Abstract
Increased membrane fluidity, which causes cofactor leakage and loss of membrane potential, has long been documented as a cause for decreased cell growth during exposure to ethanol, butanol, and other alcohols. Reinforcement of the membrane with more complex lipid components is thus thought to be beneficial for the generation of more tolerant organisms. In this study, organisms with more complex membranes, namely, archaea, did not maintain high growth rates upon exposure to alcohols, indicating that more complex lipids do not necessarily fortify the membrane against the fluidizing effects of alcohols. In the presence of alcohols, shifts in lipid composition to more saturated and unbranched lipids were observed in most of the organisms tested, including archaea, yeasts, and bacteria. However, these shifts did not always result in a decrease in membrane fluidity or in greater tolerance of the organism to alcohol exposure. In general, organisms tolerating the highest concentrations of alcohols maintained membrane fluidity after alcohol exposure, whereas organisms that increased membrane rigidity were less tolerant. Altered lipid composition was a common response to alcohol exposure, with the most tolerant organisms maintaining a modestly fluid membrane. Our results demonstrate that increased membrane fluidity is not the sole cause of growth inhibition and that alcohols may also denature proteins within the membrane and cytosol, adversely affecting metabolism and decreasing cell growth.
INTRODUCTION
The intimate interaction of microorganisms with their surrounding environment requires constant sensing and response to changes and perturbations, many of which occur at the cell's outer membrane surface. The cell membrane is involved in a variety of physiological functions, including solute and electron transport, ATP synthesis, and intercellular signaling, and is designed to withstand turgor pressure to protect the cells from bursting. In the case of bacteria, Gram-positive cells have a single cell wall composed mostly of peptidoglycan, whereas Gram-negative bacteria have two layers consisting of a thinner underlying layer of peptidoglycan and an outer membrane composed of lipopolysaccharides, phospholipids, and protein. Archaeal membranes are composed of ether-linked mono- or diglycerol di- or tetraethers that can span the entire membrane, potentially contributing to tolerance of extreme conditions. These ether lipids may contain highly branched and cyclical fatty acid tails. The membranes of eukaryotes such as yeasts are composed primarily of glycerophospholipids containing saturated or cis-unsaturated acyl chains along with sterols, such as ergosterol, which make up the majority of the nonpolar lipids in eukaryotic membranes.
Cell membranes can confer tolerance and protection for the cell under various environmental stresses. One such stress is that of alcohol toxicity, and a significant body of literature has focused on how alcohols affect membrane integrity (1, 2, 13, 23, 24, 27, 29). This topic has recently reemerged in light of renewed interest in cellulosic biofuels as an alternative transportation fuel. Ethanol in particular is known to fluidize the membrane, resulting in uncontrolled transport of solutes that can decrease the proton flux across the membrane and cause leakage of important cofactors such as Mg2+ (9, 23). Ethanol can also inactivate membrane and cytosolic enzymes, for example, ATPase and glycolytic enzymes, causing a decrease in cell growth (4, 12, 23). Butanol, another promising biofuel, is even more toxic than ethanol. Its greater toxicity may be due to its longer carbon chain that can further intercalate into the membrane and break hydrogen bonds between lipid tails (29). Hence, a compromised cell membrane is assumed to be primarily responsible for the decline in growth rate and cell viability when a microorganism is exposed to alcohols (24).
The detrimental effects that alcohols have on cell membranes suggest that reinforcement of the plasma membrane may be crucial for increasing tolerance to potential biofuels. Indeed, the insertion of more complex lipids, such as ergosterol in the case of yeasts, has been shown to reinforce the membrane, potentially producing a more viable candidate for biofuel production (11). Archaea, which often live in extreme environments, synthesize membranes that contribute to their resilience and could thus serve as more tolerant biofuel producers (5, 20). The present study was designed to establish the effects of ethanol, butanol, and isobutanol on the membranes of several types of organisms, including archaea, yeasts, and bacteria, and to determine whether unique lipid profiles conferred resilience. No correlation was observed between the degree of saturation of lipids within the membrane and either the degree of membrane fluidity or the cells' tolerance to alcohols. Each organism had a unique response to alcohol exposure, and the lipid composition did not appear to influence the tolerance of the organism.
MATERIALS AND METHODS
Growth conditions.
The organisms used in the present study are listed in Table 1 and were purchased from either the American Type Culture Collection (ATCC) or the German Resource Center for Biological Material (DSMZ), except for Methanocaldococcus jannaschii and Escherichia coli KO11, which were obtained from Frank Robb at the University of maryland in Baltimore and Lonnie Ingram at the Univerity of Florida, respectively. The growth conditions and the medium used for each organism are summarized in Table 1. Defined medium was used when possible, and organisms requiring glucose were grown at a concentration of 4 g liter−1. Each organism was grown in the Bioscreen C (Growth Curves) with optical density at 600 nm (OD600) readings taken every 20 min using EZExperiment (v1.26) unless the microbe required a growth temperature greater than 60°C or a headspace other than nitrogen or air. Anaerobic cultures were grown in the Bioscreen C housed in an anaerobic chamber (Coy Laboratory Products, Inc.). Microbes requiring a hydrogen headspace were grown in glass Hungate tubes capped with butyl rubber stoppers (Bellco) and aerobic hyperthermophiles were grown in Hungate tubes capped with chlorobutyl-isoprene rubber stoppers (Wheaton). The optical density for these organisms was measured at 600 nm with a Spectronic 20D+ (Thermo Scientific), and growth experiments were performed in triplicate. The specific growth rate for all microbes was calculated based on the OD600 from the exponential region of the growth curve. Calibration curves of the OD600 versus dry cell weight for E. coli and Saccharomyces cerevisiae grown in the presence of alcohols confirmed the equivalence of the specific growth rate based on the OD600 and the rate based on the dry cell weight.
Table 1.
