Abstract
The global regulator CodY controls the expression of dozens of metabolic genes and genes mediating adaptation to nutrient availability in many low-G+C Gram-positive bacteria. Branched-chain amino acids l-isoleucine, l-leucine, and l-valine (ILV) activate CodY both in vivo and in vitro, and genes that direct their synthesis (ilv, ybgE, and ywaA) are highly repressed by CodY, creating a potential negative feedback loop. The nucleoside triphosphate GTP also activates CodY in vitro, but the evidence for activation by GTP in vivo is limited and indirect. We constructed a Bacillus subtilis strain (ybgE bcd ywaA) that is unable to convert branched-chain α-keto acids to ILV or to use ILV as a precursor for branched-chain fatty acid synthesis. Unexpectedly, the strain was not viable on rich medium. Supplementing rich medium with short, branched-chain fatty acids or derepressing expression of genes for de novo ILV synthesis bypassed the original lethality, restoring growth and showing that the lack of viability was due to insufficient intracellular production of the precursors of branched-chain fatty acids. Spontaneous extragenic suppressor mutants that arose in the triple mutant population proved to have additional mutations in guaA or guaB or codY. Expression of ILV biosynthetic genes in codY mutants was increased. The gua mutations caused guanine/guanosine auxotrophy and led to partial derepression of direct CodY-repressed targets, including ILV biosynthetic genes, under conditions similar to those that caused the original lethality. We conclude that a guanine derivative, most likely GTP, controls CodY activity in vivo.
INTRODUCTION
Microbes respond to fluctuations in multiple environmental conditions, such as temperature and nutrient availability, to realize their full metabolic and physiological potential. Global regulatory proteins provide an effective link between environmental cues and adaptive genetic programs by sensing small molecules and altering the expression of critical genes. The result can have profound physiologic consequences for the cell and, in microbes that cause disease, can indicate transit to and from the host.
The global transcriptional regulatory protein CodY controls the expression of dozens of metabolic genes. Many targets of CodY encode products that allow bacterial cells to adapt to changes in the nutrient availability in each of several low-G+C Gram-positive bacterial genera, including Bacillus, Listeria, Staphylococcus, Clostridium, Lactococcus, and Streptococcus (8, 15, 16, 23, 25, 26, 29, 36, 45, 57). A number of these bacteria are of major industrial importance, and others are recalcitrant human pathogens that use CodY to coordinate the expression of important virulence genes with the exhaustion of nutrients. Therefore, detailed knowledge of the mechanism of action of CodY will allow us to determine how multiple gene expression programs are connected through metabolism.
The CodY proteins of Bacillus subtilis, Staphylococcus aureus, Clostridium difficile, and Listeria monocytogenes are activated for DNA binding in vitro by the presence of GTP and the branched-chain amino acids (BCAAs; l-isoleucine, l-leucine, and l-valine [ILV]) (Fig. 1A) (8, 17, 36, 49).Lactococcus lactis and Streptococcus pneumoniae CodY proteins appear to be activated only by BCAAs (25, 43). Analysis of the crystal structure of CodY shows that the C-terminal portion of the protein contains a winged helix-turn-helix (wHTH) domain needed for binding to DNA (33), whereas the N-terminal region of the protein contains a GAF domain that binds the BCAA class of effector molecules. Changes in the rate of ILV synthesis modulate CodY activity in vivo (12), and binding of ILV in vitro causes a conformational change that is thought to potentiate the DNA-binding activity of CodY (32). Additional increases in CodY activity in vivo are achieved in the presence of other exogenously added amino acids (3, 6, 12).
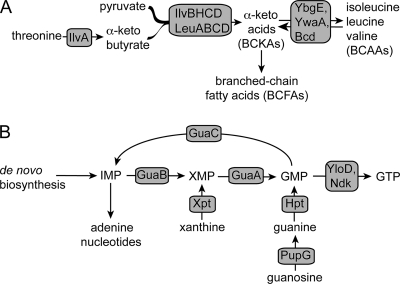
Fig. 1.
