Abstract
Polycystic kidney disease (PKD) exhibits an inflammatory component, but the contribution of inflammation to cyst progression is unknown. Macrophages promote the proliferation of tubular cells following ischemic injury, suggesting that they may have a role in cystogenesis. Furthermore, cultured Pkd1-deficient cells express the macrophage chemoattractants Mcp1 and Cxcl16 and stimulate macrophage migration. Here, in orthologous models of both PKD1 and PKD2, abnormally large numbers of alternatively activated macrophages surrounded the cysts. To determine whether pericystic macrophages contribute to the proliferation of cyst-lining cells, we depleted phagocytic cells from Pkd1fl/fl;Pkhd1-Cre mice by treating with liposomal clodronate from postnatal day 10 until day 24. Compared with vehicle-treated controls, macrophage-depleted mice had a significantly lower cystic index, reduced proliferation of cyst-lining cells, better-preserved renal parenchyma, and improved renal function. In conclusion, these data suggest that macrophages home to cystic areas and contribute to cyst growth. Interruption of these homing and proliferative signals could have therapeutic potential for PKD.
Automsomal dominant polycystic kidney disease (ADPKD) is one of the most common genetic disorders in humans.1,2 It is characterized by progressive growth of kidney cysts and atrophy of the adjacent noncystic tubules, leading to progressive loss of renal function. Mutations in PKD1 or PKD2, encoding polycystin-1 (PC1) and polycystin-2 (PC2), respectively, account for the vast majority of cases of ADPKD. Mutation or absence of these proteins can result in altered cell signaling that culminates in abnormal cell proliferation and loss of normal tubular architecture, accompanied by loss of cell polarity, increased fluid secretion, and tubular atrophy.1,3,4 These phenotypic abnormalities have become the target of newer therapies aimed at retarding cyst progression.3
Studies over the past two decades have indicated the presence of an inflammatory component in humans with PKD as well as rodent models of the disease. For example, proinflammatory cytokines such as IL-1β, TNF-alpha (TNF-α), and IL-2 are found in cyst fluid samples from human kidneys.5 Zheng et al. reported the presence of monocyte chemoattractant protein-1 (MCP1) in urine of ADPKD patients,7 and, more recently, Grantham and colleagues demonstrated that high levels of urinary MCP-1 strongly correlate with the rate of subsequent cyst growth.8 Consistent with this, Cowley et al. reported increased expression of Mcp1, accompanied by accumulation of ED-1 positive macrophages, in the kidneys of Han:SPRD polycystic rats, a nonorthologous model of ADPKD.9 Furthermore, pathway analysis of microarray data by Mrug and co-workers demonstrated that genes involved in the innate immune response were the most significantly upregulated genes in severely cystic cpk mice, a nonorthologous model of autosomal recessive PKD (ARPKD).10 Included in this innate response activation were multiple macrophage-associated factors, specifically genes such as Arg1, Ccl17, and Il-10 that are expressed by alternatively activated macrophages.
A growing number of studies indicate an important role of macrophages in kidney injury and tissue repair,11 and there has been substantial interest in the possibility that renal injury might accelerate progression of PKD.12,13 Our laboratory has recently demonstrated that following renal ischemia-reperfusion, macrophages home to the kidney and undergo a transition from classically activated, proinflammatory cells to alternative activation that promotes tubular epithelial cell proliferation during kidney repair.14 We therefore hypothesized that in polycystic kidneys, macrophage infiltration could contribute to proliferation of the cyst-lining cells and the progression of PKD.
