Abstract
A novel generic approach for stress profiling was applied to Listeria monocytogenes strain F2365. This food-borne pathogen was exposed to gradients of five different stresses of increasing intensity, typically ranging from moderate to lethal conditions. The stress factors included heat, acidic pH, a detergent disinfectant, an oxidant, and hyperosmotic conditions. In addition to CFU counts and lag time, five different molecular viability parameters were measured by fluorescence-based assays, including membrane integrity, membrane potential, esterase activity, redox activity, and intracellular pH stability. The last was measured by our recently invented real-time viability assay. Exposure to all stresses resulted in clear dose-response relationships for all viability parameters with the exception of hyperosmotic conditions. A statistical analysis showed strong correlations for (i) the growth parameters plate counts and lag times, (ii) the enzyme-associated functions redox and esterase activity, and (iii) the membrane-associated pH stability and membrane integrity. Results indicated a pronounced difference in the susceptibilities of the measured parameters depending on the stress factor applied. However, at relatively high stress intensities, all of the viability parameters became affected independent of the stress factor. Applications of the approach presented here include studies on the mechanism of action of unknown compounds with biocidal activity and a comparative analysis of the severities of the impact of stress conditions of interest. It appears that a meaningful evaluation of the impact of mild stress conditions can be obtained only through measurement of multiple viability parameters.
INTRODUCTION
Although the definition of viability is based on the ability to replicate, it is now accepted that colony formation or growth in liquid medium cannot serve as a sole indicator for whether bacteria are alive or dead (6, 13, 21, 22). Research has come up with a multitude of cellular parameters which are referred to as indirect viability criteria (3, 4, 12). These parameters include respiratory activity, enzymatic activity, ATP content, membrane potential, and membrane integrity. The presence of these activities, available energy, or integrity parameters is in many cases interpreted as viability or at least as the potential to still be alive. However, the correlation between the indirect viability parameters and the ability to replicate remains unclear. In some cases the ability to replicate might be only transiently lost and might be regained under appropriate conditions (6). In others, the measurement of indirect viability parameters can be a residual of the initial status quo, and replication potential could be irreversibly lost. The distinction between these two scenarios is hard to accomplish with the current methodology.
One approach to obtain more insight into the physiological effects of stress factors is the measurement of multiple viability parameters. So far, this approach has been performed mainly by use of flow cytometry. Hewitt and Nebe-Von-Caron proposed that the measurement of multiple vital cellular parameters allows a functional classification of a cell's physiological state beyond culturability (9). The importance of using a number of viability indicators to characterize the physiological state of stressed bacteria was also emphasized by Berney et al., indicating that each parameter reflects different levels of cellular integrity and functionality (4). The authors concluded that only the sum of multiple parameters (including culturability) can provide more certainty about the physiological state of bacteria.
In this study we made use of the versatility of a fluorescence plate reader to perform multiparameter profiling of an organism exposed to different stress gradients. Listeria monocytogenes was selected as a model organism for its tolerance to a variety of environmental stress factors (7, 23), its prominent role as a food pathogen, and the fact that it is an important target for disinfection. The measured viability parameters included CFU counts and lag phase as well as the five molecular parameters redox activity, esterase activity, membrane potential, membrane integrity, and intracellular pH stability. The presence of each of these cellular features is considered to be a prerequisite for a living cell: enzymes reducing a wide variety of substrates are essential for respiratory activity, esterases responsible for hydrolysis are among the key enzymes in metabolic pathways, membrane polarization and integrity are essential for the maintenance of the cell's energy status and the existence of a functional barrier between the extracellular environment and the cytoplasm, and the maintenance of a relatively neutral intracellular pH is required for many physiological processes in the cell (5).
This study aimed at (i) accumulating more knowledge about the behavior of various viability parameters when cells are exposed to a number of selected stress factors, (ii) correlating the different parameters, and (iii) providing a benchmark for our recently reported real-time viability (RTV) assay, with a novel viability criterion, that is, the cell's ability to maintain a neutral intracellular pH in an acidic environment (14).
MATERIALS AND METHODS
Growth conditions.
L. monocytogenes serotype 4B (strain F2365; North Carolina State University) was grown from glycerol stocks on tryptic soy agar (CM0131; Oxoid Limited, Basingstoke, Hampshire, United Kingdom) at 30°C for 48 h. Single colonies were subsequently transferred into tryptic soy broth ([TSB] M0129; Oxoid Limited, Basingstoke, Hampshire, United Kingdom) and shaken overnight at 130 rpm at 30°C. Cell growth was monitored with a spectrophotometer (Ultro Spec 2100 Pro; GE Healthcare) at 600 nm (optical density at 600 nm [OD600]). To obtain an exponentially growing culture, 1 ml of the overnight culture was transferred into a sterile flask containing 400 ml of TSB and shaken until an OD600 of 1.0 ± 0.1 was reached (this OD value corresponded to 5 × 108 CFU/ml as confirmed by plate counting). Cells were harvested by centrifugation (4,000 × g for 15 min) and washed in 15 ml of 50 mM phosphate buffer (pH 7.0), centrifuged again, and finally resuspended in 10 ml of 50 mM phosphate buffer, pH 7.
Stress exposure.
