Abstract
Among the numerous molecular methods described during the last 20 years to identify Brucella, multiplexed amplification methods offer the cheapest and simplest technical solution for molecular identification. However, one disadvantage of such methods is their need to undergo technical revalidation each time a new marker is added to the system. Moreover, polymorphic markers cannot be assessed at the single-nucleotide level in these assays. Since new Brucella species are continuously being described, open methodologies able to accommodate new markers while preserving all other system parameters have an obvious advantage. We present a ligase chain reaction (LCR)-based method that simultaneously assesses multiple genetic markers at the single-nucleotide level. Most of the selected markers originate from a multilocus sequence typing (MLST) database that has been extensively validated on hundreds of different Brucella strains. When assayed on both reference and field strains, the method yields characteristic capillary electrophoresis profiles for each of the 10 Brucella species described to date and displays discriminatory potential below the species level for some. Since the LCR methodology is insensitive to interference resulting from the use of multiple oligonucleotides in a single mixture, the way is open for smooth future updates of the proposed system. Such updates are inevitable, given the pending description of new Brucella species.
INTRODUCTION
Bacteria of the genus Brucella are causative agents of brucellosis, a widespread disease of various animal species and a zooanthroponosis characterized by chronic inflammatory lesions in the reproductive organs that may extend to joints and other organs (6). The disease remains endemic in many areas of the world and is associated with substantial economic losses (24). From a strict genotaxonomic point of view, all Brucella isolates should be considered a single species (7). However, nomenspecies designations have been assigned historically and are retained for practical reasons. There are 10 such recognized Brucella species, each having a preferential primary host or range of hosts, namely, Brucella abortus (bovine), B. melitensis (caprine and ovine), B. ovis (ovine), B. canis (canine), B. suis (porcine, rangiferine, and leporine), B. neotomae (rodent), B. microti (rodent), B. pinnipedialis (marine mammalian, preferentially pinnipedian), B.ceti (cetacean), and B. inopinata (isolated from a human breast implant). Three species are divided into biovars (B. abortus, B.melitensis, and B. suis), although some, particularly those of B. abortus, remain unresolved (7).
Biochemical typing methods are the reference methods for characterizing brucellae at both the species and the biovar levels. They involve metabolite detection, growth on dyes, sensitivity to bacteriophages, and agglutination with monospecific antisera (2). Biochemical typing is time-consuming and difficult to standardize and requires well-trained personnel operating in biocontainment level 3 facilities.
As an alternative to biotyping, the last 20 years have seen a rapid development of molecular techniques applicable to identifying brucellae and understanding their epidemiology (10, 32). The initial assays consisted of single PCRs targeting genes with genus level specificity. For species detection, both nucleotide sequence-based and amplification profile-based methods became available later. Combinations of individual PCRs reached excellent discriminatory potential at the species level but required multiple independent reactions for each analyzed strain. Multiplex PCR methods undoubtedly provided the easiest way to identify the species of an unknown Brucella strain, given the few technical steps and the lack of sophisticated instruments required. The most popular multiplex PCR assay developed initially for differentiating the five classical species of Brucella was the so-called AMOS PCR (5), which is based on the insertion sites of the IS711 element in the genomes of B. abortus, B. melitensis, B. ovis, and B. suis (hence the name AMOS). Several extended versions of the assay that used either the historical primer pairs or variants of these combined with new ones were later published. These updated assays could differentiate vaccine strains from field strains and identify new species, including the marine mammal brucellae (18, 21). The most up-to-date version of this assay displays the expected specificity at species level, except for some strains belonging to the B. canis and B. suis species (16, 21).