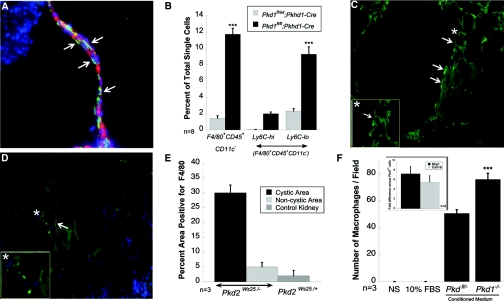
Immunostaining of kidney tissue from 24-d old C57Bl6 Pkd1fl/fl;Pkhd1-Cre mice (an orthologous model of PKD1 in which cysts grow postnatally15) with F4/80 shows macrophages (green) aligned along the cyst wall (Figure 1, A, arrows) of a collecting duct-derived cyst (identified by Dolichos biflorous, red stain). Quantitative FACS analysis of F4/80+CD45+CD11c- macrophages sorted from the kidneys of Pkd1fl/fl;Pkhd1-Cre and Pkd1fl/+;Pkhd1-Cre mice demonstrated a tenfold greater number of macrophages in the cystic kidneys as compared with their littermate controls (Figure 1, B). Further analysis of F4/80+CD45+CD11c- cells for Ly6C expression reveals that the majority of the macrophages are Ly6Clow, consistent with an alternatively activated phenotype (Figure 1, B). To determine whether the infiltration of macrophages was specific for loss of PC1, kidneys from Pkd2WS25/- mice (a model of PKD2) were stained for F4/80. Similar to the Pkd1fl/fl;Pkhd1-Cre mice, large numbers of macrophages lay closely apposed to the cyst-lining cells (Figure 1, C), whereas nearby noncystic areas had much lower numbers of macrophages (Figure 1, D; quantified in Figure 1, E), demonstrating that macrophage infiltration occurs in several murine models of PKD.
Figure 1.
Macrophages surround cysts in murine models of PKD. Kidneys were perfusion fixed with 4% PFA and cryosections stained for macrophage marker F4/80. (A) Cyst-lining cells showing closely apposed macrophages stained green. Green, F4/80; Red, DBA. (B) Quantitative analysis of FACS-sorted macrophages defined as F4/80+CD45+CD11c- cells and further analyzed for Ly6c expression; ***P < 0.0001. (C) Cystic area from Pkd2WS25/- kidney showing several F4/80+ cells (green). Inset shows magnified view of area marked with an asterisk. (D) Noncystic area from Pkd2ws25/+ kidney showing very few F4/80+ cells. Inset shows magnified view of selected area indicated by the asterisk. (E) Quantitative analysis of macrophages in kidneys from Pkd2WS25/- and Pkd2ws25/+ (control) mice. (F) Transwell migration of macrophages toward DMEM-F12, DMEM-F12 + 10%FBS, DMEM-F12 conditioned medium from Pkd1fl/- cells, and DMEM-F12 conditioned media from Pkd1-/- cells, respectively, n = 3. Inset: qRT-PCR for Mcp-1 and Cxcl16 was performed on RNA from Pkd1fl/- and Pkd1-/- cells and reported as the fold increase of Pkd1-/- as compared with Pkd1fl/-; n = 3.
We next hypothesized that the cyst-lining cells might secrete chemoattractant(s) to facilitate directed migration of macrophages to the cysts. To test this hypothesis, naïve bone-marrow-derived macrophages were cultured in the top chamber of a Transwell apparatus with conditioned medium produced from either Pkd1-/- or parental Pkd1fl/- tubular cells in the bottom chamber (cell lines as described previously16). Macrophages migrated toward the conditioned medium (CM) from both cell types; however, the number of macrophages migrating toward Pkd1-/- CM was significantly higher, 75.93 ± 4.6 versus 50.68 ± 2.77, n = 3, P < 001. In contrast, macrophages did not migrate toward serum-free DMEM/F12 (control medium) or DMEM/F12 with 10%FBS (Figure 1, F). This finding was reproduced using CM from two separate cell line clones for each genotype, indicating that Pkd1-/- tubular cells secrete increased amounts of a macrophage chemoattractant. PCR of RNA from the Pkd1fl/- and Pkd1-/- tubular cells demonstrates that both express two known macrophage chemoattractants, Mcp1 and chemokine (C-X-C) ligand-16 (Cxcl16). Quantitative RT-PCR reveals that Pkd1-/- cells expressed sevenfold greater levels of Mcp1 and fivefold more Cxcl16 than do Pkdfl/- cells (Figure 1, F, inset; n = 3). How the loss of Pc1 leads to increased expression of these cytokines remains to be determined.
