Abstract
As the putative center of origin for soybean and the second largest region of soybean production in China, the North China Plain covers temperate and subtropical regions with diverse soil characteristics. However, the soybean rhizobia in this plain have not been sufficiently studied. To investigate the biodiversity and biogeography of soybean rhizobia in this plain, a total of 309 isolates of symbiotic bacteria from the soybean nodules collected from 16 sampling sites were studied by molecular characterization. These isolates were classified into 10 genospecies belonging to the genera Sinorhizobium and Bradyrhizobium, including four novel groups, with S. fredii (68.28%) as the dominant group. The phylogeny of symbiotic genes nodC and nifH defined four lineages among the isolates associated with Sinorhizobium fredii, Bradyrhizobium elkanii, B. japonicum, and B. yuanmingense, demonstrating the different origins of symbiotic genes and their coevolution with the chromosome. The possible lateral transfer of symbiotic genes was detected in several cases. The association between soil factors (available N, P, and K and pH) and the distribution of genospecies suggest clear biogeographic patterns: Sinorhizobium spp. were superdominant in sampling sites with alkaline-saline soils, while Bradyrhizobium spp. were more abundant in neutral soils. This study clarified the biodiversity and biogeography of soybean rhizobia in the North China Plain.
INTRODUCTION
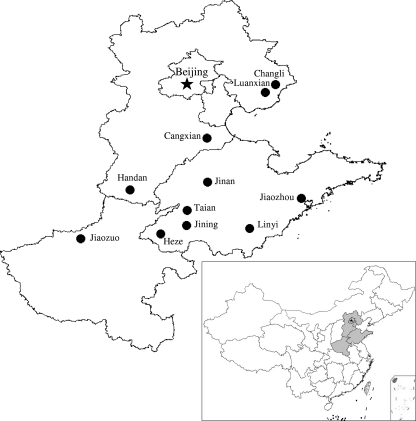
Soybean (Glycine max L.) is a major legume crop in the world, representing 50% of the global crop legume area and 68% of global legume production (11). It also plays a very important role in sustainable agriculture and in the economy for many countries because of its great nitrogen-fixing ability, which is acquired from its symbiosis with rhizobia in root nodules. In China, the soybean was domesticated approximately 4,000 years ago (29), and the region between 34 and 35°N, corresponding to the North China Plain or Huang-Huai-Hai (HHH) Plain, which refers to the downstream regions of the Huang (Yellow) River, Huai River, and Hai River (Fig. 1), was suggested to be the area of the origination of soybean based upon a comparative study of seed protein contents of cultivated (G. max) and wild (Glycine soja Sieb. and Zucc.) soybeans from different latitudes in China (46). Geographically and climatically, the North China Plain is a transit area between the northeastern, northwestern, and southern regions of China. Therefore, the North China Plain may have served as a diversification center of soybean rhizobia as estimated by Lie et al. (21), and the soybean rhizobial communities in different sites of this plain may vary in relation to their geographic locations or relative distances from other regions, since biogeographic patterns have been found in some rhizobia (22, 39), including soybean rhizobia (10, 23).
Fig. 1.
Map of the Huang-Huai-Hai (HHH) Plain showing the sampling sites (•). ★, Capital of China, Beijing. The corresponding position of the HHH Plain in China is shown in the inset. The two maps were created using DIVA-GIS software (http//www.diva-gis.org), and the sampling sites were added according to GPS records.
As the largest alluvial plain of eastern Asia, the North China Plain is based on the deposits of the Yellow River, Huai River, Hai River, Luan River, etc. The Yellow River flows through the middle of the plain into Bohai Gulf. The plain covers an area of about 409,500 km2, most of which is less than 50 m above sea level, has an average precipitation of about 500 to 800 mm from north to south, and includes three types of geographic zones: (i) alluvial fans in the north part, where the soils are fertile loam with neutral pH; (ii) alluvial plains in the central and main part of the plain, where the level of underground water is high and the soils are saline-alkaline with high clay content; and (iii) coastal plains and the delta of the Yellow River, where the soils are highly saline and mainly composed of clay. Currently, the North China Plain is the second largest region for soybean production in China, having 2,700,000 ha for soybean planting annually, which are mainly in the alluvial fans and plains. The diverse soil and climate conditions, vast area of soybean culture, transit geographic location, and the fact that it is the original center of soybean production make the North China Plain a good model for studying the biogeography and determinants of soybean rhizobia. However, the soybean rhizobia in this plain have not been sufficiently studied, although microsymbionts isolated from other regions have been studied extensively, including classification (6, 16) and the determination of phylogeny (32) and genotypic characteristics (24, 47).
To date, rhizobia nodulating with soybean in Xinjiang (a geographically isolated region surrounded by deserts and high mountains) and in subtropical and tropic regions of China have been systematically studied (4, 5, 10, 23, 47, 48), and diverse soybean rhizobia belonging to Bradyrhizobium, Mesorhizobium, and Sinorhizobium have been reported in these studies (4, 5, 10, 23, 47, 48). In addition, biogeographic patterns have been observed in the soybean rhizobia (10, 20, 23). Since the geographical distribution of symbiotic bacteria was affected by biological factors like the host plants (44) and animals (14) and by soil factors (10), similarly to the free-living bacteria (9), diverse soybean rhizobia adapted to the local conditions should be expected in the North China Plain.
Based upon the background knowledge mentioned above, we performed the present study to reveal the community composition of soybean rhizobia and to estimate the rhizobial distribution in correlation with different ecological factors in the North China Plain.
MATERIALS AND METHODS
Nodule sampling and isolation of rhizobia.
Root nodules of soybean were collected from 16 sampling sites in the major soybean production areas of the North China Plain (Fig. 1 and Table 1) during July to August of 2008. In general, the 4 sites in Hebei Province are classified as the temperate region, while the 2 sites in Henan and 10 sites in Shandong are located in subtropic regions according to their latitude and climate characteristics. All of the sampling sites were located in cultivated soybean fields without rhizobial inoculation history. In eight sites, 24 cultivars of soybean were sampled, of which 7 were found in two or four sites. In these cases, two plants were sampled for each cultivar in each site, but nine plants were sampled for cultivar Yudou 25 in the site of Jiaozuo1 for checking the effect of sampling size on the estimation of rhizobia diversity. In another eight sites in which the soybean cultivars were not recorded, five plants were sampled from each site. Roots of soybean plants excavated from soil were washed immediately with tap water to eliminate attached soils. Healthy and complete nodules dissected from roots were maintained at environmental temperature in plastic tubes filled with dehydrated silica gel for transportation. For rhizobium isolation, five dehydrated nodules randomly selected from each plant were immersed in sterile deionized-distilled water (ddH2O) overnight at 4°C and were surface sterilized by immersion in 95% ethanol for 30 s and then in 0.2% mercuric chloride for 4 to 5 min (depending on the nodule diameter). They then were washed six times with sterile ddH2O (41). The surface-sterilized nodules were crushed separately in sterilized microtubes, and the nodule juice was stroked on plates of yeast-mannitol agar (YMA) (41), which were incubated at 28°C for the isolation of the rhizobia. The obtained bacterial colonies were purified by being repeatedly struck on the same medium. The nodulation capability of each isolate was tested by inoculating seedlings of the soybean cultivar Zhonghuang 13 (a popular cultivar in the HHH Plain) grown in Leonard jars filled with vermiculite as reported previously (41). Cultures of pure isolates were maintained on YMA slants at 4°C for short-term storage or in YM broth supplied with 20% (wt/vol) glycerol at −80°C for long-term storage.
