Abstract
Although fibroblasts are responsible for the production and deposition of extracellular matrix in renal fibrosis, their origin is controversial. Circulating fibroblast precursors may contribute to the pathogenesis of renal fibrosis, but the signaling mechanisms underlying the recruitment of bone marrow-derived fibroblast precursors into the kidney in response to injury are incompletely understood. Here, in the unilateral ureteral obstruction model of renal fibrosis, tubular epithelial cells upregulated the chemokine CXCL16 in obstructed kidneys, and circulating fibroblast precursors expressed the CXCL16 receptor, CXCR6. Compared with wild-type mice, CXCL16-knockout mice accumulated significantly fewer bone marrow-derived fibroblast precursors in obstructed kidneys. CXCL16-knockout mice also exhibited significantly fewer CD45-, collagen I-, and CXCR6-triple-positive fibroblast precursors in injured kidneys. Furthermore, targeted deletion of CXCL16 inhibited myofibroblast activation, reduced collagen deposition, and suppressed expression of collagen I and fibronectin. In conclusion, CXCL16 contributes to the pathogenesis of renal fibrosis by recruiting bone marrow-derived fibroblast precursors.
Renal fibrosis is a hallmark of chronic kidney disease, and the degree of interstitial fibrosis correlates well with the prognosis of kidney disease, regardless of the underlying etiology.1,2 Furthermore, interstitial fibrosis is a key structural component of obstructive nephropathy, which is the major cause of chronic kidney disease in children.3 Renal interstitial fibrosis is characterized by massive fibroblast activation and excessive production and deposition of extracellular matrix (ECM), which leads to the destruction and collapse of renal parenchyma and progressive loss of kidney function. Because fibroblasts are the principal effector cells that are responsible for ECM production in the fibrotic kidney, their activation is regarded as a key event in the pathogenesis of renal fibrosis.4–6 However, the origin of these fibroblasts remains controversial. They are traditionally thought to arise from resident renal fibroblasts. Recent evidence indicates that they may originate from epithelial/endothelial-to-mesenchymal transition7–10 and bone marrow-derived progenitor cells.7,11–13
The bone marrow-derived fibroblast precursor cells termed “fibrocytes” were first identified in the peripheral circulation in 1994.14 These cells express mesenchymal markers such as collagen I and vimentin and hematopoietic markers such as CD45, CD11b, and CD34.14–17 These cells in culture display an adherent, spindle-shape morphology and express α-smooth muscle actin (α-SMA) that is enhanced when cells are treated with TGF-β1, consistent with the concept that they can differentiate into myofibroblasts.15–17 Recent studies have shown that these cells are involved in the pathogenesis of renal fibrosis.11,18 However, the molecular mechanisms underlying the recruitment of these cells into injured kidneys are not fully understood.
Chemokines are classified based on the relative position of cysteine residues near the amino terminus into four major families: CC, CXC, C, and CX3C.19,20 Chemokines activate their seven-transmembrane G-protein-coupled receptors and play primary roles in mediating the trafficking of circulating cells during inflammation.21 CXCL16 is a recently discovered cytokine belonging to the CXC chemokine family.22 There are two forms of CXCL16. The soluble form generated by its cleavage at the cell surface functions as a chemoattractant to recruit circulating cells. The transmembrane form has a transmembrane structure that functions as an adhesion molecule for CXCR6-expressing cells and a scavenger receptor for oxidized LDL.
In this study, we investigated the role of CXCL16 in the recruitment of bone marrow-derived fibroblast precursors into the kidney and renal fibrosis in a well established model of tubulointerstitial injury induced by unilateral ureteral obstruction (UUO) using CXCL16-knockout (KO) mice. Our results show that targeted disruption of CXCl16 prevents the development of renal fibrosis by suppressing fibroblast precursor infiltration into the kidney.
RESULTS
CXCL16 Is Induced in a Mouse Model of Renal Fibrosis
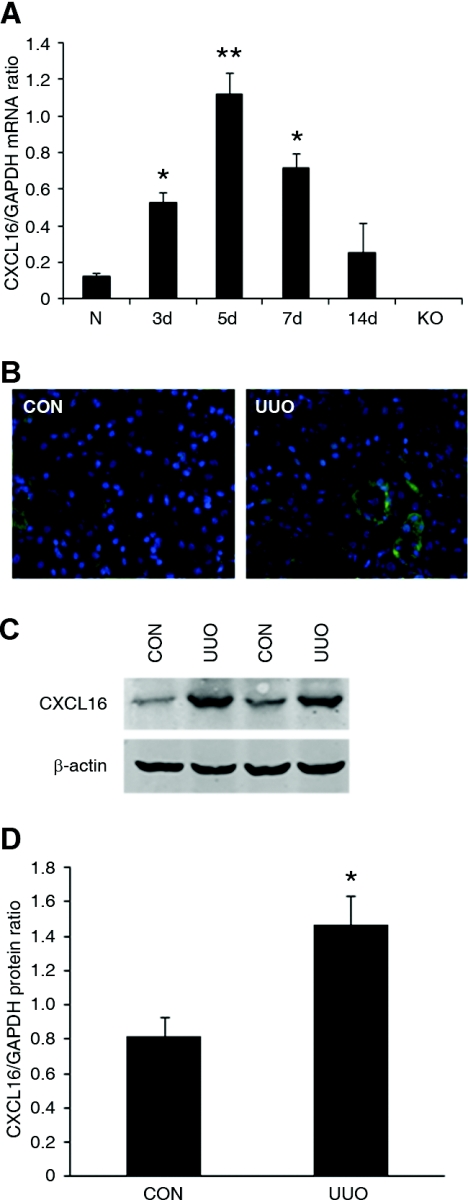
We first characterized the induction of CXCL16 in the kidney in a mouse model of tubulointerstitial fibrosis induced by UUO. Using real-time reverse transcription-PCR (RT-PCR), we found that the mRNA level of CXCL16 was upregulated in a time-dependent manner, reaching >10-fold increases in injured kidneys compared with that of control kidneys after 5 days of UUO (Figure 1A). Of note, CXCL16 mRNA was not detected in CXCL16-KO mice, which confirms the complete gene inactivation of CXCL16 in the KO mice. Serial sections of kidneys stained with anti-CXCL16 antibody revealed that CXCL16 protein was expressed at a low level in epithelial cells in control kidneys and its level was significantly induced in the epithelial cells in obstructed kidneys (Figure 1B). Consistent with the immunohistochemical findings, Western blot analysis revealed that the protein levels of CXCL16 were elevated in obstructed kidneys of wild-type (WT) mice compared with control kidneys (Figure 1, C and D).
