O157:H7, and MY on radish seeds treated with water or ClO2 (500 μg/ml) for 5 min, dried at 45°C and 23% RH for 24 h, and dry heated at 80°C and 23% RH for 6, 12, 24, or 48 h. The initial populations of TAB, E. coli O157:H7, and MY on radish seeds were 6.3, 5.9, and 5.2 log CFU/g, respectively. The populations of TAB, E. coli O157:H7, and MY on seeds after treatment with water and drying for 24 h at 45°C decreased significantly to 4.8, 4.1, and 3.3 log CFU/g, respectively. Dry-heat treatment at 80°C and 23% RH for 48 h reduced the population of TAB to 2.2 log CFU/g; E. coli O157:H7 and MY populations were reduced to levels below the detection limit (<0.95 log CFU/g) by direct plating but were detected by enrichment (≥1 CFU/5 g). The number of TAB on seeds treated with ClO2 for 5 min and dried for 24 h significantly decreased from 6.3 log CFU/g to 1.9 log CFU/g. E. coli O157:H7 and MY counts were significantly decreased from 5.9 and 5.2 log CFU/g, respectively, to below the detection limit for direct plating; however, both were detected by enrichment. When seeds were subsequently dry heated at 80°C and 23% RH for 6, 12, 24, or 48 h, TAB and MY populations decreased to numbers approaching the detection limit by direct plating. E. coli O157:H7 was detected by enrichment of seeds dry heated at 80°C for 6, 12, or 24 h. When the dry-heating time was extended to 48 h, the pathogen was not detected by enrichment.
Table 4.
Populations of TAB, E. coli O157:H7, and MY on radish seeds after treatment with water or ClO2 (500 μg/ml) for 5 min, dried at 45°C and 23% RH for 24 h, and dry heated at 80°C and 23% RH for up to 48 h
| Microorganism | Water or ClO2 treatment | Population ± SD (log10 CFU/g)a |
||||||
|---|---|---|---|---|---|---|---|---|
| Treatment time |
Drying at 45°C for 24 h | Dry-heat treatment |
||||||
| 0 h | 5 min | 6 h | 12 h | 24 h | 48 h | |||
| TAB | Water | a 6.3 ± 0.4 A | a 6.2 ± 0.2 A | a 4.8 ± 0.3 B | a 4.4 ± 0.5 BC | a 4.4 ± 0.3 BC | a 4.0 ± 0.6 C | a 2.2 ± 0.2 D |
| ClO2 | a 6.3 ± 0.4 A | a 4.9 ± 0.6 B | b 1.9 ± 0.9 C | b <1.0 (3/3)b D | b <1.0 (3/3) D | b <1.1 ± 0.2 D | b <1.0 (1/3) D | |
| E. coli O157:H7 | Water | a 5.9 ± 0.3 A | a 5.6 ± 0.3 A | a 4.1 ± 0.4 B | a 3.5 ± 0.4 B | a 3.0 ± 0.7 B | a 3.0 ± 1.4 B | a <1.0 (3/3) C |
| ClO2 | a 5.9 ± 0.3 A | a 4.5 ± 0.6 B | b <1.0 (3/3) C | b <1.0 (2/3) C | b <1.0 (0/3) C | a <1.0 (1/3) C | a <1.0 (0/3) C | |
| MY | Water | a 5.2 ± 0.2 A | a 3.6 ± 0.2 B | a 3.3 ± 0.4 B | a 2.9 ± 0.4 BC | a 2.5 ± 0.3 C | a <1.3 ± 0.6 D | a <1.0 (3/3) D |
| ClO2 | a 5.2 ± 0.2 A | a 3.0 ± 1.3 B | b <1.0 ± 0.0 C | b <1.1 ± 0.1 C | b <1.1 ± 0.2 C | a <1.1 ± 0.2 C | a <1.1 ± 0.2 C | |
Values in the same row that are not followed by the same uppercase letter are significantly different (P ≤ 0.05). Within the same microorganism, values in the same column that are not preceded by the same lowercase letter are significantly different (P ≤ 0.05).