Table 1.
Characteristics of soil factors, number of strains in genospecies, and diversity indexes of soybean rhizobia from 16 sampling sites in the HHH Plain (23.5 to 40°N)
| Site | Characteristics of soil factorsa |
No. of strains in genospeciesb: |
Sumc |
Indexd |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A_N | A_P | A_K | pH | S1 | S2 | S3 | B1 | B2 | B3 | B4 | B5 | B6 | B7 | S | B | Spp | H′ | D | J | |
| Hebei (36.6-39.6°N, temperate region) | ||||||||||||||||||||
| Cangxian | 69.5 | 6.6 | 210 | 7.94 | 4 | 16 | 4 | 20 | 4 | 3 | 0.87 | 0.50 | 0.79 | |||||||
| Changli | 80.2 | 91.2 | 597 | 7.67 | 1 | 4 | 1 | 1 | 5 | 3 | 0.87 | 0.50 | 0.79 | |||||||
| Luanxian | 32.1 | 5.6 | 54.8 | 7.13 | 3 | 5 | 2 | 1 | 0 | 11 | 4 | 1.24 | 0.68 | 0.89 | ||||||
| Handan | 56.6 | 41.6 | 290 | 8.16 | 4 | 1 | 5 | 0 | 2 | 0.50 | 0.32 | 0.72 | ||||||||
| Henan (35.9-36.25°N, subtropic region) | ||||||||||||||||||||
| Jiaozuo1 | 97.2 | 19.2 | 373 | 7.81 | 39 | 4 | 2 | 1 | 39 | 7 | 4 | 0.57 | 0.27 | 0.41 | ||||||
| Jiaozuo2 | 106 | 28.2 | 218 | 7.88 | 11 | 1 | 11 | 1 | 2 | 0.29 | 0.15 | 0.41 | ||||||||
| Shangdong (30.65°-36.65°N, subtropic region) | ||||||||||||||||||||
| Heze1 | 60.1 | 15.3 | 120 | 7.95 | 21 | 1 | 22 | 0 | 2 | 0.18 | 0.09 | 0.27 | ||||||||
| Heze2 | 95.2 | 39.6 | 362 | 8.07 | 9 | 9 | 0 | 1 | 0.00 | 0.00 | Ne | |||||||||
| Jinan | 83.3 | 24.1 | 245 | 7.97 | 34 | 1 | 1 | 4 | 2 | 34 | 8 | 5 | 0.72 | 0.33 | 0.45 | |||||
| Jining | 64.0 | 8.4 | 96.6 | 8.12 | 46 | 46 | 0 | 1 | 0.00 | 0.00 | N | |||||||||
| Jiaozhou | 85.2 | 23.2 | 106 | 6.91 | 4 | 4 | 0 | 8 | 2 | 0.69 | 0.50 | 1.00 | ||||||||
| Linyi | 57.9 | 14.0 | 108 | 7.73 | 8 | 3 | 4 | 4 | 1 | 1 | 0 | 21 | 6 | 1.57 | 0.76 | 0.87 | ||||
| Taian1 | 60.1 | 25.8 | 180 | 8.09 | 4 | 1 | 4 | 1 | 2 | 0.50 | 0.32 | 0.72 | ||||||||
| Taian2 | 67.2 | 28.0 | 248 | 8.09 | 6 | 6 | 0 | 1 | 0.00 | 0.00 | N | |||||||||
| Taian3 | 103.0 | 37.0 | 396 | 7.85 | 24 | 4 | 24 | 4 | 2 | 0.41 | 0.24 | 0.59 | ||||||||
| Taian4 | 134.0 | 57.1 | 476 | 7.69 | 8 | 6 | 1 | 3 | 8 | 10 | 4 | 1.19 | 0.66 | 0.86 | ||||||
| Total (ratiof) | 211 (68.3) | 1 (0.3) | 17 (5.5) | 22 (7.1) | 18 (5.8) | 8 (2.6) | 9 (2.9) | 15 (4.9) | 4 (1.3) | 4 (1.3) | 229 (74.1) | 80 (25.9) | ||||||||
A_N, available N; A_P, available P; A_K, available K. The unit of measurement is mg kg−1.
S1, S. fredii; S2, Sinorhizobium sp. I; S2, Sinorhizobium sp. II; B1, B. elkanii; B2, B. japonicum USDA 6T; B3, B. japonicum USDA 110; B4, B. liaoningense; B5, B. yuanmingense; B6, Bradyrhizobium sp. I; B7, Bradyrhizobium sp. II.
S, total number of strains in the genus Sinorhizobium; B, total number of strains in the genus Bradyrhizobium; Spp, number of genomic species in each sampling site.
H′, Shannon-Weiner's index; D, Simpson's index; J, Pielou's evenness index.
N, null.
Ratios are given as percentages.
Soil sampling and characterization.
Soil (about 50 g) was sampled from the root zone (0 to 20 cm in depth) when the nodules were collected. Air-dried soil samples were ground and passed through 2-mm mesh screens for determining the chemical properties. Contents of available N, available P, and available K and the pH of soil were analyzed at the Plant Nutrient and Resource Research Institution, Beijing Academy of Agriculture and Forestry Sciences, using methods described previously (1, 13, 26, 34).
Amplification and RFLP of 16S rRNA gene.
Total template DNA was extracted from each isolate and reference strain using the GUTC method described by Terefework et al. (38). Primers P1 and P6 (37) were used for the amplification of the 16S rRNA gene. The PCR amplification was performed with the procedure of Weisburg et al. (45). Amplification products were digested separately with each of the restriction endonucleases MspI, HinfI, HaeIII, and AluI (5 U per reaction) at 37°C for 6 h. The restriction fragments were separated and visualized by electrophoresis in 2.5% (wt/vol) agarose gels containing 0.5 μg ml−1 ethidium bromide (43). The restriction fragment length polymorphism (RFLP) patterns (with fragments of more than 100 bp) were combined and used in cluster analysis with the Dice coefficient and the method of unweighted pair grouping with mathematic averages (UPGMA) by using the Gelcompar II software package (Applied Maths, Belgium) (40). Isolates sharing the same RFLP patterns were designated a single rRNA type.
