Abstract
The traditional genetic procedure for random or site-specific mutagenesis in Escherichia coli K-12 involves mutagenesis, isolation of mutants, and transduction of the mutation into a clean genetic background. The transduction step reduces the likelihood of complications due to secondary mutations. Though well established, this protocol is not tenable for many pathogenic E. coli strains, such as uropathogenic strain CFT073, because it is resistant to known K-12 transducing bacteriophages, such as P1. CFT073 mutants generated via a technique such as lambda Red mutagenesis may contain unknown secondary mutations. Here we describe the isolation and characterization of transducing bacteriophages for CFT073. Seventy-seven phage isolates were acquired from effluent water samples collected from a wastewater treatment plant in Madison, WI. The phages were differentiated by a host sensitivity-typing scheme with a panel of E. coli strains from the ECOR collection and clinical uropathogenic isolates. We found 49 unique phage isolates. These were then examined for their ability to transduce antibiotic resistance gene insertions at multiple loci between different mutant strains of CFT073. We identified 4 different phages capable of CFT073 generalized transduction. These phages also plaque on the model uropathogenic E. coli strains 536, UTI89, and NU14. The highest-efficiency transducing phage, ΦEB49, was further characterized by DNA sequence analysis, revealing a double-stranded genome 47,180 bp in length and showing similarity to other sequenced phages. When combined with a technique like lambda Red mutagenesis, the newly characterized transducing phages provide a significant development in the genetic tools available for the study of uropathogenic E. coli.
INTRODUCTION
Uropathogenic E. coli (UPEC) is the primary cause of urinary tract infections (UTIs), and treatment of these infections is estimated to cost in excess of 1.6 billion dollars annually in the United States (8, 10). Over 70% of women will contract a UTI in their lifetime, and a significant portion of these women will suffer from recurrent infections (15). Insight into the pathogenesis of UPEC through application of molecular Koch's postulates has led to a solid understanding of many of the factors needed for colonization of the urinary tract (1, 5, 9, 13, 18, 27). These advances in our understanding have come about because UPEC is genetically tractable via homologous recombination methods, such as lambda Red mutagenesis (6, 16, 20). This particular technique uses exogenous expression of the phage lambda genes bet, exo, and gam to induce a hyper-recombinative state within the host cell that aids the replacement of a chromosomal region with an antibiotic resistance cassette (6, 19). Though lambda Red is an efficient method of generating mutants, the hyper-recombinative state renders the host bacterium susceptible to secondary mutations at unknown sites (11, 20). There is a 10-fold increase in the generation of spontaneous mutants attributable to expression of the lambda Red genes (20). To overcome this complication in E. coli K-12, generalized transduction is used to transfer the lambda Red mutation into a clean genetic background. However, many nonlaboratory E. coli strains, including the UPEC strain CFT073, the prototypic UPEC strain (18), are resistant to infection by P1, P22, and other characterized transducing phages. This places a significant limitation on the quality of genetics performed with CFT073 because of the potential presence of unknown mutations that may contribute to fitness, colonization, or virulence phenotypes.
Several techniques have been used to generate or identify novel transducing phages for other bacterial pathogens. In Pseudomonas aeruginosa, temperate phages were isolated from different clinical isolates, and spontaneous lytic variants of these phages that were capable of generalized transduction were isolated (2). Other studies isolated phages from the environment capable of generalized transduction in Bordetella avium, Citrobacter rodentium, Mycobacterium smegmatis, Streptomyces coelicolor, and Serratia marcescens (3, 17, 23, 24, 26).
Here we report the isolation, purification, and physical characterization of a novel CFT073 generalized transducing phage, ΦEB49. The discovery of this phage represents a significant addition to the genetic tools available for UPEC studies.
MATERIALS AND METHODS
Strains.
A list of strains used in this study is provided in Table 1. Kanamycin (Kan) or chloramphenicol (Cm) resistance gene replacement mutations of target genes were generated by the lambda Red method of homologous recombination (6).