From the start of the new millennium, whole-genome sequences became available and were used to develop new typing tools characterizing genetic diversity. Multilocus sequence typing (MLST) confirmed the genetic individuality of Brucella species defined by biochemical typing (29). Canonical single-nucleotide polymorphisms (SNPs) retrieved from compiled MLST sequences or from related markers that could be exploited as the basis of diagnostic tests were identified. Such assays could rapidly identify Brucella isolates to the species level (8, 11, 31), identify vaccine strains (12), or even identify to the biovar level where biovars reflect true genetic entities (9). VNTR (variable-number tandem repeat)-based typing schemes that reached the highest resolution ever reported in the context of Brucella molecular characterization assays have been described (15, 17, 30). However, both MLST- and VNTR-based analyses questioned the validity of some of the biovars established by classical biotyping, particularly those of B. melitensis (1, 29).
In the present work, we describe the setup of a multiplex assay able to identify 16 genetic signatures at once. Fifteen of the investigated markers were selected within genes previously characterized for their ability to identify Brucella at the species level according to the present list of recognized names (23), and one was used for genus assignment. Moreover, the assay was designed to display discriminatory potential to the biovar level for B. suis and to an intermediate level for B. abortus, B. melitensis, and B. ceti.
MATERIALS AND METHODS
Bacterial strains and DNA extraction.
All bacterial strains used in this study are listed in Table S1 in the supplemental material. Purified genomic DNA was obtained from agar-grown material with the DNeasy blood and tissue kit according to the manufacturer's instructions for Gram-negative bacteria (Qiagen, Valencia, CA).
Primers and probes.
The primers and probes used are listed in Table 1. Sixteen “padlock-shaped” probes (PLPs) were designed that contained a number of common features, including (i) a 5′-terminal phosphate group, (ii) a 3′-terminal nucleotide corresponding to the polymorphic nucleotide in the template sequence, (iii) a hybridization site for the universal reverse primer, and (iv) a nucleotide sequence stretch matching the universal forward primer but in the opposite orientation. The 5′ and 3′ arms of the PLPs were designed asymmetrically to display melting temperature (Tm) values differing by about 5°C, as described previously (27). The PLPs were engineered in such a way as to return free energy (ΔG) scores of intramolecular folding not exceeding −10 kcal mol−1 upon assessment with the standard settings of the MFOLD algorithm (33). To allow size-based identification of the amplified probes by capillary electrophoresis, each PLP was designed in such a way as to display unique sizes chosen within a range of 75 to 112 nucleotides. All PLPs were purified by polyacrylamide gel electrophoresis.
Table 1.
Properties of primers and probes
| Code | Target gene/SNPa | Nucleotide sequence | Size (nt) |
|---|---|---|---|
| URb | GACGATGAGTCCTGAGTAA | 19 | |
| UFc | CCGAGATGTACCGCTATCGT | 20 | |
| cUR | TTACTCAGGACTCATCCTC | 19 | |
| A | glk-1403G | GCCGACAAGATCACGCCCA-cUR-AA-UF-CTGGGCATCTGCGCG | 75 |