Organisms in this study
| Organism | Type | Temp (°C) | Medium (reference) | Oxygen levela |
|---|---|---|---|---|
| Methanocaldococcus jannaschii (DSMZ 2661) | Archaeon | 75 | Defined minimal (25) | AN |
| Natronomonas pharaonis (ATCC 35678) | Archaeon | 37 | APB (44) | AE |
| Aeropyrum pernix (DSMZ 11879) | Archaeon | 75 | Marine broth (44) | AE |
| Halorubrum lacusprofundi (ATCC 49239) | Archaeon | 23 | Artificial deep lake (16) | AE |
| Methanosarcina acetivorans (ATCC 35395) | Archaeon | 37 | Defined minimal (41) | AN |
| Geobacillus thermoglucosidasius M10EXG (ATCC BAA-1067) | Bacterium | 60 | TMM (14) | MA |
| Escherichia coli MG1655 (ATCC 47076), K-12 (ATCC 10798), and KO11 (ATCC 55124) | Bacterium | 37 | Defined minimal (45) | AN |
| Zymomonas mobilis (ATCC 31821) | Bacterium | 30 | Semidefined (17) | AN |
| Clostridium beijerinckii (ATCC 51743) | Bacterium | 37 | P2 (36) | AN |
| Thermoanaerobacter ethanolicus JW200 (ATCC 31937) | Bacterium | 60 | TYEG (50) | AN |
| Thermoanaerobacter pseudoethanolicus 39E (ATCC 33223) | Bacterium | 60 | TYEG (50) | AN |
| Thermoanaerobacterium saccharolyticum B6A-RI (ATCC 49915) | Bacterium | 60 | TYEG (50) | AN |
| Kluyveromyces marxianus (ATCC 10606) | Yeast | 30 | Mineral (15) | AE |
| Saccharomyces cerevisiae (DSMZ 70449) | Yeast | 30 | Defined dropout (26) | AN |
| Dekkera bruxellensis (DSMZ 3429) | Yeast | 30 | CDM (21) | MA |
AN, anaerobic; AE, aerobic; MA, microaerophilic.
Lipid extraction and methylation.
Lipids from the organisms were extracted as described by the method of Bligh and Dyer (6) with the following modifications. The organisms in Table 1 were grown in 10 ml of medium and inoculated with a mid-exponential phase culture to an OD600 of 0.01. In some tubes, an amount of ethanol, butanol, or isobutanol was added that caused a 50 or 85% reduction in the specific growth rate. Cells were harvested by centrifugation after four doubling times or when the cell density reached an OD600 of 0.4. The cell pellet was washed with 3 ml of phosphate-buffered saline (PBS) and pelleted via centrifugation. The cells were then suspended in 4.5 ml of PBS and decanted into 15-ml screw-cap glass vials. An internal standard of pentadecanoic acid was dissolved in acetic acid and added to each sample to give a final concentration of 42 μM. A 1:1 solution of methanol and chloroform was added to give a final volume of 15 ml, and the sample solution was mixed vigorously. The two phases were allowed to separate, and the entire solution was dried down overnight using a Dri-Block heater DB-3D and sample concentrator (Techne). To prepare the lipids for gas chromatography-mass spectrometry (GC-MS), the lipids were methylated according to the method of Voelker and Davies (47). Briefly, the lipid powder was suspended in methanol containing 5% H2SO4, followed by incubation at 90°C for 2 h. Once the samples cooled, an aqueous solution of 0.9% (wt/vol) NaCl was added, along with 300 μl of hexane. The solutions were mixed for 30 s, and the hexane layer was removed and placed in a crimp-top GC vial for analysis.
Archaeal samples were prepared as described above with the following modifications (46). After the wash steps, the pellets were suspended in 3 ml of PBS, transferred to 10-ml conical, screw-cap tubes, and incubated at 4°C overnight after the addition of chloroform, methanol, and the internal standard. After drying, lipid samples were suspended in 5 ml of acetone and incubated at −20°C overnight to precipitate polar lipids. The samples were centrifuged at 240 × g, and the acetone was carefully removed. A 1-ml aliquot of methanol, chloroform, and hydrochloric acid (10:1:1 by volume) was added, and the samples were incubated at 95°C for 1 h. After methylation, the hexane layer was removed and placed in a clean conical tube. The hexane was removed with nitrogen, and the dried lipid layer was silylated with 50 μl of N,O-bis(trimethylsilyl)trifluoroacetamide and 50 μl of hexane. The silylated lipids were placed in crimp-top GC vials for analysis.
GC-MS.
All GC samples were analyzed with a Varian CP-3800 gas chromatograph and 320 mass spectrometer fitted with a CombiPAL autosampler. The method used for separation of bacterial and eukaryotic lipids set the injector temperature to 300°C, the column oven at 140°C for 3 min, followed by an increase at a rate of 10°C min−1 to 320°C, which was held for 5 min. The MS detector was fixed at 1,175 V, and compounds with molecular masses between 50 and 600 Da were collected with a scan time of 0.25 s. The peaks that contained ion 74, which is indicative of a methylated lipid, were identified using the NIST database, and the area of each peak was calculated. For each sample, the ratio of the sums of the unsaturated-lipid peak areas to the saturated-lipid peak areas was calculated. If the organism does not produce unsaturated lipids, the ratio of branched to unbranched lipids was calculated.
Aeropyrum pernix samples were analyzed using the same GC-MS, while Natronomonas pharaonis, Halorubrum lacusprofundi, and Methanosarcina acetivorans samples were analyzed with an Agilent GC 7890A and MS 5975C. Both instruments used the following method. The injector temperature was set to 280°C, and the column oven was set to 140°C for 3 min, followed by an increase at a rate of 10°C min−1 to 340°C, which was held for 5 min. The MS detector parameters were kept the same as those for bacterial and eukaryotic lipid samples. The peaks that contained ion 117, which is indicative of a silylated lipid, were identified using the NIST database, and the area of each was calculated as described.
Fluorescence anisotropy.
Fluorescence anisotropy uses polarized light to excite a hydrophobic probe and measures the amount of emitted light that returns as polarized light. The following ratio indirectly measures the mobility of lipids within a membrane structure:
where Ivv is the intensity of vertically polarized light that is emitted as vertically polarized light, Ivh is the intensity of vertically polarized light that is emitted as horizontally polarized light, and G is the grating factor to correct for photomultiplier sensitivity.