Table 1.
Strains used
| Strain | Descriptiona | Source or reference |
|---|---|---|
| Strain | Descriptiona | Source or reference |
| WAM2267 | WT CFT073 | Gift from Harry Mobley |
| WAM2625 | WT E. coli K-12 | Gift from Fred Blattner |
| WAM2909 | CFT073 ΔdsdA::cm | Our laboratory |
| WAM3686 | CFT073 ΔlacZ::kan | Our laboratory |
| WAM3403 | CFT073 Δlrp::kan | Our laboratory |
| WAM4248 | CFT073 ΔcycA::kan | Our laboratory |
| WAM4227 | CFT073 ΔmppA::kan | Our laboratory |
| WAM3847 | CFT073 Δaer::kan | Our laboratory |
| WAM3432 | CFT073 ΔphoA::kan | Our laboratory |
| WAM2039 | UPEC strain 536 | Gift from Werner Goebel |
| WAM1218 | UPEC strain J96 | Gift from Barbara Minshew |
| WAM2645 | UPEC strain NU14 | Gift from Scott Hultgren |
| WAM4054 | UPEC strain UTI89 | Gift from Scott Hultgren |
| WAM3244 | E. coli K1 cystitis clinical isolate | 12 |
| WAM3229 | E. coli K1 cystitis clinical isolate | 12 |
| ECOR 2 | A:ON:H32 | 22 |
| ECOR 5 | A:O79:NM | 22 |
| ECOR 14 | A:OM:HN | 22 |
| ECOR 21 | A:O121:HN | 22 |
| ECOR 27 | B1:O104:NM | 22 |
| ECOR 29 | B1:O150:H21 | 22 |
| ECOR 35 | D:O1:NM | 22 |
| ECOR 36 | D:O79:H25 | 22 |
| ECOR 41 | D:O7:NM | 22 |
| ECOR 47 | D:OM:H18 | 22 |
| ECOR 51 | B2:O25:HN | 22 |
| ECOR 56 | B2:O6:H1 | 22 |
| ECOR 58 | B1:O112:H8 | 22 |
| ECOR 62 | B2:O2:NM | 22 |
| ECOR 64 | B2:O75:NM | 22 |
| ECOR 71 | B1:O78:NM | 22 |
WT, wild type.
Media and growth conditions.
Cultures were grown in Luria-Bertani (LB) broth or on LB agar (Difco, Sparks, MD) at 37°C. Kan (50 μg/ml) and Cm (20 μg/ml) were added when appropriate. Phages were propagated in top agar overlays (L broth with 0.7% [wt/vol] agar) on LB agar plates at room temperature and stored in phage buffer (10 mM Tris-HCl [pH 7.4], 10 mM MgSO4, 0.01% [wt/vol] gelatin) at 4°C.
Isolation and enrichment of bacteriophage.
Primary effluent samples were collected from the Nine Springs wastewater treatment plant in Madison, WI. A 19-ml aliquot from these samples was centrifuged at 600 × g for 10 min to remove large particulate matter and sterilized with a 0.45-μm filter (Millipore). The filtered effluent was concentrated to 150 μl by the use of a low-binding-affinity 30-kDa concentration filter (Amicon). A 50-μl aliquot of the concentrate was mixed with 200 μl of an overnight liquid culture of CFT073 and 4 ml top agar, laid over L-agar plates, and incubated at 37°C overnight. A total of 77 individual plaques were picked from several plates and stored in 1 ml of phage buffer at 4°C.
Titer enrichment.