None detected by direct plating. Values in parentheses represent the number of samples out of three analyzed in three replicate trials that were positive for E. coli O157:H7 as determined by enrichment. Detection limit by direct plating was 9 CFU/g of seeds, and detection limit by enrichment was 1 CFU/5 g of seeds.
Figure 2B shows the germination rates of radish seeds previously inoculated with E. coli O157:H7 and subjected to sequential treatments (water or ClO2 [500 μg/ml, 5 min], drying [45°C, 23% RH, 24 h], and dry heating [80°C, 23% RH, 6, 12, 24 or 48 h]). The germination rates of seeds exposed to water, drying, and dry-heat treatment were 82.7 to 91.3% and were not significantly different from that of untreated seeds (89.3%). However, when radish seeds were treated with ClO2, dried, and dry heated, the germination rates were significantly lower (63.3 to 77.0%) than those of untreated seeds and seeds exposed to sequential treatments with water, drying, and dry heating.
Populations of TAB, E. coli O157:H7, and MY on radish sprouts cultivated at 25°C for 5 days after treatment of seeds with water or ClO2 (500 μg/ml) for 5 min, dried at 45°C and 23% RH for 24 h, and dry heated at 80°C and 23% RH for 6, 12, 24, or 48 h are shown in Table 5. The number of TAB on radish sprouts was ca. 7.9 to 8.3 log CFU/g, regardless of treatment with water or ClO2 or dry-heat time. When sprouts were produced using seeds which had been subjected to sequential treatments with ClO2 (500 μg/ml, 5 min), drying (45°C, 23% RH, 24 h), and dry heat (80°C, 23% RH, 48 h), E. coli O157:H7 was not detected by enrichment. When radish seeds were treated with ClO2, dried, and dry heated for 24 h or less, cultivated radish sprouts contained E. coli O157:H7 at populations of 1.7 to 3.3 log CFU/g. The population of MY was 1.6 log CFU/g of sprouts produced from seeds treated with ClO2 (500 μg/ml, 5 min), followed by drying (45°C, 23% RH, 24 h) and heating (80°C, 23% RH, 48 h). Populations of MY on sprouts produced from seeds exposed to other dry-heat treatments were 6.1 to 8.2 log CFU/g.
Table 5.
Populations of TAB, E. coli O157:H7, and MY on radish sproutsa
| Microorganism | Water or ClO2 treatment | Population ± SD (log10 CFU/g)b after dry-heat treatment |
|||
|---|---|---|---|---|---|
| 6 h | 12 h | 24 h | 48 h | ||
| TAB | Water | a 8.2 ± 0.2 AB | a 7.9 ± 0.2 B | a 8.1 ± 0.2 AB | a 8.3 ± 0.1 A |
| ClO2 | a 8.3 ± 0.2 A | a 8.3 ± 0.2 A | a 8.3 ± 0.2 A | a 8.2 ± 0.0 A | |
| E. coli O157:H7 | Water | a 6.9 ± 0.8 A | a 6.6 ± 0.2 A | a 6.4 ± 0.2 AB | a 5.5 ± 0.6 B |
| ClO2 | a 3.3 ± 2.5 A | a 3.1 ± 3.6 A | b 1.7 ± 1.1 A | b <1.0 (0/3)c A | |
| MY | Water | a 8.0 ± 0.1 A | a 7.9 ± 0.2 A | a 8.1 ± 0.2 A | a 8.2 ± 0.2 A |
| ClO2 | a 7.8 ± 0.5 A | a 6.1 ± 3.4 A | a 6.7 ± 1.6 A | b 1.6 ± 0.4 B | |
Radish seeds inoculated with E. coli O157:H7 (5.9 log CFU/g) were treated with water or ClO2 (500 μg/ml) for 5 min, dried at 45°C and 23% RH for 24 h, and dry heated at 80°C and 23% RH for up to 48 h. After dry-heat treatment for 6, 12, 24, or 48 h, radish seeds were cultivated at 25°C for 5 days.
