Abstract
Among various lactic acid bacterial strains tested, cocoa-specific strains of Lactobacillus fermentum were best adapted to the cocoa pulp ecosystem. They fermented glucose to lactic acid and acetic acid, reduced fructose to mannitol, and converted citric acid into lactic acid and 2,3-butanediol.
TEXT
Fermented dry cocoa beans are the basic raw material for chocolate production. Cocoa beans are the seeds of the cocoa tree, Theobroma cacao L. The key microorganisms for successful cocoa bean fermentation processes are yeasts, lactic acid bacteria (LAB), and acetic acid bacteria (AAB) (1, 3–5, 14, 18–21). Although the LAB species diversity involved in the onset of any spontaneous cocoa bean fermentation is wide, not much is known about how cocoa-specific LAB species, such as Lactobacillus fermentum, adapt physiologically and what their targeted functional roles are during fermentation (1, 3, 4, 14, 19–21). The present study aimed at the kinetic investigation of carbohydrate fermentation and citric acid conversion by various LAB strains to unravel this.
The LAB strains used throughout this study are listed in Table 1. Monoculture fermentations were performed in 1.5 liters of a cocoa pulp simulation medium (CPSM) for LAB (16) in Biostat B-DCU fermentors (Sartorius AG/B. Braun Biotech International, Melsungen, Germany) anaerobically for 48 h. Inoculum build-up, fermentor setup, online control of temperature (Table 2), pH profile, agitation, and sampling were as described previously (13, 16). All fermentations were performed in duplicate. The results and figures presented hereinafter are representative for both fermentations.
Table 1.
Overview of the cocoa-specific and cocoa-nonspecific strains of lactic acid bacteria (LAB) used throughout this study
| LAB straina | Source | Reference(s) |
|---|---|---|
| Cocoa-specific strains | ||
| E. casseliflavus M484 | Brazilian cocoa bean box fermentation | 21 |
| F. pseudoficulneus M83 | Brazilian cocoa bean box fermentation | 21 |
| L. cacaonum LMG 24285T | Ghanaian cocoa bean heap fermentation | 4, 6 |
| L. fabifermentans LMG 24284T | Ghanaian cocoa bean heap fermentation | 4, 6 |
| L. fermentum 222 | Ghanaian cocoa bean heap fermentation | 3 |
| L. fermentum M103 | Brazilian cocoa bean box fermentation | 21 |
| L. fermentum M158 | Brazilian cocoa bean box fermentation | 21 |
| L. fermentum M332 | Brazilian cocoa bean box fermentation | 21 |
| L. plantarum 80 | Ghanaian cocoa bean heap fermentation | 3 |
| Lc. pseudomesenteroides 22 | Brazilian cocoa bean box fermentation | 21 |
| W. fabaria LMG 24289T | Ghanaian cocoa bean heap fermentation | 3, 7 |
| W. ghanensis LMG P-23179 | Ghanaian cocoa bean heap fermentation | 3, 8 |
| Cocoa-nonspecific strains | ||
| L. amylovorus DCE 471 | Corn steep liquor | 9 |
| L. fermentum IMDO 130101 | Belgian sourdough fermentation | 22 |
LMG, Belgian Coordinated Collections of Microorganisms/Laboratory for Microbiology Ghent (BCCM/LMG; Ghent, Belgium); IMDO, Research Group of Industrial Microbiology and Food Biotechnology (Vrije Universiteit Brussel, Brussels, Belgium); DCE, Department of Chemistry and Engineering (Vrije Universiteit Brussel, Brussels, Belgium).
Table 2.
