Abstract
We report the construction of a series of replicating shuttle vectors that consist of a low-copy-number cloning vector for Escherichia coli and functional components of the origin of replication (oriC) of the chromosome of the hyperthermophilic archaeon Pyrococcus furiosus. In the process of identifying the minimum replication origin sequence required for autonomous plasmid replication in P. furiosus, we discovered that several features of the origin predicted by bioinformatic analysis and in vitro binding studies were not essential for stable autonomous plasmid replication. A minimum region required to promote plasmid DNA replication was identified, and plasmids based on this sequence readily transformed P. furiosus. The plasmids replicated autonomously and existed in a single copy. In contrast to shuttle vectors based on a plasmid from the closely related hyperthermophile Pyrococcus abyssi for use in P. furiosus, plasmids based on the P. furiosus chromosomal origin were structurally unchanged after transformation and were stable without selection for more than 100 generations.
INTRODUCTION
Pyrococcus species are hyperthermophilic marine archaea that grow anaerobically at temperatures near and above 100°C (19). Their interesting biology, evolutionary history, and potential commercial utility make them an important group to study (9, 47). We recently reported an efficient method for DNA transformation for Pyrococcus furiosus (27), which provides the basis for the development of methods for genetic manipulation. A markerless deletion of the orotidine monophosphate (OMP) decarboxylase (pyrF) locus was constructed in the P. furiosus genome, generating a mutant that is a uracil auxotroph and resistant to 5-fluroorotic acid (5FOA). Complementation by the wild-type pyrF allele restored uracil prototrophy and 5FOA sensitivity to this strain. We took advantage of this marker to construct replicating shuttle vectors for use in P. furiosus.
Replicating vectors facilitate a variety of genetic manipulations, and vectors capable of shuttling between any host of interest and Escherichia coli, perhaps the most convenient biological host for DNA manipulation, are especially useful. Naturally occurring plasmids have been identified in archaeal species (7, 10–12, 16, 22, 51), and some of them have been used to develop shuttle vectors in the haloarchaea (25) and methanogens (49). A shuttle vector between E. coli and Thermococcus kodakarensis, a close relative of P. furiosus (4, 36), was reported, which combined a commercial E. coli cloning vector with a naturally occurring plasmid, pTN1, from Thermococcus nautilus (44, 46). This plasmid was used successfully to express a hemagglutinin (HA) epitope-tagged version of RpoL, a subunit of T. kodakarensis RNA polymerase, illustrating the utility of such vectors (44). Plasmid pGT5, a naturally occurring plasmid in Pyrococcus abyssi (17), was used to create a shuttle vector capable of replicating in both P. abyssi and E. coli (29), and most recently, a shuttle vector based on pGT5 was used to express a gene encoding His6-tagged subunit D of RNA polymerase in P. furiosus (50). Our attempts to construct a stable shuttle vector for P. furiosus based on pGT5 were unsuccessful.
Pyrococcus species are predicted to have a single origin of replication with both bacterial and eukaryotic features (28, 37). Other archaea (5, 30, 42) have multiple replication origins, and eukaryotes have hundreds or even thousands of replication origins (41). Eukaryotic replication origins are poorly defined in terms of sequence as well as the number and nature of auxiliary proteins that facilitate their function, and there is increasing evidence that origin maintenance in many eukaryotes is controlled by epigenetic factors whose function is also poorly understood (3, 45). Saccharomyces cerevisiae is a notable exception in that it has well-defined origins called autonomously replicating sequence (ARS) elements, generally 100 to 200 bp in length, containing multiple cis-acting sequence elements (38) and binding sites for the origin recognition complex (6). This complex is composed of origin recognition complex (Orc) proteins 1 to 6 (6) and a DNA binding protein, Cdc6, first identified in yeast as a gene whose product is involved in cell cycle control (15, 23). Most bacteria have a single origin sequence flanked by polymerases and dnaA (35), which encodes a protein that binds DNA within the origin (20) and functions to recruit DnaB and DnaC to form the replication initiation complex. While DnaA is only distantly related to Cdc6, it provides the same function in nucleating the protein complex. Replication origin regions in both bacteria and archaea are AT rich (5, 41).