Pathways for BCAA, BCFA, and guanine nucleotide synthesis in B. subtilis. (A) Pyruvate and threonine serve as sources of carbon skeletons for branched-chain fatty acids and branched-chain amino acids. IlvA, threonine deaminase; IlvBH, acetolactate synthase; IlvC, ketol-acid reductoisomerase; IlvD, dihydroxy acid dehydratase; LeuA, 2-isopropylmalate synthase; LeuB, 3-isopropylmalate dehydrogenase; LeuCD, isopropylmalate isomerase. YbgE and YwaA are BCAA aminotransferases, and Bcd is leucine dehydrogenase. (B) IMP is the branch point for adenine and guanine nucleotide synthesis. IMP is converted to xanthine 5′-monophosphate (XMP) by GuaB (IMP dehydrogenase), followed by conversion to GMP by GuaA (GMP synthetase). The putative guanylate kinase YloD and nucleoside diphosphokinase Ndk act sequentially to synthesize GTP. GuaC, GMP reductase; Hpt, hypoxanthine-guanine phosphoribosyltransferase; PupG, guanosine/deoxyguanosine-inosine/deoxyinosine phosphorylase; Xpt, xanthine phosphoribosyltransferase.
GTP also binds to CodY in vitro and enhances binding of CodY to DNA targets (24, 46). The effects of ILV and GTP are synergistic. That is, the affinity of CodY for a given target is higher when exposed to both ILV and GTP than it is in the presence of either effector alone (24, 49). The evidence that GTP is a physiologically meaningful effector of CodY in living cells is indirect and is based on a reduction in CodY-dependent repression in conjunction with conversion of GTP to guanosine tetra/pentaphosphate [(p)ppGpp] during the stringent response (51) or as a result of inhibition of GMP synthetase by the nucleoside analog decoyinine (8, 27, 28, 34, 40, 46, 54). Decoyinine and the accumulation of (p)ppGpp may have additional, incompletely defined effects on cellular physiology.
The common purine biosynthetic pathway generates IMP, a branch point that provides the skeleton for both adenine and guanine nucleotides (Fig. 1B). IMP is converted to xanthine 5′-monophosphate (XMP) by IMP dehydrogenase (encoded by guaB). This first committed step in guanine nucleotide synthesis is inhibited by (p)ppGpp (42). XMP is aminated to GMP by the action of GMP synthetase, the product of guaA (34). The synthesis of GTP from GMP proceeds in two steps by the actions of guanylate kinase (presumably encoded by yloD) and nucleoside diphosphokinase (encoded by ndk) (52).
De novo synthesis of the BCAAs requires enzymes encoded by ilvA, ilvD, and the ilv-leu operon (here referred to as the ilvB operon), leading to the synthesis of the branched-chain α-keto acids (BCKAs; 3-methyl-2-oxopentanoate, 2-oxoisovalerate, and 4-methyl-2-oxopentanoate) (Fig. 1A) (19). The BCKAs are then transaminated to their corresponding amino acids by the combined effects of YbgE and YwaA (Fig. 1A) (10, 53).
The α-keto acids are also the precursors for synthesis of branched-chain fatty acids (BCFAs) (Fig. 1A). BCFAs serve as the principal fatty acids in B. subtilis membranes under standard growth conditions and also tailor membrane integrity by altering ratios of BCFAs in response to thermal perturbation, a multifaceted process known as homeoviscous adaptation (1, 14, 38, 44, 50). To synthesize BCFAs, BCKAs are converted first to their acyl-coenzyme A (acyl-CoA) derivatives and then to their carboxylic acid derivatives by functions encoded by the bkd operon (13, 20). BCAAs can also be precursors of BCFAs. Leucine dehydrogenase (encoded by bcd, a gene located at the bkd locus and whose expression is triggered by isoleucine and valine [13]) converts BCAAs to BCKAs, which can then be used as substrates for BCFA synthesis (Fig. 1A) (13, 20).