Carbohydrate and citric acid consumption and metabolite production of cocoa-specific and cocoa-nonspecific LAB strains in a cocoa pulp simulation medium for lactic acid bacteria
| Strain | Fermentation temp (°C) | Mean consumption ± SD (mM) of substrate (after 48 h of fermentation) |
Mean production ± SD (mM) of metabolites (after 48 h of fermentation) |
Carbon recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Glucose | Fructose | Citric acid | Lactic acid | Acetic acid | Carbon dioxide | Mannitol | Flavor compounda | |||
| L. fermentum 222 | 37 | 63.4 ± 13.1 | 107.2 ± 10.7 | 55.4 ± 5.6 | 75.3 ± 1.8 | 106.7 ± 2.0 | 147.1 ± 0.1 | 98.3 ± 2.0 | 22 | 93 |
| L. fermentum M103 | 37 | 62.1 ± 8.1 | 142.9 ± 9.6 | 60.2 ± 4.4 | 97.5 ± 1.2 | 131.0 ± 1.3 | 153.8 ± 0.1 | 140.6 ± 9.6 | 16 | 102 |
| L. fermentum M158 | 37 | 78.1 ± 9.6 | 145.0 ± 8.1 | 46.9 ± 0.0 | 88.4 ± 1.9 | 125.0 ± 2.7 | 142.1 ± 0.1 | 140.0 ± 8.5 | 18 | 97 |
| L. fermentum M332 | 37 | 87.2 ± 10.1 | 130.1 ± 12.9 | 54.1 ± 1.5 | 95.3 ± 2.4 | 135.5 ± 3.8 | 154.2 ± 0.1 | 135.1 ± 0.3 | 20 | 98 |
| L. plantarum 80 | 37 | 24.2 ± 16.2 | 38.3 ± 10.0 | 14.3 ± 6.9 | 140.3 ± 0.6 | 15.2 ± 0.5 | 9.2 ± 0.1 | 0 | 0 | 100 |
| L. fabifermentans LMG 24284T | 30 | 9.9 ± 15.9 | 39.3 ± 13.9 | 0 | 98.0 ± 1.4 | 0 | 0 | 0 | 0 | 101 |
| L. fermentum IMDO 130101 | 37 | 62.5 ± 5.3 | 143.7 ± 7.9 | 0 | 70.2 ± 1.1 | 65.5 ± 1.2 | 64.2 ± 0.1 | 138.0 ± 5.7 | 0 | 100 |
| Lc. pseudomesenteroides 22 | 30 | 27.0 ± 5.6 | 85.5 ± 7.3 | 16.6 ± 4.5 | 54.1 ± 1.2 | 51.2 ± 1.2 | 41.7 ± 0.1 | 82.8 ± 6.0 | 0 | 104 |
| F. pseudoficulneus M83 | 28 | 52.4 ± 15.5 | 81.7 ± 19.5 | 0 | 50.4 ± 0.2 | 46.9 ± 0.3 | 44.2 ± 0.1 | 89.0 ± 6.9 | 0 | 102 |
| W. ghanensis LMG P-23179 | 30 | 2.9 ± 7.5 | 0 | 52.7 ± 3.3 | 10.0 ± 0.6 | 57.8 ± 5.0 | 111.1 ± 0.1 | 0 | 26 | 108 |
| W. fabaria LMG 24289T | 30 | 21.7 ± 17.1 | 0 | 46.6 ± 1.2 | 18.7 ± 0.6 | 66.0 ± 1.6 | 108.2 ± 0.1 | 0 | 24 | 96 |
| L. cacaonum LMG 24285T | 30 | 0 | 28.3 ± 24.5 | 47.7 ± 2.1 | 63.4 ± 0.4 | 51.4 ± 1.2 | 88.1 ± 0.1 | 0 | 20 | 101 |
Theoretical, estimated flavor compound production by the cocoa-specific LAB strains, according to the pathway proposed by Mayo et al. (17). In all cases, the flavor compound produced was 2,3-butanediol, except for the L. cacaonum LMG 24285T monoculture fermentations, where the flavor compound was acetoin.
