Abstract
Colony-stimulating factor-1 (CSF-1), the principal growth factor for macrophages, is increased in the kidney, serum, and urine of patients with lupus nephritis, and eliminating CSF-1 suppresses lupus in MRL-Faslpr mice. CSF-1 has three biologically active isoforms: a membrane-spanning cell surface glycoprotein (csCSF-1), a secreted proteoglycan (spCSF-1), and a secreted glycoprotein (sgCSF-1); the role of each isoform in the circulation and kidney in autoimmune disease is not well understood. Here, we constructed mutant MRL-Faslpr mice that only express csCSF-1 or precursors of the spCSF-1 and sgCSF-1 isoforms. Both csCSF-1 and spCSF-1 shifted monocytes toward proinflammatory, activated populations, enhancing their recruitment into the kidney during lupus nephritis. With advancing lupus nephritis, spCSF-1 was the predominant isoform responsible for increasing circulating CSF-1 and, along with the csCSF-1 isoform, for increasing intrarenal CSF-1. Thus, csCSF-1 appears to initiate and promote the local activation of macrophages within the kidney. Intrarenal expression of csCSF-1 and spCSF-1 increases with advancing nephritis, thereby promoting the intrarenal recruitment of monocytes and expansion of Ly6Chi macrophages, which induce apoptosis of the renal parenchyma. Taken together, these data suggest that the three CSF-1 isoforms have distinct biologic properties, suggesting that blocking both circulating and intrarenal CSF-1 may be necessary for therapeutic efficacy.
Macrophage (Mø)-rich inflammation is a cardinal feature of spontaneous autoimmune disease in MRL-Faslpr mice, a model sharing features with human lupus. As in human lupus, multiple tissues are targeted for destruction in MRL-Faslpr mice,1–3 and these mice succumb to rapidly progressive Mø-rich kidney disease.2 Activated Mø mediate renal destruction4–7; therefore, limiting the accumulation of activated Mø in the kidney provides a potential therapeutic approach for lupus nephritis.
Colony-stimulating factor-1 (CSF-1), the principal Mø growth factor, regulates Mø survival, proliferation, and activation. Through a series of mechanistic studies in MRL-Faslpr mice, we determined that CSF-1 is central to lupus. CSF-1 expression increases in the serum and kidney before nephritis and rises with advancing disease.8 Releasing CSF-1 into the kidney by implanting cells constitutively expressing CSF-1 incites Mø-rich nephritis in the area adjacent to CSF-1.9,10 Eliminating CSF-1 expression using CSF-1-deficient mice suppresses lupus nephritis and the systemic illness.11 Increasing the systemic expression of CSF-1, most notably generated by renal tubular epithelial cells (TEC) using transgenic mice, hastens the tempo of lupus nephritis and the systemic illness.5 Does CSF-1 mediate lupus nephritis in humans? We detect a striking increase in CSF-1 in the kidney (largely expressed by TEC), blood, and urine in patients with serologically-active lupus nephritis.5 Thus, CSF-1 is an attractive potential therapeutic target for lupus nephritis.
There are three biologically active, homodimeric isoforms of CSF-1 (Figure 1A). The full-length CSF-1 transcript encodes a membrane-spanning precursor protein from which either the secreted glycoprotein (sgCSF-1) or the secreted proteoglycan (spCSF-1) are cleaved in secretory vesicle. Splicing out of the region in exon 6 encoding the proteolytic cleavage sites and the glycosaminoglycan addition site from this transcript creates a truncated mRNA that encodes the biologically active membrane-spanning, cell surface glycoprotein (csCSF-1) (reviewed in reference 12). Our previous studies indicated that the individual csCSF-1 and the spCSF-1 and sgCSF-1 isoforms have distinct functions in nonautoimmune incited renal inflammation on the basis of stability, availability, and the large (approximately 18,000 Mr) chondroitin sulfate chain (ChS) on spCSF-1, but not sgCSF-1.13 To design a CSF-1 targeted therapeutic approach for lupus nephritis, it is essential to decipher whether blocking CSF-1 expression in the circulation and/or the kidney is necessary for efficacy. In this study, we have dissected the spatiotemporal expression and function of the membrane-spanning, cell surface CSF-1 and its secreted proteoglycan and glycoprotein isoforms in lupus nephritis, to clarify the role of circulating and intrarenal CSF-1 in the disease pathogenesis and to assist in tailoring a CSF-1-directed therapeutic approach for lupus nephritis.
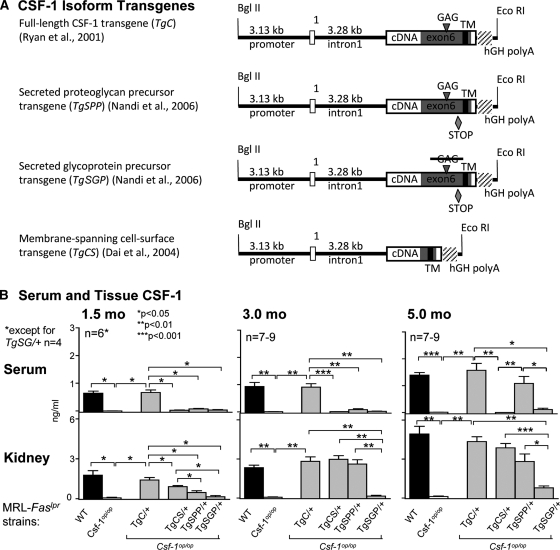
Figure 1.
spCSF-1, but not csCSF-1, contributes to the increase in serum CSF-1, and both are upregulated in the kidney with advancing disease in MRL-Faslpr mice. (A) CSF-1 isoform transgenes driven by Csf1 promoter (3.13 kb) and first intron (3.28 kb). TgC contains the full-length cDNA (exons 1 to 9). The other transgenes are altered as follows. Both TgSPP and TgSGP contain a stop codon at amino acid 456 to ensure that they are secreted. TgSGP has, in addition, a mutation (S276L-G277A) in the unique glycosaminoglycan (GAG) addition site (SGXG/A) to prevent addition of the chondroitin sulfate chain. csCSF-1, incorporated in TgCS results from alternative splicing in exon 6 that removes the GAG addition site and sequences encoding the proteolytic cleavage sites that release the secreted forms from their transmembrane tether (reviewed in reference 12). (B) Serum and kidney CSF-1 levels in TgC/+, TgCS/+, TgSPP/+, and TgSGP/+ mice with advancing lupus nephritis (1.5, 3.0, and 5.0 months of age); MRL-Faslpr mice (WT, wild type) and Csf1op/op mice served as controls. CSF-1 levels in serum and in kidney homogenates determined by ELISA. The values are the means ± SEM.