Cell suspensions were diluted in solutions containing stress factors to a concentration of 108 CFU/ml (in total volumes ranging from 10 to 50 ml). All experiments were performed at room temperature (except in the experiment where cells were subjected to temperature stress). For osmotic stress, NaCl (Merck, Darmstadt, Germany) was dissolved in 50 mM phosphate buffer to obtain a concentration range between 85 and 4,800 mM, and cells were exposed for 3 h. For detergent disinfectant stress, Suma Bac D10 (Johnson Diversey, Utrecht, Netherlands) was diluted in water, at concentrations ranging from the manufacturer's recommendation of 1% (vol/vol) up to 500 times the dilution of this concentration. Suma Bac is a commercial disinfectant and cleaning agent which, according to the manufacturer, contains a combination of at least three ingredients: a quaternary ammonium compound ([QAC] in this case, alkyldimethylbenzylammonium chloride), sodium carbonate, and the nonionic surfactant alkyl alcohol ethoxylate. Cells were exposed to Suma Bac for 30 min, followed by neutralization with inactivation solution (per liter: 3 g of lecithin, 1 g of l-histidine, 5 g of thiosulfate, 30 ml of Tween 80, 0.34 g of KH2PO4, pH 7.2; referred to as neutralizer). For oxidative stress, Halamid (chloramine-T; Thaly Medical and Safety, Voorschoten, The Netherlands), which contains sodium p-toluenesulfonchloramide as an active ingredient, was diluted in water to obtain a range between 0.01 to 0.5 mM with an exposure time of 30 min, followed by neutralization with either thiosulfate or inactivation solution (see above). For pH stress, cells were added to 50 mM phosphate buffer adjusted to different pH values in a range between 2 and 7 by addition of HCl; the exposure time was 30 min. This treatment was followed by two wash steps with 50 ml of 50 mM phosphate buffer (pH 7). Temperature stress was performed by exposing five aliquots of 10 ml each (cells resuspended in phosphate buffer, pH 7) to different temperatures (40°C, 50°C, 55°C, 60°C, and 65°C) in water baths for 10 min. Identical aliquots were pooled after heat treatment. After each stress exposure, cells were harvested by centrifugation (4,000 × g for 15 min), washed twice in 50 mM phosphate buffer, pH 7, and resuspended in 10 mM phosphate buffer to obtain a final concentration of approximately 109 cells/ml. For molecular assays, 1-ml aliquots of these suspensions were transferred to microcentrifuge tubes for further processing. Cells which were subjected to identical conditions but in the absence of stress factors served as positive controls. Negative controls were obtained by heat killing of unstressed cells at 85°C for 10 min using a standard laboratory heat block.
Plate counting and lag times.
For plate counting, 100-μl aliquots of serially diluted cell suspensions were spread on standard tryptic soy agar (TSA; Oxoid), followed by incubation at 30°C for 24 to 48 h prior to enumeration of colonies. For lag phase assays, aliquots of 200 μl from the dilutions containing approximately 103, 104, and 105 cells per ml were transferred into the wells of a Bioscreen plate (Bioscreen C; Labsystems, Helsinki, Finland). This assay measures the lag time inflicted by stress exposure when cells are returned to a growth-permissive environment. Plates were incubated at 30°C, with measurements being performed with a wide band filter and a 10-s shaking step (medium intensity) prior to each OD600 reading. Values were obtained every 15 min. The individual lag times of L. monocytogenes cells were estimated through the time of detection (Td), which is the time required for the microbial population to generate a 0.05 increase of the initial baseline value of the OD600. This optical density corresponds to a cell density estimated by a viable count of approximately 1.8 × 107 L. monocytogenes cells per well. Assuming an exponential bacterial growth at a constant specific growth rate (μ) until the detection time, Td is related to the lag time of the culture (lag) by the following equation as proposed by Baranyi and Pin (2): Td = lag + [ln(Nd) − ln(N0)]/μ, where Nd is the bacterial number at Td and N0 is the number of cells initiating growth. The N0 values were determined at the start of each incubation period for every experiment and exposure condition.
Colorimetric assays.
Molecular assays were performed in microtiter format using a Tecan Infinite M200 plate reader (Tecan Benelux BVBA, Giessen, The Netherlands). Black, chimney-style, flat-bottom 96-well plates (catalog number 655096; Greiner Bio-One, Frickenhausen, Germany) were used for measurement of membrane integrity, membrane potential, and esterase and redox activities, whereas transparent UV-Star, flat-bottom 96-well plates (catalog number 655801; Greiner Bio-One) were used for the RTV assay.
Membrane integrity.
Membrane integrity was measured using a Live/Dead BacLight bacterial viability kit (Invitrogen, Breda, The Netherlands). Three μl of each of the two dyes SYTO9 (3.34 mM) and propidium iodide (20 mM) was mixed before water was added to 1 ml. Volumes of 100 μl of this 2× staining solution were aliquoted into the wells of a microtiter plate before the addition of an identical volume of cell suspension (in 50 mM phosphate buffer, pH 7). Each well contained approximately 1 × 108 cells. Plates were incubated in the dark for 15 min with occasional manual shaking before measurement of fluorescence. SYTO9 fluorescence was measured at 538 nm, and propidium iodide fluorescence was measured at 620 nm. The excitation wavelength for both dyes was 485 nm.