During fermentation, bacterial growth (CFU per ml) was quantified through plating of 10-fold serial dilutions of the samples in saline (0.85% [wt/vol] NaCl solution) on CPSM agar (CPSM containing 1.5% [wt/vol] agar, pH 5.5) that was incubated at the appropriate fermentation temperature for 24 h. Metabolite concentrations were determined through high-performance anion-exchange chromatography using a standard addition protocol (glucose, fructose, mannitol, and citric acid) (15, 23) and high-performance liquid chromatography using external standards (lactic acid, acetic acid, and ethanol) (16). Cell count and metabolite (with external standards) measurements were performed on three independent samples. The errors on the measurements are represented as standard deviations. Gas chromatography (GC) was used for the qualitative determination of 2,3-butanediol and acetoin (15). Concentrations of carbon dioxide in the fermentor gas effluents were determined online through GC (13). The carbon recovery (CR, expressed as percentage) was calculated by dividing the total amount of carbon recovered in the metabolites by the total amount of carbon present in the carbon sources.
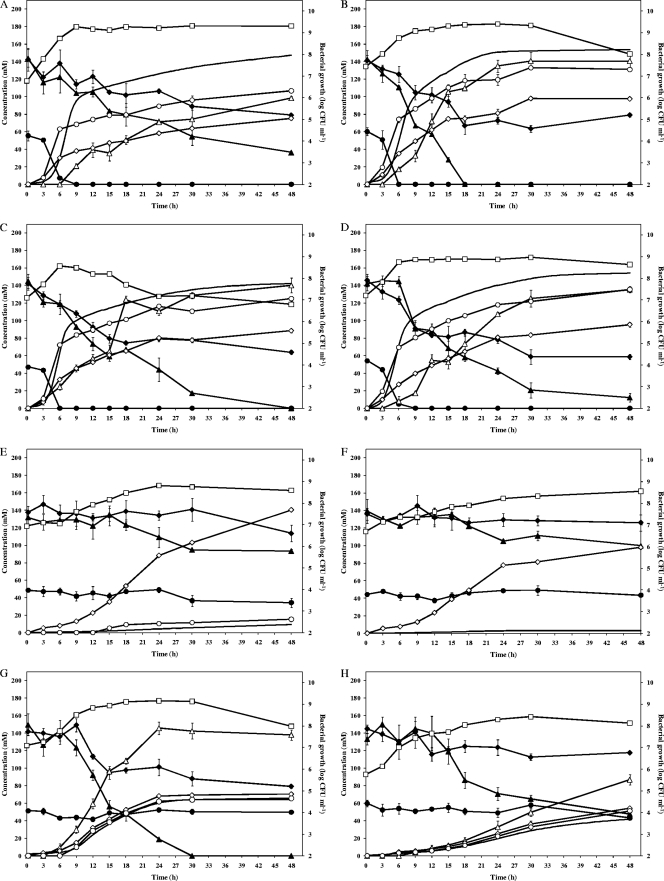
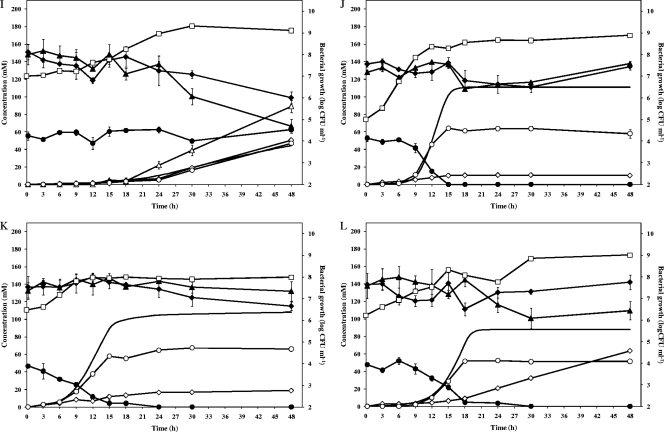
All LAB strains tested were able to grow in CPSM (Fig. 1 and Table 2). Strictly heterofermentative LAB strains (all L. fermentum and Weissella strains, Leuconostoc pseudomesenteroides 22, and Fructobacillus pseudoficulneus M83) fermented glucose, converted citric acid (not in the case of Lc. fermentum IMDO 130101 and F. pseudoficulneus M83 and only at the end of the fermentation in the case of Lc. pseudomesenteroides 22), and reduced fructose (not in the case of Weissella ghanensis LMG P-23179 and Weissella fabaria LMG 24289T). The facultative heterofermentative Lactobacillus plantarum 80 (hardly converted citric acid) and Lactobacillus fabifermentans LMG 24284T (did not convert citric acid) strains fermented glucose and fructose simultaneously, with a preference for fructose. The facultative heterofermentative Lactobacillus cacaonum LMG 24285T fermented fructose but not glucose and converted citric acid. Due to the initial low pH (3.5), Enterococcus casseliflavus M484 and Lactobacillus amylovorus DCE 471 were not able to grow in CPSM.
