Abstract
A Myxococcus xanthus cytoplasmic bacterial tyrosine kinase, BtkA, showed phosphorylation activity in the presence of Exo. Phosphorylated BtkA was expressed late after starvation induction and early after glycerol induction. The btkA mutant was unable to complete maturation to heat- and sonication-resistant spores under both starvation- and glycerol-induced developmental conditions.
TEXT
Myxococcus xanthus is a Gram-negative soil bacterium which exhibits a complex life cycle and social behavior (22). Upon nutritional starvation, more than 105 cells migrate to an aggregation center and form a fruiting body, within which rod-shaped cells differentiate into spherical myxospores. A survey of the M. xanthus genome database indicated the presence of two bacterial protein tyrosine kinases (BY kinases), a Gram-negative BY kinase (MXAN_1025) and a Gram-positive BY kinase (MXAN_3228), the latter of which is here called BtkA. BY kinases from Gram-negative bacteria are usually large, membrane-spanning, multidomain proteins composed of an N-terminal transmembrane region and a C-terminal cytosolic tyrosine kinase domain (5). In Gram-positive bacteria, these BY kinases are often split into two distinct proteins, which are encoded by genes that are usually located next to each other (19). BY kinases are involved in the biosynthesis and transport of exopolysaccharide (1, 21). In this study, we found that a cytosolic BY kinase, BtkA, of M. xanthus is required for the formation of mature spores.
Functional motifs of BtkA.
BtkA consists of 231 amino acid residues with a calculated molecular mass of 24.7 kDa. BtkA exhibited 22% to 31% identity with these BY kinases (Fig. 1). BtkA contains amino acid sequences that resemble the Walker A (GXGK[T/S]) and Walker B (hhhD, where h represents a hydrophobic amino acid) motifs normally found in nucleotide-binding proteins but not in eukaryotic tyrosine kinases (13). In addition, the Walker A′ (DXDXR) motif, important for Mg2+ binding, was located in BtkA. A tyrosine cluster containing multiple tyrosine residues was not located at the C-terminal end of BtkA, but two tyrosine residues existed at the C-terminal end of BtkA.
Fig. 1.
Amino acid sequence alignment of homologous regions (highlighted) in Staphylococcus aureus CapB, Streptococcus pneumoniae CpsD, Escherichia coli K-12 Wzc, and M. xanthus BtkA. Walker A, A′, and B motifs are indicated by overlines.
Protein kinase activity of BtkA is stimulated by Exo.
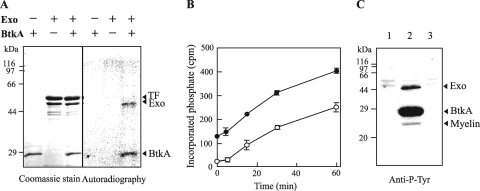
In Gram-positive BY kinases, two distinct proteins, a periplasmic receptor and a cytoplasmic protein kinase, encoded by two different genes are both required for phosphorylation (19). These proteins are encoded by genes that are usually located next to each other. An upstream gene (exo; MXAN_3227) of the btkA gene encodes a chain length determinant family protein (465 amino acids), with which it shares homology with the transmembrane adaptor for cytoplasmic BY kinase. Exo had two transmembrane domains and shared 21% and 17% identity with Staphylococcus aureus CapA2 and Streptococcus pneumoniae CpsC, respectively, suggesting that Exo may be able to interact with BtkA and promote autophosphorylation of BtkA. To confirm this hypothesis, both recombinant proteins were prepared from Escherichia coli and the activity of BtkA was assayed. The exo and btkA genes were amplified by PCR using exoEN and exoEC primers and btkAEN and btkAEC primers, respectively (Table 1), and were cloned into pCold TF and pCold vectors (Takara Bio Inc.), respectively. Purified BtkA and Exo were incubated with [γ-32P]ATP and analyzed by SDS-PAGE and autoradiography. As shown in Fig. 2A, BtkA alone did not autophosphorylate; however, autophosphorylation of BtkA was activated by the addition of Exo and led to the phosphorylation of Exo. This reaction led to the phosphorylation of BtkA and Exo in a ratio of approximately 1.5:1 to 2:1 (Fig. 2B). When BtkA, Exo, and myelin were incubated with ATP, phosphorylation of these proteins was detected by Western immunoblotting with an antiphosphotyrosine monoclonal antibody (PY20), indicating that BtkA has tyrosine protein kinase activity (Fig. 2C). A synthetic peptide (RRLIEDADYAARG; AnaSpec) for tyrosine protein kinase (18) was not phosphorylated by BtkA in the presence of Exo.