To increase the titer of each phage isolate, 50 μl of the primary phage stock was mixed with 200 μl of overnight liquid CFT073 culture and 4 ml of top agar and laid over L-agar plates. After overnight incubation at room temperature, the top agar was harvested, suspended in 3 ml of phage buffer containing 6.6% (vol/vol) chloroform, and then shaken vigorously for 2 min. The lysates were centrifuged for 20 min at 600 × g to remove the remnants of soft agar. The supernatants were collected and stored over chloroform at 4°C. Dilution series ranging from 10−1 to 10−12 were generated for each phage stock, and 10 μl of each was spotted on a top agar overlay containing 200 μl of overnight CFT073 culture. Plaques were counted to determine the overall titer of the stock. This process was repeated to generate titers ≥109 PFU/ml.
Phage host sensitivity typing.
In order to eliminate potentially redundant and sibling bacteriophages, each isolate was assayed for its ability to produce plaques on 23 different E. coli strains comprising 16 strains from the ECOR collection (22), UPEC strains NU14, J96, CFT073, 536, and UTI89 and 2 clinical isolates from patients presenting with clinical UTI. A host strain plaquing “fingerprint” was generated for each phage. A hierarchical cluster analysis was performed on the fingerprints, and the results were visualized by generating a dendrogram using the phylogenetic analysis programs Gene Cluster 3.0 and Java TreeView (7, 25).
Test for genetic transduction.
For each unique phage, a high-titer lysate (number of PFU/ml was ≥109) was prepared by growing the phage on the CFT073 mutant strain WAM3686 (ΔlacZ::kan) and harvested as outlined above. A 100-μl aliquot of the high-titer sample was mixed with 500 μl of overnight culture of the recipient CFT073 strain WAM2909 (ΔdsdA::cm) and incubated at room temperature for 20 min. Sodium citrate was then added to a final concentration of 50 mM and then incubated at 37°C for 60 min with shaking. The resulting mixtures were pelleted, resuspended in 100 μl of L broth, plated on L-agar Kan/Cm plates, and incubated overnight at 37°C. Putative transductants were streaked on L-agar Kan/Cm plates and examined for the presence of both antibiotic resistance gene insertions via PCR with primer pairs flanking the specific insertion sites.
Generalized transduction.
High-titer lysates were generated for 5 different Kan resistance gene replacement mutations of target genes located around the CFT073 chromosome using the lysate preparation and transduction conditions described above (Table 1). The Kan gene replacement mutations were transduced into the CFT073 strain WAM2909 (ΔdsdA::cm). Putative transductants were confirmed using PCR and primer pairs flanking the specific gene insertion sites.
MOI determination.
The titer of a high-titer lysate was determined as described above in triplicate. Transductions were then performed at approximate multiplicities of infection (MOIs) ranging from 1 × 10−5 phage/cell to 1 × 102 phage/cell. Aliquots of the liquid culture were plated to determine the number of CFU/ml in each transduction and facilitate precise determination of the actual MOI in each reaction. Transductants were counted after overnight growth at 37°C.
Phage DNA isolation.
DNA was isolated from 12 ml of high-titer (≥109 PFU/ml) lysate from each transducing phage. In order to remove contaminating nucleic acids, the lysate was treated with DNase (1 μg/ml) and RNase (10 μg/ml) for 60 min at 37°C with shaking. The resulting samples were passed through a 0.45-μm filter; PEG-8000 (10% [wt/vol]) and NaCl (1 M final) were added and incubated at 4°C overnight. Samples were then centrifuged at 600 × g for 20 min. The supernatant was removed, and the resulting pellet was suspended in 1 ml of phage buffer. The pellet was extracted twice with 1 volume of chloroform. An equal volume of phenol was added to the sample, mixed by inversion, and centrifuged at 20,000 × g for 3 min. The aqueous phase was removed and placed in a clean microcentrifuge tube. This step was repeated twice, once with a 1:1 mixture of phenol and chloroform and again with a 24:1 mixture of chloroform and isoamyl alcohol. Phage DNA was precipitated by adding a one-third volume of 3 M NaOAc and 1 ml of ice-cold 100% ethanol. After incubating the DNA on ice for 10 min, the DNA was pelleted by centrifugation for 2 min at 20,000 × g. The DNA pellet was washed twice with 70% ethanol, dried with a Vacufuge Plus (Eppendorf) at 60°C for 15 min, and suspended in Tris-EDTA (TE) buffer, pH 8.0.