Fig. 1.
Bacterial growth, carbohydrate and citric acid consumption, and metabolite production of Lactobacillus fermentum 222 (A), Lactobacillus fermentum M103 (B), Lactobacillus fermentum M158 (C), Lactobacillus fermentum M332 (D), Lactobacillus plantarum 80 (E), Lactobacillus fabifermentans LMG 24284T (F), Lactobacillus fermentum IMDO 130101 (G), Leuconostoc pseudomesenteroides 22 (H), Fructobacillus pseudoficulneus M83 (I), Weissella ghanensis LMG P-23179 (J), Weissella fabaria LMG 24289T (K), and Lactobacillus cacaonum LMG 24285T (L) in a cocoa pulp simulation medium for lactic acid bacteria. Glucose, ♦; fructose, ▴; citric acid, •; lactic acid, ⋄; acetic acid, ○; mannitol, ▵; carbon dioxide, —; and bacterial growth, □.
This study showed that cocoa pulp was actually an ideal substrate for strictly heterofermentative (e.g., L. fermentum) and fructophilic LAB species (e.g., F. pseudoficulneus), because it contains a high concentration of fructose (energy source and/or alternative external electron acceptor) and citric acid (additional source of pyruvate). These substrates are used for the oxidation of NADH + H+ to bypass the energy-limiting ethanol pathway and, so, to maximize their growth rate on glucose, thereby producing mannitol and lactic acid plus acetic acid, respectively (16). They are to be consumed under low-pH and anaerobic conditions in the beginning of the cocoa bean fermentation. Citric acid conversion by strictly heterofermentative L. fermentum strains seems to be source dependent, because the sourdough-specific L. fermentum IMDO 130101 strain lacked the ability to convert citric acid. As L. fermentum is strictly heterofermentative and heat, acid, and ethanol tolerant, it usually dominates successful cocoa bean fermentation processes, independent of the cocoa-producing region (3, 4, 10, 14, 19–21). Also, fructophilic LAB species seemed to be well-adapted to the cocoa pulp ecosystem and, indeed, F. pseudoficulneus has been recovered from cocoa bean fermentations (19, 20). They are generally associated with fructose-rich niches and grow on fructose (preferentially) or on glucose in the presence of alternative external electron acceptors (12). Up to now, only strictly heterofermentative LAB species have been reported as fructophilic LAB species. In this study, the facultative heterofermentative L. plantarum 80, L. fabifermentans LMG 24289T, and L. cacaonum LMG 24284T strains were characterized as fructose-loving LAB strains also. This indicates their adaptation to the cocoa pulp habitat. These cocoa-specific strains fermented fructose essentially to lactic acid. Hence, citric acid-converting, mannitol-producing (fructose-reducing), heterolactic, and/or fructose-loving LAB strains are particularly adapted to the cocoa pulp matrix. They represent interesting starter cultures to be exploited for enhanced and controlled cocoa bean fermentations.
In the present study, citric acid conversion by cocoa-specific LAB strains led to the production of the butterlike flavor compounds acetoin (L. cacaonum LMG 24285T, due to fructose homolactate fermentation) and 2,3-butanediol (all cocoa-specific L. fermentum strains, W. fabaria LMG 24289T, and W. ghanensis LMG P-23179, due to the need for extra NAD+ recuperation) (17). These compounds form part of the flavor profile of certain cocoa-based products (2, 11).