Table 1.
Oligonucleotides used in this study
| Primer | Purpose | Sequence |
|---|---|---|
| exoEN | Amplification of exo for expression | 5′-GAAGGTAGGCATATGACGGCAGACCAGCTTCTGGGAG-3′ |
| exoEC | Amplification of exo for expression | 5′-GACAAGCTTGAATTCCGTCGGGTTGTCATCCACTC-3′ |
| btkAEN | Amplification of btkA for expression | 5′-TCGAAGGTAGGCATATGGATGACAACCCGACGGGCGGCAAC-3′ |
| btkAEC | Amplification of btkA for expression | 5′-GACAAGCTTGAATTCTCGGCGAGGAAGAACGTCAGTTTC-3′ |
| btkAMN | Amplification of btkA for mutation | 5′-TTCGAGCTCACCTACGCCAACCGCG-3′ |
| btkAMC | Amplification of btkA for mutation | 5′-CAGCGTCCCGGTGGAAGCTGCATC-3′ |
| RTbtkAN | Amplification inside btkA | 5′-CAAGACGGTGACGAGCGTC-3′ |
| RTbtkAC | Amplification inside btkA | 5′-GCCAGGCCCATCTTGTTGC-3′ |
| RT3229N | Amplification inside MXAN_3229 | 5′-GTGAGGCGCTGGAGGAGAC-3′ |
| RT3229C | Amplification inside MXAN_3229 | 5′-GCCCGTGGCCTCATACACC-3′ |
Fig. 2.
Phosphorylation activity of BtkA. (A) Exo and BtkA with an N-terminal hexahistidine tag were purified by affinity chromatography on a Talon CellThru column (Clontech), and a fusion enzyme (trigger factor [TF]-Exo) was cleaved with human rhinovirus (HRV) 3C protease (Novagen) at 4°C for 12 h. The phosphorylation assay was performed in 20 μl of 50 mM Tris-HCl buffer (pH 7.5), 1 mM dithiothreitol (DTT), 5 mM MgCl2, and 0.15 MBq [γ-32P]ATP at 37°C for 30 min. The reaction mixture was loaded on a 12.5% SDS-PAGE gel, and the gel was autoradiographed and analyzed using an image analyzer (BAS1800; Fuji Film). (B) Time course of the phosphorylation of Exo and BtkA. A 0.5 μM concentration (each) of Exo and BtkA was incubated for 0 to 60 min. After SDS-PAGE analysis, the gel portions containing Exo or BtkA were cut, and the incorporation of [32P]phosphate into Exo (open circles) or BtkA (filled circles) was determined with a liquid scintillation counter (Aloka). Data are the means of the results of triplicate experiments. Standard deviations are shown by error bars. (C) Immunoblot analysis with PY20. Exo and myelin (lane 1), Exo, myelin, and BtkA (lane 2), and Exo, myelin, and 1 unit of protein kinase A (PKA; Promega) (lane 3) were incubated with ATP, separated by SDS-15% PAGE, and analyzed by immunoblotting with antiphosphotyrosine antibody PY20 (Santa Cruz Biotechnology). Cyclic AMP (cAMP)-activated PKA, a Ser/Thr protein kinase, was used as the negative control.
Developmental phenotype of btkA mutant.
To study the biological function of BtkA, the btkA gene was disrupted by insertion of the kanamycin resistance (Kmr) gene. A DNA fragment containing the btkA gene was amplified from the M. xanthus FB (IFO13542) genome using btkAMN and btkAMC primers (Table 1), and then the PCR product was cloned into a pBluescript vector. The 0.6-kb EheI fragment of the btkA gene was replaced by a 1.2-kb Kmr gene. The disrupted gene was amplified by PCR using the above-mentioned primers, and the PCR product was introduced into M. xanthus FB cells by electroporation (17).