Values in the same row that are not followed by the same uppercase letter are significantly different (P ≤ 0.05). Within the same microorganism, values in the same column that are not preceded by the same lowercase letter are significantly different (P ≤ 0.05).
None detected by direct plating. Values in parentheses represent number of samples out of three analyzed in three replicate trials that were positive for E. coli O157:H7 as determined by enrichment. Detection limit by direct plating was 10 CFU/g of sprouts; detection limit by enrichment was 1 CFU/10 g of sprouts.
DISCUSSION
This study evolved from earlier observations that treatment of radish seeds with ClO2 followed by drying and dry-heat treatment has a synergistic effect in killing E. coli O157:H7 (1, 2, 14). Kim et al. (14) reported that ClO2 treatment had a synergistic lethal effect on E. coli O157:H7 when combined with air drying. However, the treatment did not achieve the 5-log CFU/g reduction in pathogens recommended by NACMCF (20). Thus, an additional dry-heat treatment was applied in a follow-up study to increase lethality (1). We examined dry-heat treatment for its efficacy in killing E. coli O157:H7 because it is known to be less detrimental to seed germination than wet-heat treatment (8, 26). Also, lethality of dry heat may not be affected by crevices or wrinkles on seed testae, which are thought to protect pathogens from contact with chemical treatment solutions (8). Bang et al. (1) reported that treatment of radish seeds with ClO2 (500 μg/ml) for 5 min, air drying at 25°C for 2 h, and dry heating at 55°C and 23% RH for 36 h reduced TAB and E. coli O157:H7 counts by 5.1 log CFU/g and >4.8 log CFU/g, respectively. The E. coli O157:H7 population on radish seeds was reduced to levels below the detection limit by direct plating (0.8 log CFU/g). However, sprouts grown from treated seeds at 25°C for 5 days contained E. coli O157:H7 at ca. 4.3 log CFU/g. In a follow-up study, we attempted to enhance the lethality of sequential treatments by increasing the dry-heat temperature from 55°C to 60°C (2). We treated radish seeds containing E. coli O157:H7 at a population of 5.5 log CFU/g with ClO2 (500 μg/ml) for 5 min, without drying, followed by dry-heat treatment at 60°C and 23% RH for up to 48 h (2). Using these treatments, E. coli O157:H7 was eliminated from radish seeds and sprouts produced from them; however, the germination rate of seeds was decreased significantly. We suspected that the high moisture content of ClO2-treated seeds may have caused loss in germinability during the early stages of dry-heat treatment.
In the study reported here, we increased the lethality of sequential treatments (ClO2, drying, and dry-heat treatments) without decreasing seed viability by optimizing conditions for drying and dry-heat treatment. We hypothesized that, when ClO2 and dry-heat treatments are sequentially applied to radish seeds, the drying treatment after ClO2 treatment (before dry-heat treatment) is critical to minimizing the adverse effect of wet heat on seed viability during the early stage of dry-heat treatment. To test this hypothesis, the influence of drying conditions on seed viability was further investigated. When radish seeds with high aw were stored at 25 or 45°C with 23% RH, it took 48 h or 24 h, respectively, to decrease the aw to <0.30. Because an extended drying period may be undesirable by sprout producers, we decided to dry seeds at 45°C for 24 h.