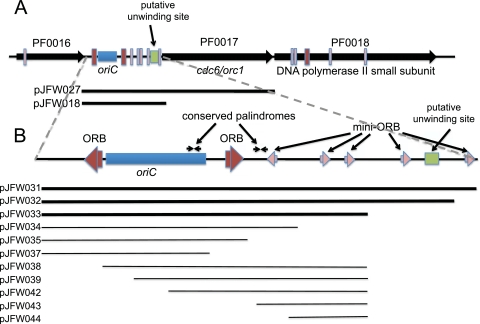
The origin of replication in several archaeal genera was first predicted by the cumulative GC skew, an early bioinformatic method used to find prokaryotic replication origins (21). In P. abyssi the location of the chromosomal replication origin was predicted to be within an ∼80-kb region that contained a large intergenic space and genes for several putative replication proteins (28). This intergenic space, neighboring genes, and features are conserved in P. furiosus (Fig. 1). While the organization of putative protein binding sites and replicative proteins around the P. furiosus oriC is bacterial in nature, the proteins that likely bind the sequences are eukaryotic. In the location where dnaA often resides in bacteria, there is a single gene homologous to the cdc6/orc1 eukaryotic replication proteins (40). Previous studies of the function of the origin sequences of Halobacterium sp. strain NRC-1 in vivo showed that a 750-bp region containing oriC requires the adjacent orc7 gene in cis for autonomous plasmid replication (8). In Sulfolobus solfataricus, there are three origins of replication (30, 42), and for each origin of replication, a cdc6 gene is adjacent but is not required in cis for the origin to function in plasmid replication (14). Evidence for the location of the P. furiosus origin and for the role of putative replication proteins is based entirely on bioinformatic and in vitro analyses. Cells emerging from replication arrest were shown previously to incorporate radiolabeled nucleotides in the DNA sequence at the predicted origin (37). Chromatin immunoprecipitation of the Cdc6/Orc1 protein showed a significant enrichment of the predicted oriC DNA region (32), and DNA binding studies showed that the Sulfolobus Cdc6/Orc1 protein binds this region of P. abyssi as well as Sulfolobus DNA in vitro (33, 42). The sequence of the sites of protein binding (referred to as origin recognition boxes [ORBs]) suggested that inverted repeat elements flanking the predicted origin, conserved among archaea, were involved in replication complex formation and binding. The binding of the Cdc6/Orc1 protein to the intergenic space resulted in an unwinding of the DNA, supporting the notion that this was the location of the origin (34). While the intergenic space is AT rich, the DNA sequence is not conserved across Pyrococcus species. Further evidence for the position of the origin comes from the fact that DNA at the origin contains a transient replication bubble produced by bidirectional DNA polymerization, and the structure may be isolated by displaying digested total genomic DNA, from actively dividing cells, on two-dimensional gels. Sequences containing a replication bubble were located to a 1-kb fragment that included the intergenic space, possibly overlapping the cdc6 gene (37). Bioinformatic analyses identified several 13-bp mini-ORB repeats in and around the intergenic space suggested previously to be involved in origin recognition and potential Cdc6/Orc1 binding (26, 33).
Fig. 1.
Region of the P. furiosus chromosome predicted to contain the origin of replication, oriC (A), with an expanded view of the intergenic space (B). ORBs are indicated in red, mini-ORBS are shown in pink, and the putative unwinding site is shown in green. The chromosomal regions that were cloned into pJFW017 to produce various plasmids are indicated by black lines below the diagrams. Conserved palindromes are marked by inverted black arrows. Inserts that resulted in plasmids capable of autonomous replication in P. furiosus are indicated by thick black lines.
Here we present an in vivo analysis of sequences at the origin of DNA replication in the P. furiosus chromosome and their function in autonomous plasmid replication. DNA fragments containing the putative chromosomal origin as well as predicted protein binding sites and the cdc6/orc1 gene were cloned into an E. coli plasmid vector and tested for the ability to promote autonomous DNA replication in P. furiosus. The cloning of the origin sequence without the cdc6/orc1 gene did not affect its ability to function, suggesting that this protein, while likely required for DNA replication, is not required in cis. The location of the origin predicted from in vitro analyses was confirmed. Only two of the predicted ORB sequences, however, are required for autonomous plasmid replication. We used this origin sequence in combination with the pSC101 origin from Salmonella enterica serovar Panama (13) for replication in E. coli to construct a replicating shuttle vector for P. furiosus that is stable in a single copy without selection for more than 100 generations and is structurally unchanged after transformation into P. furiosus and back-transformation into E. coli.
MATERIALS AND METHODS
Strains, media, and growth conditions.
E. coli strain DH5α was used for plasmid DNA constructions and preparations. Standard techniques for E. coli were performed as described previously (43). Apramycin was used for selection at 50 mg/ml. Wild-type strain P. furiosus DSM 3638 (19) and the P. furiosus COM1 ΔpyrF strain (27) were grown anaerobically in a defined medium with cellobiose as the carbon source (27) at 90°C for 16 to 20 h in 100-ml serum bottles containing 50 ml of liquid medium or on medium solidified with Phytagel (1%, wt/vol; Sigma) for 60 h. The P. furiosus COM1 ΔpyrF strain was used as a host for all DNA transformation experiments. P. abyssi strain GE5 (16, 18) was grown in a liquid base salts medium (1) containing 0.5% (wt/vol) casein hydrolysate and 0.2% (wt/vol) elemental sulfur for 40 to 48 h at 90°C under anaerobic conditions. Total genomic DNA was isolated as described previously (27), except that DNA was precipitated with isopropanol and resuspended with 50 μl TE buffer (10 mM Tris, 1 mM EDTA) containing RNase A (100 ng/ml).
Construction of vectors and transformation of P. furiosus.