Figure 1.
The expression of CXCL16 is induced in the kidney after obstructive injury. (A) Graphic presentation shows the time course of CXCL16 mRNA induction. N indicates normal kidney and KO indicates kidney of CXCL16-KO mice. *P < 0.05 and **P < 0.01 versus normal control kidney. n = 3 to 4. (B) Representative photomicrographs of kidney sections stained for CXCL16 (green) and DAPI (blue) (original magnification, ×400). (C) Representative Western blots show the protein levels of CXCL16 in control kidney and UUO kidney of WT mice. (D) Quantitative analysis of CXCL16 protein expression in the control kidney and UUO kidney of WT mice. *P < 0.05 versus WT controls. n = 4.
CXCL16 Deficiency Impairs Fibroblast Precursor Migration into the Injured Kidney
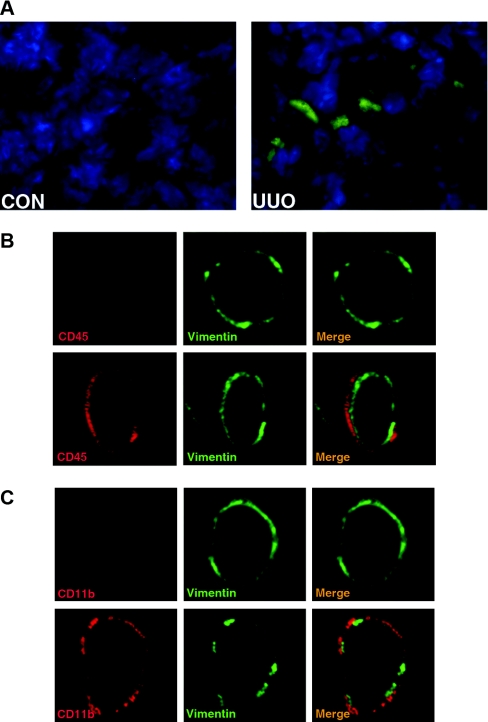
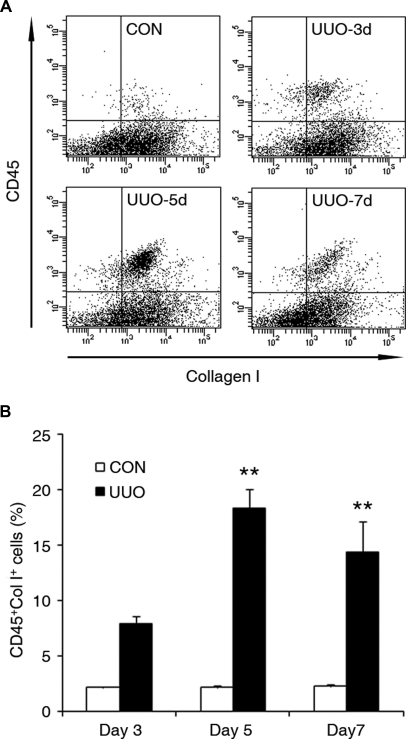
Bone marrow-derived fibroblast precursors play a critical role in the pathogenesis of tissue fibrosis, and chemokines are involved in the recruitment and differentiation of bone marrow-derived fibroblast precursors. To examine if CXCL16 plays a role in the recruitment of bone marrow-derived fibroblast precursors into the obstructed kidney, we used chimeric mice that express green fluorescence protein (GFP) driven by collagen α1(I) promoter (CoI-GFP mice). Two months after bone marrow transplantation, chimeric mice were subjected to UUO for 5 days. Bone marrow-derived collagen α1(I) promoter-GFP cells were detected in obstructed kidneys but not in the contralateral unobstructed kidneys (Figure 2A). To further characterize the bone marrow-derived fibroblasts, WT mice were subjected to UUO for 5 days. Immunofluorescence studies using specific antibodies against CD45 and vimentin or CD11b and vimentin were performed on frozen sections of kidneys. Our results showed that CD45 and vimentin dual-positive cells or CD11b and vimentin dual-positive cells accumulated in the kidneys in response to obstructive injury (Figure 2, B and C). To quantify the number of fibroblast precursors in injured kidneys, freshly dispersed renal cells were stained for CD45 and vimentin, CD11b and vimentin, CD45 and collagen I, or CD11b and collagen I. Our results confirmed that dual-positive fibroblast precursors were present in injured kidneys, whereas none of these dual-positive fibroblast precursors were observed in control kidneys (Supplemental Figure S1 A through D). We then performed flow cytometry to objectively quantify the number of bone marrow-derived fibroblast precursors in the kidney in response to obstructive injury. WT mice were subjected to UUO for 3, 5, or 7 days. Freshly dispersed renal cells were stained for CD45 and collagen I and examined by flow cytometry. Our results showed that CD45 and collagen I dual-positive fibroblast precursors accumulated in injured kidneys in a time-dependent manner, reaching maximum at day 5 and persisting for at least 7 days (Figure 3, A and B).
Figure 2.
Bone marrow-derived fibroblast precursors accumulate in the kidney in response to obstructive injury. (A) Representative photomicrographs show GFP-positive cells in the kidney in response to obstructive injury. Cryosections of the kidneys were fixed and stained with DAPI and examined with a fluorescence microscope. GFP, green; DAPI, blue. (B) Representative photomicrographs show that CD45- and vimentin-positive fibroblast precursors are present in the kidney in response to obstructive injury. Frozen kidney sections were stained for CD45 (red) and vimentin (green) and were examined with a confocal microscope (original magnification, ×1000). Upper panel shows representative photomicrographs of CD45 and vimentin staining of the control kidney. Lower panel shows representative photomicrographs of CD45 and vimentin staining of the obstructed kidney. CD45 and vimentin dual-positive fibroblast precursors are observed only in the kidney with obstructive injury. (C) Representative photomicrographs show that CD11b- and vimentin-positive fibroblast precursors are present in the kidney in response to obstructive injury. Frozen kidney sections were stained for CD11b (red) and collagen I (green) and were examined with a confocal microscope (original magnification, ×1000). Upper panel shows representative photomicrographs of CD11b and vimentin staining of the control kidney. Lower panel shows representative photomicrographs of CD11b and vimentin staining of the obstructed kidney. CD11b and vimentin dual-positive fibroblast precursors are observed only in the kidney with obstructive injury.