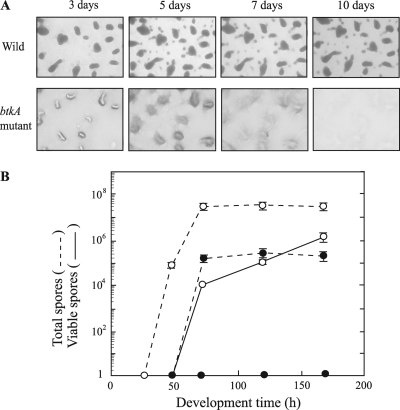
The wild-type and btkA mutant strains were cultured on starvation medium (clone fruiting [CF] agar) (10). The wild-type cells moved to aggregation centers by 24 to 32 h and then formed fruiting bodies by 48 to 72 h on CF agar (Fig. 3A). Within the fruiting bodies, rod-shaped vegetative cells of the wild type were converted into spherical myxospores. The mature wild-type myxospores were resistant to elevated temperatures and sonic vibration and were optically refractile (Fig. 3B). The btkA mutant also glided toward aggregation centers and formed discrete mounds, but its fruiting bodies were not as dark as those of the wild-type strain and these mounds disappeared by 10 days, suggesting that the mutant did not form mature, spore-filled fruiting bodies. When harvested cells were sonicated and heat treated, the total number of myxospores formed by the btkA mutant at 72 to 168 h, counted by microscopy with a hemocytometer, was approximately 0.5% of the number of wild-type myxospores. When viable spores were quantified by counting the emergence of colonies from the spores, no colonies could be observed for the mutant.
Fig. 3.
Fruiting body and spore formations of M. xanthus wild-type and btkA mutant strains. (A) Fruiting body development on CF agar. These strains (5 × 109 cells/ml) were starved on CF agar at 28°C for the indicated time periods. (B) Spore formation of wild-type (open circles) and btkA mutant (filled circles) strains on CF agar. After sonication for 1 min and incubation at 60°C for 15 min, the total numbers of spores (dashed lines) were counted with a hemocytometer. The numbers of viable spores (solid lines) were determined by plating serial dilutions on Casitone-Tris (CTT) agar plates. Data are the means of the results of triplicate experiments. Standard deviations are shown by error bars.
Glycerol-induced sporulation was examined by adding 0.5 M glycerol to vegetative Casitone-yeast extract (CYE) (3) cultures of wild-type and mutant strains. In glycerol-induced sporulation, cellular differentiation proceeds without the formation of fruiting bodies and spores are observed after 1 h of induction. The total spore numbers produced by the btkA mutant at 3 and 24 h were approximately 70% of those of the wild-type strain (Table 2); however, more sonicated and heat-treated mutant spores than wild-type spores lost their viability. These results indicate that BtkA contributes to the maturation of starvation- and glycerol-induced spores. An Ω7536 mutant strain, which contains a Tn5lac insertion in the exo gene, was also defective in both starvation- and glycerol-induced sporulation (14), suggesting that the phenotype of the Ω7536 mutant may be due to the nonphosphorylation of BtkA.
Table 2.
Glycerol-induced sporulation of wild-type and btkA mutant strainsa
| Strain | Incubation (h) | Total no. of spores induced | No. of viable spores |
|---|---|---|---|
| Wild type | 3 | 6.5 × 107 ± 1.1 × 107 | 5.5 × 102 ± 1.0 × 102 |
| btkA mutant | 3 | 4.6 × 107 ± 0.7 × 107 (71)b | 0 (0) |
| Wild type | 24 | 14.0 × 107 ± 3.8 × 107 | 2.1 × 104 ± 1.0 × 104 |
| btkA mutant | 24 | 9.8 × 107 ± 1.0 × 107 (70) | 1.0 × 102 ± 0.2 × 102 (0.5) |
The total numbers of spores were counted with a hemocytometer. After sonication for 1 min and heat treatment (15 min at 50°C), the numbers of viable spores were determined by plating serial dilutions on CTT agar plates and incubating them at 27°C. The data are the means ± standard errors of triplicate determinations.
Values in parentheses are percentages relative to the values for the wild type.
Expression of phosphorylated BtkA in M. xanthus.