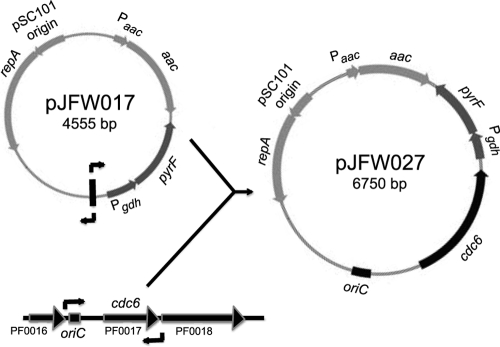
To construct pJFW027 and pJFW018, PCR products containing the indicated regions of the chromosome (Fig. 1) were ligated into a linear DNA fragment containing the entire pJFW017 plasmid (Fig. 2), also generated by PCR using primers JF266 and JF267. To generate plasmids pJFW031 to pJFW044, primers with restriction sites added to the 5′ ends were used to allow the digestion and subsequent directional cloning of origin-containing fragments into pJFW017. The 5′ end of each fragment contained a BamHI site, and the 3′ end contained a ClaI site. The PCR amplification of pJFW017 was done by use of primers JF266.2 and JF267.2 with the same restriction sites. Primers used in these constructions are listed in Table 1, and DNA sequences of the primers are shown in Table S1 in the supplemental material. E. coli strain DH5α cells were transformed by electroporation in a 2-mm-gap cuvette at 2.5 V. Plasmid DNA was isolated from liquid cultures by using QIAprep Spin Miniprep columns (Qiagen Inc.). For DNA transformations, the P. furiosus COM1 ΔpyrF strain was grown for 16 to 20 h in defined liquid medium containing 20 μM uracil. Plasmid DNA (100 to 200 ng) was added to 100 μl of culture and plated onto the defined medium without uracil. Prototrophic colonies were inoculated into liquid medium for DNA isolation. The presence of plasmid sequences in P. furiosus was confirmed by PCR amplification of the aac gene, present only on the plasmid, from P. furiosus total genomic DNA by using primers JF263 and JF264 (Table 1).
Fig. 2.
Construction of pJFW027. A linear DNA fragment containing the entire sequence of pJFW017 was generated by PCR amplification using primers JF266 and JF267 and ligated into the origin fragment indicated in Fig. 1, also generated by PCR amplification using primers JF268 and JF282. Plasmids containing the various origin fragments described in the legend of Fig. 1 were cloned into pJFW017 for testing.
Table 1.
Plasmid transformation efficienciesa
| Plasmid | oriC insert position | 5′ primer | 3′ primer | Transformation efficiency (no. of transformants/μg of plasmid DNA) |
|---|---|---|---|---|
| pJFW017 | None | 8.2 × 102 | ||
| pJFW018 | 15382–16226 | JF268 | JF269 | 9.1 × 105 |
| pJFW027 | 15382–17576 | JF268 | JF282 | 5.8 × 105 |
| pJFW031 | 15382–16228 | JF268.2 | JF269.2 | 6.6 × 105 |
| pJFW032 | 15382–16187 | JF306.2 | JF269.2 | 8.0 × 104 |
| pJFW033 | 15382–16034 | JF305.2 | JF269.2 | 6.5 × 105 |
| pJFW034 | 15382–15890 | JF304.2 | JF269.2 | 5.7 × 102 |
| pJFW035 | 15382–15786 | JF303.2 | JF269.2 | 1.4 × 103 |
| pJFW037 | 15382–15705 | JF301.1 | JF269.2 | 1.4 × 102 |
| pJFW038 | 15492–16034 | JF305.2 | JF339 | 4.7 × 101 |
| pJFW039 | 15561–16034 | JF305.2 | JF345 | <4.7 × 101 |
| pJFW042 | 15746–16034 | JF305.2 | JF348 | <4.7 × 101 |
| pJFW043 | 15813–16034 | JF305.2 | JF349 | <4.7 × 101 |
Genomic locations are based on the numbering convention of the Pyrococcus furiosus (accession number NC_003413.1) genome sequence deposited in GenBank. The detection threshold was 4.7 × 102 transformants per μg of plasmid DNA.
Assessment of plasmid maintenance, stability, and copy number.
To assess plasmid maintenance, P. furiosus transformants were serially subcultured every 24 h for 10 days in selective and nonselective liquid media. After each passage, the culture was diluted 100-fold with base salts, and 30 μl of diluted culture was plated onto selective medium to determine the number of prototrophic colonies, i.e., those maintaining the plasmid. The cell density of the liquid culture was determined by direct cell counting using a Petroff-Hausser counting chamber. To assess the structural stability of the plasmid, total genomic DNA isolated from five independent P. furiosus transformants containing pJFW027 was used to back-transform E. coli for plasmid isolation and restriction digestion analysis. To determine plasmid copy numbers, total genomic DNA was isolated from P. furiosus plasmid transformants and digested twice with 10 U of HpaI for 120 min at 37°C. The restriction fragments were separated by electrophoresis in a 1.0% (wt/vol) agarose gel and transferred onto nylon membranes (Roche, Manheim, Germany). Primers GL021 and GL023 (27) were used to amplify the glutamate dehydrogenase (gdh) promoter from wild-type P. furiosus total genomic DNA to generate a digoxigenin (DIG)-labeled probe by random priming with DIG High Prime DNA Labeling and Detection Starter Kit I (Roche, Manheim, Germany). The membrane was incubated at 42°C and washed at 65°C. Band intensities were determined by using a Storm 840 PhosporImager (GE Healthcare) equipped with ImageQuant v.5.4 software (Molecular Dynamics).