While studying the transaminases that interconvert BCKAs and BCAAs, we discovered, quite surprisingly, that knocking out the aminotransferase genes ybgE and ywaA and the leucine dehydrogenase gene bcd resulted in mutant cells (ybgE bcd ywaA) that could not grow on rich medium containing a relatively high concentration of ILV and other amino acids. In this report, we show that the growth defect is due to insufficient BCFA synthesis and that additional mutations in the genes coding for guanine nucleotide synthetic enzymes or CodY suppress the growth defect.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All strains constructed during this study are derivatives of Bacillus subtilis strain SMY and are listed in Table 1. Lennox (L) medium (31) lacking glucose or titration with NaOH was used as rich medium to routinely cultivate nonsynthetic lethal B. subtilis strains, except for transformation crosses plated onto rich medium, where DS medium (21) was used. Transformation crosses for strains not viable on rich medium were plated on TSS medium (2) supplemented with 0.02% (wt/vol) MgSO4 and 0.04 mg ml−1 each of FeCl3-6H2O and trisodium citrate. Glucose (28 mM) and NH4Cl (37.5 mM) were used as the sole sources of carbon and nitrogen, respectively. When necessary, media were solidified with agar at 15% (wt/vol) for DS medium and at 17% (wt/vol) for TSS medium. To maintain antibiotic selection, media were supplemented with antibiotics at the following concentrations: neomycin (Nm), 2.5 μg ml−1; spectinomycin (Spc), 50 μg ml−1; and erythromycin/lincomycin (Em), 1 μg ml−1/12.5 μg ml−1. Growth behavior analyses of guanine/guanosine and amino acid auxotrophs were performed in TSS medium with the indicated supplements in microtiter plate format. Briefly, single colonies were used to inoculate culture tubes containing 2 ml of minimal glucose-ammonium medium supplemented with 1.5 mM ILV and, if necessary, 200 μM guanosine. During exponential growth, cells were collected by centrifugation, washed, and resuspended in medium lacking ILV and guanosine to an optical density at 600 nm (OD600) of 1.0. Each well of a Cellstar 96-well plate (Greiner Bio-One) was filled with 190 μl of fresh medium and 10 μl of inoculum at an OD600 of 1.0, giving an initial OD of 0.05. Experiments were performed in a computer-controlled Synergy HT plate reader (Bio-Tek) set to 37°C. Kinetic parameters included vigorous shaking (intensity of 3), and the optical density was read every 15 min, with shaking between readings. Growth was monitored as an increase in absorbance at 600 nm.
Table 1.
