Abstract
We investigated butanol production from crystalline cellulose by cocultured cellulolytic Clostridium thermocellum and the butanol-producing strain, Clostridium saccharoperbutylacetonicum (strain N1-4). Butanol was produced from Avicel cellulose after it was incubated with C. thermocellum for at least 24 h at 60°C before the addition of strain N1-4. Butanol produced by strain N1-4 on 4% Avicel cellulose peaked (7.9 g/liter) after 9 days of incubation at 30°C, and acetone was undetectable in this coculture system. Less butanol was produced by cocultured Clostridium acetobutylicum and Clostridium beijerinckii than by strain N1-4, indicating that strain N1-4 was the optimal strain for producing butanol from crystalline cellulose in this coculture system.
INTRODUCTION
Biobutanol is an attractive biofuel because it has an energy density that is similar to that of gasoline (2, 8, 37). Although several Clostridium spp., including Clostridium acetobutylicum, Clostridium beijerinckii, and Clostridium saccharoperbutylacetonicum, produce butanol from various substrates such as agricultural waste (38) and soy molasses (26), these microorganisms cannot directly ferment crystalline cellulose, which is particularly abundant (6). Since the ability of butanol-producing bacteria to utilize cellulose is limited, an expensive hydrolysis step is required before fermentation to degrade the cellulose into simpler sugars.
While expression of the celA and celD genes of Neocallimastix patriciarum in C. beijerinckii has been applied to produce butanol from cellulose in a single step (20), the resulting transformants did not produce butanol from cellulosic substrates without the addition of the cellulase mixture Celluclast 1.5L (Novozymes, Bagsvaerd, Denmark). Furthermore, although butanol-producing Clostridium strains have been isolated (1, 31), the efficiency of butanol production using a single Clostridium strain on cellulosic substrates is low.
The primary advantage of using a coculture system is that it eliminates the costly enzymatic hydrolysis step and thus has a potential for cost-effective consolidated bioprocessing (CBP) (21). An example of this type of system is cocultured C. acetobutylicum and the cellulolytic Clostridium cellulolyticum or Clostridium thermocellum (24, 25, 34). The high rates of cellulose hydrolysis achieved by these cellulolytic clostridia can be attributed to the presence of a large, cell surface-bound protein complex referred to as the cellulosome (5, 11, 32). Although these coculture systems produced small quantities of butanol using simple carbon sources, they mainly produce butyrate (24, 25). Even so, butanol production using a coculture system requires butyric acid to induce the solventogenic phase of C. acetobutylicum (34). Among four groups of butanol-producing clostridia, divided on the basis of 16S rRNA gene sequences and DNA hybridization information (16, 18, 27), the C. saccharoperbutylacetonicum strain N1-4 is phylogenetically distantly related to C. acetobutylicum. We also reported that quorum-sensing-like substances found in the culture supernatant of strain N1-4 induced solventogenesis, suggesting that the induction mechanism of butanol production somewhat differs from that of other butanol-producing clostridia (19). Therefore, strain N1-4 has the potential to produce butanol from crystalline cellulose in coculture without the need for butyric acid.
We selected strain N1-4 as the butanol-producing strain and C. thermocellum as the cellulose-utilizing strain because the latter exhibits high cellulase activity against crystalline cellulose (3, 11). We produced butanol from crystalline cellulose by coculture with these two strains without the need for butyric acid.
MATERIALS AND METHODS
Bacterial strains and culture media.
C. thermocellum NBRC103400 (ATCC 27405) was cultured anaerobically at 60°C on National Institute of Technology and Evaluation (NITE) Biological Resource Center (NBRC) medium 979 containing 1.3 g of (NH4)2SO4, 2.6 g of MgCl2·6H2O, 1.43 g of KH2PO4, 7.2 g of K2HPO4·3H2O, 0.13 g of CaCl2·2H2O, 6 g of sodium glycerophosphate, 1.1 mg of FeSO4·7H2O, 0.25 g of glutathione, 4.5 g of yeast extract, 1 mg of resazurin, and 5 g of cellobiose or arbitrary concentrations of Avicel cellulose/liter (pH 7.0). C. saccharoperbutylacetonicum strain N1-4 (ATCC 13564), C. beijerinckii NCIMB 8052, and C. acetobutylicum ATCC 824 were cultured anaerobically at 30°C in clostridial growth medium (CGM) containing 4% glucose and 0.75 g of KH2PO4, 0.982 g of K2HPO4, 1 g of NaCl, 0.01 g of MnSO4, 0.004 g of p-aminobenzoic acid, 0.348 g of MgSO4, 0.01 g of FeSO4, 2 g of asparagine, 5 g of yeast extract, 2 g of (NH4)2SO4, and 40 g of glucose/liter (pH 6.5). The strains were cultured in test tubes stoppered with butyl rubber stoppers after the headspace was replaced with nitrogen gas.