RESULTS AND DISCUSSION
The P. furiosus chromosomal replication origin functions for stable autonomous plasmid replication.
Attempts to construct a stable replicating shuttle vector based on plasmid pGT5 from P. abyssi for use in P. furiosus were unsuccessful. Plasmids based on pGT5 exist in high copy numbers in P. abyssi (29, 50) but show a significantly reduced copy number in P. furiosus (50) and cannot be used for the selection of transformants in the closely related Thermococcus kodakarensis (44). In an attempt to construct a shuttle vector based on pGT5, the entire plasmid was cloned into pJFW017 that contained a pSC101 origin for replication in E. coli, an apramycin resistance gene for selection in E. coli, and a wild-type copy of the P. furiosus pyrF gene for the selection of transformants in the P. furiosus COM ΔpyrF strain. A fragment containing the entire pGT5 plasmid sequence was produced by PCR amplification with primers JF254 and JF270, linearizing the plasmid at a site within pGT5 previously shown not to interfere with replication functions (17, 31), to produce pJFW019 (see Fig. S1 in the supplemental material). This plasmid readily transformed P. furiosus but was rapidly lost without selection (Table 2) and showed internal deletions after transformation into P. furiosus and subsequent back-transformation into E. coli (Fig. S2). Other attempts to use pGT5 for the construction of shuttle vectors in T. kodakarensis were similarly unsuccessful (44).
Table 2.
Maintenance of plasmids in P. furiosusa
| Passage | No. of cells |
|||||
|---|---|---|---|---|---|---|
| pJFW018 |
pJFW027 |
pJFW019 |
||||
| +ura | −ura | +ura | −ura | +ura | −ura | |
| 1 | 187 | 256 | 190 | 194 | 73 | 112 |
| 2 | 132 | 217 | 97 | 203 | 54 | 268 |
| 3 | 112 | 232 | 132 | 143 | 11 | 138 |
| 4 | 146 | 117 | 154 | 165 | 6 | 83 |
| 5 | 87 | 276 | 100 | 113 | 0 | 77 |
| 6 | 138 | 197 | 151 | 201 | 0 | 104 |
| 7 | 144 | 263 | 111 | 122 | 0 | 91 |
| 8 | 118 | 242 | 87 | 131 | 0 | 94 |
| 9 | 93 | 213 | 114 | 218 | 0 | 97 |
| 10 | 107 | 183 | 112 | 169 | 0 | 87 |
Transformants containing each plasmid were serially passaged in liquid medium with uracil (+ura) or without uracil (−ura). Following each passage, a diluted culture was plated onto selective medium to determine the number of prototrophic cells remaining.
To test whether the predicted P. furiosus chromosomal origin of replication could promote stable autonomous plasmid replication, a fragment of the chromosome containing the predicted origin sequence and the gene encoding Cdc6/Orc1 (Fig. 1) was cloned into an E. coli plasmid, pJFW017 (Fig. 2), to make pJFW027. We used the transformation efficiency as an assay for plasmid replication (8). As shown in Table 1, transformants of pJFW027 were observed at a frequency of 5.8 × 105 transformants per μg of plasmid DNA. No transformants were observed in the absence of added plasmid DNA, and while some transformants were obtained in experiments with pJFW017, which does not contain an origin sequence (8.2 × 102 transformants per μg of plasmid DNA), this is most likely due to integration by homologous recombination between the gdh promoter region (283 bp), driving the transcription of the pyrF gene on the plasmid, and the gdh locus in the chromosome. In fact, we have observed the integration of nonreplicating plasmid DNA by homologous recombination at the same frequency (27). The transformation frequency of pJFW027 was a thousandfold greater than that of pJFW017, indicating that the plasmid was replicating autonomously. PCR amplification of the apramycin resistance gene, contained only on the plasmids, was used to confirm the presence of plasmid DNA in the transformants. A 950-bp product containing this sequence was obtained from transformant total genomic DNA but not from the wild-type or the P. furiosus COM1 ΔpyrF strain (see Fig. S3 in the supplemental material).
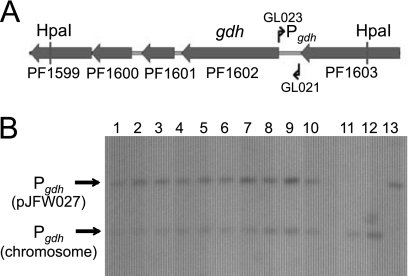
Attempts to isolate a significant quantity of pJFW027 plasmid DNA from P. furiosus were unsuccessful. This is perhaps not surprising, since quantities of plasmids based on the chromosomal origin would be expected to be low or 1 copy per chromosome. In lieu of direct plasmid isolation, we chose to rescue the plasmid by back-transformation to E. coli. That transformants contained a replicating copy of the plasmid was shown by using total genomic DNA isolated from 5 independent plasmid transformants to back-transform E. coli strain DH5α selecting for apramycin resistance. Back-transformants were obtained at frequencies of 104 transformants per μg of DNA, an underestimate of the plasmid transformation, since this frequency is based on the number of transformants per microgram of total genomic DNA, and only covalently closed circular plasmid DNA is capable of transforming E. coli strain DH5α at this frequency (24). Plasmid DNA isolated from these back-transformants was indistinguishable from the pJFW027 plasmid DNA used to transform P. furiosus by using restriction digestion analysis, indicating that there were no gross rearrangements during transformation and replication in P. furiosus or subsequent back-transformation to E. coli. When the Pgdh fragment, specific to plasmid pJFW027, was used as a probe for the Southern hybridization of total genomic DNA from the P. furiosus transformants with DNA digested with either EcoRV (data not shown) or HpaI (Fig. 3), which have a single cleavage site within the plasmid, a single band was detected, showing that the plasmid DNA was not integrated into the chromosome and existed as an autonomously replicating molecule.
