Abstract
In two siblings of consanguineous parents with intermittent nephrotic-range proteinuria, we identified a homozygous deleterious frameshift mutation in the gene CUBN, which encodes cubulin, using exome capture and massively parallel re-sequencing. The mutation segregated with affected members of this family and was absent from 92 healthy individuals, thereby identifying a recessive mutation in CUBN as the single-gene cause of proteinuria in this sibship. Cubulin mutations cause a hereditary form of megaloblastic anemia secondary to vitamin B12 deficiency, and proteinuria occurs in 50% of cases since cubilin is coreceptor for both the intestinal vitamin B12-intrinsic factor complex and the tubular reabsorption of protein in the proximal tubule. In summary, we report successful use of exome capture and massively parallel re-sequencing to identify a rare, single-gene cause of nephropathy.
Protein-encoding exons constitute only 1% of the human genome but harbor 85% of mutations in single-gene disorders.1 Among about 2800 Mendelian diseases, for which the causative gene has been identified, mutations affect the coding region or canonical splice sites and thereby the function of the encoded proteins 1. However, PCR amplification of thousands of candidate exons is costly and impractical and has hampered discovery of single-gene disease causes. Recently, re-sequencing of entire coding regions of the human genome, the “exome,” with consecutive massively parallel (MP) re-sequencing has dramatically changed this situation.2 This approach has been successfully applied to identify single-gene causes of rare diseases including Miller syndrome,2 Kabuki syndrome,3 Schinzel-Giedion syndrome,4 Bartter syndrome,5 and nephronophthisis type 10.6
Nephrotic syndrome (NS) is a common kidney disease characterized by proteinuria, hypoalbuminemia, generalized edema, and hyperlipidemia. Identification of recessive single-gene causes of NS has provided important insights into the pathogenesis of this enigmatic disorder. Whereas NPHS2 mutations explain 10 to 25% of childhood NS, and two thirds of all NS in the first year of life can be explained by mutations in four genes only (NPHS1, NPHS2, WT1, and PLCE1),7 most other recessive causes of NS are very rare (1 to 3% of cases).8–13 Whereas single-gene causes of NS occur in childhood and adolescence, in more than about 70% of cases the causative gene mutation is still unknown.7 This forbids the use of cohort studies for gene identification and necessitates the ability to identify disease-causing genes in single families. We therefore combined whole genome homozygosity mapping with consecutive whole human exome capture (WHEC) and massively parallel re-sequencing to overcome this limitation.6 In this way we here identify a homozygous deleterious frameshift mutation within the cubilin gene (CUBN) as a novel unexpected cause of proteinuria in two siblings with intermittent nephrotic-range proteinuria. This approach will likely be very useful to rapidly solve cases of rare nephropathies.
RESULTS
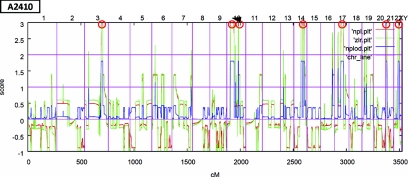
Two siblings (A 2410-21 and A2410-22) from family A2410 were discovered to have proteinuria on routine examination. The amount of proteinuria in both siblings had been fluctuating, sometimes reaching up to 2 g/d and then decreasing without any treatment (see Patients in the Concise Methods section). Given consanguinity of the parents of the two siblings with proteinuria, we hypothesized that its cause was a recessively inherited mutation. Therefore, we performed linkage analysis and homozygosity mapping in both affected siblings (A2410-21 and A2410-22) using Affymetrix 250K SNP StyI arrays. Nonparametric LOD score analysis14 was calculated for both siblings together, yielding seven segments of homozygosity by descent on chromosomes 3, 10, 14, 17, 21, and 22 with a total cumulative genomic length of 130 Mb (Figure 1). One segment of homozygosity on chromosome 10 coincided with PLCE1, a known locus for nephrotic syndrome. However, Sanger re-sequencing of all exons and adjacent exon-intron boundaries of PLCE1 yielded no mutation in this gene.
Figure 1.