Coculture of C. thermocellum NBRC103400 and strains producing butanol.
Coculture experiments proceeded in test tubes. C. thermocellum cells grown in NBRC medium 979 containing cellobiose at 60°C were collected by centrifugation. Cell pellets were washed and suspended in the same medium without any added carbon source and adjusted to an optical density at 600 nm (OD600) of 0.2 or 2. Six milliliters of the cell suspension was then used to inoculate 6 ml of NBRC medium 979 containing Avicel cellulose (final OD600, 0.1 or 1) and cultured at 60°C. Unless otherwise stated, only the butanol-producing Clostridium strains that grew exponentially were collected after 24 h by centrifugation, washed, and suspended in CGM containing 4% glucose and adjusted to an OD600 of 1.3 or 13. The cell suspension (1 ml) was then added to test tubes containing C. thermocellum cultures after the incubation temperature was decreased to at 30°C (final OD600 of 0.1 or 1). Subsequent cocultures were incubated at 30°C unless otherwise stated.
Analytical procedure.
Fermentation products were quantified by high-performance liquid chromatography (HPLC) using an Aminex HPX-87H column (Bio-Rad Laboratories K.K., Japan) with a differential refractive index detector (Shimadzu Corporation, Japan) and an aqueous solution of 5 mM H2SO4 as the solvent. Residual cellulose concentrations were determined as described by Desvaux et al. (4).
Preparation of cell extracts.
Cell extracts were prepared with slight modifications of the procedure described by Vasconcelos et al. (30). Briefly, cell samples were harvested by centrifugation, washed, and suspended in 100 mM oxygen-free Tris-hydrochloride buffer (pH 7.6) containing 2 mM dithiothreitol (DTT). Cells were disrupted in 2-ml microtubes with 0.5-mm glass beads using a Multi-Beads Shocker (Yasui Kikai Co., Japan) at 3,500 rpm for 2 min. The glass beads and cell debris were removed from resulting homogenates by centrifugation at 20,000 × g for 10 min, and the supernatant was used as a crude enzyme solution. One unit of specific activity is expressed as 1 nmol of substrate change/min/mg of protein.
The protein content of the cell extracts was determined using a Coomassie (Bradford) protein assay reagent (Pierce, Rockford, IL) with bovine serum albumin as the standard.
Enzyme assays.
Enzymes were assayed using spectrophotometry (U-2001; Hitachi Ltd., Japan) according to Vasconcelos et al. (30), except that the carbon dioxide generated by acetoacetate decarboxylase was measured using a gas chromatograph equipped with a thermal conductivity detector ([TCD] GC323; GL Science Inc., Japan) and a Molecular Sieve 13× column (GL Science Inc., Japan).
The activity of thiolase, which is specific to strain N1-4 and which converts acetyl-coenzyme A (CoA) to acetoacetyl-CoA, was used as an internal standard to normalize enzyme activities because the crude extracts prepared from cocultures contained enzymes produced by both strains. The actual thiolase activities prepared from mono- and cocultures were 66.42 ± 5.59 and 25.19 ± 0.10 U/mg, respectively.
RESULTS
Effect of C. thermocellum culture period on butanol production.