RESULTS
spCSF-1 Is the Primary Contributor to the Rise in Circulating CSF-1 in Lupus
Increasing systemic CSF-1 hastens the onset and tempo of lupus nephritis in MRL-Faslpr mice,5 whereas local delivery of CSF-1 within the kidney triggers Mø-rich inflammation in the adjacent area.9 To identify the spatial-temporal expression of individual CSF-1 isoforms in the serum and kidney during the progression of lupus nephritis, we constructed individual CSF-1 isoform transgenic MRL-Faslpr mice (Figure 1A). For this purpose, we backcrossed the CSF-1 isoform transgenes expressing all three CSF-1 isoforms (TgC/+),14 the cell surface CSF-1 isoform (TgCS/+),15 the secreted proteoglycan isoform precursor (TgSPP/+),16 and the secreted glycoprotein isoform precursor (TgSGP/+),16 as well as the CSF-1 inactivating osteopetrotic (Csf1op) mutation, onto the MRL-Faslpr background.13 The CSF-1 promotor/first intron driving expression of full-length CSF-1 was previously shown on the Csf1op/Csf1op (Csf1op/op) background to rescue the osteopetrotic phenotype14 and on the Csf1op/op;MRL-Faslpr background to restore disease-related tissue expression.5 Because the same CSF-1 promotor/first intron was used to construct the individual CSF-1 isoform transgenic strains,15,16 we were confident that the individual CSF-1 isoform transgenic MRL-Faslpr mice expressed the CSF-1 isoforms in a normal disease-related and CSF-1 tissue-specific manner during the progression of lupus nephritis. We analyzed serum CSF-1 in MRL-Faslpr mice before onset (1.5 months of age) and at the initial (3.0 months of age) and advanced (5.0 months of age) phases of lupus nephritis.17 spCSF-1 and sgCSF-1 isoforms contributed equally to serum CSF-1 because the values in the TgSPP/+ and TgSGP/+ mice were similar before, and during the initial phase of lupus nephritis (Figure 1B). This is consistent with our previous findings indicating that spCSF-1 and sgCSF-1 contribute equally to serum CSF-1 levels in normal mice (FVB background),13,16 although the levels achieved in each case were less than 50% of those of wild-type (WT) mice. However, serum CSF-1 levels rose dramatically in TgSPP/+ (P = 0.01), but not TgSGP/+, mice with advanced lupus nephritis (Figure 1B). In keeping with our prior findings, we detected a similar rise in serum CSF-1 in the TgC/+ (P = 0.001) and WT (P = 0.02) mice during advancing lupus nephritis.5 We did not detect serum CSF-1 in Csf1op/op mice13 or in Csf1op/op; TgCS/+ mice, previously shown not to express circulating CSF-1 (Figure 1).15 (Figure 1). Taken together, the spCSF-1 isoform, not the sgCSF-1 or csCSF-1 isoforms, is predominantly responsible for increasing circulating CSF-1 with advancing lupus nephritis in MRL-Faslpr mice.
csCSF-1 and spCSF-1, not sgCSF-1, Are Responsible for Increasing Intrarenal CSF-1 during Lupus Nephritis
To identify the time-related expression of the individual CSF-1 isoforms in the kidney during lupus nephritis, we probed for the expression of CSF-1 in kidney homogenates (ELISA assay) in CSF-1 transgenic, WT, and CSF-1-deficient MRL-Faslpr strains. Intrarenal CSF-1 similarly rose in TgC/+ mice (P = 0.001) and WT (P = 0.01) mice (expressing all three CSF-1 isoforms) during advancing lupus nephritis (Figure 1). By comparison, we detected a time- and disease-related rise in intrarenal CSF-1 in TgCS/+ mice (P = 0.0004) that was as rapid and robust as in TgC/+ and WT mice. Moreover, intrarenal CSF-1 in TgSPP/+ mice rose with advancing lupus nephritis (P = 0.007) and was as robustly expressed as in TgCS/+ mice. Because intrarenal CSF-1 expression in TgCS/+ mice alone or TgSPP/+ mice alone was similar to WT, this suggests that each of these isoform strains generates sufficient CSF-1 to compensate for the absence of the other CSF-1 isoforms involved in advancing lupus nephritis. In contrast, intrarenal CSF-1 in TgSGP/+ mice did not increase until 5.0 months of age (P = 0.01), and the rise was modest compared with TgCS/+ and TgSPP/+ mice (Figure 1B). Taken together, the spCSF-1 and csCSF-1 isoforms are predominantly responsible for increasing intrarenal CSF-1 in MRL-Faslpr mice with advancing lupus nephritis.
csCSF-1 or spCSF-1, but not sgCSF-1, Restore Kidney Disease and the Systemic Illness to WT Levels in CSF-1-deficient MRL-Faslpr Mice
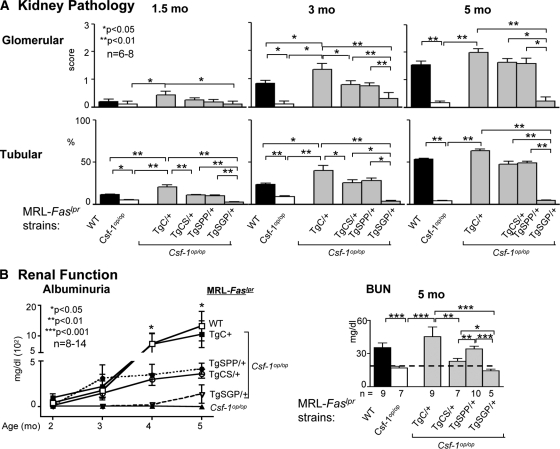
To determine whether the expression of cell surface or secreted CSF-1 promotes lupus nephritis, we compared the time-related renal pathology and loss of function in MRL-Faslpr mice: expressing individual CSF-1 isoforms (TgCS/+, TgSPP/+, TgSGP/+), expressing all three CSF-1 isoforms (TgC/+ and WT) and deficient in CSF-1 expression (Csf1op/op). The time-related severity of renal tubular and glomerular pathology in these MRL-Faslpr strains was restored in TgCS/+ (P = 0.001) and TgSPP/+ (P = 0.01) mice, but not in TgSGP/+ mice, as compared with WT mice (Figure 2A). This finding is consistent with the magnitude of intrarenal CSF-1 expression in TgCS/+ and TgSPP/+ mice. In keeping with renal pathology, we detected a similar pattern in the loss of renal function (albuminuria, blood urea nitrogen) in TgCS/+ and TgSPP/+ mice, as compared with WT mice during advancing lupus nephritis (Figure 2B). Consistent with renal pathology, the TgSGP/+ mice maintained normal renal function (Figure 2B). Taken together, the csCSF-1 and spCSF-1 isoforms, abundantly expressed and upregulated in the kidney during advancing lupus nephritis, each restored renal disease in MRL-Faslpr mice deficient in CSF-1. This suggests that the membrane-spanning csCSF-1 isoform expressed in the kidney and the secreted spCSF-1 isoform expressed in the circulation and in the kidney are similarly capable of mediating the progression of lupus nephritis.
Figure 2.
csCSF-1 or spCSF-1, but not sgCSF-1, restored kidney disease in CSF-1-deficient MRL-Faslpr mice. (A) We analyzed tubular and glomerular pathology in TgC/+, TgCS/+, TgSPP/+, and TgSGP/+ mice with advancing disease (1.5, 3.0, and 5.0 months of age); WT and Csf1op/op mice served as controls. The data are the means ± SEM, n = 6 to 8/group. (B) Loss of renal function (albuminuria and blood urea nitrogen [BUN]) is accelerated in WT mice in comparison with TgCS/+ and TgSPP/+ mice. In contrast, TgSGP/+ and Csf1op/op mice remain protected from loss of renal function. Serum control BUN for MRL-++ and B6 mice are denoted by a dotted line. The values are the means ± SEM, n = 8 to 14/group.