Figure 3.
Bone marrow-derived fibroblast precursor accumulates in obstructed kidneys in a time-dependent manner. (A) Representative cytometric diagrams showing CD45- and collagen-I-positive fibroblast precursors in the kidney of WT mice with or without UUO for 3, 5, or 7 days. Freshly isolated renal cells were stained with CD45 and collagen I and analyzed with flow cytometry. (B) Quantitative analysis of CD45 and collagen I dual-positive fibroblast precursors in the kidney of WT mice with or without UUO for 3, 5, or 7 days as determined by flow cytometry. **P < 0.01. n = 3 per group.
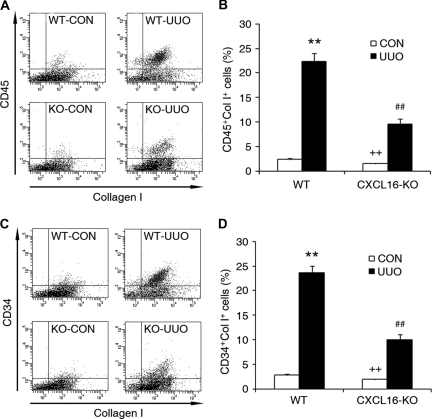
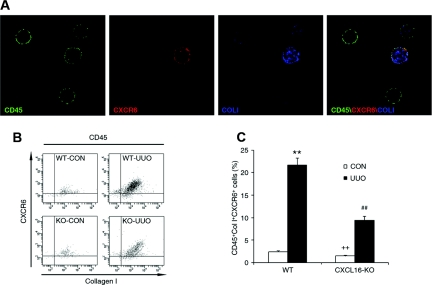
To examine the role of CXCL16 in the recruitment of bone marrow-derived fibroblast precursors into kidneys, WT and CXCL16-KO mice were subjected to obstructive injury for 5 days. Freshly dispersed renal cells were stained for CD45, CD34, and collagen I and examined by flow cytometry. Our results showed that the number of CD45- and collagen-I-positive fibroblast precursors or CD34- and collagen-I-positive fibroblast precursors was markedly increased in the injured kidneys of WT mice, whereas the number of CD45- and collagen-I-positive fibroblast precursors or CD34- and collagen-I-positive fibroblast precursors was significantly reduced in the injured kidneys of CXCL16-KO mice (Figure 4, A through D). To determine if deletion of CXCL16 affects the development of circulating fibroblast precursors, peripheral blood nucleated cells were stained for CD45 and collagen I and analyzed by flow cytometry. The results showed that targeted deletion of CXCL16 did not affect the number of CD45 and collagen I dual-positive fibroblast precursors in the peripheral circulation (Supplemental Figure S2, A and B). These data indicate that CXCL16 mediates the recruitment of bone marrow-derived fibroblast precursors into the kidney in response to obstructive injury.
Figure 4.
Targeted disruption of CXCL16 inhibits the accumulation of bone marrow-derived fibroblast precursors in obstructed kidneys. (A) Representative cytometric diagrams showing the effect of CXCL16 deficiency on the accumulation of CD45 and collagen I dual-positive fibroblast precursors in the kidney in response to UUO. (B) Quantitative analysis of CD45 and collagen I dual-positive fibroblast precursors in the kidney in response to UUO. **P < 0.01 versus WT control, ++P < 0.01 versus KO UUO, and ##P < 0.01 versus WT UUO. n = 4 per group. (C) Representative cytometric diagrams showing the effect of CXCL16 deficiency on the accumulation of CD34 and collagen I dual-positive fibroblast precursors in the kidney in response to UUO. (D) Quantitative analysis of CD34 and collagen I dual-positive fibroblast precursors in the kidney in response to UUO. **P < 0.01 versus WT control, ++P < 0.01 versus KO UUO, and ##P < 0.01 versus WT UUO. n = 4 per group.
Bone Marrow-Derived Fibroblast Precursors Express Functional CXCR6
To determine if CXCR6, the only known receptor for CXCL16, is expressed in circulating fibroblast precursors, peripheral blood nucleated cells were stained for CD45, CXCR6, and collagen I. Our results showed CD45, CXCR6, and collagen I triple-positive cells are present in the peripheral circulation (Figure 5A). These data indicate that circulating fibroblast precursors express CXCR6.
Figure 5.
Bone marrow-derived fibroblast precursors express functional CXCR6. (A) Bone marrow-derived fibroblast precursors express CXCR6. Peripheral blood cells were stained for CD45 (green), CXCR6 (red), and collagen I (blue) and identified by a deconvolution fluorescence microscope (original magnification, ×600). CD45, CXCR6, and collagen I triple-positive fibroblast precursors were detected in the circulation. (B) Representative cytometric diagrams showing the CXCR6- and collagen-I-positive cell distribution of all CD45-positive cells. Freshly isolated cells from the whole kidney of WT and CXCL16-KO mice with or without UUO for 5 days were stained with FITC-conjugated anti-CD45 antibody, PE-conjugated anti-CXCR6 antibody, and biotin-conjugated anti-collagen I antibody followed by APC-conjugated streptavidin; fluorescence intensities were measured by flow cytometry. (C) Quantitative analysis of CD45-, CXCR6-, and collagen-I-positive fibroblast precursors in the kidney of WT and CXCL16-KO mice with or without UUO as determined by flow cytometry. **P < 0.01 versus WT control, ++P < 0.01 versus KO UUO, and ##P < 0.01 versus WT UUO. n = 4 per group.
To examine if CXCR6 is involved in the recruitment of bone marrow-derived fibroblast precursors into the kidney, WT and CXCL16-KO mice were subjected to UUO for 5 days. Freshly dispersed renal cells were stained for CD45, collagen I, and CXCR6 and analyzed by flow cytometry. Our results showed that there was a significant increase in the number of CD45, collagen I, and CXCR6 triple-positive fibroblasts in the injured kidneys of WT mice, whereas the number of these triple-positive fibroblasts was significantly reduced in the injured kidneys of CXCL16-KO mice (Figure 5, B and C). Furthermore, over 90% of CD45- and collagen-I-positive fibroblast precursors expressed CXCR6. These data indicate that CXCL16 recruits bone marrow-derived fibroblast precursors into the kidney through interaction with its receptor, CXCR6.