Esterase activity.
Five mg of esterase substrate 5,6-carboxyfluorescein diacetate (CFDA) (Invitrogen, Breda, The Netherlands) was dissolved in 1.086 ml of dimethyl sulfoxide (DMSO), and the resulting 10 mM stock solution was stored at −20°C. Before use, the stock was diluted 40× in water to obtain a working solution of 250 μM, which was added in 20-μl aliquots to a microtiter plate. Suspensions of 180 μl of stressed and unstressed cells (in 50 mM phosphate buffer, pH 7) were added to the dye solution and mixed by pipetting up and down. Each well contained approximately 2 × 108 cells. The plate was incubated for 30 min in the dark with occasional shaking, followed by measurement of fluorescence (excitation, 485 nm; emission, 535 nm).
Membrane potential.
An aliquot of 25 mg of the voltage-sensitive dye DiBAC4(3) (oxonol bis-1,3-dibutylbarbituric acid trimethine oxonol; Anaspec, Fremont) was dissolved in 2.42 ml of ethanol to obtain a stock concentration of 20 mM. This stock was stored at −20°C. Before use, an aliquot of the stock was diluted 1,600-fold in water to obtain a detection solution of 12.5 mM which was added in 20-μl aliquots into the wells of a 96-well microplate. Approximately 180 μl of cell suspensions (in 50 mM phosphate buffer, pH 7) was added to the detection solution and mixed. Each well contained approximately 2 × 108 cells. The plate was incubated for 5 min in the dark before fluorescence was measured (excitation, 485 nm; emission, 535 nm).
Redox activity.
WST-8 (Quick Cell Proliferation Assay Kit II; ITK Diagnostics, Uithoorn, The Netherlands) and menadione (2-methyl-1,4-naphthoquinone; Acros Organics, Fischer Scientific, United Kingdom) were dissolved in water and DMSO, respectively, to obtain stocks of 10 mM and 8 mM. Both stocks were stored at −20°C. WST-8, menadione, and water were mixed in ratios of 9:1:10. This detection reagent was prealiquoted in 20-μl volumes in a 96-well flat-bottom, black-housing microplate. Cell aliquots of 1 ml (in 50 mM phosphate buffer, pH 7) were transferred into microcentrifuge tubes and harvested at 5,000 × g for 5 min, and pellets were resuspended in 1 ml of TSB. Volumes of 180 μl of this cell suspension (approximately 2 × 108 cells per well) were mixed with prealiquots of 20 μl of the detection reagent. Plates were immediately transferred into the plate reader to start the assay. Absorbance at 460 nm was measured every 2 min for a total of 30 to 45 min, until the absorption of the first samples exceeded the plate reader's detection limit of 3.0. Before every measurement, the plate was shaken for 5 s (linear shaking, amplitude of 3).
Real-time viability (RTV).
A stock of 1 M salicylic acid (Acros Organics, Fischer Scientific, United Kingdom) was prepared by dissolving 0.138 g in 1 ml of DMSO. A volume of 50 μl of this stock was added to 25 ml of 100 mM maleate buffer, pH 2, to obtain a probe solution. Cells were resuspended in sterile MilliQ water and added in aliquots of 100 μl into a 96-well plate (UV-compatible UV-Star plates; Greiner Bio-One, Frickenhausen, Germany) to have approximately 108 cells per assay. The identical volume of acidic probe solution was injected into each well (using the plate reader's syringe injection function; injection speed, 300 μl/s), immediately followed by real-time measurement of fluorescence. The excitation wavelength was 295 nm; emission was measured at 402 nm.
Scaling of molecular data.
All raw data from molecular viability assays were transformed into percentage values to be able to compare data from different assays. The value 100% was defined as the highest value obtained for each stress, whereas 0% was defined as the lowest value obtained. In the case of the membrane potential probe DiBAC4(3), where cell death coincides with higher fluorescence values (as the probe enters cells only when membrane potential is reduced or lost), all values were background corrected and inverted by subtraction of the value obtained without stress. The resulting numbers were used for calculation of percentages, and the value from the unstressed sample was defined as 100% signal intensity.
Statistical analysis.
Correlation analysis was performed with the data from all seven assays as listed above: log CFU, lag time, BacLight, membrane potential, redox activity, esterase activity, and RTV. Each measured parameter was range scaled (minimum to maximum, 0 to 1), and an increment factor of 0.00001 was added to all values to prevent error messages by the software caused by zero values. The overall viability parameter was calculated as the average of the seven range-scaled values per condition. The following missing values were interpolated: lag time with 0% Suma Bac plus neutralizer, 1.0; lag time with 0 mM Halamid plus thiosulfate, 1.0; lag time with 0 mM Halamid plus neutralizer, 1.0; lag time with 0.2 mM Halamid, 0.00001; lag time with 1 mM Halamid, 0.00001; membrane potential with 0.5 mM Halamid, 0.00001; and membrane potential with 1 mM Halamid, 0.00001. The correlation coefficients of the Halamid viability assay results were calculated with the following equation:
where x and y are the sample means for the range of values for viability parameter 1 and the range of values for viability parameter 2, respectively. The hierarchical clustering (Euclidian distance and complete linkage) was calculated with TIGR MeV, version 4.3.01, software (http://www.tm4.org/mev.html).