Amplification and RFLP of 16S-23S IGS.
The analysis of the internally transcribed spacer (IGS) sequences has been used to identify closely related species (37). In the present study, the ribosomal IGS between 16S and 23S rRNA genes was amplified using the primers FGPS6 and 23S-38 (25) with the PCR protocol of Rasolomampianina et al. (28). The PCR products of IGS were digested separately with each of the restriction endonucleases MspI, HinfI, and HaeIII (5 U per reaction) at 37°C for 6 h. The restriction fragments were separated, visualized, and clustered as described above for the RFLP analysis of 16S rRNA genes. Isolates sharing the same RFLP patterns were designated a single IGS type.
Sequence analyses of 16S rRNA, recA, glnII, atpD, nifH, and nodC genes.
Based on the results of RFLP analyses of 16S rRNA genes and 16S-23S IGS, representative isolates of different clusters were chosen for the analysis of multiple gene sequencing. The 16S rRNA gene was amplified with the same primers and procedures used in RFLP analysis. Partial housekeeping genes recA, glnII, and atpD were amplified using primer pairs recA41F/recA640R, glnII12F/glnII689R, and atpD255F/atpD782R, respectively, with protocols described by Vinuesa et al. (42). A fragment of the nifH gene (about 800 bp) was amplified with primer pair nifHF/nifHR and the protocol of Laguerre et al. (17). A fragment of the nodC gene (about 700 bp) was amplified with primer pair nodCF540/nodCR1160 using the protocol of Sarita et al. (33). All of the acquired nucleotide sequences were deposited in the GenBank database, and the accession numbers are individually specified in the corresponding phylograms. The sequences acquired in this study and the related sequences obtained from the GenBank database by BLASTn searching were aligned, and phylogenetic trees were constructed using the software MEGA 4.0.1 (36) with the neighbor-joining method and the Kimura two-parameter model for the 16S rRNA gene, nodC, and nifH and for multilocus sequence analysis (MLSA) (combined sequences of recA, glnII, and atpD). The phylogenetic trees were bootstrapped with 1,000 bootstrap replications.
Data analyses.
To estimate the community structure and species richness of soybean rhizobia, genospecies were defined based mainly upon the MLSA in this study. Soybean rhizobial diversity, species richness, and evenness in different sampling sites were estimated by three popular alpha ecological indexes (12): the Shannon-Wiener (H′) index and Simpson (D) index to represent diversity considering both the species richness and evenness in a community, and the Pielou (J) index to show the species evenness in the community. These indexes of biodiversity were implemented in the Vegan package (version 1.17-4; http://ftp.ctex.org/mirrors/CRAN/) and calculated by the R statistical language (version 2.12.0; http://www.r-project.org/). The program Bioenv (7) was used to find the best subset of environmental variables, so that the Euclidean distances of scaled environmental variables have the maximum (rank) correlation with community dissimilarities in biodiversity and the best subset of environmental variables, which also is implemented in the Vegan package using the R statistical language. Redundancy analysis (RDA) (27), the canonical version of principal component analysis (PCA), was used to examine the multiple relationships between soil factors (available N, P, and K and soil pH) and genospecies of soybean rhizobia of 16 sampling sites in the North China Plain. Community data of rhizobia (Table 1) were preanalyzed by detrended correspondence analysis (DCA) using CANOCO software 4.5 (Microcomputer Power, Ithaca, NY) (18); the length of gradient (first axis) was 3.872, so the data were analyzed by RDA.
Correspondence analysis also was performed to estimate the correlation among soybean cultivars and rhizobial genospecies by using SPSS software (PASW statistics 18.0; IBM Corporation). Twenty-four cultivars were treated as 24 separate levels for the cultivar variable, while the nine rhizobial genospecies (except of Bradyrhizobium sp. II) were used as levels for species variable.
Nucleotide sequence accession numbers.
The GenBank database numbers of the sequences determined in the course of this work are listed in Fig. 2 to 4 as described in the figure legends.
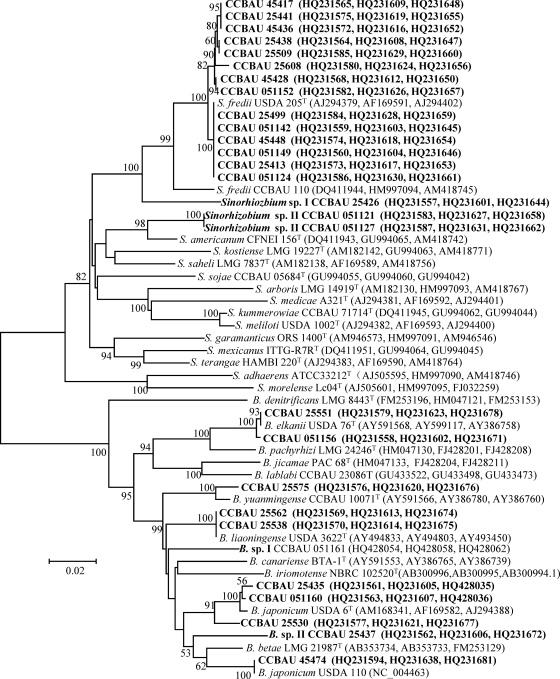
Fig. 2.
Phylogenetic tree of 16S rRNA gene sequences showing the relationships between the representative isolates (in boldface) and the reference strains for defined rhizobial species. GenBank accession numbers in boldface were newly determined as a result of this study. The neighbor-joining (NJ) tree was derived from a 16S rRNA gene sequence distance matrix (Kimura two parameter). Bootstrap confidence levels of ≥50% are indicated at the internodes. The scale bar represents 1% nucleotide substitutions.