After establishing drying conditions, the effect of the drying procedure in preserving seed viability was confirmed, and the optimum dry-heat temperature and time were established. Dry-heat treatment of radish seeds with high aw significantly decreased the germination rate. However, when seeds were dried at 45°C preceding dry heating, the germination rate was not compromised, even after treatment at 70°C for 48 h or at 80°C for 24 h. This indicated that drying seeds between ClO2 and dry-heat treatments is essential to preserve seed viability. Based upon these results, 70°C for 24 and 48 h and 80°C for 6, 12, 24, and 48 h were selected as conditions for dry-heat treatment. Sequential treatments were applied to radish seeds containing E. coli O157:H7 (5.9 log CFU/g). When radish seeds were treated with ClO2 (500 μg/ml, 5 min), dried (45°C, 23% RH, 24 h), and dry heated (70°C, 23% RH, 48 h), the pathogen was not detected in seeds, even after enrichment.
The germination rate (84.7%) of radish seeds that had been exposed to the sequential treatments was not significantly different from that of untreated radish seeds (89.3%). E. coli O157:H7 was not detected in sprouts produced from those seeds. However, when the dry-heating time was reduced to 24 h, sprouts contained E. coli O157:H7 at 7.2 log CFU/g. These results indicated that, even if the population of E. coli O157:H7 on radish seeds was reduced to a very low level, the population after cultivation of those seeds could be high. Similar results have been reported by several researchers (1, 12, 19, 20). This emphasizes the need to eliminate E. coli O157:H7 from seeds used to produce sprouts. As the number of MY on radish seeds was reduced, final populations on sprouts also decreased significantly.
Based on these results, we conclude that sequential treatment of radish seeds with ClO2 (500 μg/ml, 5 min), drying (45°C, 23% RH, 24 h), and dry heating (70°C, 23% RH, 48 h) inactivates E. coli O157:H7 at populations of at least 5.9 log CFU/g without significantly decreasing the germination rate. To determine if an increase in dry-heating temperature could be used without lowering the germination rate, seeds were dry heated at 80°C. Results suggest that that the increased temperature did not substantially decrease the time required to eliminate E. coli O157:H7 on radish seeds. In addition, the germination rate of radish seeds treated with ClO2 decreased significantly by dry-heat treatment at 80°C compared to that of seeds that had been treated with water. We concluded that treatment at 70°C was superior to 80°C as a dry-heating temperature to preserve seed viability.
In summary, sequential treatments to eliminate E. coli O157:H7 from radish seeds without decreasing the germination rate have been developed. The optimum conditions for drying radish seeds with high aw were established. The effects of drying in combination with treatments with aqueous ClO2 and dry heat in preserving seed viability were determined. Finally, we confirmed that high numbers of E. coli O157:H7 (5.9 log CFU/g) were eliminated on radish seeds and sprouts produced from them by applying sequential treatments of ClO2 (500 μg/ml, 5 min), drying (45°C, 23% RH, 24 h), and dry heating (70°C, 23% RH, 48 h).
In future studies, conditions of ClO2 treatment, such as concentration and treatment time, should be optimized. Decreasing the concentration of ClO2 may allow the use of a higher dry-heat temperature to eliminate E. coli O157:H7 on radish seeds without substantially decreasing the germination rate. The use of organic acid-based ClO2 solution at pH 5 to 6 should also be considered, since its lethality may be better than that of HCl-based ClO2 (15). The efficacy of the decontamination procedure developed in this study should be validated using commercial-scale sprout production practices. The treatment should also be tested for efficacy in eliminating Salmonella on radish seeds.
ACKNOWLEDGMENTS
This study was carried out with the support of the Cooperative Research Program for Agricultural Science and Technology Development (project no. PJ007387), Rural Development Administration, Republic of Korea, and a Korea University grant.
Footnotes
Published ahead of print on 29 July 2011.