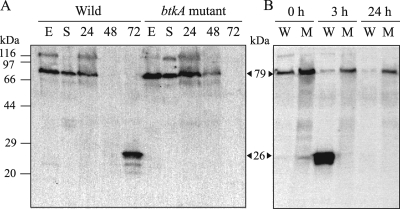
Tyrosine-phosphorylated proteins in wild-type and btkA mutant cells during the growth phase and starvation development were detected by SDS-PAGE and Western blotting using PY20 (Fig. 4A). A band at 79 kDa was detected mainly in the exponential and stationary phases and in the aggregation stage (24 h) of both protein extracts. The value of 79 kDa obtained by Western blot analysis corresponded well with the molecular mass (78.4 kDa) of the Gram-negative BY kinase gene (MXAN_1025), calculated from the predicted amino acid sequence. The 79-kDa protein disappeared in the fruiting formation phase (48 to 72 h). Frasch and Dworkin also reported that an 83-kDa tyrosine-phosphorylated membrane protein disappeared after induction of development (8). On the other hand, a 95-kDa eukaryotic tyrosine kinase (MasK) was detected in the inner membrane fraction on immunoblots probed with antiphosphotyrosine antibody (20). A new band at 26 kDa appeared in place of the 79-kDa band in wild-type cells in the late stage of development (72 h), but the 26-kDa tyrosine-phosphorylated protein was not expressed in mutant strain cells. The value of 26 kDa corresponded well with the molecular mass of BtkA. These data suggest that the 26-kDa tyrosine-phosphorylated protein was BtkA.
Fig. 4.
Expression of BtkA during development. (A) Starvation-induced development. Cells were grown in CYE medium and harvested in the exponential growth phase (E) and stationary phase (S). Cells were also starved on CF agar and harvested at 24 h, 48 h, and 72 h. (B) Glycerol-induced development. Wild-type (W) and btkA mutant (M) cells were incubated in CYE medium containing 0.5 M glycerol for the indicated time periods. Harvested cells were boiled in SDS sample buffer, separated by SDS-12.5% PAGE, and analyzed by immunoblotting with PY20. The estimated molecular mass (kDa) of each protein is shown in angle brackets.
As shown in Fig. 4B, 24- to 26-kDa tyrosine-phosphorylated proteins in wild-type protein extract were observed after 3 h of induction by 0.5 M glycerol and disappeared after 24 h of incubation. In contrast, these proteins were not observed in btkA mutant extracts during glycerol-induced spore formation. While the 79-kDa tyrosine-phosphorylated protein in wild-type protein extracts decreased during glycerol-induced spore formation, the 79-kDa protein was always observed in the mutant. These results suggest that phosphorylated BtkA was expressed in late-stage cells in fruiting body formation and at the stage of glycerol-induced spore formation and that BtkA played an important role in the maturation of myxospores.
It is reported that the exo gene was expressed late after starvation induction and early after glycerol induction (14). The timing and level of expression of the btkA gene were determined by quantitative reverse transcriptase PCR (qRT-PCR) analysis. Total RNA was isolated from growth-phase cells and during cell development at 24, 48, and 72 h. cDNA was synthesized with PrimeScript II RTase (Takara Bio Inc.), and PCR was performed with Kapa SYBR Fast qPCR master mix (KAPABiosystems), the RTbtkAN and RTbtkAC primers (Table 1), and the synthesized cDNA with the ABI 7300 real-time cycler. qRT-PCR analysis using cycle threshold values showed that the btkA gene was maximally expressed in the early stage of fruiting body formation (48 h). The relative cDNA quantities at 24 h and 72 h were 20% and 67%, respectively, of that at 48 h (defined as 100%). We also confirmed that a gene (MXAN_3229) located downstream of btkA was transcribed at similar levels at 24 to 72 h of development and was maximally transcribed at 48 h of development in wild-type and btkA mutant cells (estimated initial cDNA copy numbers at 48 h were 27,000 and 24,800 copies/reaction, respectively) using qRT-PCR with the RT3229N and RT3229C primers. The cDNA quantities at 24 and 72 h were 31% and 74%, respectively, for the wild-type cells and 28% and 72%, respectively, for the btkA mutant cells relative to those at 48 h (defined as 100%). These results suggest that the phenotypes of the btkA mutant were not due to polar effects.
Fibril polysaccharide analysis.