Strains used during this study
| Strain | Genotype | Reference or source |
|---|---|---|
| B. subtilis strains | ||
| 168 | trpC2 | |
| Derivatives of 168 | ||
| 1A619 | trpC2 liv-1::Tn917 (erm) | Bacillus Genetic Stock Center |
| MGNA-A917 | trpC2 pMUTIN4 (yebA′ lacI lacZ erm) | National BioResource Project |
| MGNA-B889 | trpC2 pMUTIN4 (yaaH′ lacI lacZ erm) | National BioResource Project |
| PS258 | trpC2 codY::erm | P. Serror |
| SMY | Prototroph, 168 lineage | P. Schaeffer |
| Derivatives of SMY | ||
| BB2833 | lacA::tet amyE::spc codY(R61A) | 5 |
| BB2834 | lacA::tet amyE::spc codY(R61K) | 5 |
| BB2836 | lacA::tet amyE::spc codY(F71A) | 5 |
| BB2837 | lacA::tet amyE::spc codY(F71R) | 5 |
| SRB4 | SMY | P. Schaeffer |
| SRB10 | codY::(erm::spc) | 12 |
| SRB44 | ΔybgE1 Ω pSRB19 (ΔybgE1 neo) | SRB4 × DNA pSRB19 |
| SRB50 | ΔguaA4::erm | SRB4 × DNA pSRB23 |
| SRB58 | ΔybgE1 | Second crossover recombinant of SRB44 |
| SRB74 | ΔguaA4::erm codY::(erm::spc) | SRB10 × DNA SRB50 |
| SRB94 | ilvBp4 | 12 |
| SRB102 | liv-1::Tn917 (erm) | SMY × DNA 1A619 |
| SRB109 | ΔflgB2::erm | SRB4 × DNA pSRB30 |
| SRB118 | Δbcd1 | SRB4 × DNA pSRB32 |
| SRB142 | ΔywaA2::spc | 12 |
| SRB146 | ybgE1 ΔywaA2::spc | SRB58 × DNA SRB142 |
| SRB150 | ilvDpΔCBS Ω pSRB36 (ilvDpΔCBS neo) | 12 |
| SRB155 | Δbcd1 Ω pSRB19 (ΔybgE1 neo) | SRB118 × DNA pSRB19 |
| SRB159 | Δbcd1 ΔybgE1 | Second crossover recombinant of SRB155 |
| SRB163 | Δbcd1 ΔybgE1 ΔywaA2::spc | SRB159 × DNA SRB142 |
| SRB166 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-1 | Spontaneous suppressor of SRB163 |
| SRB167 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-2 | Spontaneous suppressor of SRB163 |
| SRB179 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-2 Ω pMUTIN4 (yaaH′ lacI lacZ erm) | SRB167 × DNA MBNA-B889 |
| SRB189 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-3 | Spontaneous suppressor of SRB163 |
| SRB190 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-4 | Spontaneous suppressor of SRB163 |
| SRB191 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-5 | Spontaneous suppressor of SRB163 |
| SRB192 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-6 | Spontaneous suppressor of SRB163 |
| SRB253 | Δbcd1 ΔybgE1 ΔywaA2::spc codY::erm | SRB163 × DNA PS258 |
| SRB263 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-7 | Spontaneous suppressor of SRB163 |
| SRB264 | Δbcd1 ΔybgE1 liv-1::Tn917 (erm) | SRB159 × DNA 1A619 |
| SRB265 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-7 Ω pMUTIN4 (yebA′ lacI lacZ erm) | SRB263 × DNA MGNA-A917 |
| SRB268 | ΔflgB2::erm codY::(spc::erm) | SRB109 × DNA SRB10 |
| SRB277 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-1 ΔflgB2::erm | SRB166 × DNA pSRB30 |
| SRB278 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-3 ΔflgB2::erm | SRB189 × DNA pSRB30 |
| SRB279 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-4 ΔflgB2::erm | SRB190 × DNA pSRB30 |
| SRB288 | Δbcd1 ΔybgE1 ilvDpΔCBS Ω pSRB36 (ilvDpΔCBS neo) | SRB159 × SRB150 |
| SRB294 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-1 ΔflgB2::erm | SRB163 × DNA SRB277 |
| SRB295 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-3 ΔflgB2::erm | SRB163 × DNA SRB278 |