Fluorescence anisotropy was performed as described previously (33, 34) with modifications. Mid-exponential-phase cultures of each organism in Table 1 were used to inoculate fresh medium containing the concentration of alcohol required to inhibit growth by 50 and 85%. The cells were incubated for four doubling times and collected by centrifugation. The cell pellet was washed twice in 15 mM Tris-HCl buffer, suspended to an OD600 of 0.2, and incubated with 1.4 μl of 12 mM diphenylhexatriene (DPH) dissolved in tetrahydrofuran for 20 min to allow intercalation of the probe into the plasma membrane (40). The anisotropy was measured on a HORIBA Jobin Yvon Fluorolog-3 fluorometer using excitation and emission wavelengths of 358 and 428 nm, respectively, and a slit width of 5 nm. The temperature was set to that required for growth and biological triplicates were tested for all samples. The background fluorescence values of the cell suspension without DPH for all intensities were measured and subtracted from the DPH-labeled samples. All measurements were recorded with FluorEssence (v2.5.2.0).
RESULTS
Growth inhibition.
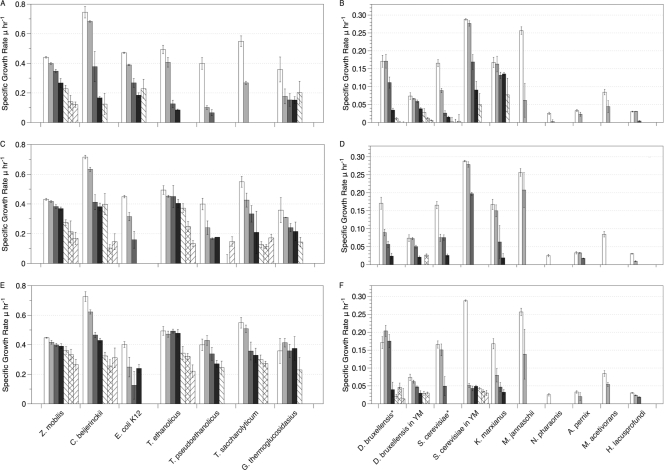
Growth studies were performed for each organism under optimal growth conditions (i.e., temperature and aeration), in a defined medium when possible, to determine the inhibitory concentration of ethanol, butanol, and isobutanol. Specific growth rates (μ) were calculated as a function of alcohol concentration for bacteria (Fig. 1A, C, and E) and for yeasts, and archaea (Fig. 1B, D, and F). Among the bacteria, Zymomonas mobilis and Clostridium beijerinckii showed the best ability to maintain a relatively high specific growth rate in the presence of all three alcohols (Fig. 1A, C, and E). As the alcohol concentration increased, their specific growth rates decreased slightly. In contrast, other bacteria exhibited a more pronounced reduction in growth rates at low concentrations of alcohols, with more subtle changes occurring at higher concentrations. E. coli K-12 followed this trend in butanol and isobutanol (Fig. 1C and E) but followed the growth patterns of Z. mobilis and C. beijerinckii in ethanol (Fig. 1A). Interestingly, the engineered ethanologenic species E. coli KO11 did not grow as well as K-12 or E. coli MG1655 in the presence of alcohols (data not shown). The thermophiles, which include Thermoanaerobacter ethanolicus, Thermoanaerobacter pseudoethanolicus, Thermoanaerobacterium saccharolyticum, and Geobacillus thermoglucosidasius, exhibited similar growth profiles in butanol and isobutanol (Fig. 1C and E), but G. thermoglucosidasius was the only thermophile moderately tolerant to ethanol (Fig. 1A). Interestingly, T. saccharolyticum showed the lowest tolerance of the bacteria tested toward ethanol (Fig. 1A) but was less affected by butanol and isobutanol (Fig. 1C and E).
Fig. 1.
Specific growth rates based on OD600 values for bacteria (A, C, and E) and for yeasts and archaea (B, D, and F) grown in the presence of ethanol (□, 0 g liter−1; light gray shading, 10 g liter−1; dark gray shading, 25 g liter−1; ■, 35 g liter−1; ▧, 45 g liter−1; ▩, 55 g liter−1; ▫, 65 g liter−1), butanol (□, 0 g liter−1; light gray shading, 2 g liter−1; dark gray shading, 4 g liter−1; ■, 6 g liter−1; ▧, 8 g liter−1; ▩, 10 g liter−1; ▫, 12 g liter−1), and isobutanol (□, 0 g liter−1; light gray shading, 2 g liter−1; dark gray shading, 4 g liter−1; ■, 6 g liter−1; ▧, 8 g liter−1; ▩, 10 g liter−1; ▫, 12 g liter−1). Panels A and B depict growth in ethanol, panels C and D depict growth in butanol, and panels E and F depict growth in isobutanol. *, minimal yeast medium.
With yeasts, butanol and isobutanol caused significant decreases in specific growth rates despite the additional nutrients present in the rich medium used (Fig. 1D and F). Dekkera bruxellensis performed best in minimal medium when exposed to alcohol; Saccharomyces cerevisiae maintained its growth better when grown on a rich medium. Kluyveromyces marxianus, which grows only in an aerobic environment, was able to tolerate up to 25 g/liter of ethanol before a decrease in its specific growth rate occurred. Overall, D. bruxellensis tolerated the alcohols better than the other two yeasts. The growth profiles of this yeast matched most closely those of C. beijerinckii and E. coli in ethanol. In general, Z. mobilis grew in higher concentrations of alcohol than any other bacteria or yeast. K. marxianus and E. coli were the most tolerant to ethanol compared to the other microorganisms, while T. saccharolyticum and C. beijerinckii withstood the highest concentrations of butanol and isobutanol.
Five archaeal species were also tested for their tolerance to alcohols. The extremophiles M. jannaschii, A. pernix, H. lacusprofundi, M. acetivorans, and N. pharaonis were selected for study because they can withstand high temperatures (>80°C), low temperature (<15°C), or extreme salt concentrations (200 g liter−1). The mechanisms used by these extremophiles to survive in their natural environments, such as using stabilizing intracellular solutes, altering the proton motive force, and favoring certain molecular interactions within proteins, may also improve their tolerance to non-native conditions (18). Therefore, it is plausible that these archaea can tolerate alcohols. However, Fig. 1B, D, and F show that the archaea had little growth tolerance to alcohols, particularly ethanol. The growth profiles show a dramatic decrease in growth rates with the lowest concentration tested and little growth was observed beyond that concentration. The results indicate that even though these archaea can withstand certain extreme conditions, the factors that effect tolerance to those conditions do not confer alcohol tolerance.