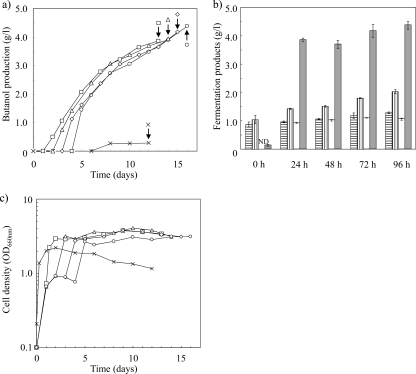
Since butanol-producing clostridia can barely utilize crystalline cellulose, butanol production on a cellulose substrate requires hydrolysis of the cellulose. Thus, we designed the coculture system so that butanol would be produced after the hydrolysis of crystalline cellulose by C. thermocellum at 60°C. To optimize hydrolysis of crystalline cellulose, strain N1-4 (final OD600, 0.1) was added to the medium after inoculation and cultivated with C. thermocellum for 0, 24, 48, 72, and 96 h (adjusted to an OD600 of 0.1), after which the butanol concentration in the culture supernatant was measured (Fig. 1 a and b).
Fig. 1.
Effect of C. thermocellum culture period on butanol production. (a) Time course of butanol production. Strain N1-4 was added to cultures after inoculation with C. thermocellum at 0 h (×), 24 h (□), 48 h (▵), 60 h (⋄), and 72 h (○). Arrow, cultivation endpoint (12 days after addition of strain N1-4). (b) Fermentation products at 12 days after the addition of strain N1-4. Bars are as follows: vertical lines, acetate; horizontal lines, butyrate; white, ethanol; and gray, butanol. ND, not detected. (c) Time course of cell densities. Symbols are as described for panel a.
Since clostridia that produce butanol cannot utilize crystalline cellulose (37), the extremely small amount (0.3 g/liter) of butanol generated after 12 days of incubation after the simultaneous addition of both C. thermocellum and strain N1-4 was predictable. In contrast, when strain N1-4 was added to the medium after C. thermocellum had been cultured for at least 24 h, all of the cultures produced over 3.9 g/liter of butanol from 20 g/liter of crystalline cellulose, that is, 40% of the theoretical yield (maximum theoretical yield, 0.41 g/g of glucose). We therefore demonstrated that crystalline cellulose can be hydrolyzed by inoculating the substrate with C. thermocellum for at least 24 h before coculturing with strain N1-4.
In cocultures in which C. thermocellum and strain N1-4 were simultaneously added, the maximum OD600 was 2.2, and this value decreased to 1.1 at 12 days of cultivation (Fig. 1c). On the other hand, the cell density of cocultures that produced butanol exceeded an OD600 of 3.0 after strain N1-4 was added and remained constant during cultivation. Although butanol production continued for over 12 days, cocultured cells did not grow.
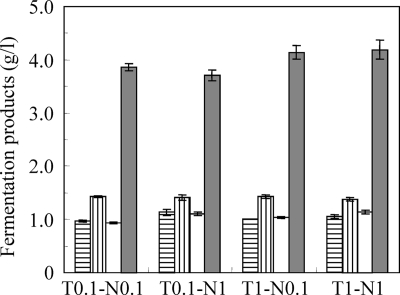
Effect of cell density on butanol production.
Since a high cell density would probably increase the hydrolysis of cellulose and butanol production, the densities of both strains were increased to an OD600 of 1.0 before coculture (Fig. 2). In response to increasing the density of C. thermocellum, butanol production slightly increased from 3.9 to 4.2 g/liter after 12 days of incubation. On the other hand, the addition of strain N1-4 had no marked effect on butanol production (Fig. 2). Taken together, these results showed that the combination of C. thermocellum and strain N1-4 cells at a density greater than an OD600 of 0.1 is sufficient to produce butanol from crystalline cellulose. However, to ensure high titers of butanol production, the cell densities of both strains were adjusted to an OD600 of 1.0 for all subsequent coculture experiments.
Fig. 2.
Effect of C. thermocellum and strain N1-4 cell density on fermentation products. Bars indicate the amounts of acetate (vertical lines), butyrate (horizontal lines), ethanol (white), and butanol (gray). T0.1 and T1, cell densities of C. thermocellum at OD600s of 0.1 and 1.0, respectively. N0.1 and N1 indicate cell densities of strain N1-4 at OD600s of 0.1 and 1.0, respectively. Values represent mean density of samples assayed in triplicate, and error bars indicate standard errors.