| B | glk-1344G | GACACGCCCTTCGATGCGT-cUR-AA-UF-AGAATTTGCTCGCCGGC | 77 |
| C | trpE-2858A | CCAGACGGGCGCCAAG-cUR-AA-UF-CATACGCTTGCCAATTATTTCCA | 80 |
| D | omp25-3627A | TAGCCAAGGTAAAGACCGGTATAGCC-cUR-AA-UF-GGCCTTGTTCCAGCCA | 83 |
| E | cobQ-3445A | AAGCCTCGCTGGATATTGATGGC-cUR-TT-UF-ATTATCTGGCTGAAGGGCTGA | 85 |
| F | glk-1557A | GCGGCGTTTATCTTTCGGGTAGCTA-cUR-AA-UF-GCTCATTTTCATGGCGCATA | 86 |
| G | aroA-677A | ACCCGCACCGGCCTG-cUR-AA-UF-TGATACTACTATGCAATGTGCTGATGAACCCA | 88 |
| H | dnaK-1654A | CATCGACCTGAAGAACGACAAGC-cUR-TT-UF-TATCCGAGTTCAAGAAGGAAAGTGA | 89 |
| I | ptsP-1677Gd | GCCTTCAATAGCGCGCGC-cUR-AA-UF-CGTCGCGTTAGACAGCTCATGGCCACCCGCC | 90 |
| J | cobQ-3224A | CACCAGCGGGCCGGA-cUR-AA-UF-TAGTCACATATCATGCTATGAAATCCACATCGGGCA | 92 |
| K | pyrH-817Gd | TTCTCGATCGCGGGC-cUR-AA-UF-GGTTCGCTTACGTTGCATAGTGCTCACCCACAAGGAAG | 94 |
| L | dnaK-1928T | ACCAGAACCACTTCGTCAATTTCG-cUR-AA-UF-ATCCGGTCTCATCGCTGAATGGTCATGCCGCCA | 98 |
| M | trpE-2796A | GTTTCGATCCTGCTGGTCGATCA-cUR-T-UF-ATGGTCGCCTATACTTATATCAAAGGTGGCTGAGGGA | 100 |
| N | omp25-3715A | CTGGAAGTTCCAGCCAGCAAACG-cUR-AA-UF-CGATCCGATTACAGGCCGATCCGTATACGATCTGGTCCTT | 104 |
| O | IS711 | ACTGTCCGCAAGCTTCAAGC-cUR-TT-UF-AAAATTTAACGTTCCTAAAGCTGAGTCTGCCCGGCCATTATGGTG | 106 |
| P | rpoB-265Ae | ATGAATGCCGTCAGCGCG-cUR-TT-UF-ATTTGACGAACGTATGCCGCTTAACTCAAATCATCCACCGAAGTTGGATGTTA | 112 |
Unless otherwise indicated, marker position refers to the polymorphic nucleotide assessed with each PLP according to the nucleotide numbering used in reference 29.
UR, universal reverse primer.
UF, universal forward primer.
Marker position refers to the nucleotide numbering used in reference 9.
Marker position refers to the nucleotide numbering used in reference 20.
LCR procedure.
A ligase chain reaction (LCR) was conducted in three successive steps according to the procedure of Gaszczyk et al. (K. Gaszczyk, E. Verstappen, O. Mendes, C. Schoen, and P. Bonants, unpublished data) with minor modifications. Briefly, the first step (ligation) was conducted in a 10-μl mixture containing 10 ng purified genomic DNA, 4 U of Pfu DNA ligase (Agilent, Santa Clara, CA), 20 mM Tris-HCl (pH 7.5), 20 mM KCl, 10 mM MgCl2, 0.1% Igepal, 0.01 mM ATP, 1 mM dithiothreitol (DTT), and 100 pM of each PLP except the IS711 probe (50 pM) and the ptsP probe (300 pM). Reaction mixtures were made up on ice and transferred rapidly onto a thermal cycler. After 3 min at 95°C, 25 cycles of 30 s at 95°C and 5 min at 65°C were performed, followed by a 2-min final denaturation at 98°C. The second step (exonuclease treatment) started with the addition of 15 μl of exonuclease mixture consisting of 67 mM glycine-KOH, pH 9.4, 2.5 mM MgCl2, 50 μg/ml bovine serum albumin (BSA), and 1 U exonuclease λ (New England BioLabs, Ipswich, MA). The resulting samples (25 μl) were incubated at 37°C for 45 min, followed by inactivation at 95°C for 10 min. Upon completion of the second step, a 3.5-μl sample was pipetted into a new microtube and supplemented with universal reverse primer and 5′ Cy5-labeled universal forward primer (8 pmol each), 12.5 μl of 2-fold real-time PCR mix (ABGene, Epsom, United Kingdom), and MiliQ water up to 25 μl. After 10 min at 95°C, 30 cycles of 30 s at 95°C, 30 s at 55°C, and 1 min at 72°C were performed, followed by a 15-min final elongation at 72°C and denaturation at 98°C for 2 min.