Bone Marrow-Derived Fibroblast Precursors Can Differentiate into Myofibroblasts
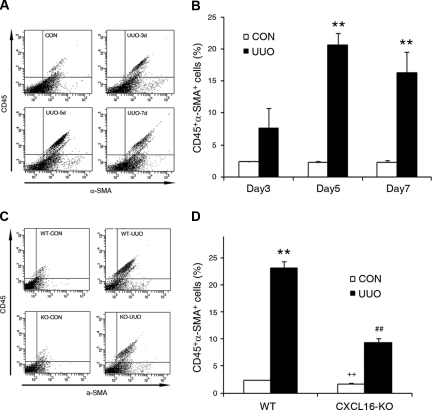
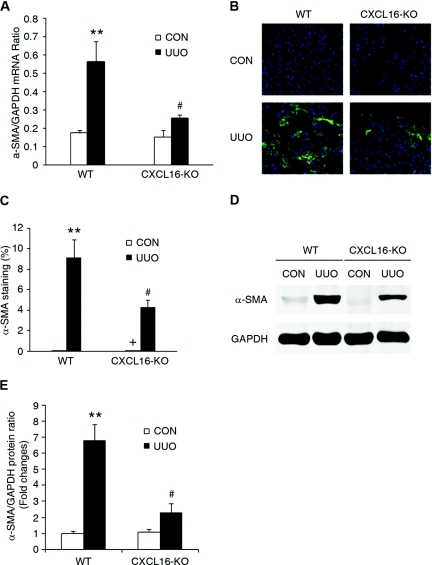
To determine if the bone marrow-derived fibroblast precursors are capable of differentiating into myofibroblasts, WT mice were subjected to UUO for different periods of time. Freshly dispersed renal cells were stained for CD45 and α-SMA and analyzed by flow cytometry. Our data showed that CD45 and α-SMA dual-positive cells accumulated in the kidneys in a time-dependent manner in response to obstructive injury (Figure 6, A and B), indicating that bone marrow-derived fibroblasts are capable of differentiating into myofibroblasts. We next examined if CXCL16 deficiency influences the number of bone marrow-derived myofibroblasts in the kidney in response to injury. Our results revealed that targeted deletion of CXCL16 resulted in a significant reduction in the number of bone marrow-derived myofibroblasts in obstructed kidneys (Figure 6, C and D). Consistent with these findings, real-time RT-PCR showed that CXCL16 deficiency significantly reduced the mRNA expression of α-SMA compared with the level in WT mice with UUO (Figure 7A). These findings were confirmed at the protein levels by immunostaining and Western blot analysis (Figure 7, B through E). Therefore, CXCL16 plays an important role in the activation and differentiation of bone marrow-derived fibroblasts.
Figure 6.
CD45 and α-SMA dual-positive myofibroblasts accumulate in the injured kidney in a time- and CXCL16-dependent manner. (A) Representative cytometric diagrams showing CD45- and α-SMA-positive cells in the kidneys of WT mice with or without UUO at day 3, 5, and 7. Freshly isolated cells from the whole kidney of WT mice with or without UUO for 3, 5, or 7 days were stained with FITC-conjugated anti-CD45 antibody and PE-conjugated anti-α-SMA antibody; fluorescence intensities were measured by flow cytometry. (B) Quantitative analysis of CD45- and α-SMA-positive cells in the kidney of WT mice with or without UUO at day 3, 5, and 7 as determined by flow cytometry. **P < 0.01. n = 3 per group. (C) Representative cytometric diagrams showing CD45- and α-SMA-positive cell distribution in the kidney of WT and CXCL16-KO mice with or without UUO. Freshly isolated cells from whole kidney of WT and CXCL16-KO mice with or without UUO for 5 days were stained with FITC-conjugated anti-CD45 antibody and PE-conjugated anti-α-SMA antibody; fluorescence intensities were measured by flow cytometry. (D) Quantitative analysis of CD45- and α-SMA-positive cells in the kidney of WT and CXCL16-KO mice with or without UUO as determined by flow cytometry. **P < 0.01 versus WT control, ++P < 0.01 versus KO UUO, and ##P < 0.01 versus WT UUO. n = 4 per group.
Figure 7.
Targeted disruption of CXCL16 reduces α-SMA expression in obstructive nephropathy. (A) The mRNA levels of α-SMA in the kidney of WT and CXCL16-KO mice in response to injury as determined by real-time RT-PCR. **P < 0.01 versus WT controls and #P < 0.05 versus WT UUO. n = 3 to 4 per group. (B) Representative photomicrographs of α-SMA immunostaining in the kidney of WT and CXCL16-KO mice at day 7 after surgery. (C) Quantitative measurements of α-SMA protein expression in the kidney of WT and CXCL16-KO mice. **P < 0.01 versus WT controls and #P < 0.05 versus WT UUO. n = 5 to 6 per group. (D) Representative Western blots show the levels of α-SMA protein expression in the kidney of WT and CXCL16-KO mice. (E) Quantitative analysis of α-SMA protein expression in the kidney of WT and CXCL16-KO mice. **P < 0.01 versus WT controls and #P < 0.05 versus WT UUO. n = 3 per group.
CXCL16 Deficiency Suppresses Renal Fibrosis
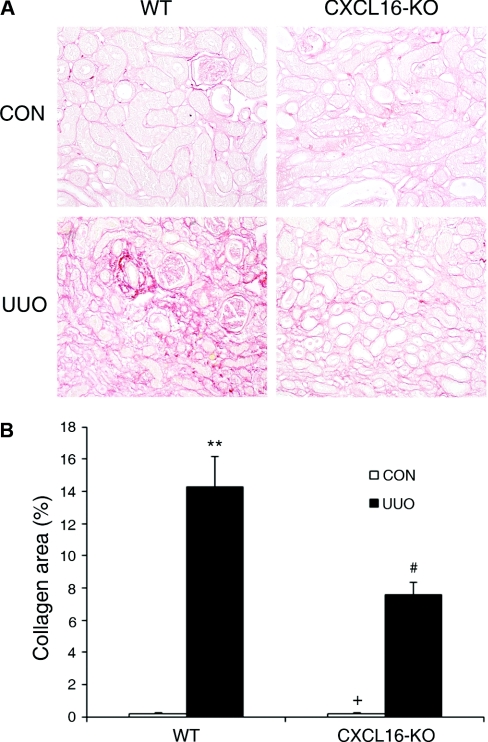
Because CXCL16 regulates the recruitment of bone marrow-derived fibroblast precursors into the kidney in response to obstructive injury, we examined if CXCL16 influenced the development of renal fibrosis. WT and CXCL16-KO mice were subjected to UUO for 14 days. WT mice developed significant collagen deposition in obstructed kidneys as demonstrated by picrosirius red staining, whereas these responses were significantly attenuated in the obstructed kidneys of CXCL16-KO mice (Figure 8, A and B). These data indicate that CXCL16 plays a critical role in the pathogenesis of renal fibrosis.