To determine whether the csCSF-1 and spCSF-1 isoforms are instrumental in the systemic illness, we evaluated systemic lymphoid features (lymphadenopathy and splenomegaly) characteristic of lupus in MRL-Faslpr mice. We detected an increase in time-related lymphadenopathy (lymph node wt/body wt) and splenomegaly (spleen wt/body wt) similar to WT mice in TgCS/+ and TgSPP/+ mice but not TgSGP/+ mice (Supplemental Figure 1). Thus, the csCSF-1 and the pgCSF-1 isoforms mediate systemic features along with kidney disease in MRL-Faslpr mice.
csCSF-1 and, to a Lesser Degree, spCSF-1 Mediate Mø Accumulation in the Kidney during Lupus Nephritis
Previous results indicate that during development, the restoration of the individual csCSF-1 and spCSF-1 isoforms in Csf1op/op mice restored Mø densities in some tissues including the renal cortex, but not in others, such as the renal medulla.15,16 However, Mø phenotypes that accumulate during normal development and diseases are distinct.18–20 To identify the individual CSF-1 isoforms that restore intrarenal Mø accumulation in Csf1op/op MRL-Faslpr mice during advancing lupus nephritis, we probed for the magnitude of the time-related (1.5, 3.0, 5.0 months of age) Mø within the areas of the kidneys in which Mø are notable (interstitial > perivascular > glomeruli) in WT mice. The number of intrarenal Mø in TgCS/+, TgSPP/+, and TgSGP/+ mice progressively rose with advancing age and disease (interstitial, Figure 3A and perivascular and glomerular, Supplemental Figure 2). We detected complete intrarenal Mø restoration in the TgCS/+ mice as compared with WT mice. However, whereas intrarenal Mø restoration in TgSPP/+ mice was robust, it was not complete. In keeping with the minimal expression of CSF-1 in TgSGP/+ mice, the number of intrarenal Mø increased only slightly above the levels detected in Csf1op/op mice (Figure 3A and Supplemental Figure 2). Notably, in contrast to Mø accumulation during normal development, we detected a similar pattern of Mø restoration in the renal cortex and medulla in the CSF-1 transgenic strains (Supplemental Figure 3).
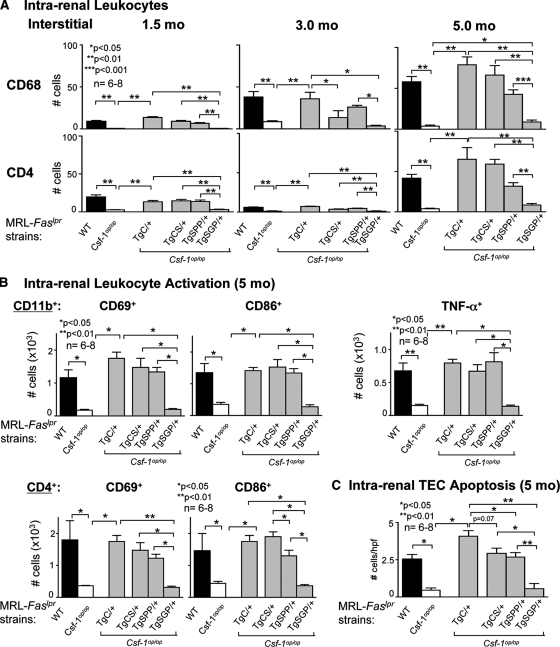
Figure 3.
csCSF-1 and spCSF-1 increase intrarenal Mø and promote activation resulting in Mø mediated TEC apoptosis. (A) Number of intrarenal CD68+ cells (Mø, DC) and T cells (CD4+) in TgC/+, TgCS/+, TgSPP/+, and TgSGP/+ mice with advancing lupus nephritis (1.5, 3.0, and 5.0 months of age). The values are means ± SEM. (B) Mø (CD11b+) activation (CD86+, CD69+, and TNF-α) and T cell activation (CD4+; CD86+/CD69+) in the kidney analyzed by flow cytometry in TgCS/+, TgSPP/+, TgSG/+, wild-type (WT), and Csf1op/op mice at 3.0 and 5 months of age. The data are representative of three separate experiments. (C) TEC apoptosis by anti-cleaved caspase-3 staining for apoptosis and TEC using morphologic criteria is increased in TgCS/+ and TgSPP/+ mice compared with TgSGP/+ and Csf1op/op mice. The values are the means ± SEM.
Because T cells accompany Mø during the progression of lupus and are central to the pathogenesis of lupus nephritis,21 we probed for the time-related accumulation of T cell populations in MRL-Faslpr mice. We detected an increase in CD4+ (Figure 3A), CD8+ and B220+ T cells (data not shown) that paralleled the rise in intrarenal Mø for each individual CSF-1 isoform. Thus, intrarenal csCSF-1, and to a lesser degree spCSF-1, fostered intrarenal Mø and T cell accumulation during advancing lupus nephritis.
Mø Activation and Intrarenal TEC Apoptosis Are Mediated by the csCSF-1 and spCSF-1 during Lupus Nephritis
Activated Mø, but not resting, induced apoptosis in renal TEC,5,22 and CSF-1 activates Mø that, in turn, induced TEC apoptosis during nonautoimmune incited renal injury (unilateral ureteral obstruction).13 Therefore, we tested the hypothesis that csCSF-1 and spCSF-1, robustly increased in the kidney with advancing lupus nephritis, are responsible for intrarenal Mø activation and Mø-mediated TEC apoptosis in MRL-Faslpr mice. We detected a number of activated Mø expressing CD69, CD86, and TNF-α (CD68+, Figure 3B and F4/80+, Supplemental Figure 4) in TgCS/+ and TgSPP/+ mice that was indistinguishable from the WT level. Thus, the individual csCSF-1 and spCSF-1 isoforms alone were sufficient to restore intrarenal activated Mø to WT levels in MRL-Faslpr mice deficient in CSF-1. Consistent with this finding, we detected a substantial rise in apoptotic TEC in TgCS/+ and TgSPP/+ mice as compared with Csf1op/op mice (Figure 3C). Moreover, the number of apoptotic TEC in TgCS/+ and TgSPP/+ mice was similar to WT mice. In contrast, we did not detect a rise in activated Mø (Figure 3B) and apoptotic TEC (Figure 3C) in TgSGP/+ mice above the level in Csf1op/op mice. Thus, the csCSF-1 and spCSF-1 isoforms are instrumental in fostering Mø activation and, in turn, Mø-mediated TEC apoptosis during advancing lupus nephritis.
csCSF-1 and spCSF-1 Shift Circulating Monocytes toward the “Inflammatory” Phenotype in Lupus Nephritis
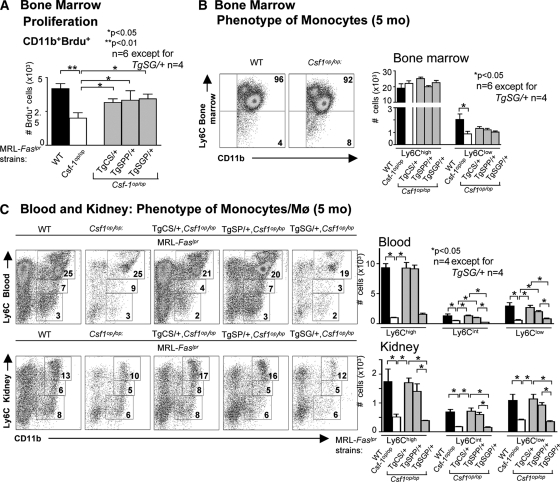
The accumulation of Mø in the kidney with advancing lupus nephritis may result from a rise in the number of circulating monocytes and a shift toward monocytes that are more readily recruited to inflamed tissues. We previously established that overexpression of CSF-1 in the circulation and kidney enhanced proliferation of bone-marrow monocytes and increased the number of circulating monocytes that, in turn, accumulated in the kidney during lupus nephritis.5 To probe for the origin of Mø accumulation in the kidney of TgCS/+ and TgSPP/+ mice, we investigated whether Mø were proliferating in the bone marrow in TgCS/+, TgSPP/+, TgSGP/+, WT, and Csf1op/op mice (5 months of age). We detected equal numbers of proliferating monocytes in the bone marrow of each individual CSF-1 isoform transgenic strain that was equivalent to WT (Figure 4A). Thus, the cell surface and both secreted CSF-1 isoforms each similarly regulated monocytes proliferation in the bone marrow.
Figure 4.
csCSF-1, spCSF-1 and sgCSF-1 heightened bone-marrow monocyte proliferation, but only csCSF-1 and spCSF-1 shift circulating Mø to the inflammatory phenotype. (A) Proliferation of BM CD11b+ leukocytes evaluated by flow cytometry 24 h after intraperitoneal administration of Brdu to TgCS/+, TgSPP/+, TgSGP/+, wild-type (WT), and Csf1op/op mice. (B and C) Flow cytometric analysis of circulating SSClowCD11b+Ly6Chi, Ly6Ciint, and Ly6Clo leukocytes in BM, blood, and kidney. The mice were 5 months of age. The values are the means ± SEM. Representative FACS plots are shown.