Nonparametric lod (NPL) scores across the human genome in two siblings with intermittent gross proteinuria of consanguinous family A2410 reveal four regions of homozygosity by descent. The x-axis shows Affymetrix 250K SNP StyI array SNP positions on human chromosomes concatenated from p-ter (left) to q-ter (right). Genetic distance is given in centimorgan (cM). Seven maximum NPL peaks (red circles) indicate candidate regions of homozygosity by descent. The arrow shows the region of homozygosity in chromosome 10, which includes CUBN.
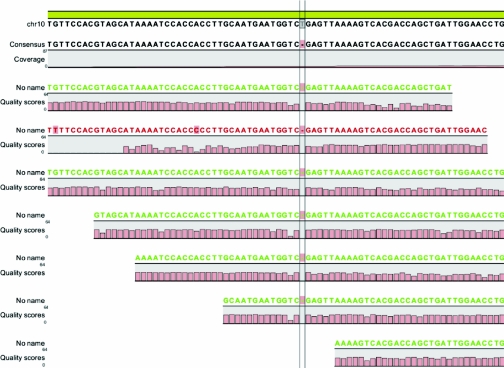
We then performed whole human exome capture in one sibling (A2410-22) using NimbleGen 2.1M Human Exome Array with consecutive massively parallel re-sequencing on two lanes of an Illumina-GAIIx sequencing platform to identify the underlying disease-causing mutation. Sequencing generated 29.5 million single-end reads of 78 bases. Quality-filtered sequence reads were aligned to the human reference genome assembly hg19 (NCBI build 37) using “CLC Genomics Workbench” software (CLC-bio, Aarhus, Denmark). About 20.1 million of these reads (68%) matched the targeted exons contributing to a median coverage of 11-fold (mean: 14-fold). No coverage was obtained for about 2.2% of all 180,000 targeted exons. CLC Genomics Workbench software was further used to call single nucleotide variants or small insertions/deletions. By filtering for homozygous-only variants, we identified 1968 variants from the reference sequence with at least fivefold sequence coverage. Of these, only 48 were not known single nucleotide polymorphisms, either reported in the database dbSNP130 or the 1000 genomes project. When examining the segments of homozygosity (132 Mb total), for which we had initially generated a positional candidate hypothesis by whole genome homozygosity mapping, only 11 nonsynonymous exonic variants remained. One of these changes was a novel 1-bp homozygous deletion (c.8355delA) in exon 53 of the gene CUBN (cubilin), resulting in a frameshift and a predicted premature truncation of the encoded protein (p.S2785fsX19) (Figure 2). We confirmed this mutation by exon-PCR and Sanger sequencing homozygously in both affected siblings and heterozygously in both parents (Figure 3). We then searched for additional CUBN mutations in a large worldwide cohort of 540 families with NS in whom we had generated homozygosity mapping data by WGHM. Four affected individuals (A155-21, A849-21, A1605-21, and A2591-21) of these families showed a genomic segment of >2 Mb of homozygosity at the CUBN locus. However, direct Sanger sequencing of all exons of CUBN in these individuals did not yield any additional mutations (data not shown).
Figure 2.
The novel single-base homozygous deletion (c.8355delA) in exon 53 of the cubilin (CUBN) gene that causes nephrotic syndrome in family A2410. Note that the sequence shown depicts the plus strand of chromosome 10 and that CUBN is encoded on the reverse strand, therefore showing a deletion of a T (thymine) rather than A (adenine).
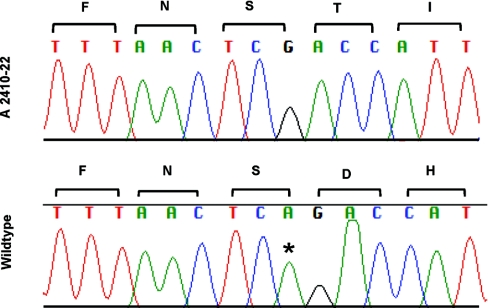
Figure 3.