Genome sequencing.
Purified DNA for ΦEB49 was sequenced using an Illumina GAIIx sequencer. A 36-cycle single-direction reaction and de novo assembly were performed at the UW Biotechnology Center's Next Generation Sequencing Facility. The remaining sequence gaps were closed by generating PCR products using primers specific to the regions flanking the gaps and dideoxy-chain termination sequencing.
Electron microscopy (EM).
Phage samples were processed by the UW Medical School Electron Microscope Facility with the two-step negative-staining method using Nano-W (Nanoprobes Inc.) on pioloform-coated Ni thin-bar 300-mesh grids. The stained samples were viewed with a Philips CM120 at 80 kV and documented using an SIS (Olympus Soft Imaging Solutions) MegaView III digital camera.
RESULTS AND DISCUSSION
Phage isolation and typing.
In order to identify potential transducing phages for UPEC, we collected primary effluent samples from the Nine Springs wastewater treatment plant in Madison, WI, a likely environmental source of E. coli-infecting phages. To isolate the phage within the samples, the large particulates and bacteria were removed with centrifugation and subsequent filter sterilization. The filtrate was then concentrated approximately 100-fold, and this material was used to infect CFT073. We isolated phage from 77 separate plaques, and these were labeled as isolates EB1 to EB77.
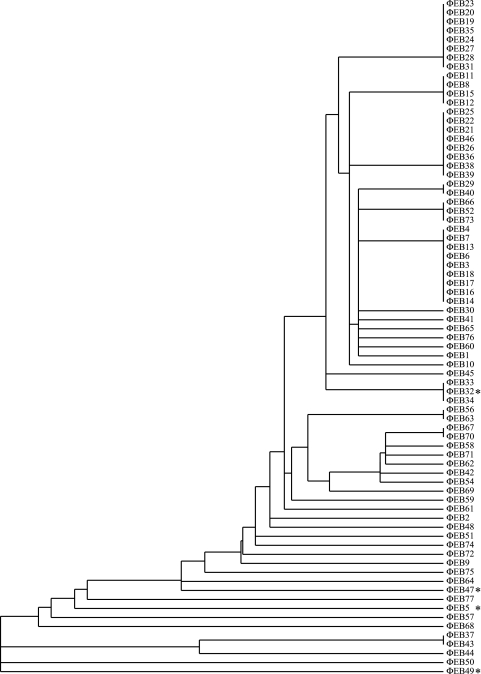
To avoid processing and testing closely related or sibling phages, a typing scheme was devised based on E. coli host range determination. A host range fingerprint was generated for each phage by testing its ability to plaque on 23 different E. coli strains. The strains included 16 evolutionarily divergent members from the ECOR collection (22), 5 laboratory UPEC strains, and 2 clinical isolates from patients with diagnosed UTIs (Table 1). An evolutionarily broad spectrum was chosen to decrease the likelihood of redundant phage types. A cluster analysis was performed on the fingerprint data to identify phages with identical host strain patterns by the use of the program Gene Cluster 3.0, and a dendrogram was constructed to visualize the clustering by the use of the program Java TreeView (7, 25) (Fig. 1). The clustering analysis identified, in addition to unique isolates, 15 phage clusters, each with identical infection patterns. For these groups, a single representative was chosen at random, and the remaining members were excluded from further evaluation. The final group consisted of 49 apparently unique phage isolates.
Fig. 1.
Dendrogram of the 77 phage isolates generated from a hierarchical cluster analysis of the plaque fingerprints of each phage on 23 different E. coli strains. Clusters represent groups of phage with identical host plaque patterns. The starred isolates are capable of generalized transduction in CFT073.
Identification of UPEC transducing phages.