Sample analysis.
LCR products were analyzed by capillary electrophoresis on a CEQ8000 instrument (Beckman Coulter, Fullerton, CA). One microliter of a 20-fold dilution of the LCR product made in MiliQ water was added to 39 μl of sample loading solution (Beckman Coulter, Fullerton, CA) containing 0.3 μl of internal size standard 400, which consists of a proprietary cocktail of 22 DNA molecular size standards with sizes ranging from 60 to 420 bases (Beckman Coulter, Fullerton, CA).
RESULTS
SNPs were selected within (i) DNA fragments included in a 9-locus MLST scheme reported previously (29), (ii) the ptsP and pyrH genes (9), (iii) the rpoB gene (20), and (iv) the IS711 insertion element (14). Some of the selected markers allow B. suis characterization up to the biovar level (ptsP, pyrH, and rpoB) or genus confirmation (IS711). The developed assay thus involves the interrogation of the bases present at 15 different SNP sites. Six of these are specific for a particular species. They are a T at position 151 of omp25, i.e., omp25-151T, for B. abortus (equivalent to omp25-3627T in reference 29); glk-427A for B. ovis (equivalent to glk-1557A); omp25-239A for B. canis (omp25-3715A); glk-255G for B. melitensis (equivalent to glk-1403G); aroA-377A for B. microti (equivalent to aroA-617A); and cobQ-3224A for B. inopinata. A seventh SNP, trpE-290A (equivalent to trpE-2858A), is group specific, as it identifies Brucella strains of marine mammalian origin. This group comprises B. ceti and B. pinnipedialis. These two species, together with B. suis and B. neotomae, are further identified on the basis that they possess an overall multiplex profile that is unique for the Brucella species considered. An eighth SNP, glk-196G (equivalent to glk-1344G), is found only in B. neotomae and in the reference strain of B. abortus biovar 3. The remaining seven SNPs assayed in our multiplex LCR are not specific for a particular species or group but rather are intended for B. suis biovar differentiation (ptsP-1677G, pyrH-817G, dnaK-1928T, and rpoB-265A), for B. ceti differentiation below the species level (cobQ-3445A and dnaK-1654A), or for the identification of Brucella strains of marine origin (strains F5/02 and F5/99) that cluster poorly with other marine mammal-derived Brucella isolates and to which a species name has not yet been formally assigned (trpE-2796A) (13, 19, 28).
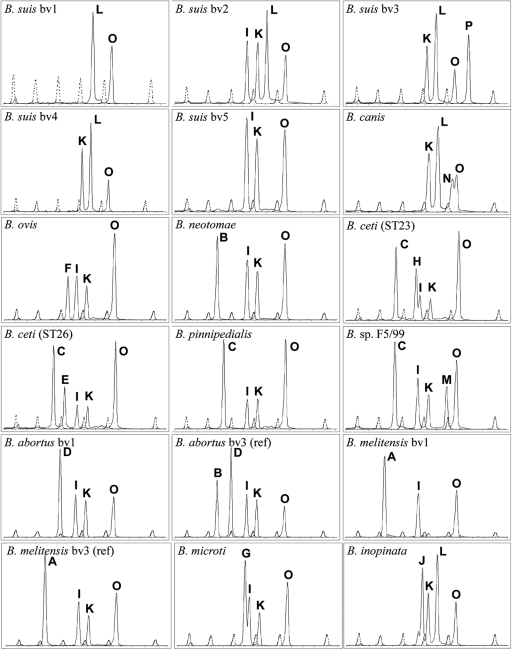
The PCR products derived from the amplification of the ligated PLPs range from 73 to 110 bp in size, although migration does not exactly correspond to the PCR fragment size (Table 2 ). For ease of identification, the assay was engineered as far as possible to display electrophoresis profiles made of peaks differing in size by at least 4 bp. This rule is not fulfilled for the profiles generated for B. canis, B. ceti ST23 (29), and B. microti, for which a 2-bp offset is displayed, and for B. inopinata (3-bp offset). Nevertheless, overall profiles are readily distinguished. Figure 1 shows typical reaction profiles obtained with the multiplex LCR procedure, demonstrating each of the 18 specific profiles that the assay generates. To validate the assay, we examined the profiles of 103 cultured Brucella isolates, including all species and biovar reference strains, as well as a number of field isolates. In total, 18 B. abortus, 22 B. melitensis, 20 B. suis, 5 B. canis, 4 B. ovis, 14 B. ceti, 16 B. pinnipedialis, 1 B. neotomae, 1 B. microti, and 1 B. inopinata strains and 2 Brucella strains with no defined species name (Brucella sp.) were examined and found to give the predicted genotype. In order to assess the specificity of the assay, it was applied to 19 non-Brucella species, including Ochrobactrum intermedium, which belongs to a genus closely related to Brucella. The assay gave no signal on those species, confirming its specificity for the genus Brucella as currently defined.