Lipid analysis.
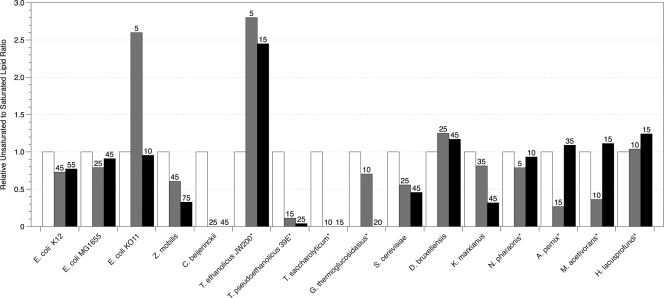
Figures 2, 3, and 4 depict the changes in lipid composition when the microbes tested were exposed to ethanol, butanol, or isobutanol. In general, the ratios of unsaturated to saturated (U/S) or of branched to unbranched (B/UB) lipids decreased in response to the fluidizing effect of alcohols on the membranes. When grown in the presence of ethanol and butanol, the majority of bacteria and yeasts examined decreased the ratio of U/S or B/UB by at least 20%. E. coli species K-12 and MG1655 maintained a constant U/S in ethanol and decreased U/S in butanol, but E. coli KO11 increased U/S by 5% in 10 g liter−1 ethanol and 20% in 2 g liter−1 butanol. Dombek and Ingram (13) showed that the membrane of K-12 contained increased amounts of oleic acid (C18:1) when grown aerobically in ethanol, but we did not observe this change under anaerobic conditions. In the presence of isobutanol, all E. coli species responded with a decrease of at least 20% in the U/S ratio.
Fig. 2.
Relative unsaturated to saturated lipid ratios of organisms grown in the presence of ethanol determined by GC-MS. The asterisk indicates that branched lipids were used in place of unsaturated lipids because unsaturated lipids were not detected in the samples. Numbers above bars correspond to the concentrations of alcohol in g liter−1 that reduced specific growth rates (μ) by 50% (▩) and 85% (■). The fold change of unsaturated to saturated lipids was calculated based on the unsaturated to saturated lipid ratio of the unexposed cells for each organism. The error of the biological triplicates for each condition was <10%.
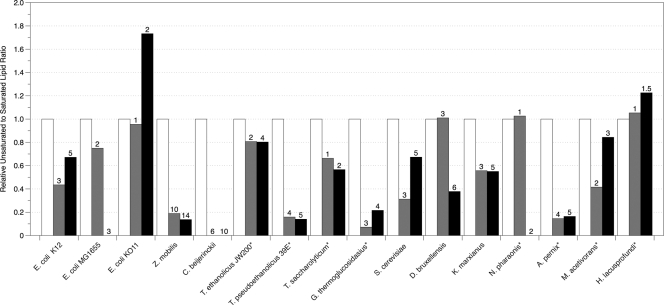
Fig. 3.
Relative unsaturated to saturated lipid ratios of organisms grown in the presence of butanol determined by GC-MS. The asterisk indicates that branched lipids were used in place of unsaturated lipids because unsaturated lipids were not detected in the samples. Numbers above bars correspond to the concentrations of alcohol in g liter−1 that reduced specific growth rates (μ) by 50% (▩) and 85% (■). The fold change of unsaturated to saturated lipids was calculated based on the unsaturated to saturated lipid ratio of the unexposed cells for each organism. The error of the biological triplicates for each condition was <10%.
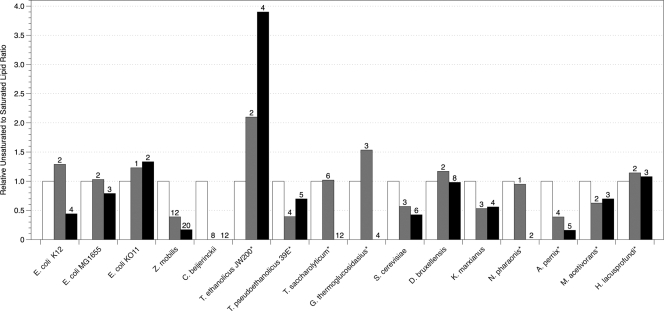
Fig. 4.
Relative unsaturated to saturated lipid ratios of organisms grown in the presence of isobutanol determined by GC-MS. The asterisk indicates that branched lipids were used in place of unsaturated lipids because unsaturated lipids were not detected in the samples. Numbers above bars correspond to the concentrations of alcohol in g liter−1 that reduced specific growth rates (μ) by 50% (▩) and 85% (■). The fold change of unsaturated to saturated lipids was calculated based on the unsaturated to saturated lipid ratio of the unexposed cells for each organism. The error of the biological triplicates for each condition was <10%.
The membranes of the alcohol producers Z. mobilis and C. beijerinckii decreased the U/S ratio when grown in the presence of all three alcohols, with C. beijerinckii eliminating all unsaturated lipids. Z. mobilis has been reported to increase the amounts of vaccenic acids and hopanoids, which are sterol-like molecules, to modulate fluidity within the membrane upon ethanol exposure (8, 22). Our results did not show an increase in vaccenic acids, and hopanoids were not measured within the present study. Hence, shifts to other saturated lipids such as hexadecanoic acid caused the U/S ratio to decrease by at least 60% in Z. mobilis.
In ethanol, T. saccharolyticum, T. pseudoethanolicus, and G. thermoglucosidasius decreased the proportion of branched lipids in their membranes by 90%, whereas T. ethanolicus increased the amount of branched lipids by 45%. It has been shown that T. pseudoethanolicus produces the transmembrane lipid α,ω-dicarboxylic acid (C30), which normally represents over 50% of the total lipids, and that low levels of ethanol exposure (<2% [vol/vol]) cause the percentage of α,ω-dicarboxylic acid to decrease to 6% (7). During our study, the amount of α,ω-dicarboxylic acid decreased by 60% during ethanol exposure but increased by 90% in both butanol and isobutanol. In butanol, T. ethanolicus and T. saccharolyticum decreased their B/UB ratios by at least 20%, while T. pseudoethanolicus and G. thermoglucosidasius decreased their B/UB ratios by as much as 85%.