There are two well documented, functional populations of monocytes in the blood: a short-lived Ly6Chi CX3CR1lowCCR2+ subset that is actively recruited to inflamed tissues and is referred to as inflammatory and a Ly6ClowCX3CR1hiCCR2− “resident” subset characterized by CCR1-dependent recruitment to noninflamed tissues.19,23 To determine the effect of each individual isoform in shifting monocytes to an inflammatory phenotype, we probed for the expression of these monocytes subsets in the bone marrow, circulation, and kidney of TgCS/+, TgSPP/+, and TgSGP/+ mice with lupus nephritis (5 months of age). We compared our findings with those for Csf1op/op and WT mice, which express all three CSF-1 isoforms. We determined that most (92 to 97%) bone-marrow monocytes populations in TgCS/+, TgSPP/+, TgSGP/+, WT, and Csf1op/op mice expressed Ly6Chi (Figure 4B). Because we did not identify differential expression of Ly6Chi in these mice, this suggests that CSF-1 did not regulate the expression of inflammatory monocytes in the bone marrow. However, we identified numbers of Ly6Chi monocytes in TgCS/+ and TgSPP/+ mice, but not in TgSGP/+ mice, in the blood and kidney that were equivalent to WT levels on the basis of total cell number (Figure 4C) and cell frequency (Supplemental Figure 5). Moreover, the levels of blood and kidney Ly6Cint and Ly6Clo monocytes in TgCS/+ and TgSPP/+ mice, but not in TgSGP/+ mice, were similar to WT levels. Because Ly6ChiCX3CR1lo monocytes are preferentially recruited to the inflamed tissue, these findings are consistent with the central role for csCSF-1 and spCSF-1 in promoting the intrarenal accumulation of Ly6Chigh Mø during lupus nephritis.
Intrarenal csCSF-1 and spCSF-10 Mediate Monocyte Recruitment into the Kidney during Lupus Nephritis
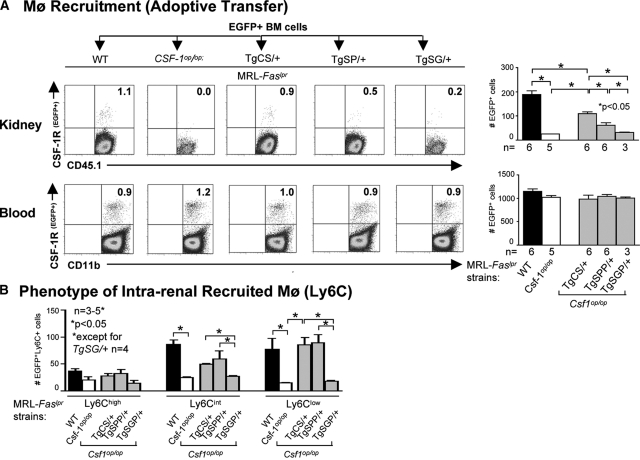
To evaluate the effect of each individual CSF-1 isoform to recruit monocytes into the kidney, we injected eGFP+ bone-marrow cells (derived from MacGreen;MRL-Faslpr mice) into TgCS/+, TgSPP/+, TgSGP/+, Csf1op/op, and WT mice with lupus nephritis (5 months of age). The majority of transferred bone-marrow cells were Ly6Chi (88 ± 5.2%), a finding that is similar in non-lupus-susceptible mice.23 Once monocytes egress from the bone marrow into the circulation, the milieu is responsible for whether Ly6C expression on monocytes is downregulated and, in turn, less readily recruited to inflamed tissue.23 After transfer of eGFP+ bone-marrow cells, we detected more eGFP+ cells in the kidney of TgCS/+ mice as compared with TgSPP/+ mice (Figure 5A). However, there were fewer eGFP+ bone-marrow cells in TgCS/+ mice as compared with WT mice. In fact, only after combining the eGFP+ cells recruited to the kidney in TgCS/+ and TgSPP/+ mice did we reach WT levels. In contrast, the number of eGFP+ cells recruited into the kidney of TgSGP/+ and Csf1op/op mice was minimal (Figure 5). Moreover, because we detected similar numbers of eGFP+ cells in the blood for each CSF-1 transgenic strain, we verified that each group received similar numbers of eGFP+ cells. Because the transferred monocytes were almost all Ly6Chi, we determined whether the Ly6C expression of these transferred bone-marrow monocytes changed within the kidney. After transfer, the Ly6Chi expression on eGFP+ monocytes downregulated, resulting in more Ly6Clow and Ly6Cint than Ly6Chi cells (Figure 5B). Of note, because we perfused kidneys before analysis, the eGFP+ cells are in the tissue and not within vessels. Taken together, the recruitment of Mø and their phenotypic expression during lupus nephritis may be dependent on the combined intrarenal expression of csCSF-1 along with spCSF-1.
Figure 5.
Intrarenal csCSF-1 and spCSF-1 mediate the recruitment of activated monocytes into the kidney during lupus nephritis. (A) BM cells from MacGreen(eGFP+);MRL-Faslpr mice were transferred into TgCS/+, TgSPP/+, TgSG/+, wild-type (WT), and Csf1op/op; MRL-Faslpr mice and the recruitment of eGFP+ cells into the kidney 24 hours after transfer of cells were evaluated by flow cytometry. WT and Csf1op/op mice served as positive and negative controls, respectively. The data are representative of three experiments. The mice were 5 months of age. The values are the means ± SEM. Representative FACS plots are displayed. (B) Flow cytometric analysis of the recruited eGFP+ Mø for there Ly6Chi, Ly6Ciint, and Ly6Clo expression in kidney. The mice were 5 months of age. The values are the means ± SEM.
Intrarenal csCSF-1 and, to a Lesser Degree, spCSF-1 Promote Mø Proliferation and Preferentially Expanded the Ly6Chi Mø Population
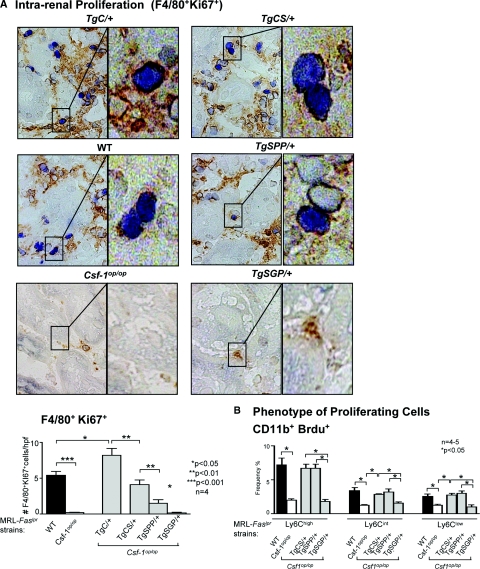
To identify the individual CSF-1 isoforms that mediate Mø proliferate within the kidney, we evaluated the intrarenal proliferating cells in TgCS/+, TgSPP/+, and TgSGP/+ mice in comparison with WT mice with advanced lupus nephritis (5 months of age) and with age-matched Csf1op/op mice deficient in CSF-1. We detected a substantial increase in proliferating Mø (F4/80+, Ki67+) in the kidney of TgCS/+ as compared with Csf1op/op mice that was equivalent to WT levels (Figure 6A). By comparison, the proliferation of intrarenal Mø in TgSPP/+ mice was less robust as compared with TgCS/+ mice. In contrast, the number of intrarenal proliferating Mø in TgSGP/+ mice did not rise above the level in Csf1op/op mice. We then determined whether preferential expansion of Ly6Chi cells contributed to the increase in the accumulation of these Mø in the kidney during lupus nephritis (Figure 4B). csCSF-1 and spCSF-1 increased the number of proliferating Ly6Chi, Ly6Cint, and Ly6Clo Mø in the kidney to the same level as in WT mice, with the number of Ly6Chi predominating in each case (Figure 6B). Of note, because monocytes in the blood do not proliferate, we are confident that our results reflect intrarenal proliferation. Moreover, our findings were similar using bromodeoxyuridine (Brdu) as a proliferation index (data not shown). Taken together, the csCSF-1 and spCSF-1 isoforms are responsible for recruiting Ly6Chi cells into the kidney. Ly6C expression is initially downregulated on Mø that were recruited into the kidney (Figure 5B); however, the preferential expansion of Ly6Chi Mø population (Figure 6B) results in a greater accumulation of Ly6Chi than of Ly6Clow and Ly6Cint Mø with advancing disease (Figure 4C), which, in turn, mediates the progression of lupus nephritis.