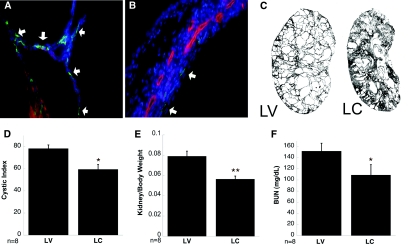
It is well established that increased proliferation is associated with cyst growth.3,4,16–18 Recent data from our laboratory shows that alternatively activated macrophages increase tubular cell proliferation,14 thus raising the possibility that macrophages surrounding the cysts may accelerate proliferation of cyst-lining cells and thereby augment cyst growth. This was tested in Pkd1fl/fl;Pkhd1-Cre mice in which cyst growth begins in the postnatal period by P10, followed by accelerated cyst expansion from P16-P24, after which the kidneys are markedly enlarged and cystic. Macrophages were depleted by intraperitoneal administration of liposomal clodronate (LC) on alternate days beginning postnatal day 10 through day 24. Control mice received an equal volume of liposomal vehicle (LV). Liposomes containing clodronate are readily engulfed by phagocytic cells such as macrophages and polymorphonuclear cells, leading to cell death within 24 h.14,19,20 On day 24, LV-treated mice exhibited large numbers of F4/80+ macrophages adjacent to the cyst-lining cells (Figure 2, A, arrows), whereas remarkably fewer macrophages are observed in the kidneys from LC-treated mice (Figure 2, B, arrows). The percentage of circulating monocytes 24 h after LC injection was reduced from an average of 5.5% in LV-treated to 0.4%. FACS analysis of macrophage numbers in Pkd1fl/fl;Pkhd1-Cre kidneys after alternate day LC treatment revealed a 95% decrease in total macrophages (0.3% of total single cells), with no significant change in the CD45+F480+CD11c–Ly6chi (0.04% of total single cells; 14% of remaining macrophages) or in the CD45+F480+CD11c–Ly6clo fraction (0.28% of total single cells; 85% of remaining macrophages). Sagittal sections of whole kidneys were then scanned and the cystic-index calculated. Macrophage depletion resulted in partial preservation of the renal parenchyma (representative images shown in Figure 2, C), with a significant decrease in the cystic index (Figure 2, D). The kidneys from LC-treated mice were smaller in size that paralleled an improved kidney/body weight ratio (Figure 2, E). Reduced cyst growth correlated with significantly improved renal function, as indicated by lower BUN values in the LC-treated group (Figure 2, F).
Figure 2.
Clodronate treatment reduces macrophage load and improves renal function. Cystic kidneys from Pkd1fl/fl;Pkhd1-Cre mice treated with LC show (B) fewer F4/80+ macrophages (arrows) along the cyst-lining cells compared with LV-treated mouse kidneys (A, arrows). (C) Scan of a sagittal section of a representative kidney from LV- and LC-treated mice, respectively, shows (D) better-preserved parenchyma in LC-treated mice and lowered cystic index, (E) improved kidney/body weight ratio, and (F) significantly improved renal BUN values. A, B: Green, F4/80; Red, DBA. *P < 0.01; **P < 0.001.
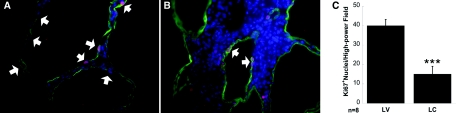
We next determined whether macrophage depletion had reduced cyst growth by influencing cell proliferation. Staining kidney sections with Ki67 (a nuclear protein expressed during cell division) revealed significantly more proliferation of cyst-lining cells in the vehicle-treated Pkd1fl/fl;Pkhd1-Cre mice (Figure 3, A, arrows) as compared with the macrophage-depleted mice (Figure 3, B; quantified in Figure 3, C). In contrast, TUNEL staining revealed no change in cyst-lining cell apoptosis between the two groups (data not shown). Taken together, these data demonstrate that macrophages populate cystic areas in orthologous mouse models of both PKD1 and PKD2. The presence and depletion of macrophages, respectively, correlates with increase or decrease in proliferation of the cyst-lining cells.