In all three alcohols tested, S. cerevisiae and K. marxianus decreased their U/S ratio by at least 40% in the highest concentrations of alcohols. The relationship between lipid composition and ethanol tolerance of S. cerevisiae has been previously studied in detail but generally under aerobic growth conditions (10, 28, 48). For example, You et al. (49) showed that the ethanol tolerance of aerobically grown S. cerevisiae was dependent on the cellular oleic acid content. Although our study showed small increases in oleic acid (data not shown), this change was not enough to increase the U/S ratio. Octadecanoic acid increased slightly in ethanol, while the amount of hexadecanoic acid decreased in S. cerevisiae in all alcohols tested. D. bruxellensis increased its U/S ratio in both ethanol and isobutanol by 20% but maintained or slightly decreased its U/S ratio in butanol. K. marxianus significantly increased the amount of hexadecanoic acid in butanol and isobutanol. Little has been reported on the effect of various alcohols on the lipid composition of D. bruxellensis or K. marxianus.
The archaea tested in the present study showed different responses. The halophiles N. pharaonis and H. lacusprofundi maintained or slightly increased their B/UB ratio in ethanol and initially maintained this ratio in butanol and isobutanol concentrations that inhibited growth by 50%. At higher concentrations of butanol and isobutanol, the presence of branched lipids in N. pharaonis decreased significantly, while the B/UB ratio of H. lacusprofundi increased. The hyperthermophilic aerobe A. pernix decreased its B/UB ratio by 70% in ethanol at 50% growth inhibition and by 80% in butanol and isobutanol at 50% inhibition. At the higher ethanol concentration, A. pernix maintained its B/UB ratio similar to the uninhibited levels. Unlike N. pharaonis, A. pernix sustained decreased levels of its branched lipids in butanol and isobutanol concentrations that inhibited growth by 85%. In the presence of ethanol and butanol, M. acetivorans decreased its B/UB ratio at 50% growth inhibition, but this ratio returned to the unexposed level at 85% growth inhibition. M. acetivorans decreased B/UB ratios in both concentrations of isobutanol. These are the first data on lipid profiles reported for these organisms during growth in the presence of alcohols.
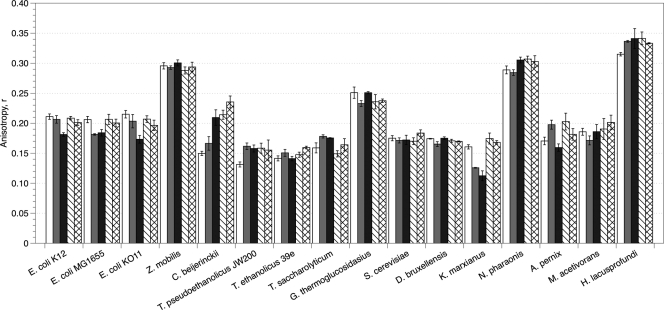
Anisotropy.
Figure 5 shows the DPH anisotropy values for organisms grown in ethanol and butanol at concentrations that reduced their specific growth rates by 50 and 85%. The anisotropy values measured for unexposed cells differed significantly (i.e., some organisms initially displayed higher or lower membrane fluidity) due to membrane protein/lipid ratios, the presence of unique lipid molecules such as sterols, and possibly the growth conditions used. Measurements were made in the absence of alcohols; however, the time required for sample preparation was minimized to prevent changes in membrane integrity from occurring after the samples were removed from alcohol-containing media. After incubation in growth-inhibiting concentrations of alcohols, most organisms that were unable to grow at concentrations of greater than 25 g liter−1 ethanol or 2 g liter−1 butanol appeared to shift their membrane fluidity by at least 10% of the unexposed membrane fluidity. The three E. coli strains increased membrane fluidity an average of 15% in ethanol, while K. marxianus increased its fluidity by 30%. The thermophiles tested, except for G. thermoglucosidasius, decreased their membrane fluidity, which correlates well with a general decrease in unsaturated lipids. Moreover, C. beijerinckii, which was able to grow in higher concentrations of alcohol, decreased its membrane fluidity by as much as 55% in butanol.
Fig. 5.
Anisotropy values of DPH in organisms grown in the presence of ethanol (solids) and butanol (patterned) at concentrations that caused a 50% (▩ and ▧) or 85% (■ and ▩) decrease in the specific growth rate, μ.
Z. mobilis maintained a constant membrane fluidity even after growth in alcohol concentrations well above those tolerated by other bacteria. S. cerevisiae also maintained a constant membrane fluidity in ethanol, which is similar to the result observed with S. cerevisiae strain 3079 (1). Consistent membrane fluidity was observed in D. bruxellensis and G. thermoglucosidasius as well. From these anisotropy results, it appears that the ability to maintain constant membrane fluidity, as opposed to increasing membrane rigidity, is more important for tolerance in higher concentrations of alcohols.
Like Z. mobilis, N. pharaonis and H. lacusprofundi had much higher initial anisotropy values than the other organisms tested, and growth in all alcohols caused a further increase in membrane rigidity. This increase was induced by very small amounts of alcohols compared to the other organisms, demonstrating that N. pharaonis and H. lacusprofundi were very sensitive to alcohols. A. pernix increased its anisotropy in ethanol and butanol concentrations that caused a 50% decrease in growth rate. In the higher alcohol concentrations, however, membrane fluidity returned to the uninhibited level. Ethanol slightly fluidized the membrane of M. acetivorans at 50% growth inhibition, while the presence of butanol slightly rigidified the membrane at 85% growth inhibition. Overall, the membrane fluidity of M. acetivorans was not drastically affected by the presence of alcohols.
DISCUSSION
Microorganisms have many defenses to combat against external stress. In the case of alcohol exposure, much research has focused on the membrane and its fluidity in response to this stress. It has been shown that ethanol and butanol exposure increase the amount of saturated lipids, transmembrane lipids, sterols, and hopanoids in the membranes of Clostridium acetobutylicum, E. coli, Z. mobilis, T. pseudoethanolicus 39E, and S. cerevisiae (3, 7, 12, 37). Given these results, our study was designed to determine whether a relationship exists between membrane fluidity, lipid composition, and the ability of an organism to maintain growth upon alcohol exposure.