Novel homozygous mutation in CUBN. A novel homozygous of a single-bp deletion is shown that leads to a frameshift mutation in exon 53 of CUBN (c.8355delA; p.S2785fsX19) in patient A2410-22 who has intermittent nephrotic range proteinuria. Nucleotide sequence traces and deduced amino acid sequence are shown for mutated (top) and wild-type (bottom) sequences. Adenine deleted in A2410-22 is marked with an asterisk in the wild-type sequence. Mutation numbering is based on CUBN human reference sequence NM_001081.3, where +1 corresponds to the A of the ATG translation start codon.
DISCUSSION
We here detected a novel 1-bp homozygous deletion of the cubilin (CUBN) gene as the cause of proteinuria in two siblings with NS using a combined strategy of homozygosity mapping and WHEC with massively parallel re-sequencing. The finding represented an unexpected cause of NS because intermittent gross proteinuria is rare in patients with CUBN mutations who are known to have Imerslund-Grasbeck syndrome (IGS; OMIM ID #261100), a form of congenital megaloblastic anemia due to vitamin B12 deficiency caused by a defect in the vitamin B12/intrinsic factor receptor (CUBN; OMIM ID #602997).
The renal effects of genetic ablation of cubilin in a mouse model has been reported previously.15 It was observed that proximal tubule cells did not localize the receptor protein “amnionless” to the plasma membrane in the absence of cubilin, indicating a mutual dependency of cubilin and amnionless to form a functional membrane receptor complex. Furthermore, cubilin-deficient mice exhibited markedly decreased uptake of albumin by proximal tubule cells, leading to proteinuria. It was shown that cubilin is essential for albumin reabsorption by proximal tubule cells.15 Recently, an association between a missense variant (I2984V) in the CUBN gene and both,urinary albumin-to-creatinin ratio and microalbuminuria has been identified in a genome-wide association study.16
In this study we detected by total human exome capture a novel homozygous deleterious CUBN mutation as the cause of proteinuria in a family in whom absence of megaloblastic anemia did not elicit any suspicion of IGS, most likely because of their young age. IGS represents the full clinical picture of CUBN mutations. This is an example of molecular genetics providing a diagnostic tool that is more sensitive and accurate than clinical diagnosis, a possibility that will be strongly enhanced as WHEC and massively parallel re-sequencing become more established.
Moreover, genetic diagnostics might inform therapy: Recently, another case with IGS and CUBN mutation was reported. The patient was a 15-year-old girl of German descent who presented with megaloblastic anemia, selective proteinuria, and funicular myelosis. After intravenous vitamin B12 therapy and consecutive intermittent intramuscular injection of vitamin B12, the patient's neurologic symptoms ceased completely after 2 months, hematologic parameters normalized within 5 months, and although proteinuria was persistent, renal function did not deteriorate.17 Likewise, the siblings described here might benefit from vitamin B12 replacement therapy.
At the cellular level, IGS, a monogenic cause of megaloblastic anemia (MGA1: OMIM ID #261100), is due to defective intestinal B12 malabsorption and/or renal tubular protein reabsorption. IGS occurs worldwide, but its prevalence is higher in several Middle Eastern countries and in Norway, and highest in Finland (0.8 in 100,000 individuals). By genetic mapping, Finnish-type IGS has been mapped to the CUBN gene locus, whereas Norwegian-type IGS has been mapped to the AMN gene locus.18 In families from the Mediterranean an Israeli Jewish family of Tunusian origin,18 a Tunisian family,19 and Turkish families20 two different AMN mutations and three different CUBN mutations were detected. Outside these geographic regions, only scattered cases have been described, for example, South Africa,21 France,22 the United States,23 and Taiwan.24 The Scandinavian cases were typical examples of founder effects, whereas in the Mediterranean region, instead of a founder effect, high degrees of consanguinity exposed rare homozygous mutations in both genes.
We here describe a novel homozygous frameshift mutation in two siblings from family A2410. Sibling A2410-21 had intermittent nephrotic range proteinuria and sibling A2410-22 had intermittent nephrotic range proteinuria along with epilepsia as the only clinical presentations. Anemia was not a part of their clinical course. CUBN mutations represent a recessive single-gene cause of proteinuria. Recessive single-gene disease causes convey full penetrance of a disease. They are thereby distinct from genetic variants that are found only to be associated with disease because associated variants usually explain only a low percentage of the phenotypic variance, as is the case for instance in the MYH9 and APOL1 variants that have been found in nephrotic syndrome.25
In conclusion, the combination of homozygosity mapping in consanguineous siblings together with WHEC and massively parallel re-sequencing provides a tool for the evaluation of patients with undiagnosed genetic diseases. Our findings also indicate that IGS should be considered when diagnosing individuals with proteinuria to identify potentially treatable variants.