RESULTS
Temperature stress.
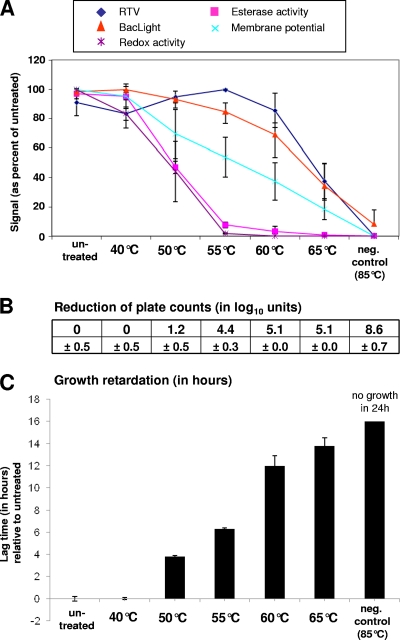
As a general trend, a dose-response relationship was observed for all measured parameters, as listed in Table 1, although the effect occurred at different stress intensities (Fig. 1). The first molecular parameters showing a decrease were redox activity and esterase activity. The drop in these parameters coincided with a decrease in plate counts and increasing lag phase. Higher temperatures were needed to result in loss of membrane potential and membrane integrity (the latter being measured by BacLight Live/Dead staining and by RTV assay). The membrane parameters reached their minimal values only at 85°C, whereas plate counts had already dropped by more than 5 log10 per ml at 60°C. In the lower temperature range, the RTV signal, in contrast to BacLight, underwent a slight rise with an increase in temperature to 55°C, whereas a further increase in temperature resulted in a sharp drop in signal. BacLight, on the other hand, suggested a continuous decline of membrane integrity with increasing temperatures.
Table 1.
Viability assays applied in this study, their underlying detection principles, and changes along the stress gradient
| Assay | Probe | Measured parameter and detection principle | Change with decreasing cell function |
|---|---|---|---|
| BacLight Live/Dead | SYTO9/PIa | Membrane integrity: SYTO9 is membrane permeant, and PI is not; strong signal increase upon dye binding to nucleic acids | Increasing entry upon PI and therefore lower signal ratios of SYTO9/PI (green/red) |
| Esterase | CFDA | Esterase activity: fluorescence upon enzymatic probe cleavage | Change in expression of esterase activity (lower or higher depending on bacterial species) |
| Membrane potential | DiBAC4(3) | Transmembrane potential: fluorescence upon entry in depolarized cells and binding to intracellular proteins and membrane lipids | Membrane depolarization resulting in increased probe uptake and higher signals |
| Redox | WST-8 | Redox activity: fluorescence upon reduction of dye | Increasing loss of reduction potential resulting in signal decrease |
| RTV | Salicylic acid | Membrane integrity and maintenance of neutral pH under acidic conditions: fluorescence upon protonation of probe | Increasing acidification of cytoplasm resulting in lower signals of probe upon entry into cells |
| Plate count | None | Ability to replicate on plates: counting of CFU | Fewer CFU |
| Growth lag time | None | Ability to grow in liquid culture after recovery/damage repair: fise in optical density | Increasing growth lag time with increasing cell damage |
PI, propidium iodide.
Fig. 1.
Effect on viability parameters of exposure of L. monocytogenes to different temperatures. Samples that were left untreated or were heat killed (85°C for 10 min) served as controls. Data obtained from heat-exposed samples were related to the data obtained without temperature treatment. Error bars indicate standard deviations as seen in three independent experiments. (A) Effect of stress exposure on RTV, BacLight, membrane potential, and esterase and redox activities. Values are expressed as a percentage of the corresponding value obtained without stress. (B) Effect of stress exposure on culturability. Numbers represent the reduction of plate counts (in log10 per ml) compared to the counts obtained without stress. (C) Effect of stress on growth in a Bioscreen assay. The diagram shows lag times compared to cells which were not exposed to stress. neg contr, negative control.
Acidic pH stress.
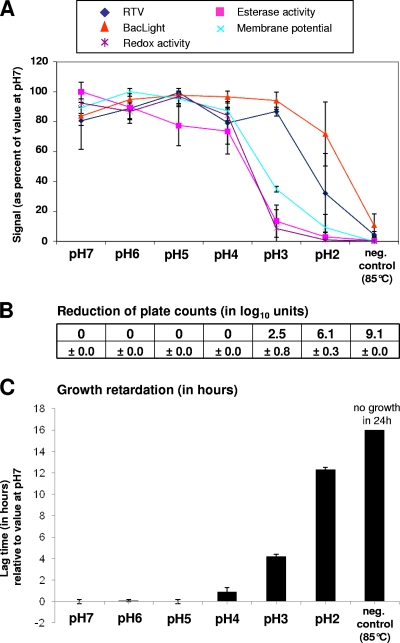
When L. monocytogenes was exposed to acidic conditions for 30 min, esterase activity and redox activity underwent a sharp decline, and membrane potential showed a more moderate decline when the pH was lowered from 4 to 3 (Fig. 2). This drop coincides with a 2.5 log loss in culturability and a substantial increase in lag time. When the pH was decreased to 2, membrane integrity parameters began dropping, as measured by RTV and BacLight assays. At pH 2, plate counts were reduced by 6.1 log10 per ml, and the lag time was more than 12 h relative to the sample kept at pH 7.