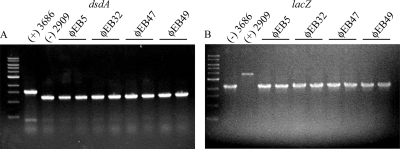
Each of the 49 phage isolates was examined for its ability to transduce a single antibiotic resistance marker. The phages were individually grown on the CFT073 donor strain WAM3686 (ΔlacZ::kan), and high-titer lysates were incubated in the presence of the recipient CFT073 strain WAM2909 (ΔdsdA::cm). Any phages capable of packaging and transducing the ΔlacZ::kan insertion into the recipient strain would result in a doubly resistant ΔlacZ::kan ΔdsdA::cm strain. No colonies were found on plates inoculated with 100 μl of the high-titer (>109-PFU/ml) lysates, indicating that the lysates were bacteria free. Of the 49 phages tested, ΦEB5, ΦEB32, ΦEB47, and ΦEB49 were able to transduce the Kan marker into WAM2909, and the proper integration of both antibiotic cassettes was confirmed by PCR (Fig. 2).
Fig. 2.
Colony PCR with dsdA-specific (A) and lacZ-specific (B) primers on transductants from each phage in duplicate. WAM3686 (ΔdsdA::kan) and WAM2909 (ΔlacZ::cm) were included as controls.
To determine if the 4 phages were capable of generalized transduction, a series of strains with Kan gene replacement mutations of target genes positioned throughout the CFT073 chromosome were selected from our laboratory stocks, and we attempted to transduce these different markers into WAM2909. With the exception of ΦEB5, each phage was capable of packaging and transducing the different mutations (Table 2). Additionally, ΦEB49 consistently generated the greatest number of transductants. An empirical examination of transduction efficiency, with no further optimization of the reaction conditions, indicated the following order of transduction efficiency from highest to lowest: ΦEB49 > ΦEB47 > ΦEB32 > ΦEB5 (data not shown).
Table 2.
Test for generalized transductiona
| Mutation | CFT073 gene location | ΦEB5 | ΦEB32 | ΦEB47 | ΦEB49 |
|---|---|---|---|---|---|
| lacZ | 448924 | + | + | + | + |
| phoA | 475723 | − | + | + | + |
| lrp | 986723 | + | + | + | + |
| mppA | 1636492 | + | + | + | + |
| aer | 3658151 | + | + | + | + |
| cycA | 5046982 | + | + | + | + |
Ability to perform generalized transduction shown as “+” for yes and “−” for no.
UPEC host range.
The plaquing patterns used for the isolate typing doubled as host range determinations for the 4 transducing phages. Of particular interest is the ability to plaque on prototrophic E. coli K-12 strain MG1655 and classic-model UPEC strains (Table 3). None of the four CFT073 transducing phages were able to plaque on E. coli K-12, though each was able to plaque on at least one other well-studied UPEC strain, including UTI89, NU14, and 536. Their ability to infect other UPEC strains suggests that they could be used to transduce mutations between strains, and interstrain transductions were successful between CFT073 and 536 with ΦEB49 (data not shown). Further optimization of transduction conditions in order to move genetic markers among different strains is the subject of further work in our laboratory.
Table 3.
UPEC host rangea
| Strain | ΦEB5 | ΦEB32 | ΦEB47 | ΦEB49 |
|---|---|---|---|---|
| CFT073 (WAM2267) | + | + | + | + |
| UTI89 (WAM4054) | + | + | + | − |
| NU14 (WAM2645) | − | + | + | − |
| J96 (WAM1218) | − | − | − | − |
| 536 (WAM2039) | − | − | + | + |
| MG1655 (WAM2625) | − | − | − | − |
Plaquing ability shown as “+” for plaques observed and “−” for no plaques observed.
Phage morphology.