Figure 3.
LC administration reduces cystic-lining cell proliferation. Representative kidney sections are shown from (A) LV-treated and (B) LC-treated mice stained for Ki67 (arrows). Green, DBA, Red/purple-Ki67; Blue, DAPI. (C) Ki67 positive nuclei quantitated within DBA positive tubules from kidneys of LV- and LC-treated mice. Data pooled from eight individual mice is shown. ***P < 0.0001.
The association of macrophages with cell proliferation was suggested almost three decades ago when Polverini and co-workers reported vascular proliferation by activated macrophages in vivo and in vitro.21 More recently, macrophages have been found to home to injured tubules of the mouse kidney following ischemic injury20 in response to inflammatory cytokines such as MCP-1, IL-6, and SDF-1 produced by epithelial and/or endothelial cells.20 In the present study, we report that tubular cells in culture express both Mcp-1 and Cxcl16, and activate macrophage migration. Interestingly, cells that are genetically identical except for loss of Pc1 express significantly higher levels of both cytokines and induce greater macrophage chemotaxis, suggesting that the expression of Pc1 may normally serve to suppress the expression of cell injury signals such as chemotactic cytokines.
These macrophages differentiate into distinct populations that can be segregated into classically activated or alternatively activated based on the expression of markers such as Ly6C, iNos, and arginase-1 (Arg1).22 We have found that alternatively activated macrophages promote tubular cell proliferation, whereas classically activated macrophages do not.14 Additionally, Duffield and colleagues have shown that classically activated macrophages can promote apoptosis in renal mesangial cells.23 In the current study, more than 90% of the F4/80+CD45+CD11c- cells from cystic kidneys were found to be Ly6Clow, consistent with an alternatively activated phenotype, and while cyst-lining cell proliferation was reduced in macrophage-depleted animals, there was no change in apoptotic rates. Thus, our data support the model that Pc1 null cells in developing cysts secrete increased amounts of homing factor(s), such as Mcp1 and Cxcl16, that signal the accumulation of alternatively activated macrophages in cystic areas, which, in turn, promote cyst growth via secretion of macrophage-derived factors that stimulate adjacent tubular cell proliferation. Approaches that inhibit the expression or action of these homing and proliferation signals may therefore provide novel targets for slowing cyst growth in PKD.
CONCISE METHODS
Animal Models
We used Pkd1fl/fl mice that have been described previously.17 These mice were crossed with previously described Pkhd1-Cre mice13 for mediating kidney-specific deletion of the Pkd1 allele. Deletion was confirmed by standard genotypic PCR analysis. Pkd1fl/fl;Pkhd1-Cre neonates were used for the experiments. All experiments involving mice were conducted as per Yale University Institutional Animal Care and Use Committee guidelines and procedures.
Macrophage Depletion
400μls/20 g body weight of liposomal suspension containing either clodronate (LC) or saline (LV) was administered into 10-d old mice by intraperitoneal injection (intraperitoneally) on alternate days.
Cell Culture
Proximal tubule cell lines heterozygous (PH cells) or null (PN cells) for Pkd1 were cultured as described previously.16
Isolation and Culture of Bone Marrow-Derived Macrophages (BMMs)
BMMs were isolated from mouse femurs of 6- to 8-wk-old C57/Bl6 mice, as described by Cui and co-workers24 and by our laboratory.14 See detailed protocol in supplemental information online.
Macrophage Migration Assay
Naïve macrophages were plated onto the top chambers of 6-well Transwell plates and allowed to attach overnight. The next day, the medium was replaced with DMEM-F12. The bottoms of the Transwell filters were then wiped with a Q-tip to remove any macrophages that may have migrated through. The bottom wells were then filled with serum-free DMEM/F12, DMEM/F12 + 10% FBS, Pkd1fl/--conditioned medium, or Pkd1-/--conditioned medium. The plates were incubated at 37 °C for 12 h, and the top of the insert was then wiped clean with a Q-tip to remove macrophages that had not migrated through the membrane. The wells were then fixed in 4% formaldehyde at room temperature for 15 min, followed by eosin/hematoxylin staining and counting using a light microscope. Twenty random fields were counted at 20x magnification. Experiments were performed in triplicates and the data pooled from three independent experiments.