REFERENCES
- 1. Bang J., Kim H., Kim H., Beuchat L. R., Ryu J.-H. 2011. Combined effects of chlorine dioxide, drying, and dry heat treatments in inactivating microorganisms on radish seeds. Food Microbiol. 28:114–118 [DOI] [PubMed] [Google Scholar]
- 2. Bang J., et al. 2011. Reduction of Escherichia coli O157:H7 on radish seeds by sequential application of aqueous chlorine dioxide and dry heat treatment. Lett. Appl. Microbiol. [Epub ahead of print.] doi: 10.1111/j.1472-765X.2011.03125.x [DOI] [PubMed] [Google Scholar]
- 3. Bari M. L., Nei D., Enomoto K., Todoriki S., Kawamoto S. 2009. Combination treatments for killing Escherichia coli O157:H7 on alfalfa, radish, broccoli, and mung bean seeds. J. Food Prot. 72:631–636 [DOI] [PubMed] [Google Scholar]
- 4. Bari M. L., Nazuka E., Sabina Y., Todoriki S., Isshiki K. 2003. Chemical and irradiation treatments for killing Escherichia coli O157:H7 on alfalfa, radish, and mung bean seeds. J. Food Prot. 66:767–774 [DOI] [PubMed] [Google Scholar]
- 5. Beuchat L. R. 1997. Comparison of chemical treatments to kill Salmonella on alfalfa seeds destined for sprout production. Int. J. Food Microbiol. 34:329–333 [DOI] [PubMed] [Google Scholar]
- 6. Beuchat L. R., Scouten A. J. 2002. Combined effects of water activity, temperature and chemical treatments on the survival of Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Appl. Microbiol. 92:382–395 [DOI] [PubMed] [Google Scholar]
- 7. Delaquis P. J., Sholberg P. L., Stanich K. 1999. Disinfection of mung bean seed with gaseous acetic acid. J. Food Prot. 62:953–957 [DOI] [PubMed] [Google Scholar]
- 8. Feng G., Churey J. J., Worobo R. W. 2007. Thermal inactivation of Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Food Prot. 70:1698–1703 [DOI] [PubMed] [Google Scholar]
- 9. Gabriel A. A. 2005. Microbial quality of chlorine soaked mung bean seeds and sprouts. Food Sci. Technol. Res. 11:95–100 [Google Scholar]
- 10. Gandhi M., Matthews K. R. 2003. Efficacy of chlorine and calcinated calcium treatment of alfalfa seeds and sprouts to eliminate Salmonella. Int. J. Food Microbiol. 87:301–306 [DOI] [PubMed] [Google Scholar]
- 11. Guiterrez E. 1997. Japan prepares as O157 strikes again. Lancet 349:1156 [Google Scholar]
- 12. Holliday S. L., Scouten A. J., Beuchat L. R. 2001. Efficacy of chemical treatments in eliminating Salmonella and Escherichia coli O157:H7 on scarified and polished alfalfa seeds. J. Food Prot. 64:1489–1495 [DOI] [PubMed] [Google Scholar]
- 13. Jaquette C. B., Beuchat L. R., Mahon B. E. 1996. Efficacy of chlorine and heat treatment in killing Salmonella stanley inoculated onto alfalfa seeds and growth and survival of the pathogen during sprouting and storage. Appl. Environ. Microbiol. 62:2212–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H., Kim H., Bang J., Beuchat L. R., Ryu J.-H. 2010. Synergistic effect of chlorine dioxide and drying treatments for inactivating Escherichia coli O157:H7 on radish seeds. J. Food Prot. 73:1225–1230 [DOI] [PubMed] [Google Scholar]
- 15. Kim H., Kang Y., Beuchat L. R., Ryu J.-H. 2008. Production and stability of chlorine dioxide in organic acid solutions as affected by pH, type of acid, and concentration of sodium chlorite, and its effectiveness in inactivating Bacillus cereus spores. Food Microbiol. 25:964–969 [DOI] [PubMed] [Google Scholar]
- 16. Kumar M., Hora R., Kostrzynska M., Waites W. M., Warriner K. 2006. Inactivation of Escherichia coli O157:H7 and Salmonella on mung beans, alfalfa, and other seed types destined for sprout production by using an oxychloro-based sanitizer. J. Food Prot. 69:1571–1578 [DOI] [PubMed] [Google Scholar]