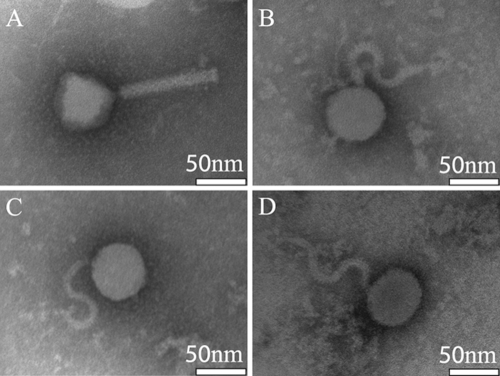
In order to further characterize the 4 CFT073 transducing phages, their morphology was determined by transmission electron microscopy (Fig. 3). ΦEB47, ΦEB32, and ΦEB5 are structurally similar, sharing a rounded head and flexible S-shaped noncontractile tails characteristic of the Siphoviridae family. The spiral tail morphology could be an artifact of EM sample preparation; however, similar structures were observed on a previously characterized phage, CVX-5, isolated from a patient with colitis (4). ΦEB49 has an icosahedral head and straight tail stalk, classifying it with the Myoviridae. Individual tails and heads were often observed, suggesting either mechanical shearing during preparation or incomplete assembly. All 4 phage have heads approximately 50 nm in diameter, suggesting that they have similar genome sizes, which was later confirmed by pulsed-field gel electrophoresis (data not shown).
Fig. 3.
Electron micrographs of the 4 confirmed transducing phages. (A) ΦEB49; (B) ΦEB5; (C) ΦEB32; (D) ΦEB47.
Transduction optimization.
Due to its enhanced transducing efficiency, ΦEB49 was selected for further optimization and characterization. The protocol developed to search for putative transducers (MOI equal to approximately 1), yielded on average 101 to 102 transductants per reaction with ΦEB49 (data not shown). Though this efficiency was above our limit of detection, we explored a range of MOIs to further increase transduction efficiency, and MOIs ranging between 10−5 and 102 phages/cell were evaluated. We could not significantly increase the efficiency by varying the MOI, but we did identify a suitable range. An MOI between 0.1 and 3 is optimal for ΦEB49 transduction of CFT073 (data not shown).
ΦEB49 temperature-dependent lysis and possible lysogen formation.
The addition of high-titer phage stocks to liquid cultures of CFT073 did not cause complete lysis of the bacterial suspension. Additionally, lytic activity of ΦEB49 is inhibited during growth at 37°C in solid and liquid media (data not shown). Temperature dependence on selection of a lytic versus lysogenic pathway has been characterized for phage lambda and Listeria phages in which colder temperatures block lysis (14, 21). However, ΦEB49 shows the opposite effect, increasing lytic activity at lower temperatures. The temperature dependence may be a result of a defect in phage adsorption or a shift in preference to a lysogenic phase, though it is not clear if ΦEB49 forms lysogens.
The lack of complete lysis and temperature effects suggested the possibility that ΦEB49 is capable of lysogeny. To examine if transduced strains were free of ΦEB49, the supernatants of liquid cultures from primary, secondary, and tertiary reisolations of ΦEB49 transductants were examined for the ability to produce plaques on CFT073. The primary isolates of transduced strains were found to produce plaques by plating on soft agar overlays, but plaques could not be generated in secondary and tertiary isolations (data not shown). In addition, southern hybridizations performed on isolated genomic DNA from secondary and tertiary isolates using probes specific to ΦEB49 were negative, indicating that infectious phage and stable lysogens are not evident past the second passage of the transductant (data not shown).
ΦEB49 genome sequence.