CONCISE METHODS
Patients
We obtained blood samples, pedigrees, and clinical information after receiving informed consent (http://www.renalgenes.org) from all patients. Approval for experiments on humans was obtained from the University of Michigan Institutional Review Board. One of the siblings of a consanguineous family from Egypt (A2410-22) was found to have proteinuria during a routine urine analysis preceeding an operation for hypospadias. A2410-22 had proteinuria of 790 mg/d. Serum creatinine was 0.4 mg/dl, serum protein 6.5 g/L, serum albumin 4.2 g/L with normal creatinine clearance, and normal C3 levels at 4 years of age. Then the other sibling of the family (A2410-21) was tested for proteinuria. Sibling A2410-21 had proteinuria of 380 mg/d. Serum creatinine was 0.4 mg/dl, total serum protein 6.8 g/L, and serum albumin 3.5 g/L. Creatinine clearance and complement 3 (C3) level were normal at 5 years of age. Both siblings were treated with ACE inhibitors with no improvement of their condition. The amount of proteinuria in both siblings had been fluctuating, sometimes reaching up to 2 g/d and then decreasing without any treatment. However, no edema developed and no immunosuppressive treatment was instituted. Renal biopsy was not performed. As for megaloblastic anemia, both siblings had normal hemoglobin levels and the red blood cell indices were also within normal range for their ages. A2410-22 has had complex partial seizures since he was 5-months old, requiring treatment with carbamazepine. He has been seizure free on carbamazepine for 1 year now.
Homozygosity Mapping
For genome-wide homozygosity mapping, the GeneChip Human Mapping 250K StyI Array from Affymetrix was used. Nonparametric LOD scores were calculated using a modified version of the program GENEHUNTER 226,27 through stepwise use of a sliding window with sets of 110 SNPs. The program ALLEGRO was employed to identify regions of homozygosity as described,28 using a disease allele frequency of 0.0001 and CEU marker allele frequencies.
Whole Human Exome Capture
Genomic DNA (10 μg) from affected sibling A2410-22 was captured using the NimbleGen 2.1M Human Exome Array (Roche/NimbleGen) according to protocol. The NimbleGen 2.1M Human Exome Array contains oligonucleotides probes that target approximately 180,000 exons of 18,673 protein-coding genes from the consensus coding sequence (CCDS) set in addition to 551 micro-RNAs. To generate random start positions and to reduce fragment sizes to be appropriate for the Illumina genome analyzer, high-throughput sequencing, captured and amplified DNA fragments in the range of 500 to 700 bp were subsequently modified using Bal31 exonuclease and DNAse-I as published previously.29
Massively Parallel Re-sequencing, Sequence Alignment, and Variant Calling
Library construction of the modified captured DNA fragments was performed using the “Genomic DNA Sample Prep Kit” according to the manufacturer's instructions (Illumina, San Diego). The library was sequenced on two lanes of an Illumina genome analyzer (GAII) as 80 base single-end reads. Image analysis and base calling were generated by the Illumina pipeline 1.5.1 using default parameters. Subsequent sequence alignment to the human genome reference genome (hg19) and variant calling was performed using CLC Genomics Workbench software. Only variants with an allele frequency of more than 80% were called to identify homozygous variants.
DISCLOSURES
None.