| SRB296 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-4 ΔflgB2::erm | SRB163 × DNA SRB279 |
| SRB298 | Δbcd1 ΔybgE1 ΔywaA2::spc Ω pMUTIN4 (yaaH′ lacI lacZ erm) | SRB163 × DNA SRB179 |
| SRB299 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-2 Ω pMUTIN4 (yaaH′ lacI lacZ erm) | SRB163 × DNA SRB179 |
| SRB300 | Δbcd1 ΔybgE1 ΔywaA2::spc Ω pMUTIN4 (yebA′ lacI lacZ erm) | SRB163 × DNA SRB265 |
| SRB301 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-7 Ω pMUTIN4 (yebA′ lacI lacZ erm) | SRB163 × DNA SRB265 |
| SRB305 | Δbcd1 ΔybgE1 ilvDpΔCBS | Second crossover recombinant of SRB288 |
| SRB308 | Δbcd1 ΔybgE1 ilvDpΔCBS liv-1::Tn917 (erm) | SRB305 × DNA 1A619 |
| SRB311 | Δbcd1 ΔybgE1 ΔywaA2::spc ΔflgB2::erm | SRB163 × DNA SRB109 |
| SRB312 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-6 ΔflgB2::erm | SRB192 × DNA pSRB30 |
| SRB313 | Δbcd1 ΔybgE1 ilvBp4 | SRB264 × DNA SRB94 |
| SRB314 | Δbcd1 ΔybgE1 ilvBp4 ilvDpΔCBS | SRB308 × DNA SRB94 |
| SRB315 | Δbcd1 ΔybgE1 ilvBp4 ΔywaA2::spc | SRB313 × DNA SRB163 |
| SRB316 | Δbcd1 ΔybgE1 ilvBp4 ilvDpΔCBS ΔywaA2::spc | SRB314 × DNA SRB163 |
| SRB319 | Δbcd1 ΔybgE1 ΔywaA2::spc sld-6 ΔflgB2::erm | SRB163 × DNA SRB312 |
| SRB351 | amyE::spc codY(R61A) ΔflgB2::erm | BB2833 × DNA pSRB30 |
| SRB352 | amyE::spc codY(R61K) ΔflgB2::erm | BB2834 × DNA pSRB30 |
| SRB353 | amyE::spc codY(F71A) ΔflgB2::erm | BB2836 × DNA pSRB30 |
| SRB355 | amyE::spc codY(F71R) ΔflgB2::erm | BB2837 × DNA pSRB30 |
| SRB356 | Δbcd1 ΔybgE1 ΔywaA2::spc codY(R61A) ΔflgB2::erm | SRB163 × DNA SRB351 |
| SRB357 | Δbcd1 ΔybgE1 ΔywaA2::spc codY(R61K) ΔflgB2::erm | SRB163 × DNA SRB352 |
| SRB358 | Δbcd1 ΔybgE1 ΔywaA2::spc codY(F71A) ΔflgB2::erm | SRB163 × DNA SRB353 |
| SRB359 | Δbcd1 ΔybgE1 ΔywaA2::spc codY(F71R) ΔflgB2::erm | SRB163 × DNA SRB355 |
| E. coli strains | ||
| DH5α | supE44 Δlac U169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 48 |
| JM107 | supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) F′ (traD36 proAB+lacIqZΔM15) | 48 |
| One Shot Top10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 araD139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
For shake flask experiments, precultures were inoculated with the strain of interest in DS medium supplemented with 0.1 mM short, branched-chain fatty acids (sBCFAs; isovaleric, isobutyric, and 2-methylbutyric acids) and, when indicated, 1 mM guanosine. Precultures were incubated overnight at 37°C and were subsequently diluted into 25 ml of fresh medium in 125-ml Erlenmeyer flasks to give an OD600 of 0.05. Experimental cultures were incubated at 37°C with vigorous agitation (setting 7; ∼280 rpm) in a G76 Gyrotory shaking water bath (New Brunswick Scientific). Growth was monitored using an Ultrospec II UV-visible spectrophotometer (LKB Biochrom). At the end of each experiment, cells were plated on DS medium with and without sBCFAs to verify phenotypes and to ensure that no additional extragenic suppressor mutants were present in the cultures.
Petri plate images were acquired at a 1,200-dpi resolution using a computer-controlled Perfection 1200 flatbed scanner (Epson) and processed using Image Capture (version 6.0.1; Apple) and Photoshop (version CS2; Adobe) software.
DNA manipulations and transformations.