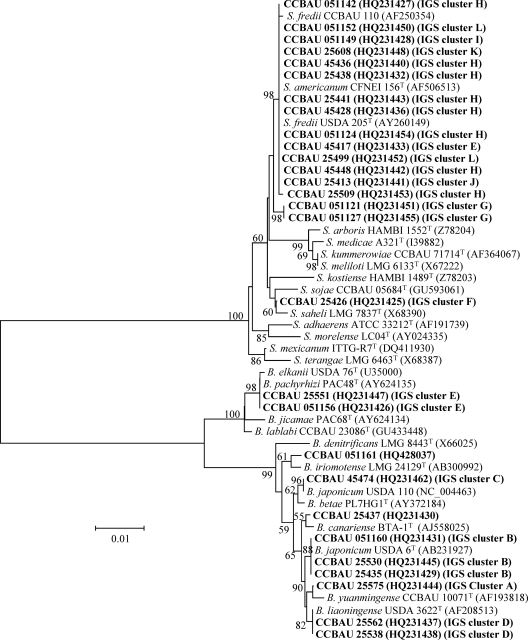
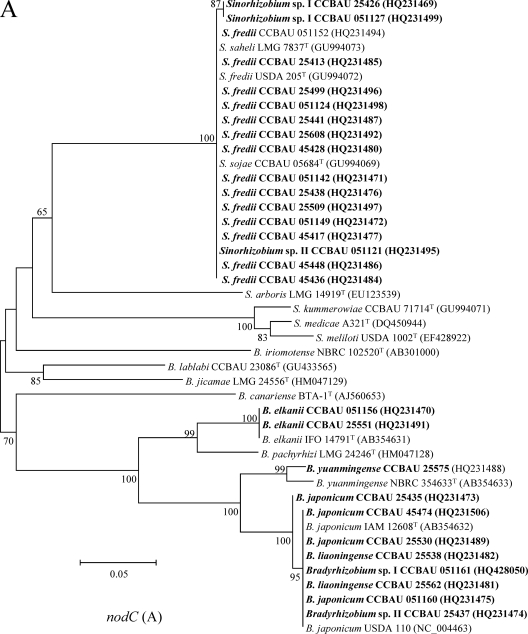
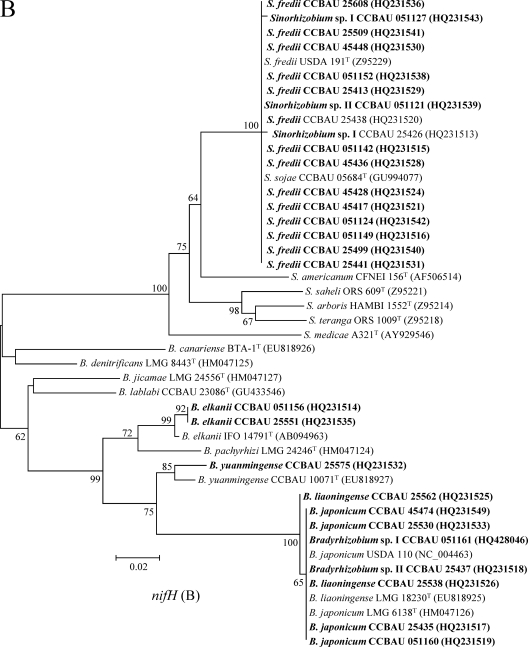
Fig. 4.
Phylogenetic tree of nodC (A) and nifH (B) gene sequences showing the relationships between the representative strains (in boldface) and the related rhizobial species. GenBank accession numbers in boldface were newly determined as a result of this study. The neighbor-joining dendrograms were derived from a sequence distance matrix (Kimura two parameter). Bootstrap confidence levels of ≥50% are indicated at the internodes. Scale bars represent 5 or 2% nucleotide divergence.
RESULTS
Isolation of root nodule bacteria.
A total of 309 pure rhizobial isolates were obtained from 625 soybean nodules, including 229 fast-growing (colonies of ≥2 mm in diameter in 3 to 5 days), acid-producing bacteria and 80 slow-growing (colonies of ≤1 mm in diameter in 7 days), alkali-producing bacteria. All of them formed effective nodules on the soybean cultivar Zhonghuang 13, as evidenced by the red color of nodules and the healthy plants (dark-green leaves). Isolation was not successful for 316 nodules, because (i) no growth was obtained in the nodule juice after 20 days of incubation in several cases, and (ii) in other cases, the isolates did not induce nodules on soybean in the nodulation test and were not included in the subsequent analyses. The numbers of isolates from different sampling sites are shown in Table 1, and detailed information is available in Table S1 in the supplemental material.
Soil characteristics.
The results of soil characterization are presented in Table 1. In general, most of the soil samples were slightly alkaline, with pH 7.67 to 8.16; only two samples, from Luanxian (pH 7.13) and Jiaozhou (pH 6.91), were neutral. The contents of the main mineral nutrients in dry soil were (in mg kg−1) 32.1 to 134.0 for available N, 5.6 to 91.2 for available P, and 54.8 to 597.0 for available K.
RFLP analyses of 16S rRNA gene and IGS.
In the PCR-RFLP analysis of the 16S rRNA gene, five rRNA types (rrs types) were identified among the 309 isolates (see Table S1 in the supplemental material), which were clustered into two groups corresponding to the genera Bradyrhizobium and Sinorhizobium. These rRNA types were identical to those of reference strains for B. yuanmingense (type I, containing 15 isolates), B. japonicum-B. liaoningense (type II, containing 43 isolates), B. elkanii (type III, containing 22 isolates), S. fredii USDA 194 (type IV, containing 24 isolates), and S. fredii USDA 205T (type V, containing 205 isolates). The IGS-RFLP analysis revealed greater genetic diversity and higher resolution among isolates than were obtained by RFLP of 16S rRNA genes. A total of 17 IGS types (patterns) were obtained among the isolates, which were grouped into 12 IGS clusters (A through L) (see Table S1 in the supplemental material) at a similarity level of 94% based on the cluster analysis. Bradyrhizobium comprised five IGS clusters (A through E), and Sinorhizobium contained seven IGS clusters (F to L). Isolates of rRNA type I all were identified as IGS cluster A. Isolates of rRNA type II were divided into three clusters (B, C, and D), which comprised 26, 8, and 9 isolates, respectively. rRNA type III was regarded as IGS cluster E (22 isolates). Isolates of rRNA type IV were divided into IGS clusters F, G, and I, containing 1, 17, and 6 isolates, respectively. rRNA type V was divided into four IGS clusters (H, J, K, and L), which comprised 85, 5, 23, and 92 isolates, respectively (see Table S1).
Phylogeny of 16S rRNA gene and housekeeping genes.
In the phylogenetic tree of the 16S rRNA gene (Fig. 2), 28 isolates representing different IGS clusters were grouped into the genera Bradyrhizobium and Sinorhizobium. The isolates of rRNA type I (IGS cluster A, represented by strain CCBAU 25575) and type III (IGS cluster E, represented by strains CCBAU 25551 and CCBAU 051156) were most similar to B. yuanmingense and B. elkanii, respectively. Isolates of rRNA type II (IGS clusters B, C, and D) were close to B. japonicum, B. liaoningense, B. canariense, and B. iriomotense reference strains. IGS clusters F and G were two lineages related to, but different from, the reference strains for S. fredii and S. saheli-S. sojae, while isolates of IGS clusters H through L were similar to S. fredii.
Based on the MLSA sequence similarities between the isolates and reference strains (Table 2) and the MLSA phylogenetic tree (Fig. 3), 10 genospecies were identified, including S. fredii, Sinorhizobium sp. I and sp. II, B. elkanii, B. japonicum (USDA 6T), B. japonicum Ia (USDA 110), B. yuanmingense, B. liaoningense, and Bradyrhizobium sp. I and sp. II.
Table 2.