LAB species are important for a successful microbial succession during cocoa bean fermentations. Actually, LAB form the link between the ethanol- and flavor-producing yeast fermentation and the acetic acid-producing AAB fermentation (10, 16). The lactic acid and mannitol they produce could serve as extra energy sources for AAB species, while their citric acid conversion results in a rise in pH and a possible contribution to cocoa flavor. So, LAB strains, either as monoculture or coculture, will be essential components of starter cultures aimed at the control of cocoa bean fermentation processes to obtain well-fermented dry cocoa beans and improved standard- and superior-tasting chocolates produced therefrom.
In summary, the kinetics of both aqueous and gaseous metabolite production by cocoa-specific and cocoa-nonspecific LAB strains revealed a deeper insight into their energy and citric acid metabolism and metabolite production patterns. The full meaning of these results for the actual control of cocoa bean fermentations by the use of these strains as appropriate starter cultures is under investigation. Nevertheless, this kinetic study contributes to a better understanding of the functional behavior of cocoa-specific LAB strains to be used as interesting starter cultures for controlled cocoa bean fermentations. In addition, the cocoa-specific L. fermentum strains can be categorized as the ones best adapted to the cocoa pulp ecosystem and show interesting functional roles for the development of a defined starter culture.
Acknowledgments
This research was funded by the Research Council of the Vrije Universiteit Brussel (OZR, GOA, and IOF projects), the Research Foundation—Flanders, the government agency for Innovation by Science and Technology (IWT-080357), and Barry Callebaut N.V.
In particular, we acknowledge the help of Barry Callebaut Belgium (Nicholas Camu and Herwig Bernaert).
Footnotes
Published ahead of print on 29 July 2011.
REFERENCES
- 1. Ardhana M. M., Fleet G. H. 2003. The microbial ecology of cocoa bean fermentations in Indonesia. Int. J. Food Microbiol. 86:87–99 [DOI] [PubMed] [Google Scholar]
- 2. Caligiani A., Acquotti D., Cirlini M., Palla G. 2010. 1H NMR study of fermented cocoa (Theobroma cacao L.) beans. J. Agric. Food Chem. 58:12105–12111 [DOI] [PubMed] [Google Scholar]
- 3. Camu N., et al. 2007. Dynamics and biodiversity of populations of lactic acid bacteria and acetic acid bacteria involved in spontaneous heap fermentation of cocoa beans in Ghana. Appl. Environ. Microbiol. 73:1809–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Camu N., et al. 2008. Influence of turning and environmental contamination on the dynamics of populations of lactic acid and acetic acid bacteria involved in spontaneous cocoa bean heap fermentation in Ghana. Appl. Environ. Microbiol. 74:86–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daniel H.-M., et al. 2009. Yeast diversity of Ghanaian cocoa bean heap fermentations. FEMS Yeast Res. 9:774–783 [DOI] [PubMed] [Google Scholar]
- 6. De Bruyne K., Camu N., De Vuyst L., Vandamme P. 2009. Lactobacillus fabifermentans sp. nov. and Lactobacillus cacaonum sp. nov., isolated from Ghanaian cocoa fermentations. Int. J. Syst. Evol. Microbiol. 59:7–12 [DOI] [PubMed] [Google Scholar]
- 7. De Bruyne K., Camu N., De Vuyst L., Vandamme P. 2010. Weissella fabaria sp. nov., from a Ghanaian cocoa fermentation. Int. J. Syst. Evol. Microbiol. 60:1999–2005 [DOI] [PubMed] [Google Scholar]
- 8. De Bruyne K., Camu N., Lefebvre K., De Vuyst L., Vandamme P. 2008. Weissella ghanensis sp. nov., isolated from a Ghanaian cocoa fermentation. Int. J. Syst. Evol. Microbiol. 58:2721–2725 [DOI] [PubMed] [Google Scholar]
- 9. De Vuyst L., Callewaert R., Crabbe K. 1996. Primary metabolite kinetics of bacteriocin biosynthesis by Lactobacillus amylovorus and evidence for stimulation of bacteriocin production under unfavourable growth conditions. Microbiology 142:817–827 [DOI] [PubMed] [Google Scholar]