The ΦEB49 genome was sequenced in order to further characterize its genetic potential and relatedness to previously described bacteriophages. ΦEB49 DNA was sequenced using an Illumina GAIIx sequencer, generating 3.1 × 107 36-bp reads. A de novo assembly of the raw sequence data produced 7 contigs ranging from 16,000× to 22,000× coverage and totaling 42 kb. Pulsed-field gel electrophoresis of the uncut DNA suggested a genome size of 48 kb, so the gap regions were examined to identify the missing sequence information. The contigs were first oriented relative to one another based on apparent sequence similarity to the phage JK06 (GenBank no. DQ121662), and adjacent contigs were joined with PCR using primers specific to the ends of the gap regions. An additional 5 kb of sequence was found in the various gaps, resulting in a complete genome of 47,180 bp (GenBank no. JF770475). The sequencing and PCR data suggest that the ΦEB49 genome is circularly permutated, and restriction digestion indicates it is composed of double-stranded DNA (data not shown). The completed sequence was annotated with DNA Master (http://cobamide2.bio.pitt.edu/), which predicted the location of 74 putative open reading frames (ORFs) and 2 tRNAs (Fig. 4). BLAST searches of the predicted ORFs showed sequence homology to the enteric bacteriophages JK06 and Rtp (29) (see Table S1 in the supplemental material). Predicted ORFs in ΦEB49 were identified with high sequence identity to tail fiber structural and assembly components (70.4% to 99.5% identical), a prohead protease (96.7% identical), a putative portal protein (98.5% identical), and several hypothetical ORFs from JK06, Rtp, and other sequenced phages. Other predicted ORFs showed limited sequence identity to 4 putative endonucleases (59.1% to 63.6% identical) and a putative DNA primase (74.9% identical). Additionally, 8 predicted ORFs appear specific to ΦEB49, showing no significant similarity to any of the queried GenBank sequences. CFT073 is known to contain components of remnant phages (28). However, the ΦEB49 genome shares no significant similarity with the published CFT073 genome sequence, suggesting that ΦEB49 did not originate from the CFT073 isolate.
Fig. 4.
Annotated map of the 47,180-bp ΦEB49 genome. The computer-based genome annotation software DNAMaster (http://cobamide2.bio.pitt.edu/) predicted the location of 74 ORFs and 2 tRNAs.
Up until our work reported here, the genetic tools available for pathogenic E. coli limited the relative quality of the genetics performed in CFT073 and other UPEC strains. We successfully identified a novel generalized transducing phage for the model UPEC strain CFT073. ΦEB49 is a generalized transducing phage capable of high-efficiency transduction among CFT073 strains and greatly reduces the complications of unknown secondary site mutations generated when performing lambda Red mutagenesis. With this phage available, we feel the standard of genetic analyses in CFT073 has been raised significantly.
Supplementary Material
Acknowledgements
This research was funded by the National Institutes of Health Grant R01 DK063250-07.
We thank Randal Massey at the UW Medical School Electron Microscope Facility for his assistance with the electron microscopy, Eric Cabot at the UW Biotechnology Center for his help with the genome sequencing and assembly, and Jason Flowers for his help in collecting the wastewater effluent samples.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 22 July 2011.