Acknowledgments
We thank the patients and their physicians for contribution of blood samples and clinical data. This work was supported by grants to F.H. from the National Institutes of Health (DK076683, RC1-DK086542), from the NephCure Foundation, and from the Thrasher Research Fund. F. Hildebrandt is an Investigator of the Howard Hughes Medical Institute, a Doris Duke Distinguished Clinical Scientist, and the Frederick G.L. Huetwell Professor for the Cure and Prevention of Birth Defects.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Cooper DN, Krawczak M, Antonorakis SE: The nature and mechanisms of human gene mutation. In: The Metabolic and Molecular Bases of Inherited Disease, 7th ed., edited by Scriver C, Beaudet al., Sly WS, Valle D. New York, McGraw-Hill, 1995, pp 259–291 [Google Scholar]
- 2. Ng SB, Buckingham KJ, Lee C, Bigham AW, Tabor HK, Dent KM, Huff CD, Shannon PT, Jabs EW, Nickerson DA, Shendure J, Bamshad MJ: Exome sequencing identifies the cause of a mendelian disorder. Nat Genet 42: 30–35, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ng SB, Bigham AW, Buckingham KJ, Hannibal MC, McMillin MJ, Gildersleeve HI, Beck AE, Tabor HK, Cooper GM, Mefford HC, Lee C, Turner EH, Smith JD, Rieder MJ, Yoshiura K, Matsumoto N, Ohta T, Niikawa N, Nickerson DA, Bamshad MJ, Shendure J: Exome sequencing identifies MLL2 mutations as a cause of Kabuki syndrome. Nat Genet 42: 790–793, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoischen A, van Bon BW, Gilissen C, Arts P, van Lier B, Steehouwer M, de Vries P, de Reuver R, Wieskamp N, Mortier G, Devriendt K, Amorim MZ, Revencu N, Kidd A, Barbosa M, Turner A, Smith J, Oley C, Henderson A, Hayes IM, Thompson EM, Brunner HG, de Vries BB, Veltman JA: De novo mutations of SETBP1 cause Schinzel-Giedion syndrome. Nat Genet 42: 483–485, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Choi M, Scholl UI, Ji W, Liu T, Tikhonova IR, Zumbo P, Nayir A, Bakkaloğlu A, Ozen S, Sanjad S, Nelson-Williams C, Farhi A, Mane S, Lifton RP: Genetic diagnosis by whole exome capture and massively parallel DNA sequencing. Proc Natl Acad Sci U S A 106: 19096–19101, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, van Reeuwijk J, Letteboer SJ, Sang L, Giles RH, Liu Q, Coene KL, Estrada-Cuzcano A, Collin RW, McLaughlin HM, Held S, Kasanuki JM, Ramaswami G, Conte J, Lopez I, Washburn J, Macdonald J, Hu J, Yamashita Y, Maher ER, Guay-Woodford LM, Neumann HP, Obermüller N, Koenekoop RK, Bergmann C, Bei X, Lewis RA, Katsanis N, Lopes V, Williams DS, Lyons RH, Dang CV, Brito DA, Dias MB, Zhang X, Cavalcoli JD, Nürnberg G, Nürnberg P, Pierce EA, Jackson PK, Antignac C, Saunier S, Roepman R, Dollfus H, Khanna H, Hildebrandt F: Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nat Genet 42: 840–850, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hinkes BG, Mucha B, Vlangos CN, Gbadegesin R, Liu J, Hasselbacher K, Hangan D, Ozaltin F, Zenker M, Hildebrandt F: Arbeitsgemeinschaft für Paediatrische Nephrologie Study Group: Nephrotic syndrome in the first year of life: Two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119: e907–e919, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Zenker M, Aigner T, Wendler O, Tralau T, Müntefering H, Fenski R, Pitz S, Schumacher V, Royer-Pokora B, Wühl E, Cochat P, Bouvier R, Kraus C, Mark K, Madlon H, Dötsch J, Rascher W, Maruniak-Chudek I, Lennert T, Neumann LM, Reis A: Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum Mol Genet 13: 2625–2632, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Hasselbacher K, Wiggins RC, Matejas V, Hinkes BG, Mucha B, Hoskins BE, Ozaltin