Fig. 2.
Effect on viability parameters of exposure of L. monocytogenes to low pH. Samples that were exposed to pH 7 or heat killed (85°C for 10 min) served as controls. Data obtained from stressed cells were related to the data obtained from cells exposed to pH 7. Error bars indicate standard deviations as seen in three independent experiments. For further explanations of the panels, see the legend of Fig. 1.
Detergent disinfectant stress.
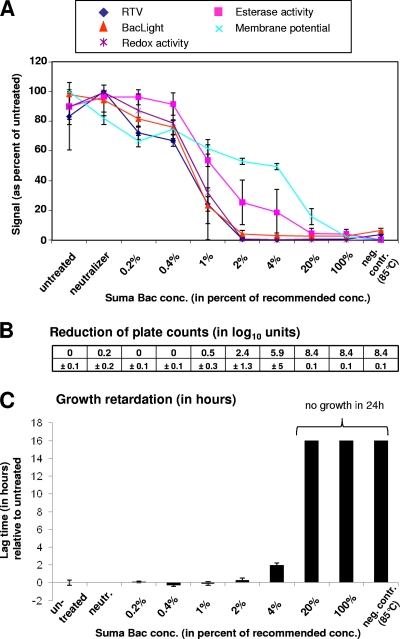
The effects of a 30-min exposure to Suma Bac followed by the addition of neutralizer is shown in Fig. 3. Suma Bac contains the quaternary ammonium compound alkyldimethylbenzylammonium chloride as an active ingredient apart from sodium carbonate and the surfactant alkyl alcohol ethoxylate. In contrast to the two previous stress factors, membrane integrity was the first parameter to drop (as measured by RTV and BacLight assays), closely followed by redox activity. At 2% disinfectant concentration, where membrane integrity parameters have reached the minimal values, culturability had undergone a decrease of 2.4 log units. An increase in lag time was seen at 4% Suma Bac and more dramatically at 20%. Membrane potential was the last parameter to drop, which does not correlate with the loss of membrane integrity and might be an experimental artifact due to an ingredient contained in the Suma Bac product.
Fig. 3.
Effect on viability parameters of exposure of L. monocytogenes to different concentrations of the detergent disinfectant Suma Bac. Samples that were left untreated or were heat killed (85°C for 10 min) served as controls. Data obtained from stressed cells were related to the data obtained from untreated cells. Error bars indicate standard deviations as seen in three independent experiments. For further explanations of the panels, see the legend of Fig. 1. conc, concentration; neutr, neutralizer.
Oxidative stress.
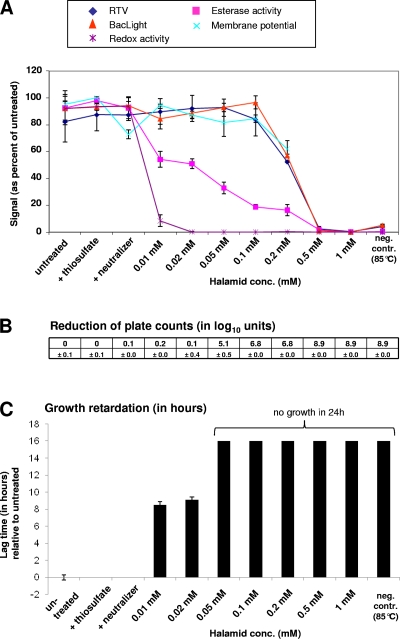
Cells were exposed to different concentrations of Halamid (in which chloramine is the active ingredient) followed by the addition of neutralizer. Untreated cells and cells exposed only to neutralizer (containing thiosulfate) or to pure thiosulfate served as controls. Stress exposure resulted in an immediate decrease in redox activity even at the lowest Halamid concentration and reached a minimum value at 0.02 mM Halamid (Fig. 4). It cannot be excluded, however, that residual Halamid, which might not have been neutralized or washed away entirely, interfered with WST-8 reduction. Halamid has a strong oxidizing effect, which might have prevented the reduction of the formazan dye by cellular activity. Esterase activity was the second parameter which dropped as a result of exposure to Halamid. A strong decrease in plate counts was seen at 0.05 mM Halamid, whereas an effect on lag time was already seen at lower concentrations. Membrane potential values are shown only up to concentrations of 0.2 mM Halamid as higher concentrations of the oxidant resulted in an unspecific signal increase, presumably due to oxidation of the DiBAC4(3) dye by residual oxidant.
Fig. 4.
Effect on viability parameters of exposure of L. monocytogenes to different concentrations of the oxidant Halamid. Samples that were left untreated or were heat killed (85°C for 10 min) served as controls. Data obtained from stressed cells were related to the data obtained from untreated cells. Error bars indicate standard deviations as seen in three independent experiments. For further explanations of the panels, see the legend of Fig. 1.
Hyperosmotic conditions.