Immunostaining
Mice were anesthetized by an intraperitoneal injection of pentobarbital sodium and fixed in vivo with 4% paraformaldehyde (PFA) via cardiac perfusion. The kidneys were harvested and processed for cryosectioning or paraffin embedding. Five-μm-thick sections of kidneys were stained with hematoxylin and eosin. Macrophages were detected by immunofluorescence (IF) staining for a pan-macrophage marker, F4/80. Cell proliferation in tissues was detected by staining for the nuclear proliferation marker Ki67. Tissue sections were incubated overnight with an anti-mouse F4/80 antibody (eBioscience Inc., San Diego, CA) or Ki67 antibody (Thermoscientific, Fremont, CA) at 1:100 dilution, followed by appropriate secondary antibody and mounting in Vectashield DAPI-containing mounting medium (Vector Laboratories, Burlingame, CA). Tissues were colabeled with a fluorescein or rhodamine conjugated lectin, Dolichos Biflorus (DBA, Sigma Aldrich, St. Louis, MO), to specifically mark collecting ducts. IF staining was analyzed using a NIKON Eclipse TE2000-U microscope and images taken with a 40x objective and processed in Adobe Photoshop CS.
FACS Analysis of Macrophages
Kidneys were harvested, minced, and homogenized, single cell suspension made by incubating with Liberase (Roche Diagnostics, IN) containing DNAse-I (Sigma Aldrich, St. Louis, MO) and cells stained with the following antibodies: anti-F4/80 FITC-conjugated (eBiosciences, San Diego, CA), anti-CD45 PERCP-conjugated, anti-Ly6C, and anti-CD11c (BD Biosciences, San Jose, CA). Cells were then analyzed using LSRII analyzer (BD Biosciences, San Jose, CA).
Cystic Index
The cyst formation was quantified from sagittal sections of whole kidneys, as described previously.17 Briefly, whole-kidney images were acquired using automated image acquisition mode of MetaMorph (Universal Imaging) and total kidney area, cystic and noncystic area measured. Cystic index is expressed as the percent of total kidney area that is occupied by cysts. In WS25 −/− mice, macrophages were quantified by counting F4/80+ cells/x40 field, as described in detailed methods.
Blood Urea Nitrogen (BUN) Measurement
Blood for BUN was collected at the time of sacrifice on postnatal day 24. BUN was measured by the Yale Mouse Phenotype core facility.
Quantitative Real-Time PCR
RNA was isolated using RNA-STAT60 (Tel-test, Inc., Friendswood, TX), transcribed into cDNA and used for qPCR analysis, as recently described.14
Statistical Analysis
Two-tailed t test was used to compare data between two groups. Significance was determined at P < 0.05 and represented by *, **, and *** to denote P < 0.05, P < 0.001, and P < 0.0001, respectively. Where appropriate, data are presented as mean ± SEM.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was funded by an NIH award to SS and LGC (DK57328) and the Yale Polycystic Kidney Disease Center.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Gallagher AR, Germino GG, Somlo S: Molecular advances in autosomal dominant polycystic kidney disease. Adv Chronic Kidney Dis 17, 118–130, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Torres VE, Harris PC: Autosomal dominant polycystic kidney disease: The last 3 years. Kidney Int 76, 149–168, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Harris PC, Torres VE: Polycystic kidney disease. Annu Rev Med 60, 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nadella R, Blumer JB, Jia G, Kwon M, Akbulut T, Qian F, Sedlic F, Wakatsuki T, Sweeney WE, Jr, Wilson PD, Lanier SM, Park F: Activator of G protein signaling 3 promotes epithelial cell proliferation in PKD. J Am Soc Nephrol 21, 1275–1280, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gardner KD, Jr, Burnside JS, Elzinga LW, Locksley RM: Cytokines in fluids from polycystic kidneys. Kidney Int 39, 718–724, 1991 [DOI] [PubMed] [Google Scholar]