F, Nürnberg G, Becker C, Hangan D, Pohl M, Kuwertz-Bröking E, Griebel M, Schumacher V, Royer-Pokora B, Bakkaloglu A, Nürnberg P, Zenker M, Hildebrandt F: Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int 70: 1008–1012, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Hinkes B, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nürnberg G, Garg P, Verma R, Chaib H, Hoskins BE, Ashraf S, Becker C, Hennies HC, Goyal M, Wharram BL, Schachter AD, Mudumana S, Drummond I, Kerjaschki D, Waldherr R, Dietrich A, Ozaltin F, Bakkaloglu A, Cleper R, Basel-Vanagaite L, Pohl M, Griebel M, Tsygin AN, Soylu A, Müller D, Sorli CS, Bunney TD, Katan M, Liu J, Attanasio M, O'toole JF, Hasselbacher K, Mucha B, Otto EA, Airik R, Kispert A, Kelley GG, Smrcka AV, Gudermann T, Holzman LB, Nürnberg P, Hildebrandt F: Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat Genet 38: 1397–1405, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Caridi G, Piemonte F, Montini G, Ghiggeri GM, Murer L, Barisoni L, Pastore A, Muda AO, Valente ML, Bertini E, Emma F: COQ2 nephropathy: A newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol 18: 2773–2780, 2007 [DOI] [PubMed] [Google Scholar]
- 12. López LC, Schuelke M, Quinzii CM, Kanki T, Rodenburg RJ, Naini A, Dimauro S, Hirano M: Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet 79: 1125–1129, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shih NY, Li J, Karpitskii V, Nguyen A, Dustin ML, Kanagawa O, Miner JH, Shaw AS: Congenital nephrotic syndrome in mice lacking CD2-associated protein. Science 286: 312–315, 1999 [DOI] [PubMed] [Google Scholar]
- 14. Hildebrandt F, Heeringa SF, Rüschendorf F, Attanasio M, Nürnberg G, Becker C, Seelow D, Huebner N, Chernin G, Vlangos CN, Zhou W, O'Toole JF, Hoskins BE, Wolf MT, Hinkes BG, Chaib H, Ashraf S, Schoeb DS, Ovunc B, Allen SJ, Vega-Warner V, Wise E, Harville HM, Lyons RH, Washburn J, Macdonald J, Nürnberg P, Otto EA: A systematic approach to mapping recessive disease genes in individuals from outbred populations. PLoS Genet 5: e1000353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amsellem S, Gburek J, Hamard G, Nielsen R, Willnow TE, Devuyst O, Nexo E, Verroust PJ, Christensen EI, Kozyraki R: Cubilin is essential for albumin reabsorption in the renal proximal tubule. J Am Soc Nephrol 21: 1859–1867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Böger CA, Chen MH, Tin A, Olden M, Köttgen A, Deboer IH, Fuchsberger C, O'Seaghdha CM, Pattaro C, Teumer A, Liu CT, Glazer NL, Li M, O'Connell JR, Tanaka T, Peralta CA, Kutalik Z, Luan J, Zhao JH, Hwang SJ, Akylbekova E, Kramer H, van der Harst P, Smith AV, Lohman K, de Andrade M, Hayward C, Kollerits B, Tönjes A, Aspelund T, Ingelsson E, Eiriksdottir G, Launer LJ, Harris TB, Shuldiner AR, Mitchell BD, Arking DE, Franceschini N, Boerwinkle E, Egan J, Hernandez D, Reilly M, Townsend RR, Lumley T, Siscovick DS, Psaty BM, Kestenbaum B, Haritunians T, Bergmann S, Vollenweider P, Waeber G, Mooser V, Waterworth D, Johnson AD, Florez JC, Meigs JB, Lu X, Turner ST, Atkinson EJ, Leak TS, Aasarød K, Skorpen F, Syvänen AC, Illig T, Baumert J, Koenig W, Krämer BK, Devuyst O, Mychaleckyj JC, Minelli C, Bakker SJ, Kedenko L, Paulweber B, Coassin S, Endlich K, Kroemer HK, Biffar R, Stracke S, Völzke H, Stumvoll M, Mägi R, Campbell H, Vitart V, Hastie ND, Gudnason V, Kardia SL, Liu Y, Polasek O, Curhan G, Kronenberg F, Prokopenko I, Rudan I, Arnlöv J, Hallan S, Navis G; the CKDGen Consortium, Parsa A, Ferrucci L, Coresh J, Shlipak MG, Bull SB, Paterson AD; Paterson on behalf of DCCT/EDIC, Wichmann HE, Wareham NJ, Loos RJ, Rotter JI, Pramstaller PP, Cupples