Fig. 3.
Determination of copy number for pJFW027 in P. furiosus. (A) Diagram of the chromosomal region, including the gdh open reading frame. HpaI sites are indicated, as are the locations of primers used to generate the gdh hybridization probe. (B) Southern blot of pJFW027 transformants. Lanes 1 to 10, DNA isolated from transformants and digested with HpaI; lanes 11 and 12, DNAs from P. furiosus wild-type and COM1 ΔpyrF strains, respectively; lane 13, pJFW027 plasmid DNA purified from E. coli.
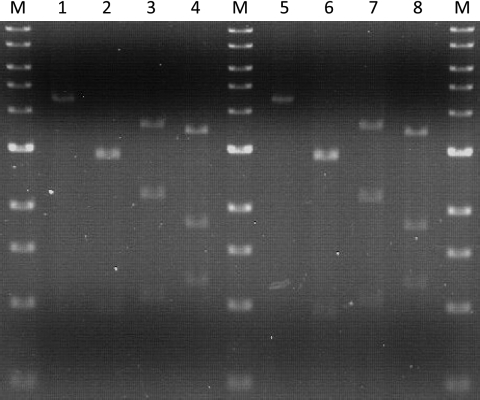
To examine plasmid maintenance, transformants of pJFW027 and pJFW018 were serially subcultured in liquid medium with or without selection (i.e., in the absence or presence of uracil) for more than 100 generations and then plated onto minimal medium without uracil to assay plasmid maintenance. No loss of plasmids with oriC was detected even without selection (Table 2). In addition, the restriction pattern of plasmid DNA isolated from E. coli after transformation into P. furiosus and subsequent transformation back into E. coli remained unchanged, indicating that no rearrangements of the plasmid DNA occurred (Fig. 4 [data for 1 of 10 transformants tested are shown]).
Fig. 4.
Restriction analysis of plasmid DNA before and after transformation of P. furiosus and back-transformation to E. coli. Lanes M, 1-kb DNA ladder; lanes 1 to 4, pJFW018 plasmid DNA isolated from E. coli DH5α (lane 1) and digested with AccI (lane 2), AvaI (lane 3), and HindIII (lane 4); lanes 5 to 8, plasmid DNA isolated from E. coli DH5α back-transformed from P. furiosus transformants (lane 5) and digested with AccI (lane 6), AvaI (lane 7), and HindIII (lane 8).
The cdc6/orc1 open reading frame is not required in cis for replication origin function.
In bacteria, oriC is often, but not always, adjacent to dnaA. In E. coli, oriC is between gidA and mioC (which encodes another replication protein), approximately 43 kb from dnaA, and can function for the autonomous replication of plasmids without cis-acting replicating components (39, 48). In the chromosome of Halobacterium NRC-1, oriC requires the adjacent orc7 gene in cis for autonomous plasmid replication (8). In Sulfolobus solfataricus, there are three origins of replication, and for each origin of replication, a cdc6 gene is adjacent but is not required in cis for the origin to function in autonomous plasmid replication (14). In the sequenced Pyrococcus species P. furiosus, P. abyssi, and P. horikoshii as well as the closely related T. kodakarensis, there is a single oriC adjacent to a cdc6/orc1 homologue, but nothing is known about the requirement of this protein for oriC function. To test whether cdc6/orc1 was required in cis for autonomous plasmid replication in P. furiosus, a fragment containing only oriC was cloned into parent plasmid pJFW017 to generate pJFW18 (Fig. 1). As shown in Table 1, plasmids containing the fragment with only the oriC sequence without the cdc6/orc1 gene transformed P. furiosus as efficiently and were maintained as stably as plasmid pJFW27 carrying the cdc6/orc1 gene, suggesting that the cdc6/orc1 gene is not required in cis for stable autonomous plasmid replication.
Only two of the predicted ORB sequences and part of the predicted chromosomal origin sequence are required for plasmid replication.