Our results show that the presence of unique lipids, specifically those found in archaea, did not confer increased growth tolerance to alcohols. Alcohol inhibition may thus reflect the lack of alcohols within the natural environments of archaea and the absence of stress responses evolved against such exposure. Interestingly, Z. mobilis, S. cerevisiae, D. bruxellensis, K. marxianus, C. beijerinckii, and E. coli maintained similar growth profiles, especially in ethanol, with growth rates at lower concentrations of alcohols close to those of unexposed cells. By comparison, G. thermoglucosidasius, T. saccharolyticum, T. ethanolicus, and T. pseudoethanolicus showed an immediate decrease in growth rates at low concentrations of ethanol, which was not as evident in butanol and isobutanol. These results suggest that the thermophilic organisms shifted immediately from energy production for growth to survival, while the mesophiles maintained a more even balance between growth and stress responses.
Our data show a slight correlation between growth and membrane fluidity. In general, organisms that sustained growth at 25 g liter−1 ethanol or higher maintained or increased membrane fluidity. These results suggest that it is crucial to maintain a fluid membrane in which solute transport and ion gradients can readily function and that increased membrane rigidity has detrimental effects. In addition, there was no correlation between membrane fluidity and changes in lipid composition. Generally, the ratio of unsaturated to saturated or of branched to unbranched lipids decreased upon exposure to alcohol with few exceptions. It was previously demonstrated that increased saturation or unbranching of lipids decreased the fluidity of liposomes, a concept commonly thought to apply to whole cells (13). Even though the trend was to decrease unsaturated and branched lipids, the membrane fluidity varied among the organisms.
The microbes Z. mobilis, S. cerevisiae, D. bruxellensis, G. thermoglucosidasius, and N. pharaonis maintained fairly constant membrane fluidity even at alcohol concentrations that inhibited their growth by 85%. These organisms apparently have the ability to increase saturated lipids to reinforce their membrane without making it too rigid. D. bruxellensis and N. pharaonis were the exceptions in that their initial lipid ratios maintained membrane fluidity in ethanol and low concentrations of butanol. In addition to increased saturated lipids fortifying the membrane, polysaccharides, including trehalose and surface glucans, have produced similar effects in organisms, such as S. cerevisiae and Z. mobilis, during ethanol exposure (4, 31). The increased presence of surface glucans stimulates flocculation in S. cerevisiae and Z. mobilis cultures, which may help mitigate the effects of toxic substances within an environment (10, 12, 35).
The results observed in E. coli, K. marxianus, and M. acetivorans were interesting given that these organisms increased or maintained the degree of saturation within their lipids, but the anisotropy slightly decreased, mainly in ethanol, indicating that the membrane is more fluid. This increase in fluidity may be due to an increase of shorter saturated lipids, such as C14 and C16, which is observed for K. marxianus and E. coli K-12, as well as to other, as yet undetermined, differences in membrane composition that allow for the fluidizing effects of alcohol. In butanol, however, the responses of K. marxianus and E. coli were similar to those seen in Z. mobilis and S. cerevisiae, which maintained a constant membrane fluidity. Because butanol can intercalate further into the membrane, K. marxianus and E. coli may counteract this fluidizing effect by significantly increasing the degree of saturation to maintain membrane fluidity. These results show that in the presence of ethanol, E. coli and K. marxianus may direct more energy to maintaining cellular functions instead of membrane stability, while butanol exposure demands greater maintenance of the membrane.
The results from C. beijerinckii, T. saccharolyticum, T. ethanolicus, T. pseudoethanolicus, and A. pernix illustrate that as the ratio of unsaturated to saturated lipids declines and the anisotropy tends to increase, the membrane becomes more rigid, possibly to prevent cofactor and ion loss. T. ethanolicus and A. pernix, however, increased or maintained their B/UB ratios in ethanol. As mentioned previously, T. pseudoethanolicus 39E produces long-chain (C30) α,ω-dicarboxylic acids, which would decrease membrane fluidity, but little research has focused on lipid profiles of these organisms. C. beijerinckii, whose major fermentative end product is butanol, only synthesized saturated lipids to rigidify its membrane in the presence of alcohols. This may be due to the natural response that C. beijerinckii has developed against the intercalation of butanol into the hydrophobic regions of the membrane (28). In general, the Thermoanaerobacter and Thermoanaerobacterium species maintained growth rates better in butanol and isobutanol than in ethanol. These results may indicate that increasing membrane rigidity is important for growth tolerance to longer-chain alcohols.
The psychrophilic halophile H. lacusprofundi exhibited decreased membrane fluidity similar to the other halophile tested, N. pharaonis. H. lacusprofundi, however, displayed a different lipid profile from N. pharaonis, increasing its B/UB ratio in ethanol and butanol. This response may be influenced by the psychrophile's low growth temperature (23°C versus 37°C) instead of the presence of alcohols. In general, the responses of archaea were different for each species in regard to both lipid profile and anisotropy. Although it would be of interest to determine how unique survival mechanisms in different classes of extremophiles may influence or contribute to their response to alcohols, none seemed to confer tolerance in the present study.
Because the variations in membrane fluidity did not fully explain growth tolerance to alcohols, other mechanisms may play a significant role during alcohol stress. The production of osmoprotectants such as l-proline, glycine, and betaine, as well as redox and ion regulators, for example, glycerol and glycogen, have been documented as a response to ethanol exposure in S. cerevisiae and E. coli (10, 19, 32, 48). These molecules maintain proton gradients and protect against cell lysis during alcohol exposure. Protein denaturation, including enzymes involved in glycolysis, is also a well-documented consequence of ethanol exposure, and glycolytic enzymes, heat shock proteins, and trehalose production are upregulated to prevent this damage (4, 12, 43). Given these results, the eventual denaturation of proteins involved with energy metabolism, not just an increase in membrane fluidity, may cause growth to decline. More tolerant organisms may therefore combat alcohol stress at the membrane, as well as within the cytosol. Further studies on protein activity and membrane leakiness would reveal whether enzyme denaturation and not membrane fluidity is the major cause of the decline in growth.