Although the major fermentation product was butanol, small quantities of acetate, butyrate, and ethanol were also produced, but acetone was undetectable.
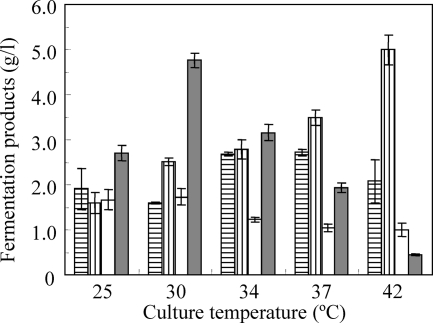
Effect of N1-4 growth temperature on cellulase activity.
The optimal temperature for cellulase activity in thermophilic C. thermocellum is over 60°C (3), implying that elevated culture temperatures might increase cellulose hydrolysis and butanol production. We therefore investigated the effect of culture temperature on butanol production by strain N1-4 (Fig. 3). The temperatures ranged from 25 to 42°C because strain N1-4 is a mesophilic bacterium.
Fig. 3.
Effect of culture temperature on fermentation products after the addition of strain N1-4. Bars show amounts of acetate (vertical lines), butyrate (horizontal lines), ethanol (whites), and butanol (gray). Values are shown as means of samples assayed in triplicate; error bars indicate standard errors.
The titer of butanol production (4.8 g/liter from 20 g/liter of crystalline cellulose) was the highest in strain N1-4 when it was cultured at 30°C for 13 days. In addition, butanol production decreased with increasing culture temperatures (0.4 g/liter of butanol at 42°C) (Fig. 3). Conversely, increasing the culture temperature to 42°C resulted in higher rates of butyrate production at concentrations above 5.0 g/liter. This finding closely correlates with findings of N1-4 in monocultures using glucose as a substrate. Strain N1-4 produced extremely low levels of butanol at 42°C in CGM (0.2 g/liter), suggesting that the optimal culture temperature for the butanol producer is more important than the optimal cellulose hydrolysis temperature for the cellulosic strain in our coculture system.
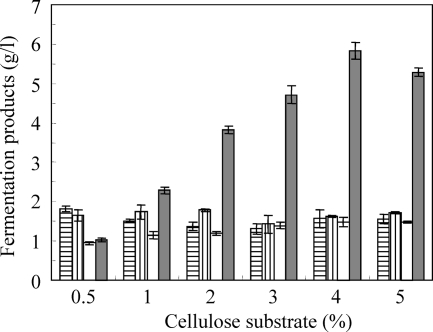
Optimizing butanol production using a cocultured C. thermocellum and strain N1-4.
To more accurately clarify butanol production, initial substrate concentrations were varied from 5 to 50 g/liter (Fig. 4). Butanol production proportionally increased in response to an increase in substrate concentration up to 40 g/liter of substrate, with 5.8 g/liter of butanol being produced from 40 g/liter of substrate. The major product was butanol, and the concentrations of acetate, butyrate, and ethanol were approximately equal under each condition.
Fig. 4.
Maximal amounts of butanol produced by increasing cellulose substrate concentrations. Bars indicate amounts of acetate (vertical lines), butyrate (horizontal lines), ethanol (white), and butanol (gray). Values are shown as mean of samples assayed in triplicate; error bars indicate standard error.
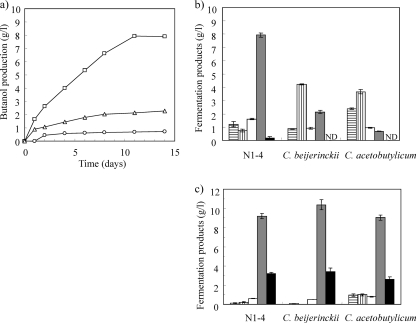
Effectiveness of strain N1-4 as a butanol producer in a coculture system.
The addition of butyric acid is necessary for the onset of butanol production by C. acetobutylicum (34). However, in our coculture system, strain N1-4 produced butanol without the addition of butyric acid to the culture medium. To assess whether strain N1-4 is optimally suited for butanol production using our coculture system, C. beijerinckii NCIMB 8052 and C. acetobutylicum ATCC 824 were also cocultured with C. thermocellum using this system, and the fermentation products were measured (Fig. 5 a and b). As reported, C. acetobutylicum produced low levels of butanol (24, 25, 34), but butanol production by C. beijerinckii was 26% of that obtained using strain N1-4. The amount of butanol produced by the three strains was notably similar when the strains were separately cultivated in CGM containing 40 g/liter glucose (Fig. 5c). These results imply that strain N1-4 can produce butanol from crystalline cellulose only when it is cocultured with C. thermocellum.