Similarities of the housekeeping gene sequences between the new isolates and the reference strains
| Representative strain(s) (CCBAUa no.) | IGS cluster | Closest species | Similarity (%) | Genospecies identified |
|---|---|---|---|---|
| 25575 | A | B. yuanmingense CCBAU 10071T | 98.5 | B. yuanmingense |
| 25435, 051160, 25530 | B | B. japonicum USDA 6T | 97.5∼99.4 | B. japonicum USDA 6T |
| 051161 | B | B. liaoningense USDA 3622T | 94.8 | Bradyrhizobium sp. I |
| 25437 | B | B. liaoningense USDA 3622T | 94.6 | Bradyrhizobium sp. II |
| 45474 | C | B. japonicum USDA 110 | 100 | B. japonicum Ia USDA 110 |
| 25562, 25538 | D | B. liaoningense USDA 3622T | 100 | B. liaoningense USDA 3622T |
| 25551, 051156 | E | B. elkanii USDA 76T | 99.7∼100 | B. elkanii |
| 25426 | F | Sinorhizobiumfredii USDA 205T | 93.6 | Sinorhizobium sp. I |
| 051121, 051127 | G | S. americanum CFNI 156T | 96.4 | Sinorhizobium sp. II |
| 051149 | I | S. fredii USDA 205T | 98.9∼100 | S. fredii |
| 25413 | J | S. fredii USDA 205T | 98.9∼100 | S. fredii |
| 25608 | K | S. fredii USDA 205T | 98.9∼100 | S. fredii |
| 45417,25441, 051152 | L | S. fredii USDA 205T | 98.9∼100 | S. fredii |
| 45436, 25438, 25009, 45428, 25499, 051142, 45448, 051124 | H | S. fredii USDA 205T | 98.9∼100 | S. fredii |
Culture Collection of Beijing Agricultural University.
Fig. 3.
Phylogenetic tree of MLSA based on concatenated sequences of recA (375 nucleotides [nt]), glnII (519 nt), and atpD (359 nt). Taxa and GenBank accesion numbers in boldface were newly determined as a result of this study. Bootstrap confidence levels of ≥50% are indicated at the internodes. The bar indicates 2% nucleotide divergence.
Sequence analyses of symbiotic genes nodC and nifH.
In the phylogenetic tree of nodC genes (Fig. 4A), the representative isolates formed four clades. All of the isolates belonging to Sinorhizobium formed a lineage harboring sequences identical or very similar to those of reference strains for S. fredii, S. sojae, and S. saheli. The representative isolates of Bradyrhizobium genospecies were divided into three clades corresponding to B. elkanii, B. yuanmingense, and B. japonicum-B. liaoningense. These grouping results were consistent in general with the relationships revealed in the MLSA, except for the cases of Bradyrhizobium sp. II CCBAU 25437 and sp. I CCBAU 051161, the representatives for two lineages distinct from B. japonicum and B. liaoningense in MLSA that had a nodC gene identical to those of B. japonicum. The phylogenetic tree of nifH genes (Fig. 4B) showed topology similar to that of nodC genes.
Distribution and diversity of soybean rhizobia in different sampling sites.
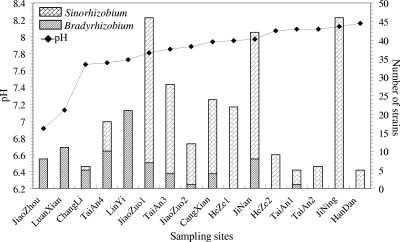
In general, S. fredii is the superdominant species (occupied 68.28% of total isolates), followed by B. elkanii (7.12%), B. japonicum (USDA 6T) (5.83%), Sinorhizobium sp. II (5.50%), and B. yuanmingense (4.85%). The relative abundance for genospecies Sinorhizobium sp. I, B. japonicum Ia (USDA 110), B. liaoningense, and Bradyrhizobium sp. I and sp. II varied from 0.32 to 2.91% (Table 1). All of the isolates from the sites of Jiaozhou, Luanxian, and Linyi belonged to Bradyrhizobium, while isolates from Heze1, Heze2, Taian2, Jining, and Handan all were Sinorhizobium species. Isolates from the other sampling sites comprise both Bradyrhizobium and Sinorhizobium (Table 1 and Fig. 5).
Fig. 5.
Influence of soil pH in different sampling sites of the HHH Plain on the distribution and relative abundance of Sinorhizobium and Bradyrhizobium associated with soybean.
The diversity index (Table 1) of Shannon-Weiner (H′) for Linyi was the highest (1.57), followed by that for Luanxian (1.24) and Taian4 (1.19). The lowest value (0) was found in Heze2, Jining, and Taian2, because only one species was isolated there. The remaining sampling sites had H′ values between 0.87 and 0.18. The values of Simpson's index (D) varied between 0.76 and 0 in the 16 sampling sites and were very consistent with the H′ values. The evenness index, Pielou (J), varied from 0.27 in the case of Heze1 to 1.0 in the case of Jiaozhou. These results demonstrated that the diversity and species composition of soybean rhizobial communities varied dramatically between different sampling sites (Table 1 and Fig. 5).
Correlation among soil characters or soybean cultivars and distribution of soybean rhizobia.
In the DCA to test the models of species response to environmental variables, the length of the gradient (first axis) was 3.872, demonstrating that both the linear model and unimodal model are suitable. After further model tests, redundancy analysis (RDA) proved to be the best method. The results of RDA demonstrated that soil pH and available N explained the largest fraction of variation of soybean rhizobia in the North China Plain (RDA axis 1, 52.1%; P = 0.004). Nearly 61% of the variation in species (P = 0.002) was explained by environmental variables of soil pH and available N, P, and K (Fig. 6). The analysis by Bioenv proved that soil pH was the best subset of environmental variables to explain the distribution of species (Spearman rank correlation coefficient of 0.45), while the climate (subtropical and temperate regions) seems not to contribute to the biogeography of soybean rhizobia in the North China Plain.
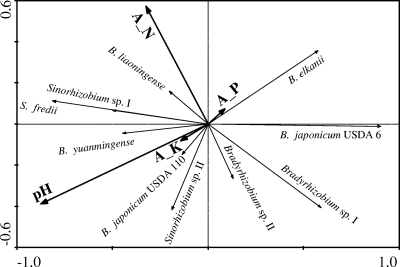
Fig. 6.
Biplot of the RDA on the 10 genospecies and their soil factors from sampling sites in the HHH Plain by CANOCO. A_N, available N; A_P, available P; A_K, available K. Canonical correspondence analyses (CCA) were used to evaluate influence. The longer the arrow is, the greater the influence it has; the smaller the angle is between two arrows, the closer their relationship.
According to the lengths of the arrows and the angles among them (Fig. 6), we could observe that high pH has strong positive correlation with the distribution of S. fredii, Sinorhizobium sp. I, Sinorhizobium sp. II, B. yuanmingense, and B. japonicum group Ia (USDA 110); strong negative correlation with the distribution of B. elkanii and B. japonicum (USDA 6T group); and no significant correlation with the distribution of B. liaoningense, Bradyrhizobium sp. I, and Bradyrhizobium sp. II.