The origin region was predicted previously to contain several ORB and mini-ORB sequences (33), suggested to be binding sites for the replication initiation protein Cdc6/Orc1, which is presumed to facilitate the nucleation of the replication complex. Our analysis using the BLASTN 2.2.24+ algorithm (2) identified three ORB repeats and several mini-ORB repeats by the self-alignment of the sequence of the genomic region containing oriC and neighboring genes. These results are similar but not identical to those described previously by Matsunaga et al. (33), in that we found a clustering of mini-ORB repeats in and around oriC, but the exact number and position of these mini-ORB repeats were different. In addition, we identified two conserved palindromic sequences (Table 3) conserved in all sequenced Pyrococcus species. One of them contains compensating changes within the sequence that retain the perfect palindromic structure, suggesting that these are not random sequences within this highly repetitive region of DNA and may potentially be binding sites for other replication proteins or have a structural role in replication. These palindromes are not present in the oriC region of the closely related Thermococcus species, however, suggesting that if they have a function, it may be specific to Pyrococcus. To test whether these sequences were required for autonomous plasmid replication, plasmids containing various portions of the region around the predicted origin were constructed and tested for the ability to replicate. The smallest insert able to promote autonomous plasmid replication was the 653-bp fragment cloned into pJFW033. As shown in Table 1, only two of the three ORB sequences, and only a part of the sequence predicted to contain the origin, were required for plasmid replication. The predicted unwinding site, for example, is apparently not required for autonomous plasmid replication.
Table 3.
Conserved palindromic sequences within the Pyrococcus oriC regiona
| Species (GenBank accession no.) | Sequence | Genomic position |
|---|---|---|
| Palindrome 1 | ||
| P. furiosus (NC_003413.1) | ATATTTAAATAT | 15641–15674 |
| P. abyssi (NC_000868.1) | TATTTAAATA | 123223–123232 |
| P. horikoshii (NC_000961.1) | TATTTAAATA | 111307–111316 |
| Palindrome 2 | ||
| P. furiosus (NC_003413.1) | ATTAgaTTAAtcTAAT | 15809–15824 |
| P. abyssi (NC_000868.1) | ATTAagTTAAccTAAT | 123072–123087 |
| P. horikoshii (NC_000961.1) | ATTAagTTAActTAAT | 111155–111170 |
Base differences are indicated by lowercase type; underlining indicates a base that deviates from the palindrome consensus. Genomic locations are based on the numbering of the genome sequences deposited in GenBank.
Replicating shuttle vectors based on the chromosomal origin exist in single copies.
To determine the approximate copy number of the oriC-based plasmids, a PCR product generated from the Pgdh promoter was used as a probe in Southern hybridization experiments with total genomic DNA from P. furiosus wild-type cells and pJFW027 transformants. Since Pgdh is present in one copy both on pJFW027 and in the P. furiosus chromosome, a densitometry analysis of the amount of DIG-labeled probe hybridized to each one allowed an estimation of the number of plasmid copies per chromosome (Fig. 3). The relative intensities of the plasmid-derived hybridization signal to the chromosomally derived hybridization signal of Pgdh for the EcoRV and HpaI digests ranged from 1.4 to 1.8 for 10 transformants tested, indicating that the oriC-based plasmids exist in a single copy per chromosome.
Conclusions.
The functional analysis of the replication origin of the P. furiosus chromosome reported here showed that only two of the three ORB sequences, those flanking an AT-rich sequence most conserved in arrangement and sequence among the Archaea (42), and no more than three of the mini-ORB sites are required for autonomous plasmid replication. In particular, the DNA-unwinding site, predicted by P1 endonuclease assays (34), is not required for autonomous plasmid replication, nor are any of the predicted ORB or mini-ORB sequences within the DNA polymerase small-subunit open reading frame. We emphasize that we have not ruled out the possibility that these sequences are important for chromosomal replication and that they may serve to promote additional Cdc6/Orc1 binding for chromosomal replication initiation. The open reading frame encoding the Cdc6/Orc1 protein present adjacent to the predicted origin sequence is not required in cis for autonomous plasmid replication. Vectors based on P. furiosus oriC were stably maintained for more than 100 generations without selection and showed no evidence of rearrangement after replication and transformation between E. coli and P. furiosus. The smallest oriC fragment identified in this study capable of conferring autonomous replication was 653 bp in length, and vectors based on the origin exist in a single copy per chromosome in the cell. Two conserved short palindromes were identified within the origin region that are conserved among Pyrococcus species but not in the closely related species Thermococcus kodakarensis, suggesting that if they have a function, it may be specific to Pyrococcus species. We anticipate that these vectors will have utility for homologous and heterologous gene expression, as well as providing a tool for the study of natural competence, and in vivo studies of replication and recombination in P. furiosus.
Supplementary Material
ACKNOWLEDGMENTS
We are especially grateful to Gina Lipscomb and Karen Stirrett for discussions and advice throughout the course of this work and to Sidney Kushner for guidance in the design of experiments testing DNA replication. We also thank Jennifer Huddleston for critical review of the manuscript.
This work was supported by a grant to M.W.W.A. and J.W. from the BioEnergy Science Center (DE-PS02-06ER64304), administered by the Oak Ridge National Laboratory, and by the Office of Biological and Environmental Research (FG02-08ER64690) in the DOE Office of Science.
Footnotes
Supplemental material for this article may be found at http://aem.asm.org/.
Published ahead of print on 22 July 2011.