REFERENCES
- 1. Bahrani-Mougeot F. K., et al. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45:1079–1093 [DOI] [PubMed] [Google Scholar]
- 2. Budzik J. M., Rosche W. A., Rietsch A., O'Toole G. A. 2004. Isolation and characterization of a generalized transducing phage for Pseudomonas aeruginosa strains PAO1 and PA14. J. Bacteriol. 186:3270–3273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burke J., Schneider D., Westpheling J. 2001. Generalized transduction in Streptomyces coelicolor. Proc. Natl. Acad. Sci. U. S. A. 98:6289–6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chandra K., Kidwai J. R., Gupta B. M. 1979. Morphology, serology and biochemical characters of phage CVX-5, isolated from a patient with colitis. Folia Microbiol. (Praha) 24:334–338 [DOI] [PubMed] [Google Scholar]
- 5. Connell I., et al. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. U. S. A. 93:9827–9832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Datsenko K. A., Wanner B. L. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Hoon M. J., Imoto S., Nolan J., Miyano S. 2004. Open source clustering software. Bioinformatics 20:1453–1454 [DOI] [PubMed] [Google Scholar]
- 8. Foxman B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 113(Suppl. 1A):5S–13S [DOI] [PubMed] [Google Scholar]
- 9. Guyer D. M., Radulovic S., Jones F. E., Mobley H. L. 2002. Sat, the secreted autotransporter toxin of uropathogenic Escherichia coli is a vacuolating cytotoxin for bladder and kidney epithelial cells. Infect. Immun. 70:4539–4546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Haley R. W., et al. 1985. The efficacy of infection survillance and control programs in preventing nosocomial infections in US hospitals. Am. J. Epidemiol. 121:182–205 [DOI] [PubMed] [Google Scholar]
- 11. Hobman J. L., et al. 2007. Comparative genomic hybridization detects secondary chromosomal deletions in Escherichia coli K-12 MG1655 mutants and highlights instability in the flhDC region. J. Bacteriol. 189:8786–8792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson J. R., et al. 2001. Clonal relationships and extended virulence genotypes among Escherichia coli isolates from women with a first or recurrent episode of cystitis. J. Infect. Dis. 183:1508–1517 [DOI] [PubMed] [Google Scholar]
- 13. Keith B. R., Maurer L., Spears P. A., Orndorff P. E. 1986. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect. Immun. 53:693–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim J. W., Kathariou S. 2009. Temperature-dependent phage resistance of Listeria monocytogenes epidemic clone II. Appl. Environ. Microbiol. 75:2433–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kunin C. M. 1994. Urinary tract infections in females. Clin. Infect. Dis. 18:1–10,11-12 [DOI] [PubMed] [Google Scholar]
- 16. Lee D. J., et al. 2009. Gene doctoring: a method for recombineering in laboratory and pathogenic Escherichia coli strains. BMC Microbiol. 9:252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee S., et al. 2004. Bxz1, a new generalized transducing phage for mycobacteria. FEMS Microbiol. Lett. 241:271–276 [DOI] [PubMed] [Google Scholar]
- 18. Mobley H. L., et al. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Murphy K. C. 1998. Use of bacteriophage lambda recombination functions to promote gene replacement in Escherichia coli. J. Bacteriol. 180:2063–2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy K. C., Campellone K. G. 2003. Lambda Red-mediated recombinogenic engineering of enterohemorrhagic and enteropathogenic E. coli. BMC Mol. Biol. 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nash H. A. 1981. Integration and excision of bacteriophage lambda: the mechanism of conservation site specific recombination. Annu. Rev. Genet. 15:143–167 [DOI] [PubMed] [Google Scholar]
- 22. Ochman H., Selander R. K. 1984. Standard reference strains of Escherichia coli from natural populations. J. Bacteriol. 157:690–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petty N. K., Foulds I. J., Pradel E., Ewbank J. J., Salmond G. P. 2006. A generalized transducing phage (phiIF3) for the genomically sequenced Serratia marcescens strain Db11: a tool for functional genomics of an opportunistic human pathogen. Microbiology 152:1701–1708 [DOI] [PubMed] [Google Scholar]
- 24. Petty N. K., et al. 2007. A generalized transducing phage for the murine pathogen Citrobacter rodentium. Microbiology 153:2984–2988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saldanha A. J. 2004. Java Treeview-extensible visualization of microarray data. Bioinformatics 20:3246–3428 [DOI] [PubMed] [Google Scholar]
- 26. Shelton C. B., et al. 2000. Discovery, purification, and characterization of a temperate transducing bacteriophage for Bordetella avium. J. Bacteriol. 182:6130–6136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Torres A. G., Redford P., Welch R. A., Payne S. M. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69:6179–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Welch R. A., et al. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 99:17020–17024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wietzorrek A., Schwarz H., Herrmann C., Braun V. 2006. The genome of the novel phage Rtp, with a rosette-like tail tip, is homologous to the genome of phage T1. J. Bacteriol. 188:1419–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.