In summary, our results show that although the membrane may play a crucial role in alcohol tolerance, the fluidity of the membrane may not be the sole determinant of a cell's response to alcohol exposure. Evidence suggests that alcohol tolerance is composed of an extensive and complex set of responses, not just those responsible for repairing the membrane. Transcriptomic and proteomic analyses have revealed increased transcripts and proteins responsible for cell wall biogenesis, osmoregulation, heat shock proteins, carbohydrate transport, glycolytic enzymes, protein turnover, and in some cases sporulation during alcohol exposure (19, 30, 38, 39, 42). Therefore, the generation of more tolerant organisms will have to involve modifications that not only maintain membrane fluidity, but also maintain redox, osmotic, and ion homeostasis, as well as protein structure and function.
ACKNOWLEDGMENTS
We thank Nadia Mykytczuk of McGill University for helpful discussions on fluorescence anisotropy. We also acknowledge William Beeson at the Energy Biosciences Institute for assistance with lipid extraction steps.
This research was supported by the Energy Biosciences Institute.
Footnotes
Published ahead of print on 22 July 2011.
REFERENCES
- 1. Alexandre H., Berlot J. P., Charpentier C. 1994. Effect of ethanol on membrane fluidity of protoplasts from Saccharomyces cerevisiae and Kloeckera apiculata grown with or without ethanol, measured by fluorescence anisotropy. Biotechnol. Techniques 8:295–300 [Google Scholar]
- 2. Alexandre H., Rousseaux I., Charpentier C. 1994. Relationship between ethanol tolerance, lipid composition and plasma membrane fluidity in Saccharomyces cerevisiae and Kloeckera apiculata. FEMS Microbiol. Lett. 124:17–22 [DOI] [PubMed] [Google Scholar]
- 3. Baer S. H., Blaschek H. P., Smith T. L. 1987. Effect of butanol challenge and temperature on lipid composition and membrane fluidity of butanol-tolerant Clostridium acetobutylicum. Appl. Environ. Microbiol. 53:2854–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Banat I. M., Nigam P., Singh D., Marchant R., McHale A. P. 1998. Ethanol production at elevated temperatures and alcohol concentrations. I. Yeasts in general. World J. Microbiol. Biotechnol. 14:809–821 [Google Scholar]
- 5. Benvegnu T., Lemiegre L., Cammas-Marion S. 2008. Archaeal lipids: innovative materials for biotechnological applications. Eur. J. Org. Chem. 2008(28):4725–4744 [Google Scholar]
- 6. Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911–917 [DOI] [PubMed] [Google Scholar]
- 7. Burdette D. S., Jung S. H., Shen G. J., Hollingsworth R. I., Zeikus J. G. 2002. Physiological function of alcohol dehydrogenases and long-chain (C30) fatty acids in alcohol tolerance of Thermoanaerobacter ethanolicus. Appl. Environ. Microbiol. 68:1914–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carey V. C., Ingram L. O. 1983. Lipid composition of Zymomonas mobilis: effects of ethanol and glucose. J. Bacteriol. 154:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cartwright C. P., et al. 1986. Ethanol dissipates the proton-motive force across the plasma membrane of Saccharomyces cerevisiae. J. Gen. Microbiol. 132:369–377 [Google Scholar]
- 10. Dake M. S., Jadhv J. P., Patil N. B. 2010. Variations of two pools of glycogen and carbohydrate in Saccharomyces cerevisiae grown with various ethanol concentrations. J. Indust. Microbiol. Biotechnol. 37:701–706 [DOI] [PubMed] [Google Scholar]
- 11. Dickey A. N., Yim W. S., Faller R. 2009. Using ergosterol to mitigate the deleterious effects of ethanol on bilayer structure. J. Phys. Chem. B 113:2388–2397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding J. M., et al. 2009. Tolerance and stress response to ethanol in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 85:253–263 [DOI] [PubMed] [Google Scholar]
- 13. Dombek K. M., Ingram L. O. 1984. Effects of ethanol on the Escherichia coli plasma membrane. J. Bacteriol. 157:233–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fong J. C., et al. 2006. Isolation and characterization of two novel ethanol-tolerant facultative-anaerobic thermophilic bacteria strains from waste compost. Extremophiles 10:363–372 [DOI] [PubMed] [Google Scholar]
- 15. Fonseca G. G., Gombert A. K., Heinzle E., Wittmann C. 2007. Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res. 7:422–435 [DOI] [PubMed] [Google Scholar]
- 16. Franzmann P. D., et al. 1988. Halobacterium lacusprofundi sp. nov., a halophilic bacterium isolated from Deep Lake, Antarctica. Syst. Appl. Microbiol. 11:20–27 [Google Scholar]
- 17. Fu N., Peiris P. 2008. Co-fermentation of a mixture of glucose and xylose to ethanol by Zymomonas mobilis and Pachysolen tannophilus. World J. Microbiol. Biotechnol. 24:1091–1097 [Google Scholar]
- 18. Gomes J., Steiner W. 2004. The biocatalytic potential of extremophiles and extremozymes. Food Technol. Biotechnol. 42:223–235 [Google Scholar]
- 19. Gonzalez R., et al. 2003. Gene array-based identification of changes that contribute to ethanol tolerance in ethanologenic Escherichia coli: comparison of KO11 (parent) to LY01 (resistant mutant). Biotechnol. Prog. 19:612–623 [DOI] [PubMed] [Google Scholar]
- 20. Hanford M., Peeples T. L. 2002. Archaeal tetraether lipids: unique structures and applications. Appl. Biochem. Biotechnol. 97:45–62 [DOI] [PubMed] [Google Scholar]
- 21. Harris V., Ford C. M., Jiranek V., Grbin P. R. 2008. Dekkera and Brettanomyces growth and utilisation of hydroxycinnamic acids in synthetic media. Appl. Microbiol. Biotechnol. 78:997–1006 [DOI] [PubMed] [Google Scholar]