REFERENCES
- 1. Adams M. W. W., et al. 2001. Key role for sulfur in peptide metabolism and in regulation of three hydrogenases in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 183:716–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Altschul S. F., et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antequera F. 2004. Genomic specification and epigenetic regulation of eukaryotic DNA replication origins. EMBO J. 23:4365–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atomi H., Fukui T., Kanai T., Morikawa M., Imanaka T. 2004. Description of Thermococcus kodakaraensis sp. nov., a well studied hyperthermophilic archaeon previously reported as Pyrococcus sp. KOD1. Archaea 1:263–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barry E. R., Bell S. D. 2006. DNA replication in the Archaea. Microbiol. Mol. Biol. Rev. 70:876–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bell S. P., Stillman B. 1992. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature 357:128–134 [DOI] [PubMed] [Google Scholar]
- 7. Benbouzid-Rollet N., et al. 1997. Isolation of new plasmids from hyperthermophilic Archaea of the order Thermococcales. Res. Microbiol. 148:767–775 [DOI] [PubMed] [Google Scholar]
- 8. Berquist B. R., DasSarma S. 2003. An archaeal chromosomal autonomously replicating sequence element from an extreme halophile, Halobacterium sp. strain NRC-1. J. Bacteriol. 185:5959–5966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blumer-Schuette S. E., Kataeva I., Westpheling J., Adams M. W. W., Kelly R. M. 2008. Extremely thermophilic microorganisms for biomass conversion: status and prospects. Curr. Opin. Biotechnol. 19:210–217 [DOI] [PubMed] [Google Scholar]
- 10. Bokranz M., Klein A., Meile L. 1990. Complete nucleotide sequence of plasmid pME2001 of Methanobacterium thermoautotrophicum (Marburg). Nucleic Acids Res. 18:363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Charbonnier F., Erauso G., Barbeyron T., Prieur D., Forterre P. 1992. Evidence that a plasmid from a hyperthermophilic archaebacterium is relaxed at physiological temperatures. J. Bacteriol. 174:6103–6108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Charlebois R. L., Lam W. L., Cline S. W., Doolittle W. F. 1987. Characterization of pHV2 from Halobacterium volcanii and its use in demonstrating transformation of an archaebacterium. Proc. Natl. Acad. Sci. U. S. A. 84:8530–8534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cohen S. N., Chang A. C., Boyer H. W., Helling R. B. 1973. Construction of biologically functional bacterial plasmids in vitro. Proc. Natl. Acad. Sci. U. S. A. 70:3240–3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Contursi P., et al. 2004. Identification and autonomous replication capability of a chromosomal replication origin from the archaeon Sulfolobus solfataricus. Extremophiles 8:385–391 [DOI] [PubMed] [Google Scholar]
- 15. Culotti J., Hartwell L. H. 1971. Genetic control of the cell division cycle in yeast. 3. Seven genes controlling nuclear division. Exp. Cell Res. 67:389–401 [DOI] [PubMed] [Google Scholar]
- 16. Erauso G., Charbonnier F., Barbeyron T., Forterre P., Prieur D. 1992. Preliminary characterization of a hyperthermophilic archaebacterium with a plasmid, isolated from a north Fiji basin hydrothermal vent. C. R. Acad. Sci. 314:387–393 [Google Scholar]
- 17. Erauso G., et al. 1996. Sequence of plasmid pGT5 from the archaeon Pyrococcus abyssi: evidence for rolling-circle replication in a hyperthermophile. J. Bacteriol. 178:3232–3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Erauso G., et al. 1993. Pyrococcus abyssi sp. nov., a new hyperthermophilic archaeon isolated from a deep-sea hydrothermal vent. Arch. Microbiol. 160:338–349 [Google Scholar]
- 19. Fiala G., Stetter K. O. 1986. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 145:56–61 [Google Scholar]
- 20. Fuller R. S., Funnell B. E., Kornberg A. 1984. The DnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell 38:889–900 [DOI] [PubMed] [Google Scholar]
- 21. Grigoriev A. 1998. Analyzing genomes with cumulative skew diagrams. Nucleic Acids Res. 26:2286–2290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hall M. J., Hackett N. R. 1989. DNA sequence of a small plasmid from Halobacterium strain GN101. Nucleic Acids Res. 17:10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hartwell L. H., Mortimer R. K., Culotti J., Culotti M. 1973. Genetic control of the cell division cycle in yeast. V. Genetic analysis of cdc mutants. Genetics 74:267–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hoekstra W. P., Bergmans J. E., Zuidweg E. M. 1980. Role of recBC nuclease in Escherichia coli transformation. J. Bacteriol. 143:1031–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lam W. L., Doolittle W. F. 1989. Shuttle vectors for the archaebacterium Halobacterium volcanii. Proc. Natl. Acad. Sci. U. S. A. 86:5478–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z., Santangelo T. J., Cubonova L., Reeve J. N., Kelman Z. 26 October 2010. Affinity purification of an archaeal DNA replication protein network. mBio 1(5):pii=e00221-10. doi:10.1128/mBio.e00221-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lipscomb G. L., et al. 2011. Natural competence in the hyperthermophilic archaeon Pyrococcus furiosus facilitates genetic manipulation: construction of multiple markerless deletions of genes encoding the two cytoplasmic hydrogenases. Appl. Environ. Microbiol. 77:2232–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lopez P., Philippe H., Myllykallio H., Forterre P. 1999. Identification of putative chromosomal origins of replication in Archaea. Mol. Microbiol. 32:883–886 [DOI] [PubMed] [Google Scholar]