Contents of available K and P had slight effects on the distribution of soybean rhizobia, because the arrows representing them were short. Based upon the direction of the arrows, the effects of K were the same as those of pH, and the effects of P were in contrast to those of pH.
Since the angle between the content of N and pH was almost 90°, these two factors had independent effects on the distribution of soybean rhizobia in the sampling area. According to the results shown in Fig. 6, high nitrogen content in soil was positively correlated with the distribution of S. fredii, Sinorhizobium sp. I, B. liaoningense, and B. yuanmingense and negatively correlated with B. japonicum (USDA 6T), Bradyrhizobium sp. I and sp. II, and Sinorhizobium sp. II, while having almost no effects on B. elkanii and B. japonicum Ia (USDA 110).
In the correlation analysis between the soybean cultivars and genospecies of rhizobia (correspondence figure not shown), only cultivar Handou 5 and Sinorhizobium sp. II had a close correlation. The remaining soybean cultivars and rhizobial genospecies did not show a close relationship, which meant that these soybean cultivars did not select their rhizobial partners strictly. And because only one isolate of Sinorhizobium sp. II was involved in the correlation analysis, the influence of soybean cultivars on rhizobial populations in the North China Plain was not significant.
DISCUSSION
In the present study, the soybean rhizobia were isolated from 16 sites, which represented the main soybean planting areas in the North China Plain. According to the soil characteristics presented in Table 1, the major proportion of the sampling sites had alkaline soil, and only two had neutral soil. This proportion is well reflected in the composition of soil types in the plain, as mentioned in the introduction. Based upon the National Norma of China, soil fertility was divided into six levels (http://www.soil17.com/news_more/1663.html). The soils in the 16 sampling sites covered levels 1 through 5 (very high to low) for available N, levels 1 through 4 for available P, and levels 1 through 5 for available K. These variations among the sampling sites demonstrated that the sampling sites were good representatives for the soil types in the plain.
In the present study, the operational taxonomic units (OTUs) were defined according to the consensus of grouping results in RFLP analyses of 16S rRNA genes and IGS, as well as sequence analyses of 16S rRNA and three housekeeping genes (Fig. 2 and 3), which have been used to distinguish other known diazotrophic organisms (30) and soybean rhizobial species (3). With these methods, 10 genospecies within the genera Sinorhizobium and Bradyrhizobium were defined among the soybean rhizobia (Tables 1 and 2; also see Table S1 in the supplemental material). Most of these genospecies, like S. fredii, B. elkanii, B. japonicum, B. japonicum Ia (USDA 110), B. liaoningense, and B. yuanmingense, have been reported as soybean rhizobia previously (2, 10, 23), but the definition of Bradyrhizobium sp. I and sp. II and Sinorhizobium sp. I and sp. II demonstrated that there may be novel rhizobial species associated with soybean in the North China Plain. The finding of novel soybean rhizobia implies that the diversity of rhizobia is far from fully evaluated, since the soybean rhizobia have been extensively studied in the world.
The community composition of soybean rhizobia in the North China Plain is quite different from those reported in other regions in China (10, 23) and in India (2). The soybean rhizobia in the North China Plain were characterized by the predominance of S. fredii (68.28%) followed by B. elkanii (7.12%) and the other eight genospecies (0.32 to 5.83%). In the subtropical and tropic regions of China, which has acidic soils in general, the most abundant soybean rhizobia was B. japonicum, followed by B. elkanii and several genospecies of Bradyrhizobium (23), while S. fredii was isolated only in some zones (4). In Xinjiang, which has saline alkaline soils, both S. fredii (45%) and B. liaoningense (43%) were the predominant soybean rhizobia, while B. yuanmingense, B. japonicum, and Rhizobium strains were the minor groups. In India, the soybean rhizobia in alkaline soils were Bradyrhizobium spp. (38%), B. yuanmingense (36%), and B. liaoningense (26%). Considering the recently described soybean rhizobial species Sinorhizobium sojae (19), at least 11 species were found in root nodules of soybean grown in the North China Plain. Therefore, the soybean rhizobia in the North China Plain were more diverse than those in other regions, which might be related to the long history of soybean cultivation and the diverse soil conditions in this region.
The diversity of soybean rhizobia in the North China Plain also was revealed by the sequence analyses of symbiotic genes (Fig. 4). As the original center of soybean production, the soybean rhizobia in the North China Plain harbored all the known types of symbiotic genes in soybean rhizobia reported previously (2, 10). The four phylogenetic lineages of symbiotic genes (S. fredii, B. japonicum, B. yuanmingense, and B. elkanii) found in the soybean rhizobia demonstrated again that vertical transfer is the main form to maintain symbiotic genes in soybean rhizobia (10, 23), and that they have divergent origins and have coevolved with chromosome genes. Similarly to previous studies (10, 19), several lateral transfers of symbiotic genes were detected in the present study, because identical nodC and nifH genes were shared by isolates of Sinorhizobium sp. I and sp. II and S. fredii and by Bradyrhizobium sp. I and sp. II and B. japonicum (Fig. 4). Horizontal transfer happened rarely, but it is an important mechanism to form novel species and to improve the biodiversity of soybean rhizobia. These results also demonstrated that the relationships between the symbiotic genes and housekeeping genes in soybean rhizobia are rather stable, although the symbiotic genes only related to their host range (17) and are located on transferable elements (symbiotic plasmids or islands) (35).
The study of the biogeography of plants and animals at continental and local scales started centuries ago, but similar studies of bacteria were impossible at that time (9), because they are too small to see by the naked eye and the definition of bacterial species is difficult. Both these difficulties now have been overcome by the development of microscopy and molecular methods. In the present study, the rhizobial community composition varied in the sampling sites in the North China Plain, as demonstrated by the presence or absence and relative abundance of the rhizobial genospecies (see Table S1 in the supplemental material) and by the diversity indexes (Table 1).
The unique community composition of soybean rhizobia in the major soybean-producing areas of the North China Plain, together with previous studies of soybean rhizobia (10) in Xinjiang in the subtropical regions of China (23) and in alkaline soils in India (2), evidenced the existence of biogeography in soybean rhizobia (Table 1 and Fig. 5). Although analyses by CANOCO (Fig. 6) and R statistical language revealed the content of available N as a main factor correlating to the distribution of B. liaoningense and Bradyrhizobium sp. I and sp. II, more study was needed to verify this correlation, because it was not reported previously.