Figure 8.
Targeted disruption of CXCL16 attenuates renal fibrosis and ECM deposition. (A) Representative photomicrographs show kidney sections stained with picrosirius red for assessment of total collagen deposition (original magnification, ×200). (B) Quantitative analysis of renal interstitial collagen in different groups as indicated. **P < 0.01 versus WT controls, +P < 0.05 versus KO UUO, and #P < 0.05 versus WT UUO. n = 5 to 6 per group.
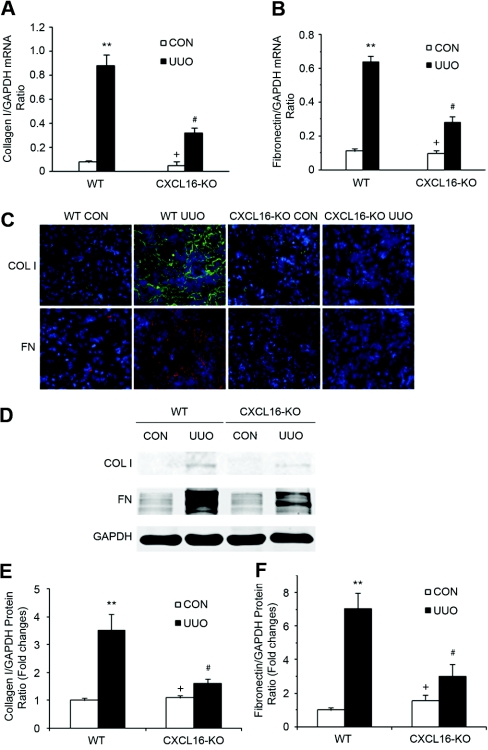
We next investigated the effect of targeted disruption of CXCL16 on expression and accumulation of collagen I and fibronectin, two major components of ECM. As shown in Figure 9, A and B, there was a marked increase in the mRNA levels of collagen I and fibronectin in the obstructed kidneys of WT mice, whereas targeted deletion of CXCL16 significantly suppressed these matrix components in the obstructed kidney. We then examined protein expression in the kidney in response to obstructive injury. There was minimal collagen I and fibronectin protein accumulation in the control kidneys of WT and CXCL16-KO mice. There was a marked increase in collagen I and fibronectin deposition in the obstructed kidneys of WT mice. This response was significantly attenuated in the obstructed kidneys of CXCK16-KO mice (Figure 9C). Consistent with the immunohistochemical findings, Western blot analysis confirmed that the protein levels of collagen I and fibronectin were increased in the obstructed kidneys of WT mice. These changes were reduced in the injured kidneys of CXCL16-KO mice (Figure 9, D through F). These data indicate that targeted deletion of CXCL16 attenuates renal fibrosis by inhibiting production and deposition of ECM proteins.
Figure 9.
Targeted disruption of CXCL16 inhibits collagen I and fibronectin expression in the obstructed kidney. (A) The mRNA levels of collagen I in the kidney of WT and CXCL16-KO mice in response to injury as determined by real-time RT-PCR. **P < 0.01 versus WT controls, +P < 0.05 versus KO UUO, and #P < 0.05 versus WT UUO. n = 3 to 4 per group. (B) The mRNA levels of fibronectin in the kidney of WT and CXCL16-KO mice in response to injury as determined by real-time RT-PCR. **P < 0.01 versus WT controls, +P < 0.05 versus KO UUO, and #P < 0.05 versus WT UUO. n = 3 to 4 per group. (C) Representative photomicrographs of collagen I and fibronectin immunostaining in the kidney of WT and CXCL16-KO mice at day 14 after surgery (original magnification, ×400). (D) Representative Western blots show the protein levels of collagen I and fibronectin in the kidney of WT and CXCL16-KO mice. (E) Quantitative analysis of collagen I protein expression in the kidney of WT and CXCL16-KO mice. **P < 0.01 versus WT controls, +P < 0.05 versus KO UUO, and #P < 0.05 versus WT UUO. n = 3 per group. (F) Quantitative analysis of fibronectin protein expression in the kidney of WT and CXCL16-KO mice. **P < 0.01 versus WT controls, +P < 0.05 versus KO UUO, and #P < 0.05 versus WT UUO. n = 3 per group.
DISCUSSION
In this study, we have demonstrated that (1) CXCL16 is induced in the kidney during the pathogenesis of renal fibrosis; (2) bone marrow-derived fibroblast precursors are recruited into the kidney in a CXCL16-dependent manner; (3) fibroblast precursors express the CXCL16 receptor, CXCR6, and targeted disruption of CXCL16 inhibits the recruitment of CD45-, collagen-I-, and CXCR6-positive fibroblast precursors in the kidney; and (4) targeted disruption of CXCL16 reduces the severity of renal fibrosis and the expression of ECM protein. These results indicate that CXCL16 plays a pivotal role in the pathogenesis of renal fibrosis by recruiting bone marrow-derived fibroblast precursors into the kidney in response to obstructive injury.