LA, Beckmann JS, Yang Q, Heid IM, Rettig R, Dreisbach AW, Bochud M, Fox CS, Kao WH: CUBN is a gene locus for albuminuria. J Am Soc Nephrol 22: 555–570, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hauck FH, Tanner SM, Henker J, Laass MW: Imerslund-Gräsbeck syndrome in a 15-year-old German girl caused by compound heterozygous mutations in CUBN. Eur J Pediatr 167: 671–675, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Tanner SM, Aminoff M, Wright FA, Liyanarachchi S, Kuronen M, Saarinen A, Massika O, Mandel H, Broch H, de la Chapelle A: Amnionless, essential for mouse gastrulation, is mutated in recessive hereditary megaloblastic anemia. Nat Genet 33: 426–429, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Bouchlaka C, Maktouf C, Mahjoub B, Ayadi A, Sfar MT, Sioud M, Gueddich N, Belhadjali Z, Rebaï A, Abdelhak S, Dellagi K: Genetic heterogeneity of megaloblastic anaemia type 1 in Tunisian patients. J Hum Genet 52: 262–270, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Tanner SM, Li Z, Bisson R, Acar C, Oner C, Oner R, Cetin M, Abdelaal MA, Ismail EA, Lissens W, Krahe R, Broch H, Gräsbeck R, de la Chapelle A: Genetically heterogeneous selective intestinal malabsorption of vitamin B12: founder effects, consanguinity, and high clinical awareness explain aggregations in Scandinavia and the Middle East. Hum Mutat 23: 327–333, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Stones DK, Ferreira M: Imerslund-Gräsbeck syndrome in an African patient. J Trop Pediatr 45: 106–107, 1999 [DOI] [PubMed] [Google Scholar]
- 22. Flechelles O, Schneider P, Lesesve JF, Baruchel A, Vannier JP, Tron P, Schaison G: Imerslund's disease. Clinical and biological aspects. Apropos of 6 cases. Arch Pediatr 4: 862–866, 1997. (French) [DOI] [PubMed] [Google Scholar]
- 23. Mackenzie IL, Donaldson RM, Jr., Trier JS, Mathan VI: Ileal mucosa in familial selective vitamin B 12 malabsorption. N Engl J Med 286: 1021–1025, 1972 [DOI] [PubMed] [Google Scholar]
- 24. Lin SH, Sourial NA, Lu KC, Hsueh EJ: Imerslund-Grasbeck syndrome in a Chinese family with distinct skin lesions refractory to vitamin B12. J Clin Pathol 47: 956–958, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Genovese G, Tonna SJ, Knob AU, Appel GB, Katz A, Bernhardy AJ, Needham AW, Lazarus R, Pollak MR: A risk allele for focal segmental glomerulosclerosis in African Americans is located within a region containing APOL1 and MYH9. Kidney Int 78: 698–704, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kruglyak L, Daly MJ, Reeve-Daly MP, Lander ES: Parametric and nonparametric linkage analysis: A unified multipoint approach. Am J Hum Genet 58: 1347–1363, 1996 [PMC free article] [PubMed] [Google Scholar]
- 27. Strauch K, Fimmers R, Kurz T, Deichmann KA, Wienker TF, Baur MP: Parametric and nonparametric multipoint linkage analysis with imprinting and two-locus-trait models: Application to mite sensitization. Am J Hum Genet 66: 1945–1957, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gudbjartsson DF, Jonasson K, Frigge ML, Kong A: Allegro, a new computer program for multipoint linkage analysis. Nat Genet 25: 12–13, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Otto EA, Ramaswami G, Janssen S, Chaki M, Allen SJ, Zhou W, Airik R, Hurd TW, Ghosh AK, Wolf MT, Hoppe B, Neuhaus TJ, Bockenhauer D, Milford DV, Soliman NA, Antignac C, Saunier S, Johnson CA, Hildebrandt F: the GPN Study Group: Mutation analysis of 18 nephronophthisis associated ciliopathy disease genes using a DNA pooling and next generation sequencing strategy. J Med Genet 48: 105–116, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]