Table 2.
Multiplex LCR profiles of the different Brucella species
| Species | Biovar | STc | Presence of peaka: |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ab (72.3) | B (73.9) | C (76.9) | Db (80.3) | E (81.7) | Fb (82.9) | Gb (85) | H (85.6) | I (87.2) | Jb (88.5) | K (91.7) | L (95.2) | Mb (99.3) | Nb (101.3) | O (103.3) | P (109) | |||
| B. suis | 1, 3 | 14, 17 | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + | − |
| B. suis | 2 | 15, 16 | − | − | − | − | − | − | − | − | + | − | + | + | − | − | + | − |
| B. suis | 3 (ref)f | 17 | − | − | − | − | − | − | − | − | − | − | + | + | − | − | + | + |
| B. suis | 4 | 17, 18 | − | − | − | − | − | − | − | − | − | − | + | + | − | − | + | − |
| B. suis | 5 | 19 | − | − | − | − | − | − | − | − | + | − | + | − | − | − | + | − |
| B. canis | 20, 21 | − | − | − | − | − | − | − | − | − | − | + | + | − | + | + | − | |
| B. ovis | 13 | − | − | − | − | − | + | − | − | + | − | + | − | − | − | + | − | |
| B. neotomae | 22 | − | + | − | − | − | − | − | − | + | − | + | − | − | − | + | − | |
| B. ceti | 23 | − | − | + | − | − | − | − | + | + | − | + | − | − | − | + | − | |
| B. ceti | 26 | − | − | + | − | + | − | − | − | + | − | + | − | − | − | + | − | |
| B. pinnipedialis | 24, 25 | − | − | + | − | − | − | − | − | + | − | + | − | − | − | + | − | |
| Brucella sp.d | 27 | − | − | + | − | − | − | − | − | + | − | + | − | + | − | + | − | |
| B. abortus | 1, 2, 3, 4, 5, 6, 9 | 1 to 5 | − | − | − | + | − | − | − | − | + | − | + | − | − | − | + | − |
| B. abortus | 3 (ref)f | 6 | − | + | − | + | − | − | − | − | + | − | + | − | − | − | + | − |
| B. melitensis | 1, 2, 3 | 7e, 8e, 10, 11e, 12 | + | − | − | − | − | − | − | − | + | − | − | − | − | − | + | − |
| B. melitensis | 2, 3 (ref)f | 7e, 8e, 9, 11e | + | − | − | − | − | − | − | − | + | − | + | − | − | − | + | − |
| B. microti | NAg | − | − | − | − | − | − | + | − | + | − | + | − | − | − | + | − | |
| B. inopinata | NA | − | − | − | − | − | − | − | − | − | + | + | + | − | − | + | − | |
| O. intermedium | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
+, present; -, absent. The experimental electrophoretic mobility (mean of 10 values obtained from independent runs) (nt) is shown in parentheses.