The soil pH as the main ecological factor to determine the distribution of different soybean rhizobia observed in the present study was similar to findings of previous reports for soybean rhizobia (2, 10) and for other soil bacterial and fungal communities (8, 31). The data reported previously (2, 10, 23) and in the present study demonstrated that (i) B. elkanii and B. japonicum (USDA 6T) were common in acidic and neutral soils, and high pH (>8.0) greatly decreased or eliminated their nodule occupation in fields; (ii) Sinorhizobium species were the predominant soybean rhizobia in saline-alkaline soils; and (iii) B. yuanmingense, B. liaoningense, and B. japonicum Ia (USDA 110) were more resistant to alkaline soils than B. elkanii and B. japonicum (USDA 6T) but may be more sensitive to salinity than the Sinorhizobium spp. (Fig. 5 and 6).
The slight correlation between the content of available P and the distribution of soybean rhizobia was similar to previous observations (10). The slight correlation of K content was not found in the previous study (10), and it is possible that this effect partially reflects the soil salinity, as revealed in the study on soybean rhizobia in Xinjiang (10).
Previously, effects of legume cultivar, including on the diversity and composition of symbiotic bacteria, has been reported, including that for soybean rhizobia (15). During the collection of nodules, at least 24 soybean cultivars were involved, but no apparent difference was found among the rhizobial populations associated with distinct cultivars. S. fredii was isolated from almost all of the cultivars, and only several Bradyrhizobium strains were isolated from them. It seems that the diversity and distribution of soybean rhizobia were determined mainly by the soil characteristics, and effects of cultivars were not important in the samples. This observation might be related to the fact that all of these cultivars were selected to fit the local conditions, especially the saline-alkaline soils, which are different from the laboratory conditions in the study of Israel et al. (15).
In the case of cultivar Yudou 25, the nodules were collected from two sites with different intensities: 37 isolates from Jiaozuo1, including 31 of S. fredii and 6 of Bradyrhizobium spp., and 4 isolates of S. fredii from Jiaozuo2. These results demonstrated that the species richness might be underestimated in some sites when the sample size (number of isolates) was small, since these two sites have similar soil conditions.
In conclusion, 10 genospecies within the genera of Sinorhizobium and Bradyrhizobium, including two novel genospecies in each, were detected from the nodules of soybean grown in the North China Plain, the putative center of origin for soybean in China. The rhizobial community was more diverse than those detected in other regions and were characterized by the absolute predominance of S. fredii, followed by B. elkanii and other Bradyrhizobium and Sinorhizobium species. The biodiversity indexes also indicated a unique distribution and composition of soybean rhizobial communities in this plain. The geographic distribution of these rhizobial species is highly influenced by the soil pH. The present study is the first systematic assessment of G. max microsymbionts in the North China Plain and contributes to clarifying the biogeography of soybean rhizobia, providing a comprehensive illustration of how these species are distributed across the country.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jun Li, Feng Ming Cao, Da Wei Guan, Jun Cheng Zhang, and Qi Lin Zhao for helping in nodule collection.
This work was financed by the Foundation of the State Key Basic Research and Development Plan of China (grant 2010CB126500); the Funds of Commercialization of Agricultural and Scientific Findings, MOST (2008GB23600460); the National Natural Science Foundation of China (project no. 30970004 and 30870004); and funds from SKLAB (2009SKLAB05-1, 2010SKLAB01-1, and 2011SKLAB06-8). E.T.W. is financially supported by grants SIP20100067 and 20110423, authorized by IPN, and PICS08-3, authorized by ICyT DF of Mexico.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 22 July 2011.
REFERENCES
- 1. AFNOR 2005. Soil quality, determination of pH. NF ISO 10390. AFNOR, Paris, France [Google Scholar]
- 2. Appunu C., N′Zoue A., Laguerre G. 2008. Genetic diversity of native bradyrhizobia isolated from soybeans (Glycine max L.) in different agricultural-ecological-climatic regions of India. Appl. Environ. Microbiol. 74:5991–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appunu C., Sasirekha N., Prabavathy V. R., Nair S. 2009. A significant proportion of indigenous rhizobia from India associated with soybean (Glycine max L.) distinctly belong to Bradyrhizobium and Ensifer genera. Biol. Fert. Soils 46:57–63 [Google Scholar]
- 4. Camacho M., et al. 2002. Soils of the Chinese Hubei province show a very high diversity of Sinorhizobium fredii strains. Syst. Appl. Microbiol. 25:592–602 [DOI] [PubMed] [Google Scholar]
- 5. Chen W. L., Huang Q. Y., Xiong X. J. 2004. Distribution and biodiversity of soybean rhizobia in the soils of Shennongjia forest reserve, China. Biol. Fert. Soils 40:306–312 [Google Scholar]
- 6. Chen W. X., Yan G. H., Li J. L. 1988. Numerical taxonomic study of fast-growing soybean rhizobia and a proposal that Rhizobium fredii be assigned to Sinorhizobium gen. nov. Int. J. Syst. Bacteriol. 38:392–397 [Google Scholar]
- 7. Clarke K. R., Ainsworth M. 1993. A method of linking multivariate community structure to environmental variables. Mar. Ecol. Prog. Ser. 92:205–219 [Google Scholar]
- 8. Fernández-Calviño D., Baath E. 2010. Growth response of the bacterial community to pH in soils differing in pH. FEMS Microbiol. Ecol. 73:149–156 [DOI] [PubMed] [Google Scholar]
- 9. Fierer N., Jackson R. B. 2006. The diversity and biogeography of soil bacterial communities. Proc. Natl. Acad. Sci. U. S. A. 103:626–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Han L. L., et al. 2009. Unique community structure and biogeography of soybean rhizobia in the saline-alkaline soils of Xinjiang, China. Plant Soil 324:291–305 [Google Scholar]
- 11. Herridge D. F., Peoples M. B., Boddey R. M. 2008. Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311:1–18 [Google Scholar]
- 12. Hill T. C. J., Walsh K. A., Harris J. A., Moffett B. F. 2003. Using ecological diversity measures with bacterial communities. FEMS Microbiol. Ecol. 43:1–11 [DOI] [PubMed] [Google Scholar]
- 13. Houba V. J. G., Novozamsky I., Huybregts A. W. M., Van der Lee J. 1986. Comparison of soil extractions by 0.01M CaCl 2, by EUF and by some conventional extraction procedures. Plant Soil 96:433–437 [Google Scholar]
- 14. Humphries M. M., Thomas D. W., Speakman J. R. 2002. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 418:313–316 [DOI] [PubMed] [Google Scholar]