Fig. 5.
Comparison of fermentation products produced in coculture system using strain N1-4, C. beijerinckii NCIMB 8052, and C. acetobutylicum ATCC 824 as butanol-producing strains. (a) Time course of butanol produced from cellulose by strain N1-4 (□), C. beijerinckii (▵), and C. acetobutylicum (○). (b) Fermentation end products obtained from three Clostridium strains cocultured on cellulose substrate. (c) Fermentation end products obtained from three Clostridium strains cultivated in CGM containing 40 g/liter glucose. For panels b and c, bars indicate production of acetate (vertical lines), butyrate (horizontal lines), ethanol (white), butanol (gray), and acetone (black). Values represent means of triplicate samples; error bars indicate standard errors. ND, not detected.
Assays of enzymes associated with acetone production.
Acetone was not detected in the culture supernatant of our coculture system. Since the acetone and butanol-producing genes are generally located on a polycistronic operon (9, 19), unless the acetone-producing gene is disrupted or its expression is repressed, acetone is produced together with butanol (15, 28). To elucidate the underlying reason for the extremely low level of acetone production, we measured the activities of the enzymes responsible for acetone production (Table 1). The activities of these enzymes in the coculture system were similar to those in monocultures of strain N1-4 (Table 1). These findings suggest that acetone production is maintained even in coculture and that other factors are apparently responsible for the minimal levels of acetone.
Table 1.
Enzymatic activities associated with acetone and butanol production
| Enzyme | Activity (U/mg) in: |
|
|---|---|---|
| Strain N1-4 | Coculturea | |
| CoA-transferase (acetate) | 29.72 ± 2.43 | 27.43 ± 2.51 |
| CoA-transferase (butyrate) | 28.64 ± 2.55 | 29.36 ± 2.75 |
| Acetoacetate decarboxylase | 140.81 ± 17.14 | 168.08 ± 23.70 |
| Butyraldehyde dehydrogenase | 1.24 ± 0.01 | 0.95 ± 0.13 |
| Butanol dehydrogenase | 1.04 ± 0.07 | 0.90 ± 0.02 |
Activities were normalized based on thiolase activity because crude extracts were prepared from cocultured strains N1-4 and C. thermocellum.
DISCUSSION
Products of the cellulosome secreted by C. thermocellum differ according to the type of substrate, for example, whether it contains cellobiose or crystalline cellulose (11, 29, 35). Since C. thermocellum was grown on medium containing cellobiose before initiating coculture, we considered that preculture might be required to induce the cellulosome components required for degrading crystalline cellulose. Indeed, when C. thermocellum and strain N1-4 were cocultivated simultaneously at 30°C in medium containing crystalline cellulose, the coculture system produced very little butanol (0.3 g/liter) (Fig. 1a and b). Conversely, when strain N1-4 was added to medium in which C. thermocellum had been incubated at 60°C for a minimum of 24 h, all cocultures incubated at 30°C produced butanol from crystalline cellulose. These findings substantiated those of a previous study (24) in which cellulose degradation was optimal only when the butanol-producing strain was added to the growth medium after the strain capable of utilizing cellulose, C. thermocellum, had been cultured on the same medium. Thus, the optimal expression of cellulosome components was essential for producing butanol from crystalline cellulose in both the present and in the previous study.
The culture temperature after the addition of the butanol-producing strain is crucial for the efficiency of butanol production (Fig. 3). The findings were similar for C. acetobutylicum NCIB 8052, with the solvent yield decreasing to 24% at 40°C (22). As in their study, butanol production in our study also decreased, even when strain N1-4 was cultivated as monocultures at 42°C (0.2 g/liter). The optimum temperature for butanol production using a coculture system such as that described in this study was thus 30°C.