- 6. Li X, Magenheimer BS, Xia S, Johnson T, Wallace DP, Calvet JP, Li R: A tumor necrosis factor-alpha-mediated pathway promoting autosomal dominant polycystic kidney disease. Nat Med 14, 863–868, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng D, Wolfe M, Cowley BD, Jr, Wallace DP, Yamaguchi T, Grantham JJ: Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 14, 2588–2595, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Torres VE, Grantham JJ, Chapman AB, Kyong Tae Bae, Tao C, Guay-Woodford LM, Harris PC, Mrug M, Bennett WM, Moxey-Mims MM, Bost JE: Urinary monocyte chemotactic protein-1 (MCP1) predicts progression in autosomal dominant polycystic kidney disease (ADPKD). in J Am Soc Nephrol (2010), pp. PO1814 [Google Scholar]
- 9. Cowley BD, Jr, Ricardo SD, Nagao S, Diamond J: Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int 60, 2087–2096, 2001 [DOI] [PubMed] [Google Scholar]
- 10. Mrug M, Zhou J, Woo Y, Cui X, Szalai AJ, Novak J, Churchill GA, Guay-Woodford LM: Overexpression of innate immune response genes in a model of recessive polycystic kidney disease. Kidney Int 73, 63–76, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Duffield JS: Macrophages and immunologic inflammation of the kidney. Semin Nephrol 30, 234–254, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel V, Chowdhury R, Igarashi P: Advances in the pathogenesis and treatment of polycystic kidney disease. Curr Opin Nephrol Hypertens 18, 99–106, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P: Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17, 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, Ruhrberg C, Cantley LG: Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol 22, 317–326, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishio S, Tian S, Gallagher AR, Yu Z, Patel V, Igarashi P, Somlo S: Loss of oriented cell division does not initiate cyst formation. J Am Soc Nephrol 21, 295–302, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wei F, Karihaloo A, Yu Z, Marlier A, Seth P, Shibazaki S, Wang T, Sukhatme VP, Somlo S, Cantley LG: Neutrophil gelatinase-associated lipocalin suppresses cyst growth by Pkd1 null cells in vitro and in vivo. Kidney Int 74, 1310–1318, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S: Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17, 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson PD, Falkenstein D: The pathology of human renal cystic disease. Curr Top Pathol 88, 1–50, 1995 [DOI] [PubMed] [Google Scholar]
- 19. van Rooijen N, Sanders A, van den Berg TK: Apoptosis of macrophages induced by liposome-mediated intracellular delivery of clodronate and propamidine. J Immunol Methods 193, 93–99, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Jo SK, Sung SA, Cho WY, Go KJ, Kim HK: Macrophages contribute to the initiation of ischaemic acute renal failure in rats. Nephrol Dial Transplant 21, 1231–1239, 2006 [DOI] [PubMed] [Google Scholar]
- 21. Polverini PJ, Cotran PS, Gimbrone MA, Jr, Unanue ER: Activated macrophages induce vascular proliferation. Nature 269, 804–806, 1977 [DOI] [PubMed] [Google Scholar]
- 22. Lin SL, Castano AP, Nowlin BT, Lupher ML, Jr, Duffield JS: Bone marrow Ly6Chigh monocytes are selectively recruited to injured kidney and differentiate into functionally distinct populations. J Immunol 183, 6733–6743, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Duffield JS, Erwig LP, Wei X, Liew FY, Rees AJ, Savill JS: Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol 164, 2110–2119, 2000 [DOI] [PubMed] [Google Scholar]
- 24. Cui W, Ke JZ, Zhang Q, Ke HZ, Chalouni C, Vignery A: The intracellular domain of CD44 promotes the fusion of macrophages. Blood 107, 796–805, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.