BY kinases have been shown to be involved mainly in the synthesis and/or export of extracellular polysaccharides (1, 21). M. xanthus fibrils are required for aggregation, social motility, and cellular agglutination and are composed of polysaccharides with roughly equivalent amounts of associated proteins (2). Fibril polysaccharide production was analyzed quantitatively by the binding of two separate dyes, Congo red and trypan blue, in colorimetric assays (12). Exponentially growing cells (5 × 108 cells/ml) in CYE medium and developing cells (8 × 108 cells/ml) at 72 h of incubation on CF agar were used for the assays, and the percentages of dye bound by cells were determined. btkA mutant cells in the growth phase and at 72 h of development bound 13% and 32%, respectively, of Congo red and 15% and 42%, respectively, of trypan blue. These levels of bound dye were similar to those in wild-type cells. To further investigate btkA mutant fibril formation, the cohesion of M. xanthus cells was measured with an agglutination assay (4). In agglutination buffer (10 mM MOPS [morpholinepropanesulfonic acid; pH 6.8], 1 mM MgCl2, and 1 mM CaCl2), wild-type and mutant cells began to agglutinate after 40 min of incubation and were precipitated within 120 min, demonstrating that BtkA is not necessary for fibril polysaccharide production.
M. xanthus cells glide on solid surfaces and periodically reverse the direction of their movement by switching the polarity of type IV pili and the adventurous motility engine with slime secretion at a certain frequency (24). When observed on CF agar, the btkA mutant strain showed a slightly shorter reversal period (3.3 min) than the wild-type strain (4.5 min). Social motility is important for cell aggregation and tight packing of spores within fruiting bodies (23). Since the btkA mutant aggregated and formed mounds under starvation conditions, the btkA mutant may have normal type IV pili and an adventurous motility engine.
Spore polysaccharides and proteins.
Myxospores were protected by a spore coat that consists mainly of polysaccharides and different proteins (11). Spore coat proteins W, C, S2, and U are synthesized during starvation-induced development, while the spore coat of glycerol-induced spores is composed of proteins S1 and U (6, 9, 15, 16). Purified wild-type and mutant spores prepared by centrifugation on sucrose step gradients (15) showed no significant difference between the numbers of wild-type and mutant spore coat proteins from starvation- and glycerol-induced spores in SDS-PAGE analysis (data not shown).
To determine the amount of spore-enveloped polysaccharide produced by developing cells, broken cell pellets from wild-type and btkA mutant strains under starvation conditions were prepared with sonication. No differences between wild-type and mutant strains were detected in the amounts of carbohydrate contained in the cell pellets at 48 h (Table 3), while mutant cell pellets at 112 h contained slightly less (70%) carbohydrate than wild-type cell pellets (defined as 100%). Correspondingly, the carbohydrate level of btkA mutant cell pellets prepared from 8-h glycerol-induced spores by sonication with glass beads was 67% of that of wild-type cell pellets (data not shown). These results did not strongly suggest that BtkA is involved in the synthesis of spore polysaccharides.
Table 3.
Carbohydrates isolated from starvation-induced sporesa
| Strain | Development time (h) | Supernatant (μg) | Pellet (μg) |
|---|---|---|---|
| Wild type | 48 | 242 ± 26 | 42 ± 10 |
| 112 | 252 ± 15 | 74 ± 13 | |
| btkA mutant | 48 | 231 ± 28 | 40 ± 12 |
| 112 | 256 ± 14 | 52 ± 15 |
Both types of cells (∼6 × 109) were inoculated onto CF agar and harvested at 48 h and 112 h. Cells were washed three times with distilled water and sonicated with glass beads 10 times for 15 s each, and then the supernatant and pellet were separated by centrifugation. The pellet was washed three times with distilled water. The sugar contents of the supernatant and pellet suspension were determined at 490 nm by the phenol-sulfuric acid method, with glucose as the standard (7).
BY kinases have been found to phosphorylate sugar dehydrogenases or transferases, sigma factors, and single-stranded DNA-binding proteins (1). To understand the role of BtkA, further work is needed to identify endogenous substrates of BtkA in M. xanthus cells.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant no. 22570187).
Footnotes
Published ahead of print on 12 August 2011.