Strain N1-4 produced the most butanol in our coculture system among three tested butanol-producing strains (Fig. 5a and b), indicating that the genes responsible for producing solvent were induced to a greater extent under coculture conditions in strain N1-4 than in either C. beijerinckii or C. acetobutylicum. The regulatory mechanism for this solventogenesis in butanol-producing strains is only partly understood. Butyric acid production is important for solvent formation because the intracellular and/or extracellular levels of butyric acid are implicated in the induction of solventogenesis in C. acetobutylicum (13, 14). In addition, levels of butyryl phosphate, but not of acetyl phosphate, correlate with the initiation of solventogenesis in C. acetobutylicum (36). In addition, butyric acid must be added to medium inoculated with C. acetobutylicum for butanol production using cocultures (34). However, since strain N1-4 produced butanol in the absence of added butyric acid, the amount of butyric acid is clearly irrelevant in cocultures using this strain.
Acetone was barely detectable in our coculture system even when the substrate concentration was increased and either C. beijerinckii or C. acetobutylicum served as the butanol-producing strain (Fig. 4 and Fig. 5b). Although several clostridial species can convert acetone to isopropanol (10), the isopropanol levels in coculture supernatants were below the detection limit, indicating that acetone was not converted. One explanation for the very low levels of acetone produced during coculture in the present study could be that C. thermocellum metabolized the acetone produced by strain N1-4. Indeed, acetone is metabolized via several pathways in some microorganisms (12). However, the absence of a decrease in the acetone concentration when C. thermocellum was incubated with acetone (1 g/liter) for 10 days (data not shown) implies that the acetone was not metabolized. Another possible explanation for the low acetone concentrations is that electron flow in strain N1-4 was directed toward NADH regeneration rather than hydrogen gas production, which arises due to the extreme reducing conditions in the coculture medium caused by the dissolved hydrogen gas produced by C. thermocellum. Such electron flow would change the acetone and butanol ratio because the production of butanol requires NADH whereas that of acetone does not (17). Indeed, butanol production is enhanced by an increase in hydrogen partial pressure (7, 33). In addition, through downregulation of the putative H2-uptake hydrogenase hupCBA gene, we obtained a transformant producing large amounts of acetone and small amounts of butanol (23), and the amount of NADH required for butanol production was insufficient. It is therefore considered that the observed decrease in acetone production is related to increased NADH regeneration in strain N1-4.
In this report, we described butanol production from crystalline cellulose by cocultured thermophilic and cellulolytic C. thermocellum and the mesophilic strain N1-4. To improve the rate of butanol production, the rate of cellulose hydrolysis should be accelerated via the expression of cellulase in both C. thermocellum and strain N1-4. In addition, the application of mesophilic and cellulolytic clostridia, such as C. cellulolyticum and C. cellulovorans, should be investigated to improve the economics of the process.
ACKNOWLEDGMENTS
This study was supported in part by the New Energy and Industrial Technology Development Organization and a Grant-in-Aid for Young Scientists (B).
Footnotes
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Berezina O. V., Brandt A., Yarotsky S., Schwarz W. H., Zverlov C. C. 2009. Isolation of a new butanol-producing Clostridium strain: high level of hemicellulosic activity and structure of solventogenesis genes of a new Clostridium saccharobutylicum isolate. Syst. Appl. Microbiol. 32:449–459 [DOI] [PubMed] [Google Scholar]
- 2. Demain A. L. 2009. Biosolutions to the energy problem. J. Ind. Microbiol. Biotechnol. 36:319–332 [DOI] [PubMed] [Google Scholar]