- 10. De Vuyst L., Lefeber T., Papalexandratou Z., Camu N. 2010. The functional role of lactic acid bacteria in cocoa bean fermentation, p. 301–326 In Mozzi F., Raya R. R., Vignolo G. M. (ed.), Biotechnology of lactic acid bacteria: novel applications. Wiley-Blackwell, Ames, IA [Google Scholar]
- 11. Ducki S., Miralles-Garcia J., Zumbe A., Tornero A., Storey D. M. 2008. Evaluation of solid-phase micro-extraction coupled to gas chromatography-mass spectrometry for the headspace analysis of volatile compounds in cocoa products. Talanta 74:1166–1174 [DOI] [PubMed] [Google Scholar]
- 12. Endo A., Futagawa-Endo Y., Dicks L. M. T. 2009. Isolation and characterization of fructophilic lactic acid bacteria from fructose-rich niches. Syst. Appl. Microbiol. 32:593–600 [DOI] [PubMed] [Google Scholar]
- 13. Falony G., et al. 2009. In vitro kinetics of prebiotic inulin-type fructan fermentation by butyrate-producing colon bacteria: implementation of online gas chromatography for quantitative analysis of carbon dioxide and hydrogen gas production. Appl. Environ. Microbiol. 75:5884–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Garcia-Armisen T., et al. 2010. Diversity of the total bacterial community associated with Ghanaian and Brazilian cocoa bean fermentation samples as revealed by a 16 S rRNA gene clone library. Appl. Microbiol. Biotechnol. 87:2281–2292 [DOI] [PubMed] [Google Scholar]
- 15. Lefeber T., Gobert W., Vrancken G., Camu N., De Vuyst L. 2011. Dynamics and species diversity of communities of lactic acid bacteria and acetic acid bacteria during spontaneous cocoa bean fermentation in vessels. Food Microbiol. 28:457–464 [DOI] [PubMed] [Google Scholar]
- 16. Lefeber T., Janssens M., Camu N., De Vuyst L. 2010. Kinetic analysis of strains of lactic acid bacteria and acetic acid bacteria in cocoa pulp simulation media to compose a starter culture for cocoa bean fermentation. Appl. Environ. Microbiol. 76:7708–7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mayo B., et al. 2009. Updates in metabolism of lactic acid bacteria, p. 3–34 In Mozzi F., Raya R. R., Vignolo G. M. (ed.), Biotechnology of lactic acid bacteria: novel applications. Wiley-Blackwell, Ames, IA [Google Scholar]
- 18. Nielsen D. S., Honholt S., Tano-Debrah K., Jespersen L. 2005. Yeast populations associated with Ghanaian cocoa fermentations analysed using denaturing gradient gel electrophoresis (DGGE). Yeast 22:271–284 [DOI] [PubMed] [Google Scholar]
- 19. Nielsen D. S., et al. 2007. The microbiology of Ghanaian cocoa fermentations analysed using culture-dependent and culture-independent methods. Int. J. Food Microbiol. 114:168–186 [DOI] [PubMed] [Google Scholar]
- 20. Papalexandratou Z. 2011. Species diversity, community dynamics, and metabolite kinetics of the spontaneous cocoa bean fermentation process worldwide. Ph.D. thesis. Vrije Universiteit Brussel, Brussels, Belgium [Google Scholar]
- 21. Papalexandratou Z., Vrancken G., De Bruyne K., Vandamme P., De Vuyst L. 2011. Spontaneous organic cocoa bean box fermentations in Brazil are characterized by a restricted species diversity of lactic acid bacteria and acetic acid bacteria. Food Microbiol. 28:1326–1338 [DOI] [PubMed] [Google Scholar]
- 22. Van der Meulen R., et al. 2007. Population dynamics and metabolite target analysis of lactic acid bacteria during laboratory fermentations of wheat and spelt sourdoughs. Appl. Environ. Microbiol. 73:4741–4750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vrancken G., Rimaux T., De Vuyst L., Leroy F. 2008. Kinetic analysis of growth and sugar consumption by Lactobacillus fermentum 130101 reveals adaptation to the acidic sourdough ecosystem. Int. J. Food Microbiol. 128:58–66 [DOI] [PubMed] [Google Scholar]