- 29. Lucas S., et al. 2002. Construction of a shuttle vector for and spheroplast transformation of the hyperthermophilic archaeon Pyrococcus abyssi. Appl. Environ. Microbiol. 68:5528–5536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lundgren M., Andersson A., Chen L., Nilsson P., Bernander R. 2004. Three replication origins in Sulfolobus species: synchronous initiation of chromosome replication and asynchronous termination. Proc. Natl. Acad. Sci. U. S. A. 101:7046–7051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marsin S., Forterre P. 1998. A rolling circle replication initiator protein with a nucleotidyl-transferase activity encoded by the plasmid pGT5 from the hyperthermophilic archaeon Pyrococcus abyssi. Mol. Microbiol. 27:1183–1192 [DOI] [PubMed] [Google Scholar]
- 32. Matsunaga F., Forterre P., Ishino Y., Myllykallio H. 2001. In vivo interactions of archaeal Cdc6/Orc1 and minichromosome maintenance proteins with the replication origin. Proc. Natl. Acad. Sci. U. S. A. 98:11152–11157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Matsunaga F., et al. 2007. Genomewide and biochemical analyses of DNA-binding activity of Cdc6/Orc1 and Mcm proteins in Pyrococcus sp. Nucleic Acids Res. 35:3214–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Matsunaga F., et al. 2010. Localized melting of duplex DNA by Cdc6/Orc1 at the DNA replication origin in the hyperthermophilic archaeon Pyrococcus furiosus. Extremophiles 14:21–31 [DOI] [PubMed] [Google Scholar]
- 35. Messer W. 2002. The bacterial replication initiator DnaA. DnaA and oriC, the bacterial mode to initiate DNA replication. FEMS Microbiol. Rev. 26:355–374 [DOI] [PubMed] [Google Scholar]
- 36. Morikawa M., Izawa Y., Rashid N., Hoaki T., Imanaka T. 1994. Purification and characterization of a thermostable thiol protease from a newly isolated hyperthermophilic Pyrococcus sp. Appl. Environ. Microbiol. 60:4559–4566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Myllykallio H., et al. 2000. Bacterial mode of replication with eukaryotic-like machinery in a hyperthermophilic archaeon. Science 288:2212–2215 [DOI] [PubMed] [Google Scholar]
- 38. Newlon C. S., Theis J. F. 2002. DNA replication joins the revolution: whole-genome views of DNA replication in budding yeast. Bioessays 24:300–304 [DOI] [PubMed] [Google Scholar]
- 39. Oka A., Sugimoto K., Takanami M., Hirota Y. 1980. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol. Gen. Genet. 178:9–20 [DOI] [PubMed] [Google Scholar]
- 40. Robb F. T., et al. 2001. Genomic sequence of hyperthermophile, Pyrococcus furiosus: implications for physiology and enzymology. Methods Enzymol. 330:134–157 [DOI] [PubMed] [Google Scholar]
- 41. Robinson N. P., Bell S. D. 2005. Origins of DNA replication in the three domains of life. FEBS J. 272:3757–3766 [DOI] [PubMed] [Google Scholar]
- 42. Robinson N. P., et al. 2004. Identification of two origins of replication in the single chromosome of the archaeon Sulfolobus solfataricus. Cell 116:25–38 [DOI] [PubMed] [Google Scholar]
- 43. Sambrook J., Russell D. W. 2006. The condensed protocols from molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 44. Santangelo T. J., Cubonova L., Reeve J. N. 2008. Shuttle vector expression in Thermococcus kodakaraensis: contributions of cis elements to protein synthesis in a hyperthermophilic archaeon. Appl. Environ. Microbiol. 74:3099–3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schwob E. 2004. Flexibility and governance in eukaryotic DNA replication. Curr. Opin. Microbiol. 7:680–690 [DOI] [PubMed] [Google Scholar]
- 46. Soler N., et al. 2007. The rolling-circle plasmid pTN1 from the hyperthermophilic archaeon Thermococcus nautilus. Mol. Microbiol. 66:357–370 [DOI] [PubMed] [Google Scholar]
- 47. Stetter K. O. 1996. Hyperthermophilic procaryotes. FEMS Microbiol. Rev. 18:149–158 [Google Scholar]
- 48. Sugimoto K., et al. 1978. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc. Natl. Acad. Sci. U. S. A. 76:575–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tumbula D. L., Bowen T. L., Whitman W. B. 1997. Characterization of pURB500 from the archaeon Methanococcus maripaludis and construction of a shuttle vector. J. Bacteriol. 179:2976–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Waege I., Schmid G., Thumann S., Thomm M., Hausner W. 2010. Shuttle vector-based transformation system for Pyrococcus furiosus. Appl. Environ. Microbiol. 76:3308–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zillig W., et al. 1994. Screening for Sulfolobales, their plasmids and their viruses in Icelandic solfataras. Syst. Appl. Microbiol. 16:609–628 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.