Figure 6.
Intrarenal csCSF-1 and, to a lesser degree, spCSF-1 regulate Mø proliferation and preferentially expanded the Ly6Chi Mø population. (A) Proliferation of TgC/+, TgCS/+, TgSPP/+, TgSGP/+, wild-type (WT), and Csf1op/op mice evaluated by dual staining kidney sections (brown reaction product (F4/80+ cells) = Mø, blue reaction product (Ki67+intracellular) = proliferating cells). Enlargement shows proliferating F4/80+ cells. The data are the means ± SEM. Representative microphotographs are shown; magnification, 40×; enlargement magnification, 100×. (B) Flow cytometric analysis of intrarenal Mø for their capacity to proliferate in the Ly6C subpopulations. The mice were 5 months of age. The values are the means ± SEM.
DISCUSSION
CSF-1 promotes Mø-rich lupus nephritis through a series of CSF-1-dependent mechanistic steps. CSF-1 emanating from the kidney and within the circulation promotes lupus nephritis and the systemic illness in a model of lupus with a multiorgan disease, the MRL-Faslpr mouse. CSF-1, expressed largely by TEC, spills over into the circulation, regulates the progenitor monocytes that are seeded from the bone marrow into the circulation, shifts the monocytes population toward an inflammatory and activated phenotype, and fosters monocyte recruitment into the kidney.5,8,24–28 The net result is a florid accumulation of Mø in the kidney. These intrarenal Mø are poised to destroy the kidney as they are activated and release mediators that induce TEC apoptosis.5,22 Prompted by these findings, we established that a rise of CSF-1 expression in the kidney, circulation, and urine is an index of serologically active lupus nephritis in humans.5 Thus, dissecting the source and function of the three distinct CSF-1 isoforms is critical to designing therapeutic approaches for lupus nephritis. Using unique mutant transgenic MRL-Faslpr mice expressing the individual membrane-spanning csCSF-1 isoform and the secreted CSF-1 precursors, spCSF-1 and sgCSF-1, we now report several novel findings. First, spCSF-1, not sgCSF, is responsible for the rise in CSF-1 in the circulation instrumental in advancing lupus nephritis. Second, csCSF-1 and spCSF-1 each shift the circulating monocyte population toward an inflammatory, activated phenotype more readily recruited to the kidney during lupus nephritis. Third, an increase in either intrarenal csCSF-1 or spCSF-1 recruits and expands activated Ly6Chi Mø, thereby leading to the destruction of renal parenchyma during lupus nephritis. Taken together, these studies provide insight into designing therapeutic strategies necessary to block the CSF-1 isoforms that drive lupus nephritis.
Intrarenal and circulating spCSF-1 isoform is dramatically increased in the kidney during lupus nephritis and facilitates renal destruction in MRL-Faslpr mice. The spCSF-1 isoform that is increased in the serum and kidney during lupus nephritis restores renal disease (pathology and loss of function), the accumulation of intrarenal Mø, and TEC apoptosis in the MRL-Faslpr mice deficient in CSF-1. The effect of spCSF-1 on lupus nephritis is particularly striking because spCSF-1 and sgCSF-1 have a short half-life (approximately 10 minutes) in vivo, at least in normal mice,29 as compared with csCSF-1 (approximately 7 hours in cultured cells).30 Moreover, our findings for the spCSF-1 isoform are even more impressive because the TgSPP/+ strain that we backcrossed with MRL-Faslpr mice only generates half the amount of serum CSF-1 compared with WT mice, and the sgCSF-1 isoform is responsible for contributing the other half. Thus, it is remarkable that spCSF-1 is so prominent in the circulation and kidney during lupus nephritis. The rise in spCSF-1 in the kidneys, evident during the initial phase of lupus nephritis, precedes the rise in serum spCSF-1 during the advanced phase of this illness. This is consistent with CSF-1 emanating from the renal TEC and perhaps other inflamed tissues such as the skin that leads to elevating CSF-1 in the circulation.5,31 The lag in the rise of serum spCSF-1 during lupus nephritis in MRL-Faslpr mice may be related to a relatively low rate of secretion of spCSF-1 into the circulation in early disease and its rapid clearance until the clearance mechanisms are saturated by the increased amount produced as disease progresses. Saturation of the physiologic clearance mechanism greatly extends the half-life of circulating CSF-1.29 Moreover, it is impressive that spCSF-1, as opposed to sgCSF-1, is retained in the kidney and is biologically central to advancing lupus nephritis. The ChS chain is central to the CSF-1 mechanisms advancing lupus nephritis, because the covalently-linked glycosaminoglycan ChS chain is the only difference between the precursors of the spCSF-1 and sgCSF-1.16 Because the ChS chain on the spCSF-1 has previously been shown to bind type V collagen, a component of extracellular matrix,32 there are several possible mechanisms by which the ChS chain may facilitate CSF-1 renal destruction. It may bind spCSF-1 to components of the kidney's extracellular matrix (e.g. collagen, perlecan, and fibronectin), which is expanded during disease progression, thereby concentrating and extending the bioavailability of the local expression of CSF-1. Additionally, the ChS chain may prevent the rapid proteolytic inactivation of CSF-1 by a membrane proteases such as CD26/dipeptidyl peptidase IV, as reported for heparan sulfate-protection of stromal cell-derived factor-1,33 a molecule released by TEC during renal injury34,35 that promotes lupus.36 Although the exact mechanism responsible for the role of intrarenal spCSF-1 advancing lupus nephritis via the ChS chain remain to be explored, our studies highlight the pivotal position of the spCSF-1 isoform in driving lupus nephritis.
Intrarenal membrane-spanning csCSF-1 and intrarenal and serum spCSF-1 mediate the recruitment of monocytes into the kidney during lupus nephritis. It is possible to envision the attraction of Mø into the kidney via spCSF-1 that sets up a concentration gradient emanating within the kidney and extending into the circulation. However, the recruitment of Mø by csCSF-1, which is more potent than spCSF-1, is more puzzling. Among the most plausible explanations is that csCSF-1 may activate intrarenal rogue Mø that initiate inflammation, thereby releasing a gradient of chemokines, largely generated by TEC, that are responsible for recruiting a stream of monocytes that amplify renal inflammation. Another possibility is that TEC expressing csCSF-1 establishes a gradient of cleaved, locally released CSF-1 that, alone or together with chemokines, attracts monocytes toward the TEC.37 Although possible, it is less likely that intrarenal csCSF-1 is proteolytically cleaved to generate a soluble growth factor released into the circulation comparable to the secreted CSF-1 isoforms,38 because, at least at the times we evaluated the serum, CSF-1 was not detectable. A fourth possibility derives from the expression of CSF-1 by endothelial cells.39 The increased expression of csCSF-1 by endothelial cells during lupus may be instrumental in the recruitment CSF-1R-expressing Mø for extravasation.