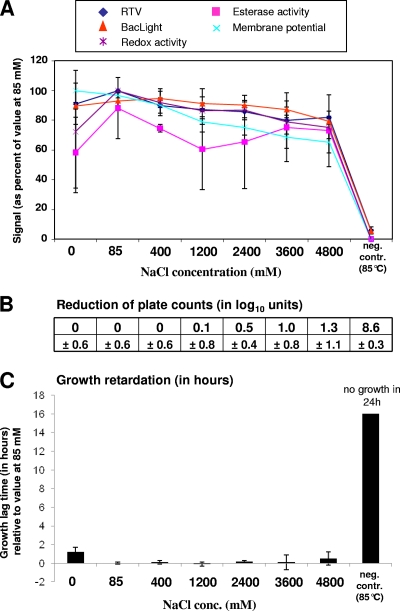
The last experiment consisted in exposing L. monocytogenes to different concentrations of NaCl up to 4.8 M (Fig. 5) (the concentration of a saturated NaCl solution is 6.14 M at 25°C). The cells exposed to a NaCl concentration of 85 mM served as a reference as this condition corresponded to the salt concentration of the tryptic soy broth used for cultivation. Exposure to neither salt-free buffer nor hyperosmotic conditions for 3 h substantially affected any of the molecular viability indicators. This correlated with the finding that growth on plates and in liquid culture medium was only very moderately impacted by the change in osmolarity. The longest lag phase (1.2 h) was observed with cells exposed to salt-free buffer.
Fig. 5.
Effect on viability parameters of exposure of L. monocytogenes to different NaCl concentrations. Samples that were exposed to 85 mM NaCl (same NaCl concentration as in growth medium) or were heat killed (85°C for 10 min) served as controls. Data obtained from stressed cells were related to the data obtained from cells exposed to 85 mM. Error bars indicate standard deviations as seen in three independent experiments. For further explanations of the panels, see the legend of Fig. 1.
Responsiveness of viability parameters.
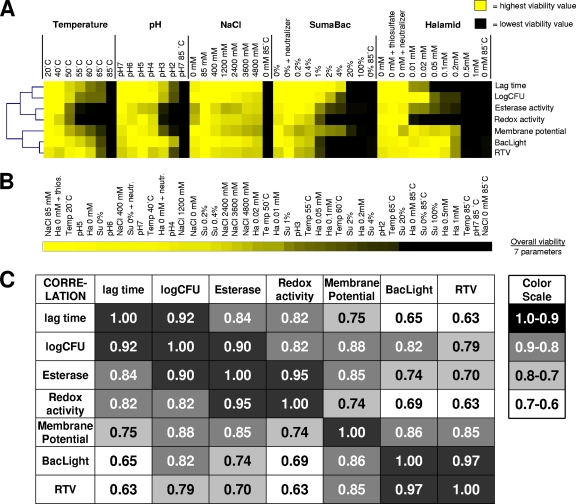
Range scaling of all parameters allowed a graphical representation of the different levels of responsiveness of measured parameters to distinct stresses, as illustrated in Fig. 6A. Substantial differences in sensitivities of different parameters can be seen. Apart from assessing the responsiveness of different parameters to different stresses, the approach was used to compare the severities of stress conditions and to align them on a viability scale (Fig. 6B). Overall, viability was calculated as the average of the seven viability parameters shown in Fig. 6A. Incubation in 85 mM NaCl had the least impact on viability. A correlation analysis for all viability parameters under all tested conditions revealed high correlations between (i) the cultivation-dependent viability assessments, i.e., plate counts and lag times, (ii) the esterase and redox activities, and (iii) the BacLight and RTV assays (Fig. 6C).
Fig. 6.
Summary of stress impact on viability of L. monocytogenes F2365 and correlation analysis of measured parameters. (A) Graphical illustration of responsiveness of different viability parameters to distinct stresses. Each viability parameter was range scaled, with yellow indicating the highest viability value and black indicating the lowest viability value. (B) Impact scale of different stress conditions on overall viability (calculated as the average of seven range-scaled viability values). Yellow indicates the highest viability value, and black indicates the lowest viability value. (C) Statistical analysis of correlations between measured parameters and conditions. Correlations are expressed as values between 0 (no correlation) and 1.0 (identical; highest correlation). Su, Suma Bac; Ha, Halamid.
DISCUSSION
Behavior of viability parameters upon stress exposure.
With the exception of hyperosmotic NaCl exposure, all stress gradients resulted in an increasing impact on all measured viability parameters. The order of decline was dependent on the stress condition. In other words, depending on the stress applied, some viability parameters were more sensitive and decreased faster, whereas others decreased only under more stringent conditions. Redox activity and esterase activity appeared to be the most responsive parameters when L. monocytogenes was exposed to temperature, pH, and Halamid stress, whereas membrane integrity parameters (as measured by BacLight and RTV assays) were the first ones to decrease when cells were exposed to Suma Bac. Suma Bac is a commercial disinfectant and cleaning agent which contains a combination of at least three ingredients. The pH of an aqueous Suma Bac solution is basic (>10) due to the presence of sodium carbonate. The supplier states the QAC as the active ingredient, which would be in agreement with the obtained data as QACs are known to target bacterial membranes (10). This example shows how the approach provides information about the mode of action of different biocidal treatments.