Species-specific marker.
ST as defined in the MLST scheme described in reference 29.
Nonascribed Brucella species of probable marine origin; includes strains F5/02 and F5/99 (28).
Marker K (pyrH-817G) is variable in ST7, ST8, and ST11 (B. melitensis).
Profile observed only for the reference strain (ref) of biovar 3 (B. abortus and B. melitensis).
NA, not applicable.
Fig. 1.
Fragment analysis of representative LCR assays. The fragments were analyzed in two fluorescence channels. The dotted line is the signal of internal molecular size standards (channel 1). They have the following sizes (from left to right): 60, 70, 80, 90, 100, and 120 nucleotides. The solid line is the signal of the amplified LCR products (channel 2). A through P refer to the amplified probe codes listed in Table 1.
DISCUSSION
The assay described here proposes a novel approach to identifying Brucella isolates to the species level. The molecular signatures addressed by the assay—polymorphic nucleotides located in genes with housekeeping functions scattered around the Brucella genome—were shown in earlier studies to display appropriate discriminatory potential for genotaxonomic characterization (9, 20, 29). Moreover, most of these signatures have been validated on >500 strains, thereby guaranteeing the accuracy of the identifications. An MLST scheme (29) was the main source of molecular signatures for our assay. It was extended to B. microti and B. inopinata (25, 26), which were not yet described at the time the initial MLST scheme was published.
The rationale behind the assay was the selection from a number of previously sequenced housekeeping genes of a series of SNPs whose nucleotide identities, when combined, allow the identification of any Brucella species. The assay is basically a three-step, single-tube assay analyzed by capillary electrophoresis and applicable to purified DNA extracts. To minimize laboratory work, reagents required for the second and third steps are adapted for successive addition to the reaction tube, allowing smooth sample processing with no intermediate extraction or purification. The assay was developed using the Beckman 8000 genetic analysis system but should be easily transferable to other fragment analysis platforms. The principle of the assay relies on the conditional ligation of a series of single-stranded DNA probes displaying SNP-specific 3′-terminal nucleotides (3). The probes are padlock shaped (PLPs) so that ligation, which is conditioned by a perfect match between the template DNA and the 3′ terminus of the probe, generates a circular molecule (22). Each PLP has a unique length and includes an annealing site for a universal amplification primer and a sequence stretch identical to another universal primer in inverted-repeat orientation. The first stage involves multiplex PLP ligation using a thermostable DNA ligase. The second step involves nuclease removal of noncircular DNA molecules, i.e., template DNA and nonligated PLPs. The third step consists of PCR amplification of the ligated (circularized) PLPs. Thus, only PLPs matching the template sequence perfectly at the critical 3′-terminal nucleotide will be PCR amplified and detected.
The final assay conditions presented in this study and described in Materials and Methods were established after performance assessment of the individual PLPs and optimization of their nucleotide sequences and relative concentrations in the multiplex assay.
Compared to biotyping techniques, the assay has substantial advantages in terms of speed, robustness, and biosafety. It reaches the same sensitivity at species level. Due to the lack of genetic evidence to support the classification of Brucella into biovars as defined by biotyping, the assay presented here can assess ranking below the species level only partially. Compared to multiplex PCR (18), identification with the new assay is more amenable to automation but requires a capillary electrophoresis instrument. Moreover, in its actual design, it cannot differentiate field strains from vaccine strains. Compared to multiple-locus variable-number tandem-repeat analysis (MLVA) (4, 17, 30), it is less sensitive but also cheaper, requires less pipetting work, and is based on genetic markers with sound phylogenetic value. Technically, it is a single-tube test performed in three steps within a working day and requiring no subsequent extraction or purification. Assuming that carefully purified, phosphorylated PLPs are available in nonlimiting quantities, other reagents and consumables are reduced, resulting in a cost per analysis not exceeding $9.00 in our laboratory. Given the low sensitivity of the assay to interference due to the use of multiple PLPs in a single mixture, it lends itself to future expansion. Such expansion may target novel Brucella species, vaccine strains, or taxonomic ranks below the species level, when such ranks can be defined genetically.