Our data indicate that Ly6Chi Mø preferentially expand and are prominent in MRL-Faslpr mice with advancing lupus nephritis. In contrast, Ly6Clow Mø preferentially expand in another lupus model, BXSB-Yaa.40 It is not surprising that our findings and those in BXSB-Yaa differ because this strain and the MRL-Faslpr have distinct pathogenic mechanisms. Lupus in BXSB-Yaa mice only occurs in males, and these mice develop a monocytosis linked to a mutant gene located on the Y chromosome (Yaa),37 whereas MRL-Faslpr females and males develop lupus nephritis and have multiple lymphoid feature abnormalities that are absent in BXSB-Yaa.41 Moreover, the expression of CD14+CD16− monocytes, the equivalent of Ly6Chi mouse monocytes, in human lupus is controversial. Although some studies report an increase in circulating CD14+CD16+ monocytes (equivalent to mouse Ly6Clow) subsets,42,43 others detect a decrease in the number of circulation monocytes without a difference in the proportion of CD14+CD16− and CD14+CD16+ monocytes subsets in lupus patients compared with healthy controls.44 Further experiments detailing the function of Ly6C-expressing Mø subsets in the kidney of distinct mouse models of lupus nephritis and studies probing CD14 and CD16 expression in intrarenal Mø in patients with lupus nephritis45 are required to clarify the role of these Mø populations in the pathogenesis of lupus nephritis.
Our findings underscore differing effects of the individual CSF-1 isoforms during normal development and disease. We have shown that the csCSF-1 isoform fully restores lupus nephritis and systemic illness in MRL-Faslpr mice deficient in CSF-1. In contrast, the secreted CSF-1 isoforms rescued most other Csf1op/op defects more efficiently than the csCSF-1 isoform during normal development in nonautoimmune (FVB/NJ) mice.16 During normal development, the csCSF-1 isoform fully restored Mø accumulation in the renal cortex but not in the medulla. In contrast, these findings indicate that csCSF-1 fully replenished the accumulation of Mø in the renal cortex and medulla during advancing lupus nephritis in MRL-Faslpr mice deficient in CSF-1. On the other hand, the relative effect of spCSF-1 and sgCSF-1 in restoring lupus nephritis in MRL-Faslpr mice deficient in CSF-1 and repairing normal developmental defects in CSF-1-deficient mice are similar. For example, spCSF-1 more fully restored the numbers of Mø in the kidney as compared with sgCSF-1 during normal development,16 a finding consistent with the relative effect of spCSF-1 and sgCSF-1 on Mø accumulation in advancing lupus nephritis. Taken together, this suggests that the role of the cell surface and secreted CSF-1 isoforms regulate distinct Mø with differing functions during normal development and autoimmune renal disease.
Intrarenal proliferation, regulated by CSF-1, is a major contributor to the intrarenal accumulation of Mø during lupus nephritis. Interestingly, the csCSF-1 isoform more fully restored intrarenal Mø proliferation than the spCSF-1 isoform, whereas the sgCSF-1 isoform was the least effective in MRL-Faslpr mice deficient in CSF-1. One possible explanation is that the membrane-spanning csCSF-1 displayed on the cell surface binds to the CSF-1 receptor on the Mø and provides a stabilizing engagement required to signal proliferation. This cell-cell contact may be more stable than spCSF-1, bound perhaps to the extracellular matrix, engaging with CSF-1 receptors on Mø. Also, because sgCSF-1 is modestly retained within the kidney during lupus nephritis, it is not surprising that the sgCSF-1 isoform does not mediate intrarenal proliferation in MRL-Faslpr mice with lupus nephritis. Thus, intrarenal csCSF-1 and pgCSF-1 are potent triggers for Mø proliferation and thereby mediate Mø accumulation in MRL-Faslpr mice during advancing lupus nephritis.
Because CSF-1 may be a potential therapeutic target for lupus nephritis, understanding the CSF-1 isoforms that regulate Mø that responsible for renal destruction are critical. We previously detailed the effect of CSF-1 in the circulation, apart from the kidney, in promoting lupus nephritis.5 Intrarenal expression of CSF-1 in the kidney is central to the recruitment and expansion of Mø within kidneys. Thus, our findings highlight the pivotal position of intrarenal CSF-1 in mediating advancing lupus nephritis. Although it may be less difficult to eliminate CSF-1 in the circulation, constructing approaches that effectively block the expression of csCSF-1 and spCSF-1 in the kidney may be necessary and more challenging for enduring therapeutic efficacy. Taken together, our findings tease apart the relative contribution of each CSF-1 isoform in the pathogenesis of lupus nephritis. Moreover, because Mø are emerging into the forefront as regulators of a broad array of diseases, clarifying the effect of circulating and intrarenal CSF-1 isoforms during lupus nephritis may be applicable to other Mø-rich kidney and immune-mediated diseases.
CONCISE METHODS
Mice
Mice heterozygous for the osteopetrotic mutation (Csf1op) on the C57BL/6JxC3Heb/FeJ-a/a background and MRL/MpJ-Faslpr/Faslpr (MRL-Faslpr) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The Csf1op mutation was backcrossed onto the MRL-Faslpr background for ten generations. The plasmid bearing the full-length CSF-1 driven by the CSF-1 promotor and the first intron14 was used in the construction of the cell surface (TgCS), secreted proteoglycan precursor (TgSPP), and secreted glycoprotein precursor (TgSGP) transgenic mice.15,16 The breeding, genotyping, and nomenclature of these transgenic mice have been described previously,14–16 and they were backcrossed onto the MRL-Faslpr background for seven generations before their introduction onto the Csf1op/Csf1op (Csf1op/op) MRL-Faslpr background. The resulting transgenic lines, in which the only source of CSF-1 is derived from the transgene, were used for experiments: (1) Csf1op/Csf1op; TgN(FLCsf1)10Ers/+ (referred to as TgC/+); (2) Csf1op/Csf1op; TgN(CSCsf1)8Ers/+ (referred to as TgCS/+); (3) Csf1op/Csf1op; TgN(SPPCsf1)7Ers/+ (referred to as TgSPP/+); and (4) Csf1op/Csf1op; TgN(SGPCsf1)4Ers/+ (referred to as TgSGP/+). Transgenic Tgfms-EGFP mice that express EGFP under the control of the CSF-1R (c-fms) promoter and first intron46 were provided by Dr. D.A. Hume (University of Edinburgh, Edinburgh, UK). These mice were backcrossed onto the MRL-Faslpr background (N7) and are referred to as MacGreen;MRL-Faslpr mice. The mice were bred and housed at Harvard Medical School. Only female mice were used. The use of mice in this study was reviewed and approved by the Standing Committee on Animals in the Harvard Medical School in adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
CSF-1 ELISA
To quantify the levels of CSF-1 in serum and tissue homogenates, we evaluated samples using an ELISA method as previously detailed.5 Briefly, we homogenized tissue samples using a MixMill 300 (Qiagen, Valencia, CA). We determined the protein concentration of each homogenate using the BCA Protein Assay Kit (Pierce, Rockford, IL) and evaluated 200 μg of protein per tissue sample. The antibodies (Abs) and reagents in this assay were purchased from BD Bioscience (San Jose, CA).
Splenomegaly/Lymphadenopathy
We assessed splenomegaly and lymphadenopathy by the ratio of the spleen and lymph node weights to body weight when the mice were sacrificed.