In contrast to the obviously biocidal effects of heat, pH, Suma Bac, and Halamid (reflected by decreases in measured viability parameters), hyperosmotic conditions did not greatly impact any of the measured viability parameters. High salt concentrations are commonly used in the food industry to achieve preservation by growth inhibition (11). The data underline that the continuous presence of salt is necessary to maintain the desired bacteriostatic effect. The minimal impact on the measured parameters is in agreement with earlier studies that reported a very high salt tolerance of L. monocytogenes (8, 16). Growth was observed at unusually high NaCl concentrations of up to 10% (equivalent to 1.7 M). A study by Liu et al. looking at the tolerance of different virulent and avirulent L. monocytogenes strains to different conditions reported that all strains tested were resistant to saturated NaCl (corresponding to approximately 6.1 M or 36%, wt/vol, at 25°C) for at least 20 h and possibly longer, as tested by enumeration of CFU (15).
It has to be pointed out that all assays have different intrinsic sensitivities, with plate counting being able to detect a few cells, whereas the detection limit of many fluorescent plate reader assays assessing membrane integrity is typically around 10E7 cells per well (14). Plate-reader-based assays measuring enzymatic activities tend to show greater sensitivity as signals from low numbers of active cells can be intensified by allowing for longer reaction times. Although such differences in sensitivities have to be taken into account and compromise comparability to some extent, Fig. 6A illustrates high overall responsiveness of redox and esterase activity, whereas more severe stress conditions are typically required to have an impact on membrane integrity. An exception is the exposure of cells to Suma Bac for the reason mentioned earlier. For the oxidative agent Halamid, the finding that higher concentrations of the oxidant are required to affect membrane permeability than to inhibit colony formation is in good agreement with earlier studies with chlorine (20, 24).
Correlation between viability parameters.
When the different viability parameters are compared, a striking correlation between the following parameters can be observed: (i) log CFU and lag time, (ii) esterase and redox activities, and (iii) BacLight and RTV assays (Fig. 6C). Whereas a good agreement between colony formation on plates and growth in liquid medium is obvious, the correlation between esterase and redox activities might result from the fact that both activities are based on enzymatic action. Discounting de novo protein synthesis during stress exposure, the involved proteins might have very similar sensitivities to exposure to different stress factors. It has to be pointed out, however, that the limited data set presented in this study recommends caution in making far-reaching conclusions. In a preliminary study with Escherichia coli, esterase activity tended, in contrast to that in L. monocytogenes, to increase upon exposure to sublethal stress, whereas other parameters followed the same trend as seen with L. monocytogenes (unpublished data). Esterase activity in E. coli seemed, therefore, to function as a stress indicator rather than as a viability parameter. This species dependence of esterase activity is in agreement with a study by Baatout et al. looking at the response of multiple viability parameters to exposure to hydrogen peroxide (1). Increasing concentrations of H2O2 resulted in a decrease in esterase activity in Shewanella oneidensis but to an increase in E. coli and Ralstonia metallidurans. More studies have to be performed with a wider spectrum of microorganisms to develop a better understanding of how and at what rate different parameters respond to different stresses and how they correlate with each other.
Benchmarking of the real-time viability assay.
The very high correlation between BacLight and RTV assays under all tested conditions is of great interest for the validation of the novel RTV technology of Kort et al. (14). Like the established Live/Dead BacLight assay, the RTV assay successfully detected loss of viability under adverse conditions and showed a clear dose-response relationship. Whereas the BacLight principle is based on selective penetration of nucleic acid binding dyes, the signals from the RTV probe, salicylic acid (administered with a low-pH buffer), reflect the intracellular pH. It is well known that only intact membranes can function as an efficient barrier (19). A low cytosolic pH resulting from the passive influx of protons from the surrounding acidic environment can be seen as a result of membrane damage, while a neutral pH can be maintained only if membranes are intact, impermeable to protons, and able to maintain the proton gradient necessary for energy conservation (17). It seems logical that the more severe the extent of membrane damage, the faster and more dramatic the acidification of the cytosol will be when cells are abruptly subjected to low pH, as is the case during the RTV assay. The method can thus be seen as an indirect measure of the membrane integrity. The advantage of the RTV assay over BacLight is that signals are obtained instantaneously after probe addition, whereas the penetration of nucleic acid stains requires an incubation period, typically in the range of minutes.
Concluding remarks.
Although “no staining technique can give a guaranteed answer about a bacterial cell's reproductive viability” (18), the measurement of multiple indirect viability parameters provides a better understanding of the physiological state of bacteria exposed to different conditions of interest. Only in this way can insight into the effect of mild stress conditions be obtained. An interesting potential application is to retrieve mechanistic information about the mode of action of new biocides. The multiparameter assay presented here could be further refined in the future by addition of more viability parameters, which would provide more detailed clues about how cell death occurred. The scaling of different conditions allows a comparative analysis with regard to their impact on microbial viability and allows comparisons for stress sensitivities among strains and species.
ACKNOWLEDGMENT
We thank Carlo Brouwer for diligent performance of part of the experimental work.
Footnotes
Published ahead of print on 15 July 2011.