- 3. Demain A. L., Newcomb M., Wu J. H. 2005. Cellulase, clostridia, and ethanol. Microbiol. Mol. Biol. Rev. 69:124–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Desvaux M., Emmanuel G., Petitdemange H. 2000. Cellulose catabolism by Clostridium cellulolyticum growing in batch culture on defined medium. Appl. Environ. Microbiol. 66:2461–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Desvaux M. 2005. Clostridium cellulolyticum: model organism of mesophilic cellulolytic clostridia. FEMS Microbiol. Rev. 29:741–764 [DOI] [PubMed] [Google Scholar]
- 6. Doi R. H., Tamaru Y. 2001. The Clostridium cellulovorans cellulosome: an enzyme complex with plant cell wall degrading activity. Chem. Rec. 1:24–32 [DOI] [PubMed] [Google Scholar]
- 7. Doremus M. G., Linden J. C., Moreira A. R. 1985. Agitation and pressure effects on acetone-butanol fermentation. Biotechnol. Bioeng. 27:852–860 [DOI] [PubMed] [Google Scholar]
- 8. Dürre P. 2007. Biobutanol: an attractive biofuel. Biotechnol. J. 2:1525–1534 [DOI] [PubMed] [Google Scholar]
- 9. Dürre P., et al. 2002. Transcriptional regulation of solventogenesis in Clostridium acetobutylicum. J. Mol. Microbiol. Biotechnol. 4:295–300 [PubMed] [Google Scholar]
- 10. George H. A., Johnson J. L., Moore W. E., Holdeman L. V., Chen J. S. 1983. Acetone, isopropanol, and butanol production by Clostridium beijerinckii (syn. Clostridium butylicum) and Clostridium aurantibutyricum. Appl. Environ. Microbiol. 45:1160–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gold N. D., Martin V. J. J. 2007. Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. J. Bacteriol. 189:6787–6795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hausinger R. P. 2007. New insights into acetone metabolism. J. Bacteriol. 189:671–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hüsemann M. H. W., Papoutsakis E. T. 1988. Solventogenesis in Clostridium acetobutylicum fermentations related to carboxylic-acid and proton concentrations. Biotechnol. Bioeng. 32:843–852 [DOI] [PubMed] [Google Scholar]
- 14. Hüsemann M. H., Papoutsakis E. T. 1990. Effects of propionate and acetate additions on solvent production in batch cultures of Clostridium acetobutylicum. Appl. Environ. Microbiol. 56:1497–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Y., et al. 2009. Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab. Eng. 11:284–291 [DOI] [PubMed] [Google Scholar]
- 16. Johnson J. L., Toth J., Santiwatanakul S., Chen J. S. 1997. Cultures of “Clostridium acetobutylicum” from various collections comprise Clostridium acetobutylicum, Clostridium beijerinckii, and two other distinct types based on DNA-DNA reassociation. Int. J. Syst. Bacteriol. 47:420–424 [DOI] [PubMed] [Google Scholar]
- 17. Jones D. T., Woods D. R. 1986. Acetone-butanol fermentation revisited. Microbiol. Rev. 50:484–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keis S., Bennett C. F., Ward V. K., Jones D. T. 1995. Taxonomy and phylogeny of industrial solvent-producing clostridia. Int. J. Syst. Bacteriol. 45:693–705 [DOI] [PubMed] [Google Scholar]
- 19. Kosaka T., Nakayama S., Nakaya K., Yoshino S., Furukawa K. 2007. Characterization of the sol operon in butanol-hyperproducing Clostridium saccharoperbutylacetonicum strain N1-4 and its degeneration mechanism. Biosci. Biotechnol. Biochem. 71:58–68 [DOI] [PubMed] [Google Scholar]
- 20. López-Contreras A. M., et al. 2001. Clostridium beijerinckii cells expressing Neocallimastix patriciarum glycoside hydrolases show enhanced lichenan utilization and solvent production. Appl. Environ. Microbiol. 67:5127–5133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lynd L. R., van Zyl W. H., McBride J. E., Laser M. 2005. Consolidated bioprocessing of cellulosic biomass: an update. Curr. Opin. Biotechnol. 16:577–583 [DOI] [PubMed] [Google Scholar]
- 22. McNeil B., Kristianse B. 1985. Effect of temperature upon growth rate and solvent production in batch cultures of Clostridium acetobutylicum. Biotechnol. Lett. 7:499–502 [Google Scholar]