- 15. Israel D. W., Mathis J. N., Barbour W. M., Elkan G. H. 1986. Symbiotic effectiveness and host-strain interactions of Rhizobium fredii USDA 191 on different soybean cultivars. Appl. Environ. Microbiol. 51:898–903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Keyser H. H., Bohlool B. B., Hu T. S., Weber D. F. 1982. Fast growing rhizobia isolated from root-nodules of soybean. Science 215:1631–1632 [DOI] [PubMed] [Google Scholar]
- 17. Laguerre G., et al. 2001. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology 147:981–993 [DOI] [PubMed] [Google Scholar]
- 18. Lepš J., Scaronmilauer P. 2003. Multivariate analysis of ecological data using CANOCO. Cambridge University Press, Cambridge, United Kingdom [Google Scholar]
- 19. Li Q. Q., et al. Ensifer (Sinorhizobium) sojae sp. nov., isolated from root nodules of Glycine max grown in saline-alkaline soils in Hebei province of China. Int. J. Syst. Evol. Microbiol., in press [DOI] [PubMed]
- 20. Li Q. Q., et al. 2011. Diversity and biogeography of rhizobia isolated from root nodules of Glycine max grown in Hebei Province, China. Microb. Ecol. 61:917–931 [DOI] [PubMed] [Google Scholar]
- 21. Lie T. A., Gktan D., Engin M., Pijnenborg J., Anlarsal E. 1987. Co-evolution of the legume-rhizobium association. Plant Soil 100:171–181 [Google Scholar]
- 22. Lu Y. L., et al. 2009. Genetic diversity and biogeography of rhizobia associated with Caragana species in three ecological regions of China. Syst. Appl. Microbiol. 32:351–361 [DOI] [PubMed] [Google Scholar]
- 23. Man C. X., et al. 2008. Diverse rhizobia associated with soybean grown in the subtropical and tropical regions of China. Plant Soil 310:77–87 [Google Scholar]
- 24. Minamisawa K., Seki T., Onodera S., Kubota M., Asami T. 1992. Genetic relatedness of Bradyrhizobium japonicum field isolates as revealed by repeated sequences and various other characteristics. Appl. Environ. Microbiol. 58:2832–2839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Normand P., Cournoyer B., Simonet P., Nazaret S. 1992. Analysis of a ribosomal-rna operon in the Actinomycete frankia. Gene 111:119–124 [DOI] [PubMed] [Google Scholar]
- 26. Olsen S. R. 1954. Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA, Washington, DC [Google Scholar]
- 27. Rao C. R. 1964. The use and interpretation of principal component analysis in applied research. Sankhyaá 26:329–358 [Google Scholar]
- 28. Rasolomampianina R., et al. 2005. Nitrogen-fixing nodules from rose wood legume trees (Dalbergia spp.) endemic to Madagascar host seven different genera belonging to alpha and beta proteobacteria. Mol. Ecol. 14:4135–4146 [DOI] [PubMed] [Google Scholar]
- 29. Risal C. P., Yokoyama T., Ohkama-Ohtsu N., Djedidi S., Sekimoto H. 2010. Genetic diversity of native soybean bradyrhizobia from different topographical regions along the southern slopes of the Himalayan Mountains in Nepal. Syst. Appl. Microbiol. 33:416–425 [DOI] [PubMed] [Google Scholar]
- 30. Roesch L. F. W., Fulthorpe R. R., Jaccques R. J. S., Bento F. M., Camargo F. A. D. 2010. Biogeography of diazotrophic bacteria in soils. World J. Microbiol. Biotechnol. 26:1503–1508 [Google Scholar]
- 31. Rousk J., et al. 2010. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 4:1340–1351 [DOI] [PubMed] [Google Scholar]
- 32. Saeki Y., et al. 2005. Phylogenetic analysis of soybean nodulating rhizobia isolated from alkaline soils in Vietnam. Soil Sci. Plant Nutr. 51:1043–1052 [Google Scholar]
- 33. Sarita S., Sharma P. K., Priefer U. B., Prell J. 2005. Direct amplification of rhizobial nodC sequences from soil total DNA and comparison to nodC diversity of root nodule isolates. FEMS Microbiol. Ecol. 54:1–11 [DOI] [PubMed] [Google Scholar]
- 34. Simonis A. D. 1996. Effect of temperature on extraction of phosphorus and potassium from soils by various extracting solutions. Commun. Soil Sci. Plant Anal. 27:665–684 [Google Scholar]
- 35. Sullivan J. T., et al. 2002. Comparative sequence analysis of the symbiosis island of Mesorhizobium loti strain R7A. J. Bacteriol. 184:3086–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tamura K., Dudley J., Nei M., Kumar S. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 37. Tan Z. Y., et al. 2001. Specific detection of Bradyrhizobium and Rhizobium strains colonizing rice (Oryza sativa) roots by 16S-23S ribosomal DNA intergenic spacer-targeted PCR. Appl. Environ. Microbiol. 67:3655–3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Terefework Z., Kaijalainen S., Lindstrm K. 2001. AFLP fingerprinting as a tool to study the genetic diversity of Rhizobium galegae isolated from Galega orientalis and Galega officinalis. J. Biotechnol. 91:169–180 [DOI] [PubMed] [Google Scholar]
- 39. Tian C. F., Wang E. T., Han T. X., Sui X. H., Chen W. X. 2007. Genetic diversity of rhizobia associated with Vicia faba in three ecological regions of China. Arch. Microbiol. 188:273–282 [DOI] [PubMed] [Google Scholar]
- 40. Vauterin L., Vauterin P. 1992. Computer-aided objective comparison of electrophoresis patterns for grouping and identification of microorganisms. Eur. Microbiol. 1:37–41 [Google Scholar]
- 41. Vincent J. M. 1970. A manual for the practical study of the root nodule bacteria. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom [Google Scholar]
- 42. Vinuesa P., et al. 2005. Molecular systematics of rhizobia based on maximum likelihood and Bayesian phylogenies inferred from rrs, atpD, recA and nifH sequences, and their use in the classification of Sesbania microsymbionts from Venezuelan wetlands. Syst. Appl. Microbiol. 28:702–716 [DOI] [PubMed] [Google Scholar]
- 43. Wang E. T., et al. 1998. Rhizobium huautlense sp. nov., a symbiont of Sesbania herbacea that has a close phylogenetic relationship with Rhizobium galegae. Int. J. Syst. Evol. Microbiol. 48:687. [DOI] [PubMed] [Google Scholar]
- 44. Wang Z., Brown J. H., Tang Z., Fang J. 2009. Temperature dependence, spatial scale, and tree species diversity in eastern Asia and North America. Proc. Natl. Acad. Sci. U. S. A. 106:13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weisburg W. G., Barns S. M., Pelletier D. A., Lane D. J. 1991. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 173:697–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu B., Zheng H. Y., Lu J. L., Zhou S. C., Shao R. C. 1984. Protein resources of soybean in China. Soybean Sci. 3:329–331 [Google Scholar]
- 47. Yang J. K., Zhang W. T., Yuan T. Y., Zhou J. C. 2006. Genotypic characteristics of the rrn operon and genome of indigenous soybean bradyrhizobia in cropping zones of China. Can. J. Microbiol. 52:968–976 [DOI] [PubMed] [Google Scholar]
- 48. Yang S. S., et al. 2001. Effect of pH and soybean cultivars on the quantitative analyses of soybean rhizobia populations. J. Biotechnol. 91:243–255 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.