- 22. Hermans M. A. F., Neuss B., Sahm H. 1991. Content and composition of hopanoids in Zymomonas mobilis under various growth conditions. J. Bacteriol. 173:5592–5595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ingram L. O. 1976. Adaptation of membrane lipids to alcohols. J. Bacteriol. 125:670–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ingram L. O. 1986. Microbial tolerance to alcohols: role of the cell membrane. Trends Biotechnol. 4:40–44 [Google Scholar]
- 25. Jones W. J., Leigh J. A., Mayer F., Woese C. R., Wolfe R. S. 1983. Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch. Microbiol. 136:254–261 [Google Scholar]
- 26. Kaiser C., et al. 1994. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 27. Littleton J. M. 1979. Adaptive changes in membrane lipid composition and fluidity as the basis for ethanol tolerance. Drug Alcohol Dependence 4:189–195 [DOI] [PubMed] [Google Scholar]
- 28. Liu S. Q., Qureshi N. 2009. How microbes tolerate ethanol and butanol. New Biotechnol. 26:117–121 [DOI] [PubMed] [Google Scholar]
- 29. Ly H. V., Longo M. L. 2004. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 87:1013–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma M. G., Liu L. Z. 2010. Quantitative transcription dynamic analysis reveals candidate genes and key regulators for ethanol tolerance in Saccharomyces cerevisiae. BMC Microbiol. 10:169–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ma M. G., Liu Z. L. 2010. Mechanisms of ethanol tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 87:829–845 [DOI] [PubMed] [Google Scholar]
- 32. Mansure J. J. C., Panek A. D., Crowe L. M., Crowe J. H. 1994. Trehalose inhibits ethanol effects on intact yeast cells and liposomes. Biochim. Biophys. Acta Biomembr. 1191:309–316 [DOI] [PubMed] [Google Scholar]
- 33. Mykytczuk N. C. S., Trevors J. T., Ferroni G. D., Leduc L. G. 2010. Cytoplasmic membrane fluidity and fatty acid composition of Acidithiobacillus ferrooxidans in response to pH stress. Extremophiles 14:427–441 [DOI] [PubMed] [Google Scholar]
- 34. Mykytczuk N. C. S., Trevors J. T., Twine S. M., Ferroni G. D., Leduc L. G. 2010. Membrane fluidity and fatty acid comparisons in psychrotrophic and mesophilic strains of Acidithiobacillus ferrooxidans under cold growth temperatures. Arch. Microbiol. 192:1005–1018 [DOI] [PubMed] [Google Scholar]
- 35. Palha M. A. P. F., Lopes C. E., Pereira N. 1997. Ethanol stimulates the flocculation of Zymomonas mobilis. Biotechnol. Lett. 19:499–501 [Google Scholar]
- 36. Qureshi N., Blaschek H. P. 1999. Production of acetone butanol ethanol (ABE) by a hyper-producing mutant strain of Clostridium beijerinckii BA101 and recovery by pervaporation. Biotechnol. Prog. 15:594–602 [DOI] [PubMed] [Google Scholar]
- 37. Rupcic J., Juresic G. C. 2010. Influence of stressful fermentation conditions on neutral lipids of a Saccharomyces cerevisiae brewing strain. World J. Microbiol. Biotechnol. 26:1331–1336 [DOI] [PubMed] [Google Scholar]
- 38. Rutherford B. J., et al. 2010. Functional genomic study of exogenous n-butanol stress in Escherichia coli. Appl. Environ. Microbiol. 76:1935–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shi Z., Blaschek H. P. 2008. Transcriptional analysis of Clostridium beijerinckii NCIMB 8052 and the hyper-butanol-producing mutant BA101 during the shift from acidogenesis to solventogenesis. Appl. Environ. Microbiol. 74:7709–7714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shinitzky M., Yuli I. 1982. Lipid fluidity at the submacroscopic level: determination by fluorescence polarization. Chem. Phys. Lipids 30:261–282 [Google Scholar]
- 41. Sowers K. R., Baron S. F., Ferry J. G. 1984. Methanosarcina acetivorans sp. nov., an acetotrophic methane-producing bacterium isolated from marine sediments. Appl. Environ. Microbiol. 47:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stanley D., Chambers P. J., Stanley G. A., Borneman A., Fraser S. 2010. Transcriptional changes associated with ethanol tolerance in Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 88:231–239 [DOI] [PubMed] [Google Scholar]
- 43. Stevens T. H., Forgac M. 1997. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu. Rev. Cell Dev. Biol. 13:779–808 [DOI] [PubMed] [Google Scholar]
- 44. Tindall B. J., Ross H. N. M., Grant W. D. 1984. Natronobacterium gen. nov. and Natronococcus gen. nov., two new genera of haloalkaliphilic archaebacteria. Syst. Appl. Microbiol. 5:41–57 [Google Scholar]
- 45. Trinh C. T., Unrean P., Srienc F. 2008. A minimal Escherichia coli cell for the most efficient production of ethanol from hexoses and pentoses. Appl. Environ. Microbiol. 74:3634–3643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Virtue P., Nichols P. D., Boon P. I. 1996. Simultaneous estimation of microbial phospholipid fatty acids and diether lipids by capillary gas chromatography. J. Microbiol. Methods 25:177–185 [Google Scholar]
- 47. Voelker T. A., Davies H. M. 1994. Alteration of the specificity and regulation of fatty acid synthesis of Escherichia coli by expression of a plant medium-chain acyl-acyl carrier protein thioesterase. J. Bacteriol. 176:7320–7327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vriesekoop F., Haass C., Pamment N. B. 2009. The role of acetaldehyde and glycerol in the adaptation to ethanol stress of Saccharomyces cerevisiae and other yeasts. FEMS Yeast Res. 9:365–371 [DOI] [PubMed] [Google Scholar]
- 49. You K. M., Rosenfield C. L., Knipple D. C. 2003. Ethanol tolerance in the yeast Saccharomyces cerevisiae is dependent on cellular oleic acid content. Appl. Environ. Microbiol. 69:1499–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zeikus J. G., Ben-Bassat A., Hegge P. W. 1980. Microbiology of methanogenesis in thermal, volcanic environments. J. Bacteriol. 143:432–440 [DOI] [PMC free article] [PubMed] [Google Scholar]