Renal fibrosis is a hallmark of progressive kidney disease, and tubulointerstitial fibrosis is central to the loss of renal function after kidney injury. However, the origin of the fibroblasts that are responsible for the excessive production of ECM is still the subject of intense debate. Recent reports provide evidence that bone marrow-derived fibroblasts are recruited into the kidney and contribute to kidney fibrosis. One study of mismatched kidney transplantation in humans has shown that the proportion of host-derived SMA-positive cells is approximately 30% in allografts undergoing chronic rejection compared with 10% in those without rejection.12 In rodent models of renal fibrosis, several studies using bone marrow transplantation have shown that bone marrow-derived fibroblasts are present in the kidney in response to injury.7,13,23–25 For example, one study using bone marrow transplantation of transgenic mice that express enhanced GFP under the control of the fibroblast-specific protein 1 promoter has demonstrated that 15% of bone marrow-derived fibroblasts are present in the kidney 10 days after obstructive injury.7 Another study using bone marrow transplantation of transgenic rats that express human placental alkaline phosphatase revealed that >30% of α-SMA-positive myofibroblasts are derived from bone marrow 7 days after ischemia-reperfusion injury.13
Bone marrow-derived fibroblast precursors play a critical role in the pathogenesis of renal fibrosis.11,18 These cells express hematopoietic markers such as CD45 and CD11b, stem cell marker CD34, and mesenchymal markers such as collagen I and vimentin. In this study, we have identified that fibroblast precursors accumulate in the kidney after obstructive injury using chimeric mice expressing GFP driven by the collagen 1α(I) promoter and on the basis of their expression of mesenchymal markers such as vimentin and collagen I and hematopoietic markers such as CD45 and CD11b. We then performed flow cytometric analyses to characterize the infiltrating fibroblast precursors on the basis of their expression of collagen I and CD45. Our results demonstrate that CD45 and collagen I dual-positive fibroblast precursors migrate into the injured kidneys of WT mice in a time-dependent manner. The recruitment of CD45 and collagen I dual-positive fibroblast precursors into the kidneys was detected as early as 3 days after obstructive injury. Maximal recruitment of CD45 and collagen I dual-positive fibroblast precursors was observed 5 days after obstructive injury, and the number of infiltrating CD45 and collagen I dual-positive fibroblast precursors appeared to decrease by day 7, which is consistent with the notion that infiltration of circulating fibroblast precursors precedes the development of tissue fibrosis.11,26,27
The signaling mechanisms underlying the recruitment of bone marrow-derived fibroblast precursors into kidney are incompletely understood. Chemokines play an important role in the regulation of fibroblast precursor infiltration in response to injury. Sakai et al.11 showed that CCL21 and its receptor, CCR7, are involved in the infiltration of fibroblast precursors in the kidney in a murine model of renal fibrosis induced by obstructive injury. Our results show that CXCL16 mRNA is induced in the kidney in response to obstructive injury in a time-dependent manner and CXCL16 protein is upregulated mainly in the kidney epithelial cells in the obstructed kidney. Consistent with our findings, it has been reported that CXCL16 protein is expressed at a low level in epithelial cells in the normal kidney and is upregulated in response to obstructive injury.28 However, the role of CXCL16 in the pathogenesis of renal fibrosis has not been reported. In this study, we demonstrate for the first time that CXCL16 is pathologically important because targeted disruption of CXCL16 causes a significant decrease in the number of bone marrow-derived fibroblast precursors in the kidney in response to obstructive injury without affecting the development of circulating fibroblast precursors. These data indicate CXCL16 plays a critical role in recruiting bone marrow-derived fibroblast precursors into the kidney.
Fibroblast precursors express certain chemokine receptors such as CCR2, CXCR4, and CCR7, and inhibition of these chemokine receptors has been shown to suppress fibrosis through suppression of fibroblast precursor infiltration into various tissues.26,27,29 In this study, we demonstrate for the first time that bone marrow-derived fibroblast precursors express CXCR6, the receptor for CXCL16. CXCR6 was first cloned as an orphan receptor in 199730–32 and was termed STRL33, BONZO, or TYMSTR. It was recently reported that bone marrow-derived mesenchymal stem cells express CXCR6, and these cells demonstrate appreciable chemotactic migration in response to CXCL16.33 Our results show that targeted disruption of CXCL16 suppresses the infiltration of CD45, CXCR6, and collagen I triple-positive fibroblast precursors into the kidney, suggesting that CXCl16 regulates fibroblast precursor trafficking by an interaction with its receptor, CXCR6. It is noted that targeted disruption of CXCL16 does not completely block the infiltration of CD45, CXCR6, and collagen I triple-positive fibroblast precursors. This can be explained by the observation that bone marrow-derived fibroblast precursors express chemokine receptors other than CXCR6.17 Indeed, CCR7 has been reported to regulate fibroblast precursor infiltration into the kidney in response to obstructive injury.11
We demonstrate that bone marrow-derived myofibroblasts identified as CD45 and α-SMA dual-positive cells accumulate in the injured kidney of WT mice, whereas their accumulation is significantly reduced in the injured kidney of CXCL16-KO mice. This finding is important because myofibroblast activation is generally considered a key event in the pathogenesis of renal fibrosis.4–6 Furthermore, experimental studies have shown that the number of interstitial myofibroblasts correlates closely with the severity of tubulointerstitial fibrosis and the progression of kidney disease.34,35 Our results strongly indicate that bone marrow-derived fibroblast precursors are activated in the kidney and may contribute to the population of renal myofibroblasts.
A key marker of renal fibrosis is the dramatic increase and deposition of ECM proteins, including collagen and fibronectin. Morphometric analysis of picrosirius red staining of kidney sections at day 14 after obstructive injury demonstrates the presence of interstitial collagen deposition. This collagen deposition is significantly attenuated in the obstructed kidneys of CXCL16-KO mice. The magnitude of collagen reduction we observed here is similar to that observed in a study of CCR7-KO mice.11 In keeping up with these findings, we further illustrate that the mRNA and protein levels of collagen I and fibronectin are markedly increased in the injured kidneys of WT mice, whereas these responses are significantly attenuated in the injured kidney of CXCL16-KO mice.
In summary, our study defines a novel mechanism by which CXCL16 participates in renal fibrosis. In response to injury, the upregulated CXCL16 leads to the recruitment of circulating fibroblast precursors into the kidney, which contribute to the pathogenesis of renal fibrosis. These data suggest that inhibition of CXCL16 could constitute a novel therapeutic approach for chronic kidney disease.
CONCISE METHODS
Animals
The Institutional Animal Care and Use Committee of the Baylor College of Medicine approved the animal experiments. WT C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The CXCL16-KO mouse on a background of C57BL/6J was generated by Dr. S. Han.36 CoI-GFP mice were a generous gift from Dr. D.A. Brenner.29 WT male C57BL/6J mice or CXCL16-KO mice at 8 to 12 weeks old weighing 20 to 30 g were anesthetized by intraperitoneal injection of ketamine (80 mg/kg) and xylazine (10 mg/kg). Through a flank incision, the left ureter was exposed and completely ligated using fine suture material (4-0 silk) at two points. Mice were allowed to recover from anesthesia and were housed in standard rodent cages with ad libitum access to water and food until sacrificed.