- 17. Lang M. M., Ingham B. H., Ingham S. C. 2000. Efficacy of novel organic acid and hypochlorite treatments for eliminating Escherichia coli O157:H7 from alfalfa seeds prior to sprouting. Int. J. Food Microbiol. 58:73–82 [DOI] [PubMed] [Google Scholar]
- 18. Lim J.-H., Jeong J.-W., Kim J.-H., Park K.-J. 2008. Efficacy of aqueous chlorine dioxide and citric acid in reducing Escherichia coli on the radish seeds used for sprout production. Food Sci. Biotechnol. 17:878–882 [Google Scholar]
- 19. Muñoz M., De Ancos B., Sánchez-Moreno C., Pilar Cano M. 2006. Evaluation of chemical and physical (high-pressure and temperature) treatments to improve the safety of minimally processed mung bean sprouts during refrigerated storage. J. Food Prot. 69:2395–2402 [DOI] [PubMed] [Google Scholar]
- 20. National Advisory Committee on Microbiological Criteria for Foods 1999. Microbiological safety evaluations and recommendations on sprouted seeds. Int. J. Food Microbiol. 52:123–153 [DOI] [PubMed] [Google Scholar]
- 21. Peñas E., Gómez R., Frías J., Vidal-Valverde C. 2009. Efficacy of combinations of high pressure treatment, temperature and antimicrobial compounds to improve the microbiological quality of alfalfa seeds for sprout production. Food Control 20:31–39 [Google Scholar]
- 22. Saroj S. D., et al. 2007. Radiation processing for elimination of Salmonella Typhimurium from inoculated seeds used for sprout making in India and effect of irradiation on germination of seeds. J. Food Prot. 70:1961–1965 [DOI] [PubMed] [Google Scholar]
- 23. Scouten A. J., Beuchat L. R. 2002. Combined effects of chemical, heat and ultrasound treatments to kill Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Appl. Microbiol. 92:668–674 [DOI] [PubMed] [Google Scholar]
- 24. Sharma R. R., Demirci A., Beuchat L. R., Fett W. F. 2003. Application of ozone for inactivation of Escherichia coli O157:H7 on inoculated alfalfa sprouts. J. Food Process. Preserv. 27:51–64 [Google Scholar]
- 25. Sharma R. R., Demirci A. 2003. Treatment of Escherichia coli O157:H7 inoculated alfalfa seeds and sprouts with electrolyzed oxidizing water. Int. J. Food Microbiol. 86:231–237 [DOI] [PubMed] [Google Scholar]
- 26. Siddiqui S. U., Ali A., Chaudhary M. F. 2008. Germination behavior of wheat (Triticum aestivum) varieties to artificial ageing under varying temperature and humidity. Pak. J. Bot. 40:1121–1127 [Google Scholar]
- 27. Taormina P. J., Beuchat L. R. 1999. Behavior of enterohemorrhagic Escherichia coli O157:H7 on alfalfa sprouts during the sprouting process as influenced by treatments with various chemicals. J. Food Prot. 62:850–856 [DOI] [PubMed] [Google Scholar]
- 28. Taormina P. J., Beuchat L. R. 1999. Comparison of chemical treatments to eliminate enterohemorrhagic Escherichia coli O157:H7 on alfalfa seeds. J. Food Prot. 62:318–324 [DOI] [PubMed] [Google Scholar]
- 29. Taormina P. J., Beuchat L. R., Slutsker L. 1999. Infections associated with eating seed sprouts: an international concern. Emerg. Infect. Dis. 5:626–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Waje C., Kwon J. H. 2007. Improving the food safety of seed sprouts through irradiation treatment. Food Sci. Biotechnol. 16:171–176 [Google Scholar]
- 31. Weissinger W. R., Beuchat L. R. 2000. Comparison of aqueous chemical treatments to eliminate Salmonella on alfalfa seeds. J. Food Prot. 63:1475–1482 [DOI] [PubMed] [Google Scholar]