Supplementary Material
Acknowledgements
This work was supported by a contractual research grant from the Belgian Ministry of Public Health (RT-06/08 EMRiSK). Brucellosis research activities at VLA are supported by the United Kingdom Department of Environment, Food and Rural Affairs (Defra).
We thank C. Schoen (Plant Research International) for his support in LCR assays.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 29 July 2011.
REFERENCES
- 1. Al Dahouk S., et al. 2007. Evaluation of genus-specific and species-specific real-time PCR assays for the identification of Brucella spp. Clin. Chem. Lab. Med. 45:1464–1470 [DOI] [PubMed] [Google Scholar]
- 2. Alton G. G., Jones L. M., Angus R. D., Verger J. M. 1988. Techniques for the brucellosis laboratory, 1st ed. Institut National de la Recherche Agronomique, Paris, France [Google Scholar]
- 3. Barany F. 1991. Genetic disease detection and DNA amplification using cloned thermostable ligase. Proc. Natl. Acad. Sci. U. S. A. 88:189–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bricker B. J., Ewalt D. R., Halling S. M. 2003. Brucella ‘HOOF-Prints’: strain typing by multi-locus analysis of variable number tandem repeats (VNTRs). BMC Microbiol. 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bricker B. J., Halling S. M. 1994. Differentiation of Brucella abortus bv. 1, 2, and 4, Brucella melitensis, Brucella ovis, and Brucella suis bv. 1 by PCR. J. Clin. Microbiol. 32:2660–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corbel M. J. 1997. Brucellosis: an overview. Emerg. Infect. Dis. 3:213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corbel M. J. 1988. International Committee on Systematic Bacteriology Subcommittee on the taxonomy of Brucella. Report of the meeting, 5 September 1986, Manchester, England. Int. J. Syst. Bacteriol. 38:450–452 [Google Scholar]
- 8. Foster J. T., et al. 2008. Real-time PCR assays of single-nucleotide polymorphisms defining the major Brucella clades. J. Clin. Microbiol. 46:296–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fretin D., et al. 2008. Brucella suis identification and biovar typing by real-time PCR. Vet. Microbiol. 131:376–385 [DOI] [PubMed] [Google Scholar]
- 10. Godfroid J., et al. 13 May 2011. Brucellosis at the animal/ecosystem/human interface in the beginning of the 21st century. Prev. Vet. Med. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 11. Gopaul K. K., Koylass M. S., Smith C. J., Whatmore A. M. 2008. Rapid identification of Brucella isolates to the species level by real time PCR based single nucleotide polymorphism (SNP) analysis. BMC Microbiol. 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gopaul K. K., Sells J., Bricker B. J., Crasta O. R., Whatmore A. M. 2010. Rapid and reliable single nucleotide polymorphism-based differentiation of Brucella live vaccine strains from field strains. J. Clin. Microbiol. 48:1461–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groussaud P., Shankster S. J., Koylass M. S., Whatmore A. M. 2007. Molecular typing divides marine mammal strains of Brucella into at least three groups with distinct host preferences. J. Med. Microbiol. 56:1512–1518 [DOI] [PubMed] [Google Scholar]
- 14. Halling S. M., Tatum F. M., Bricker B. J. 1993. Sequence and characterization of an insertion sequence, IS711, from Brucella ovis. Gene 133:123–127 [DOI] [PubMed] [Google Scholar]
- 15. Huynh L. Y., et al. 2008. Multiple locus variable number tandem repeat (VNTR) analysis (MLVA) of Brucella spp. identifies species-specific markers and insights into phylogenetic relationships, p. 47–54In St. Georgiev V., Western K. A., McGowan J. J.(ed.), National Institute of Allergy and Infectious Disease, NIH, vol. 1. Frontiers in research. Humana Press, Totowa, NJ [Google Scholar]