- 23. Nakayama S., et al. 2008. Metabolic engineering for solvent productivity by downregulation of the hydrogenase gene cluster hupCBA in Clostridium saccharoperbutylacetonicum strain N1-4. Appl. Microbiol. Biotechnol. 78:483–493 [DOI] [PubMed] [Google Scholar]
- 24. Petitdemange E., Fond O., Caillet F., Petitdemange H., Gay R. 1983. A novel one step process for cellulose fermentation using mesophilic cellulolytic and glycolytic clostridia. Biotechnol. Lett. 5:119–124 [Google Scholar]
- 25. Petitdemange E., Fond O., Raval G., Petitdemange H., Gay R. 1984. Screening of cellulolytic anaerobic bacteria, co-fermentations with methanic and acetobutylic fermentation. Part one: fermentation of cellulose by a co-culture of Clostridium cellulolyticum and Clostridium acetobutylicum, p. 223–234 In Ferrero G. L., Ferranti M. P., Naveau H. (ed.), Anaerobic digestion and carbohydrate hydrolysis of wastes. Elsevier Applied Science, London, United Kingdom [Google Scholar]
- 26. Qureshi N., Lolas A., Blaschek H. P. 2001. Soy molasses as fermentation substrate for production of butanol using Clostridium beijerinckii BA101. J. Ind. Microbiol. Biotechnol. 26:290–295 [DOI] [PubMed] [Google Scholar]
- 27. Shaheen R., Shirley M., Jones D. T. 2000. Comparative fermentation studies of industrial strains belonging to four species of solvent-producing clostridia. J. Mol. Microbiol. Biotechnol. 2:115–124 [PubMed] [Google Scholar]
- 28. Sillers R., Al-Hinai M. A., Papoutsakis E. T. 2009. Aldehyde-alcohol dehydrogenase and/or thiolase overexpression coupled with CoA transferase downregulation lead to higher alcohol titers and selectivity in Clostridium acetobutylicum fermentations. Biotechnol. Bioeng. 102:38–49 [DOI] [PubMed] [Google Scholar]
- 29. Stevenson D. M., Weimer P. J. 2005. Expression of 17 genes in Clostridium thermocellum ATCC 27405 during fermentation of cellulose or cellobiose in continuous culture. Appl. Environ. Microbiol. 71:4672–4678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vasconcelos I., Girbal L., Soucaille P. 1994. Regulation of carbon and electron flow in Clostridium acetobutylicum grown in chemostat culture at neutral pH on mixtures of glucose and glycerol. J. Bacteriol. 176:1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Virunanon C., Chantaroopamai S., Denduangbaripant J., Chulalaksananukul W. 2008. Solventogenic-cellulolytic clostridia from 4-step-screening process in agricultural waste and cow intestinal tract. Anaerobe 14:109–117 [DOI] [PubMed] [Google Scholar]
- 32. Wu J. H. D., Orme-Johnson W. H., Demain A. L. 1988. Two components of an extracellular protein aggregate of Clostridium thermocellum together degrade crystalline cellulose. Biochemistry 27:1703–1709 [Google Scholar]
- 33. Yelshalmi L., Volesky B., Szczesny T. 1985. Effects of increased hydrogen partial pressure on the acetone-butanol fermentation by Clostridium acetobutylicum. Appl. Microbiol. Biotechnol. 22:103–107 [Google Scholar]
- 34. Yu E. K. C., Chan M. K. H., Saddler J. N. 1985. Butanol production from cellulosic substrates by sequential co-culture of Clostridium thermocellum and C. acetobutylicum. Biotechnol. Lett. 7:509–514 [Google Scholar]
- 35. Zhang Y. H., Lynd L. R. 2005. Regulation of cellulase synthesis in batch and continuous cultures of Clostridium thermocellum. J. Bacteriol. 187:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao Y., Tomas C. A., Rudolph F. B., Papoutsakis E. T., Bennett G. N. 2005. Intracellular butyryl phosphate and acetyl phosphate concentrations in Clostridium acetobutylicum and their implications for solvent formation. Appl. Environ. Microbiol. 71:530–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zheng Y. N., et al. 2009. Problems with the microbial production of butanol. J. Ind. Microbiol. Biotechnol. 36:1127–1138 [DOI] [PubMed] [Google Scholar]
- 38. Zverlov V. V., Berezina O., Velikodvorskaya G. A., Schwarz W. H. 2006. Bacterial acetone and butanol production by industrial fermentation in the Soviet Union: use of hydrolyzed agricultural waste for biorefinery. Appl. Microbiol. Biotechnol. 71:587–597 [DOI] [PubMed] [Google Scholar]