REFERENCES
- 1. Baatout S., De Boever P., Mergeay M. 2006. Physiological changes induced in four bacterial strains following oxidative stress. Prikl. Biokhim. Mikrobiol. 42:418–427 [PubMed] [Google Scholar]
- 2. Baranyi J., Pin C. 1999. Estimating growth parameters by means of detection times. Appl. Environ. Microbiol. 65:732–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Berney M., et al. 2008. Rapid, cultivation-independent assessment of microbial viability in drinking water. Water Res. 42:4010–4018 [DOI] [PubMed] [Google Scholar]
- 4. Berney M., Weilenmann H. U., Egli T. 2006. Flow-cytometric study of vital cellular functions in Escherichia coli during solar disinfection (SODIS). Microbiology 152:1719–1729 [DOI] [PubMed] [Google Scholar]
- 5. Breeuwer P., Abee T. 2000. Assessment of viability of microorganisms employing fluorescence techniques. Int. J. Food Microbiol. 55:193–200 [DOI] [PubMed] [Google Scholar]
- 6. Colwell R. R., et al. 1996. Viable but non-culturable Vibrio cholerae O1 revert to a culturable state in the human intestine. World J. Microbiol. Biotechnol. 12:28–31 [DOI] [PubMed] [Google Scholar]
- 7. Cossart P., Archambaud C. 2009. The bacterial pathogen Listeria monocytogenes: an emerging model in prokaryotic transcriptomics. J. Biol. 8:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fratamico P. M., Bhunia A. K., Smith J. L.(ed.). 2005. Foodborne pathogens: microbiology and molecular biology. Caister Academic Press, Norwich, United Kingdom [Google Scholar]
- 9. Hewitt C. J., Nebe-Von-Caron G. 2004. The application of multiparameter flow cytometry to monitor individual microbial cell physiological state. Adv. Biochem. Eng. Biotechnol. 89:197–223 [DOI] [PubMed] [Google Scholar]
- 10. Ioannou C. J., Hanlon G. W., Denyer S. P. 2007. Action of disinfectant quaternary ammonium compounds against Staphylococcus aureus. Antimicrob. Agents Chemother. 51:296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Juneja V. K. 2003. Combining traditional and new preservation techniques to control pathogens: the case of E. coli, p. 207–209In Zeuthen P., Bøgh-Sørensen L. (ed.), Food preservation techniques. Woodhead Publishing, Cambridge, United Kingdom [Google Scholar]
- 12. Keer J. T., Birch L. 2003. Molecular methods for the assessment of bacterial viability. J. Microbiol. Methods 53:175–183 [DOI] [PubMed] [Google Scholar]
- 13. Kell D. B., Kaprelyants A. S., Weichart D. H., Harwood C. R., Barer M. R. 1998. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Van Leeuwenhoek 73:169–187 [DOI] [PubMed] [Google Scholar]
- 14. Kort R., et al. 2010. Real-time detection of viable microorganisms by intracellular phototautomerism. BMC Biotechnol. 10:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu D., Lawrence M. L., Ainsworth A. J., Austin F. W. 2005. Comparative assessment of acid, alkali and salt tolerance in Listeria monocytogenes virulent and avirulent strains. FEMS Microbiol. Lett. 243:373–378 [DOI] [PubMed] [Google Scholar]
- 16. McClure P. J., Roberts T. A., Oguru P. O. 1989. Comparison of the effects of sodium chloride, pH and temperature on the growth of Listeria monocytogenes on gradient plates and liquid medium. Lett. Appl. Microbiol. 9:95–99 [Google Scholar]
- 17. Mitchell P. 1961. Coupling of phosphorylation to electron and hydrogen transfer by a chemiosmotic type of mechanism. Nature 191:144–148 [DOI] [PubMed] [Google Scholar]
- 18. Nebe-von-Caron G., Stephens P. J., Hewitt C. J., Powell J. R., Badley R. A. 2000. Analysis of bacterial function by multi-colour fluorescence flow cytometry and single cell sorting. J. Microbiol. Methods 42:97–114 [DOI] [PubMed] [Google Scholar]
- 19. Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nocker A., Sossa K. E., Camper A. K. 2007. Molecular monitoring of disinfection efficacy using propidium monoazide in combination with quantitative PCR. J. Microbiol. Methods 70:252–260 [DOI] [PubMed] [Google Scholar]
- 21. Oliver J. D. 2000. The public health significance of viable but nonculturable bacteria, p. 277–300In Colwell R. R., Grimes D. J. (ed.), Nonculturable microorganisms in the environment. ASM Press, Washington, DC [Google Scholar]
- 22. Rowan N. J. 2004. Viable but non-culturable forms of food and waterborne bacteria: quo vadis? Trends Food Sci. Technol. 15:462–467 [Google Scholar]
- 23. Tortorello M. L. 2003. Indicator organisms for safety and quality—uses and methods for detection: minireview. J. AOAC Int. 86:1208–1217 [PubMed] [Google Scholar]
- 24. Virto R., Mañas P., Álvaraz I., Condon S., Raso J. 2005. Membrane damage and microbial inactivation by chlorine in the absence and presence of a chlorine-demanding substrate. Appl. Environ. Microbiol. 71:5022–5028 [DOI] [PMC free article] [PubMed] [Google Scholar]