Renal Function
We measured blood urea nitrogen and albuminuria as described previously.47
Kidney Pathology
We fixed kidneys in 10% formalin, prepared and stained paraffin sections with the periodic acid Schiff's reagent. The slides were coded before grading the renal pathology. We evaluated renal (glomerular and tubular) pathology on a scale of 0 (normal) to 3 (severe) as described previously.28
Immunostaining
Kidney and skin tissue was processed and stained for the presence of CD68, CD4, CD8 (eBioscience, San Diego, CA), and anti-cleaved caspase-3 Ab (Asp175; Cell -signaling, MA).47 The number of cells bearing CD4, CD8, and CD68 determinants and apoptotic cells was assessed in ten randomly selected high-power fields. We determined intrarenal Mø proliferation by dual staining.48 Briefly, we stained frozen kidney sections for the presence of Mø (F4/80; Serotec, Raleigh, NC) and identified proliferating cells using Ki67 (SP6; Lab Vision, Fremont, CA). We assessed Mø proliferation by counting the number of Ki67+ cells bearing F4/80+ in ten randomly-selected high-power fields.
Flow Cytometry
We prepared and stained single cell suspensions from kidneys, blood leukocytes, bone-marrow monocytes (BM) as described previously.47 We collected 0.5 to 1.0 × 106 total kidney cells and 0.5 to 1.0 × 105 blood leukocytes/BM monocytes using a FACSCalibur (Becton Dickinson, San Jose, CA) and analyzed the data using Flowjo software (Tree Star, Palo Alto, CA).
Antibodies
We used the following Abs from eBioscience (San Diego, CA) for FACS analysis: FITC-conjugated anti-CD4 (L3T4), anti-CD8 (53 to 6.7), anti-CD11b (M1/70) band anti-CD45.1; (104) PE-conjugated anti-CD4, anti-CD45.1, anti-CD69 (H1.2F3), anti-CD86 (GL1), anti-TNF-α; PE-Cy5-conjugated anti-CD8; and allophycocyanin-conjugated anti-CD4, anti-CD45.1, and anti-F4/80 (BM8). We used FITC- and allophycocyanin- conjugated anti-CD68 Ab (FA11) and PE-conjugated Ly6C (Serotec, Oxford, UK). For the secondary PE- or allophyocyanin-conjugated Ab, we used goat anti-rabbit Ab from Jackson ImmunoResearch Laboratories (West Grove, PA) and biotin-conjugated rabbit anti-goat Ab (Vector Laboratories, Burlingame, CA). To detect biotin-conjugated secondary Abs, we used streptavidin PE or allophyocyanin (Jackson ImmunoResearch Laboratories, West Grove, PA).
Brdu Treatment
In vivo, we injected mice with Brdu (2 mg/mouse intraperitoneally, every 12 hours; Sigma, St. Louis, MO) for 24 hours before sacrifice. Brdu+ cells were analyzed by flow cytometry with an anti-Brdu Ab (eBioscience, San Diego, CA).
Adoptive Transfer
We isolated BM from MacGreen;MRL-Faslpr mice and adoptively transferred these EGFP+ cells (2 × 107) by injecting (intravenously) into the tail. After 24 h, the mice were sacrificed, and we perfused the kidneys with 25 ml of ice-cold PBS. We dissociated the kidneys into single cell suspension by removing the capsule and placing kidneys into a small (2.5 cm2) 40-μm mesh bag immersed in 10 ml of medium (DMEM with FCS). While the bag was held with a pair of forceps, a 1-ml syringe plunger was used to press kidney cells through the mesh bag into the medium. The cells were collected by centrifugation. Red blood cells were lysed using ACK lysing buffer, and the remaining cells were washed in PBS. We washed the cells with FACS buffer (PBS, 5% FBS, and 0.09% NaN3). We then stained cells isolated from the kidney and blood to detect EGFP+ cells using flow cytometry as described previously.47 We analyzed 3 to 5 × 104 CD45.1+ cells using a FACSCalibur.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK 36149 (to V.R.K.), The Alliance for Lupus Research (to V.R.K.), CA32551, and CA26504 (to E.R.S.); a National Kidney Foundation Fellowship (to Y.I.), and Deutsche Forschungsgemeinschaft Grant ME-3194/1-1 (to J.M. and the Department of Nephrology and Rheumatology, Johannes-Gutenberg University, Mainz, Germany).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Theofilopoulos AN, Dixon FJ: Eito-pathogenesis of murine SLE. Immunol Rev 55: 179–216, 1981 [DOI] [PubMed] [Google Scholar]
- 2. Kelley VE, Roths JB: Interaction of mutant lpr gene with background strain influences renal disease. Clin Immunol Immunopathol 37: 220–229, 1985 [DOI] [PubMed] [Google Scholar]
- 3. Moyer CF, Strandberg JD, Reinisch CL: Systemic mononuclear-cell vasculitis in MRL/Mp-lpr/lpr mice: A histologic and immunocytochemical analysis. Am J Pathol 127: 229–242, 1987 [PMC free article] [PubMed] [Google Scholar]
- 4. Tesch GH, Maifert S, Schwarting A, Rollins BJ, Kelley VR: Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice. J Exp Med 190: 1813–1824, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, Kelley VR: Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis. J Am Soc Nephrol 20: 2581–2592, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, Madaio MP, Bottinger EP, Davidson A: Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol 180: 1938–1947, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ricardo SD, van Goor H, Eddy AA: Macrophage diversity in renal injury and repair. J Clin Invest 118: 3522–3530, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yui MA, Brissette WH, Brennan DC, Wuthrich RP, Rubin-Kelley VE: Increased macrophage colony-stimulating factor in neonatal and adult autoimmune MRL-lpr mice. Am J Pathol 139: 255–261, 1991 [PMC free article] [PubMed] [Google Scholar]
- 9. Naito T, Yokoyama H, Moore KJ, Dranoff G, Mulligan RC, Kelley VR: Macrophage growth factors introduced into the kidney initiate renal injury. Mol Med 2: 297–312, 1996 [PMC free article] [PubMed] [Google Scholar]
- 10. Moore KJ, Naito T, Martin C, Kelley VR: Enhanced response of macrophages to CSF-1 in autoimmune mice: A gene transfer strategy. J Immunol 157: 433–440, 1996 [PubMed] [Google Scholar]
- 11. Kikawada E, Lenda DM, Kelley VR: IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology. J Immunol 170: 3915–3925, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Pixley FJ, Stanley ER: CSF-1 regulation of the wandering macrophage: Complexity in action. Trends Cell Biol 14: 628–638, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Jang MH, Herber DM, Jiang X, Nandi S, Dai XM, Zeller G, Stanley ER, Kelley VR: Distinct in vivo roles of colony-stimulating factor-1 isoforms in renal inflammation. J Immunol 177: 4055–4063, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Ryan GR, Dai XM, Dominguez MG, Tong W, Chuan F, Chisholm O, Russell RG, Pollard JW, Stanley ER: Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood 98: 74–84, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Dai XM, Zong XH, Sylvestre V, Stanley ER: Incomplete restoration of colony-stimulating factor 1 (CSF-1) function in CSF-1-deficient Csf1op/Csf1op mice by transgenic expression of cell surface CSF-1. Blood 103: 1114–1123, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Nandi S, Akhter MP, Seifert MF, Dai XM, Stanley ER: Developmental and functional significance of the CSF-1 proteoglycan chondroitin sulfate chain. Blood 107: 786–795, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rubin Kelley V, Bloom RD, Yui MA, Martin C, Price D: Pivotal role of colony stimulating factor-1 in lupus nephritis. Kidney Int Suppl 45: S83–S85, 1994 [PubMed] [Google Scholar]
- 18. Geissmann F, Jung S, Littman DR: Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82, 2003 [DOI] [PubMed] [Google Scholar]