Bone Marrow Transplantation
Bone marrow transplantation was performed as described previously.27 Briefly, bone marrow cells (5 × 106) from CoI-GFP mice were transferred to lethally irradiated C57BL/6 mice. Chimeric mice were allowed to recuperate for 2 months before induction of kidney injury by UUO.
Renal Morphology
Mice were euthanized and perfused by injections of PBS into the left ventricle of the heart to remove blood. One portion of the renal tissue was fixed in 10% buffered formalin and embedded in paraffin, cut at 4-μm thickness, and stained with hematoxylin and eosin for initial evaluation or picrosirius red staining to identify collagen fiber. The picrosirius-red-stained sections were scanned using a microscope equipped with a digital camera (Nikon, Melville, NY), and quantitative evaluation was performed using NIS-Elements Br 3.0 software. The collagen-stained area was calculated as a percentage of the total area.
Immunofluorescence
Renal tissues were embedded in OCT compound and snap-frozen on dry ice, cut at 5-μm thickness using a cryostat, mounted on Superfrost Plus microscope slides, and then stored at −80°C. Sections were fixed in acetone for 10 minutes at room temperature. Nonspecific binding was blocked with protein block, serum free (DAKO). Slides were then incubated with goat anti-CXCL16 antibody (R&D Systems) followed by Alexa-488-conjugated donkey anti-goat antibody (Invitrogen), rabbit anti-collagen I antibody (Rockland) followed by Alexa-488-conjugated donkey anti-rabbit antibody (Invitrogen), rabbit anti-fibronectin antibody (Sigma) followed by Alexa-488-conjugated donkey anti-rabbit antibody (Invitrogen), or FITC-conjugated mouse anti-α-SMA antibody (Sigma). For double immunofluorescence, renal tissues or cells were fixed and stained with primary antibodies sequentially followed by the appropriate secondary antibodies. Slides were mounted with VECTASHIELD HardSet mounting medium with DAPI. Fluorescence intensity was visualized using a confocal or deconvolution microscope equipped with a digital camera (Nikon, Melville, NY). Quantitative evaluation of sections stained with antibodies to α-SMA was performed using NIS-Elements Br 3.0 software. The fluorescence-positive area was calculated as a percentage of the total area.
Cell Isolation and Flow Cytometry
Renal cells were isolated as reported with modification.11 Briefly, kidney was decapsulated, minced, and incubated at 37°C for 40 minutes in PBS containing 0.5 mg/ml Liberase TM (Roche) and 10 U/ml DNase (Roche). Cells were filtered through a 40-μm strainer, rinsed, centrifuged, and resuspended in FACS buffer. Cells (5 × 105) were incubated with PE-anti-CD34, FITC-anti-CD45 (all from BD Biosciences, San Jose, CA), PE-anti-CXCR6, PE-anti-α-SMA (R&D systems, Minneapolis, MN), or biotin-anti-collagen I (Rockland, Gilbertsville, PA)/streptavidin-APC (BD Biosciences, San Jose, CA). If necessary, cells were fixed and permeabilized using the Cytofix/Cytoperm kit (BD Biosciences, San Jose, CA) in accordance with the manufacturer's protocol. Cells incubated with irrelevant isotype-matched antibodies (BD Biosciences, San Jose, CA) and unstained cells were used as controls. The cutoffs were set according to results of controls. FITC/PE/APC fluorescence intensities were measured using a BD LSR II flow cytometer (BD Biosciences, San Jose, CA). Data were analyzed using BD FACSDiva software.
Quantitative Real-Time RT-PCR
Quantitative analysis of the target mRNA expression was performed with real-time RT-PCR by the relative standard curve method. Total RNA was extracted from snap-frozen kidney tissues with TRIzol Reagent (Invitrogen) followed by RNase-free DNase I (Roche). Aliquots (1 μg) of total RNA were reverse transcribed and amplified in triplicate using IQ SYBR green supermix reagent (Bio-Rad, Herculus, CA) with an Opticon real-time PCR machine (MJ Research, Waltham, MA), according to the manufacturer's instructions. The specificity of real-time PCR was confirmed via routine agarose gel electrophoresis and melting-curve analysis. The expression level of the target genes was normalized by the glyceraldehyde 3-phosphate dehydrogenase (GAPDH) level in each sample. The following are the primer sequences. CXCL16: forward 5′-ACCCTTGTCTCTTGCGTTCTTCCT-3′ and reverse 5′-ATGTGATCCAAAGTACCCTGCGGT-3′; collagen I: forward 5′-TGCCGCGACCTCAAGATGTG-3′ and reverse 5′-CACAAGGGTGCTGTAGGTGA-3′; fibronectin: forward 5′-CTTCTCCGTGGAGTTTTACCG-3′ and reverse 5′- GCTGTCAAATTGAATGGTGGTG-3′; α-SMA: forward 5′-ACTGGGACGACATGGAAAAG-3′ and reverse 5′-CATCTCCAGAGTCCAGCACA-3′; and GAPDH: forward 5′-TGCTGAGTATGTCGTGGAGTCTA-3′ and reverse 5′-AGTGGGAGTTGCTGTTGAAATC-3′.
Western Blot Analysis
Protein was extracted using radioimmunoprecipitation assay buffer containing cocktail proteinase inhibitors (Sigma) and quantified with a Bio-Rad protein assay. An equal amount of protein was separated on SDS-polyacrylamide gels in a Tris/HCl buffer system, transferred onto nitrocellulose membranes, and blotted according to standard procedures with primary antibodies (CXCL16, collagen I, fibronectin, and α-SMA). Membranes were then stripped and reblotted with anti-GAPDH or β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). The specific bands of target proteins were visualized by chemiluminescence, and band intensities were quantified using NIH Image/J.