- 16. Koylass M. S., et al. 2010. Comparative performance of SNP typing and ‘Bruce-ladder’ in the discrimination of Brucella suis and Brucella canis. Vet. Microbiol. 142:450–454 [DOI] [PubMed] [Google Scholar]
- 17. Le Flèche P., et al. 2006. Evaluation and selection of tandem repeat loci for a Brucella MLVA typing assay. BMC Microbiol. 6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. López-Goni I., et al. 2008. Evaluation of a multiplex PCR assay (Bruce-ladder) for molecular typing of all Brucella species, including the vaccine strains. J. Clin. Microbiol. 46:3484–3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maquart M., et al. 2009. MLVA-16 typing of 295 marine mammal Brucella isolates from different animal and geographic origins identifies 7 major groups within Brucella ceti and Brucella pinnipedialis. BMC Microbiol. 9:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Marianelli C., Ciuchini F., Tarantino M., Pasquali P., Adone R. 2006. Molecular characterization of the rpoB gene in Brucella species: new potential molecular markers for genotyping. Microbes Infect. 8:860–865 [DOI] [PubMed] [Google Scholar]
- 21. Mayer-Scholl A., Draeger A., Gollner C., Scholz H. C., Nockler K. 2010. Advancement of a multiplex PCR for the differentiation of all currently described Brucella species. J. Microbiol. Methods 80:112–114 [DOI] [PubMed] [Google Scholar]
- 22. Nilsson M., et al. 1994. Padlock probes: circularizing oligonucleotides for localized DNA detection. Science 265:2085–2088 [DOI] [PubMed] [Google Scholar]
- 23. Osterman B., Moriyon I. 2006. International Committee on Systematics of Prokaryotes; subcommittee on the taxonomy of Brucella: minutes of the meeting, 17 September 2003, Pamplona, Spain. Int. J. Syst. Evol. Microbiol. 56:1173–1175 [Google Scholar]
- 24. Pappas G., Papadimitriou P., Akritidis N., Christou L., Tsianos E. V. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91–99 [DOI] [PubMed] [Google Scholar]
- 25. Scholz H. C., et al. 2008. Brucella microti sp. nov., isolated from the common vole Microtus arvalis. Int. J. Syst. Evol. Microbiol. 58:375–382 [DOI] [PubMed] [Google Scholar]
- 26. Scholz H. C., et al. 2010. Brucella inopinata sp. nov., isolated from a breast implant infection. Int. J. Syst. Evol. Microbiol. 60:801–808 [DOI] [PubMed] [Google Scholar]
- 27. Szemes M., et al. 2005. Diagnostic application of padlock probes—multiplex detection of plant pathogens using universal microarrays. Nucleic Acids Res. 33:e70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Whatmore A. M., et al. 2008. Marine mammal Brucella genotype associated with zoonotic infection. Emerg. Infect. Dis. 14:517–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Whatmore A. M., Perrett L. L., MacMillan A. P. 2007. Characterisation of the genetic diversity of Brucella by multilocus sequencing. BMC Microbiol. 7:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whatmore A. M., et al. 2006. Identification and characterization of variable-number tandem-repeat markers for typing of Brucella spp. J. Clin. Microbiol. 44:1982–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Winchell J. M., Wolff B. J., Tiller R., Bowen M. D., Hoffmaster A. R. 2010. Rapid identification and discrimination of Brucella isolates by use of real-time PCR and high-resolution melt analysis. J. Clin. Microbiol. 48:697–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu W. L., Nielsen K. 2010. Review of detection of Brucella spp. by PCR. Croat. Med. J. 51:306–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zuker M. 2003. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31:3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.