REFERENCES
- 1. Bechet E., et al. 2009. Tyrosine-kinases in bacteria: from a matter of controversy to the status of key regulatory enzymes. Amino Acids 37:499–507 [DOI] [PubMed] [Google Scholar]
- 2. Behmlander R. M., Dworkin M. 1994. Biochemical and structural analyses of the extracellular matrix fibrils of Myxococcus xanthus. J. Bacteriol. 176:6295–6303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campos J. M., Geisselsoder J., Zusman D. R. 1978. Isolation of bacteriophage Mx4, a generalized transducing phage for Myxococcus xanthus. J. Mol. Biol. 119:167–178 [DOI] [PubMed] [Google Scholar]
- 4. Chang B.-Y., Dworkin M. 1996. Mutants of Myxococcus xanthus dsp defective in fibril binding. J. Bacteriol. 178:697–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doublet P., Grangeasse C., Obadia B., Vaganay E., Cozzone A. J. 2002. Structural organization of the protein-tyrosine autokinase Wzc within Escherichia coli cells. J. Biol. Chem. 277:37339–37348 [DOI] [PubMed] [Google Scholar]
- 6. Downard J. S., Zusman D. R. 1985. Differential expression of protein S genes during Myxococcus xanthus development. J. Bacteriol. 161:1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A., Smith F. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350–356 [Google Scholar]
- 8. Frasch S. C., Dworkin M. 1996. Tyrosine phosphorylation in Myxococcus xanthus, a multicellular prokaryote. J. Bacteriol. 178:4084–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gollop R., Inouye M., Inouye S. 1991. Protein U, a late-developmental spore coat protein of Myxococcus xanthus, is a secretory protein. J. Bacteriol. 173:3597–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hagen D. C., Bretscher A. P., Kaiser D. 1978. Synergism between morphogenic mutants of Myxococcus xanthus. Dev. Biol. 64:284–296 [DOI] [PubMed] [Google Scholar]
- 11. Kottel R. H., Bacon K., Clutter D., White D. 1975. Coats from Myxococcus xanthus: characterization and synthesis during myxospore differentiation. J. Bacteriol. 124:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lancero H., et al. 2004. Characterization of a Myxococcus xanthus mutant that is defective for adventurous motility and social motility. Microbiology 150:4085–4093 [DOI] [PubMed] [Google Scholar]
- 13. Leipe D. D., Koonin E. V., Aravind L. 2003. Evolution and classification of P-loop kinases and related proteins. J. Mol. Biol. 333:781–815 [DOI] [PubMed] [Google Scholar]
- 14. Licking E., Gorski L., Kaiser D. 2000. A common step for changing cell shape in fruiting body and starvation-independent sporulation of Myxococcus xanthus. J. Bacteriol. 182:3553–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McCleary W. R., Esmon B., Zusman D. R. 1991. Myxococcus xanthus protein C is a major spore surface protein. J. Bacteriol. 173:2141–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Otani M., et al. 1998. Protein W, a spore-specific protein in Myxococcus xanthus, formation of a large electron-dense particle in a spore. Mol. Microbiol. 30:57–66 [DOI] [PubMed] [Google Scholar]
- 17. Plamann L., Davis J. M., Cantwell B., Mayor J. 1994. Evidence that asgB encodes a DNA-binding protein essential for growth and development of Myxococcus xanthus. J. Bacteriol. 176:2013–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quijano V. J., Jr., Sheffield L. G. 1998. Prolactin decreases epidermal growth factor receptor kinase activity via a phosphorylation-dependent mechanism. J. Biol. Chem. 273:1200–1207 [DOI] [PubMed] [Google Scholar]
- 19. Soulat D., et al. 2006. Staphylococcus aureus operates protein-tyrosine phosphorylation through a specific mechanism. J. Biol. Chem. 281:14048–14056 [DOI] [PubMed] [Google Scholar]
- 20. Thomasson B., et al. 2002. MglA, a small GTPase, interacts with a tyrosine kinase to control type IV pili-mediated motility and development of Myxococcus xanthus. Mol. Microbiol. 46:1399–1413 [DOI] [PubMed] [Google Scholar]
- 21. Whitfield C. 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu. Rev. Biochem. 75:39–68 [DOI] [PubMed] [Google Scholar]
- 22. Whitworth D. E. (ed.). 2007. Myxobacteria: multicellularity and differentiation. ASM Press, Washington, DC [Google Scholar]
- 23. Yang Z., Geng Y., Xu D., Kaplan H. B., Shi W. 1998. A new set of chemotaxis homologues is essential for Myxococcus xanthus social motility. Mol. Microbiol. 30:1123–1130 [DOI] [PubMed] [Google Scholar]
- 24. Zusman D. R., Scott A. E., Yang Z., Kirby J. R. 2007. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat. Rev. Microbiol. 5:862–872 [DOI] [PubMed] [Google Scholar]