- 19. Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F: Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670, 2007 [DOI] [PubMed] [Google Scholar]
- 20. Laskin DL, Sunil VR, Gardner CR, Laskin JD: Macrophages and tissue injury: Agents of defense or destruction. Annu Rev Pharmacol Toxicol 51: 267–288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nalbandian A, Crispin JC, Tsokos GC: Interleukin-17 and systemic lupus erythematosus: Current concepts. Clin Exp Immunol 157: 209–215, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tesch GH, Schwarting A, Kinoshita K, Lan HY, Rollins BJ, Kelley VR: Monocyte chemoattractant protein-1 promotes macrophage-mediated tubular injury, but not glomerular injury, in nephrotoxic serum nephritis. J Clin Invest 103: 73–80, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, Leenen PJ: Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol 172: 4410–4417, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Bloom RD, Florquin S, Singer GG, Brennan DC, Kelley VR: Colony stimulating factor-1 in the induction of lupus nephritis. Kidney Int 43: 1000–1009, 1993 [DOI] [PubMed] [Google Scholar]
- 25. Naito T, Griffiths RC, Coffman TM, Kelley VR: Transplant approach establishes that kidneys are responsible for serum CSF-1 but require a stimulus in MRL-lpr mice. Kidney Int 49: 67–74, 1996 [DOI] [PubMed] [Google Scholar]
- 26. Wada T, Naito T, Griffiths RC, Coffman TM, Kelley VR: Systemic autoimmune nephritogenic components induce CSF-1 and TNF-alpha in MRL kidneys. Kidney Int 52: 934–941, 1997 [DOI] [PubMed] [Google Scholar]
- 27. Lenda D, Kikawada E, Stanley ES, Kelley VR: Reduced Mø recruitment, proliferation, and activation in CSF-1 deficient mice, results in decreased tubular apoptosis during renal inflammation. J Immunol 2004 [DOI] [PubMed] [Google Scholar]
- 28. Lenda DM, Stanley ER, Kelley VR: Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice. J Immunol 173: 4744–4754, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Bartocci A, Mastrogiannis DS, Migliorati G, Stockert RJ, Wolkoff AW, Stanley ER: Macrophages specifically regulate the concentration of their own growth factor in the circulation. Proc Natl Acad Sci U S A 84: 6179–6183, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Price LK, Choi HU, Rosenberg L, Stanley ER: The predominant form of secreted colony stimulating factor-1 is a proteoglycan. J Biol Chem 267: 2190–2199, 1992 [PubMed] [Google Scholar]
- 31. Menke J, Hsu MY, Byrne KT, Lucas JA, Rabacal WA, Croker BP, Zong XH, Stanley ER, Kelley VR: Sunlight triggers cutaneous lupus through a CSF-1-dependent mechanism in MRL-Fas(lpr) mice. J Immunol 181: 7367–7379, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Suzu S, Ohtsuki T, Makishima M, Yanai N, Kawashima T, Nagata N, Motoyoshi K: Biological activity of a proteoglycan form of macrophage colony-stimulating factor and its binding to type V collagen. J Biol Chem 267: 16812–16815, 1992 [PubMed] [Google Scholar]
- 33. Sadir R, Imberty A, Baleux F, Lortat-Jacob H: Heparan sulfate/heparin oligosaccharides protect stromal cell-derived factor-1 (SDF-1)/CXCL12 against proteolysis induced by CD26/dipeptidyl peptidase IV. J Biol Chem 279: 43854–43860, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Lotan D, Sheinberg N, Kopolovic J, Dekel B: Expression of SDF-1/CXCR4 in injured human kidneys. Pediatr Nephrol 23: 71–77, 2008 [DOI] [PubMed] [Google Scholar]
- 35. Togel F, Isaac J, Hu Z, Weiss K, Westenfelder C: Renal SDF-1 signals mobilization and homing of CXCR4-positive cells to the kidney after ischemic injury. Kidney Int 67: 1772–1784, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Balabanian K, Couderc J, Bouchet-Delbos L, Amara A, Berrebi D, Foussat A, Baleux F, Portier A, Durand-Gasselin I, Coffman RL, Galanaud P, Peuchmaur M, Emilie D: Role of the chemokine stromal cell-derived factor 1 in autoantibody production and nephritis in murine lupus. J Immunol 170: 3392–3400, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Izui S, Higaki M, Morrow D, Merino R: The Y chromosome from autoimmune BXSB/MpJ mice induces a lupus-like syndrome in (NZW×C57BL/6)F1 male mice, but not in C57BL/6 male mice. Eur J Immunol 18: 911–915, 1988 [DOI] [PubMed] [Google Scholar]
- 38. Horiuchi K, Miyamoto T, Takaishi H, Hakozaki A, Kosaki N, Miyauchi Y, Furukawa M, Takito J, Kaneko H, Matsuzaki K, Morioka H, Blobel CP, Toyama Y: Cell surface colony-stimulating factor 1 can be cleaved by TNF-alpha converting enzyme or endocytosed in a clathrin-dependent manner. J Immunol 179: 6715–6724, 2007 [DOI] [PubMed] [Google Scholar]
- 39. Aharinejad S, Abraham D, Paulus P, Zins K, Hofmann M, Michlits W, Gyongyosi M, Macfelda K, Lucas T, Trescher K, Grimm M, Stanley ER: Colony-stimulating factor-1 transfection of myoblasts improves the repair of failing myocardium following autologous myoblast transplantation. Cardiovasc Res 79: 395–404, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Amano H, Amano E, Santiago-Raber ML, Moll T, Martinez-Soria E, Fossati-Jimack L, Iwamoto M, Rozzo SJ, Kotzin BL, Izui S: Selective expansion of a monocyte subset expressing the CD11c dendritic cell marker in the Yaa model of systemic lupus erythematosus. Arthritis Rheum 52: 2790–2798, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Kikuchi S, Santiago-Raber ML, Amano H, Amano E, Fossati-Jimack L, Moll T, Kotzin BL, Izui S: Contribution of NZB autoimmunity 2 to Y-linked autoimmune acceleration-induced monocytosis in association with murine systemic lupus. J Immunol 176: 3240–3247, 2006 [DOI] [PubMed] [Google Scholar]
- 42. Cairns AP, Crockard AD, Bell AL: The CD14+ CD16+ monocyte subset in rheumatoid arthritis and systemic lupus erythematosus. Rheumatol Int 21: 189–192, 2002 [DOI] [PubMed] [Google Scholar]
- 43. Sumegi A, Antal-Szalmas P, Aleksza M, Kovacs I, Sipka S, Zeher M, Kiss E, Szegedi G: Glucocorticosteroid therapy decreases CD14-expression and CD14-mediated LPS-binding and activation of monocytes in patients suffering from systemic lupus erythematosus. Clin Immunol 117: 271–279, 2005 [DOI] [PubMed] [Google Scholar]
- 44. Li Y, Lee PY, Reeves WH: Monocyte and macrophage abnormalities in systemic lupus erythematosus. Arch Immunol Ther Exp 58: 355–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoshimoto S, Nakatani K, Iwano M, Asai O, Samejima K, Sakan H, Terada M, Harada K, Akai Y, Shiiki H, Nose M, Saito Y: Elevated levels of fractalkine expression and accumulation of CD16+ monocytes in glomeruli of active lupus nephritis. Am J Kidney Dis 50: 47–58, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Sasmono RT, Oceandy D, Pollard JW, Tong W, Pavli P, Wainwright BJ, Ostrowski MC, Himes SR, Hume DA: A macrophage colony-stimulating factor receptor-green fluorescent protein transgene is expressed throughout the mononuclear phagocyte system of the mouse. Blood 101: 1155–1163, 2003 [DOI] [PubMed] [Google Scholar]
- 47. Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N, Mayadas TN, Lan HY, Sharpe AH, Kelley VR: Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: Distinct roles. J Immunol 179: 7466–7477, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Le Meur Y, Tesch GH, Hill PA, Mu W, Foti R, Nikolic-Paterson DJ, Atkins RC: Macrophage accumulation at a site of renal inflammation is dependent on the M-CSF/c-fms pathway. J Leukocyte Biol 72: 530–537, 2002 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.