Statistical Analysis
All data were expressed as mean ± SEM. Multiple group comparisons were performed by one-way ANOVA followed by the Bonferroni procedure for comparison of means. Comparison between two groups was analyzed by the two-tailed t test. P < 0.05 was considered statistically significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Dr. William E. Mitch for critical reading of the manuscript and helpful discussion. We also thank the Innovative Research team at University of Shanghai Municipal Education Commission and the Integrated Microscopy Core at the Baylor College of Medicine for technical assistance. This work was supported in part by National Institutes of Health (NIH) grant K08HL92958, American Heart Association grant 09SDG2280102, and a grant from Dr. and Mrs. Harold Selzman. M.L.E. was supported by NIH grant R01HL89792.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Schainuck LI, Striker GE, Cutler RE, Benditt EP: Structural-functional correlations in renal disease. II. The correlations. Hum Pathol 1: 631–641, 1970 [DOI] [PubMed] [Google Scholar]
- 2. Nath KA: The tubulointerstitium in progressive renal disease. Kidney Int 54: 992–994, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Eddy AA: Molecular basis of renal fibrosis. Pediatr Nephrol 15: 290–301, 2000 [DOI] [PubMed] [Google Scholar]
- 4. Neilson EG: Mechanisms of disease: Fibroblasts—A new look at an old problem. Nat Clin Pract Nephrol 2: 101–108, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Strutz F, Muller GA: Renal fibrosis and the origin of the renal fibroblast. Nephrol Dial Transplant 21: 3368–3370, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Liu Y: Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG: Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110: 341–350, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A: Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest 112: 1486–1494, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zeisberg M, Kalluri R: The role of epithelial-to-mesenchymal transition in renal fibrosis. J Mol Med 82: 175–181, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R: Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S: Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci U S A 103: 14098–14103, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grimm PC, Nickerson P, Jeffery J, Savani RC, Gough J, McKenna RM, Stern E, Rush DN: Neointimal and tubulointerstitial infiltration by recipient mesenchymal cells in chronic renal-allograft rejection. N Engl J Med 345: 93–97, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Broekema M, Harmsen MC, van Luyn MJ, Koerts JA, Petersen AH, van Kooten TG, van Goor H, Navis G, Popa ER: Bone marrow-derived myofibroblasts contribute to the renal interstitial myofibroblast population and produce procollagen I after ischemia/reperfusion in rats. J Am Soc Nephrol 18: 165–175, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A: Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med 1: 71–81, 1994 [PMC free article] [PubMed] [Google Scholar]
- 15. Metz CN: Fibrocytes: A unique cell population implicated in wound healing. Cell Mol Life Sci 60: 1342–1350, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Quan TE, Cowper S, Wu SP, Bockenstedt LK, Bucala R: Circulating fibrocytes: Collagen-secreting cells of the peripheral blood. Int J Biochem Cell Biol 36: 598–606, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Abe R, Donnelly SC, Peng T, Bucala R, Metz CN: Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol 166: 7556–7562, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Niedermeier M, Reich B, Rodriguez Gomez M, Denzel A, Schmidbauer K, Gobel N, Talke Y, Schweda F, Mack M: CD4+ T cells control the differentiation of Gr1+ monocytes into fibrocytes. Proc Natl Acad Sci U S A 106: 17892–17897, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mackay CR: Chemokines: Immunology's high impact factors. Nat Immunol 2: 95–101, 2001 [DOI] [PubMed] [Google Scholar]
- 20. Rollins BJ: Chemokines. Blood 90: 909–928, 1997 [PubMed] [Google Scholar]
- 21. Murphy PM: The molecular biology of leukocyte chemoattractant receptors. Annu Rev Immunol 12: 593–633, 1994 [DOI] [PubMed] [Google Scholar]
- 22. Matloubian M, David A, Engel S, Ryan JE, Cyster JG: A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol 1: 298–304, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Lin SL, Kisseleva T, Brenner DA, Duffield JS: Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol 173: 1617–1627, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin F, Moran A, Igarashi P: Intrarenal cells, not bone marrow-derived cells, are the major source for regeneration in postischemic kidney. J Clin Invest 115: 1756–1764, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roufosse C, Bou-Gharios G, Prodromidi E, Alexakis C, Jeffery R, Khan S, Otto WR, Alter J, Poulsom R, Cook HT: Bone marrow-derived cells do not contribute significantly to collagen I synthesis in a murine model of renal fibrosis. J Am Soc Nephrol 17: 775–782, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM: Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 114: 438–446, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML: Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A 103: 18284–18289, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Okamura DM, Lopez-Guisa JM, Koelsch K, Collins S, Eddy AA: Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol 293: F575–F585, 2007 [DOI] [PubMed] [Google Scholar]
- 29. Kisseleva T, Uchinami H, Feirt N, Quintana-Bustamante O, Segovia JC, Schwabe RF, Brenner DA: Bone marrow-derived fibrocytes participate in pathogenesis of liver fibrosis. J Hepatol 45: 429–438, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Alkhatib G, Liao F, Berger EA, Farber JM, Peden KW: A new SIV co-receptor, STRL33. Nature 388: 238, 1997 [DOI] [PubMed] [Google Scholar]
- 31. Deng HK, Unutmaz D, Kewal Ramani VN, Littman DR: Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 388: 296–300, 1997 [DOI] [PubMed] [Google Scholar]
- 32. Loetscher M, Amara A, Oberlin E, Brass N, Legler D, Loetscher P, D'Apuzzo M, Meese E, Rousset D, Virelizier JL, Baggiolini M, Arenzana-Seisdedos F, Moser B: TYMSTR, a putative chemokine receptor selectively expressed in activated T cells, exhibits HIV-1 coreceptor function. Curr Biol 7: 652–660, 1997 [DOI] [PubMed] [Google Scholar]
- 33. Sordi V, Malosio ML, Marchesi F, Mercalli A, Melzi R, Giordano T, Belmonte N, Ferrari G, Leone BE, Bertuzzi F, Zerbini G, Allavena P, Bonifacio E, Piemonti L: Bone marrow mesenchymal stem cells express a restricted set of functionally active chemokine receptors capable of promoting migration to pancreatic islets. Blood 106: 419–427, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA: Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3: 349–363, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Eddy AA: Molecular insights into renal interstitial fibrosis. J Am Soc Nephrol 7: 2495–2508, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Zhang L, Ran L, Garcia GE, Wang XH, Han S, Du J, Mitch WE: Chemokine CXCL16 regulates neutrophil and macrophage infiltration into injured muscle, promoting muscle regeneration. Am J Pathol 175: 2518–2527, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.