All molecular biology techniques, including E. coli transformations, were performed as described by Sambrook and Russell (48). Preparation of B. subtilis chromosomal DNA and B. subtilis transformations with plasmid or chromosomal DNA were performed as previously described (7). Primers were obtained from Integrated DNA Technologies, and all cloned fragments and mutant alleles were verified by sequencing by the Tufts University Nucleic Acids and Protein Core Facility.
Plasmid constructions. (i) pSRB19.
The region encompassing positions −601 to +6 relative to the putative ybgE translational start was amplified from B. subtilis SMY chromosomal DNA using primers oSRB28 (5′-ATG AAG AAG TGG TAC CAT AT-3′ and oSRB29 (5′-ATT CAA TCT AAT CAG CAC ACC TTT CAC ATA ATT G-3′). Underlined bases denote a KpnI site engineered into the primer. In addition, the region encompassing the ybgE stop codon and 550 bp of downstream sequence was amplified from the SMY chromosome using primers oSRB30 (5′-GTG TGC TGA TTA GAT TGA ATT GAA AAT CGA AAA AGA ACC TG-3′) and oSRB31 (5′-GAT CCT GTC CGC GGC TAA TT-3′). Underlined bases denote a SacII site engineered into the primer, and boldfaced bases denote a region of complementarity for overlapping PCR (58). The upstream and downstream flank-containing fragments were purified to remove primers, mixed in equal proportions, and used as a template in a third PCR with primers oSRB28 and oSRB31 to create an ∼1.1-kb overlap PCR product containing the allele ΔybgE1. The fragment was cut with KpnI and SacII, gel extracted, and ligated to the same sites of pBB544 (4). This plasmid of ∼5.4 kb was named pSRB19.
(ii) pSRB23.
The region encompassing positions −501 to +3 relative to the guaA translational start was amplified from the strain SMY chromosome using primers oSRB15 (5′-AGT CCT CCT GGT ACC AGC TGT ATT-3′) and oSRB16 (5′-ATA TAT ATA TAT GTC GAC CAT GGT TGT CAC CTA ATC TCC T-3′). Underlined bases denote KpnI and SalI sites engineered into the primers, respectively. In addition, the region encompassing the guaA stop codon and 476 bp of downstream flanking sequence was amplified from the strain SMY chromosome using primers oSRB17 (5′-ATA TAT ATA TAT GGA TCC TAA GAA TCA ATT AAT GGA AAC CA-3′) and oSRB18 (5′-ACA AAA TAT ATG CCG CGG ATA AAA AAG TCG TTA AT-3′). The underlined bases denote BamHI and SacII sites engineered into the primers, respectively. Each 0.5-kb fragment was cloned in pCR2.1 (Invitrogen), according to the manufacturer's instructions. Fragments were then cloned sequentially in pBB544 to yield a ΔguaA allele. This plasmid was cut (within ΔguaA) with BamHI and SalI, to which a 1.45-kb BamHI/SalI fragment of pJPM14 (J.P. Mueller and A. L. Sonenshein, unpublished data) containing the erm cassette conferring Emr was ligated, generating allele ΔguaA4::erm. This plasmid of ∼6.6 kb was named pSRB23.
(iii) pSRB28.
Regions upstream and downstream of the B. subtilis flgB coding sequence were amplified from the strain SMY chromosome using primers oSRB108 (5′-AAT GAA AGG TAC CTA TAT CA-3′) and oSRB109 (5′-TTT TTT GTC GAC TTA CCT CCA TTT-3′) and primers oSRB110 (5′-TGG AGG TAA GTC GAC AAA AAA TTT TTA GGA TCC TGA TGA CAG CTT TTC ATA-3′) and oSRB111 (5′-TGA CAT TCA CCG CGG ATG ATC TAG T-3′). Underlined bases denote the KpnI, SalI, BamHI, and SacII sites engineered into the primers, respectively, and boldface bases denote a region of complementarity for overlapping PCR. The upstream and downstream fragments (458-bp and 432-bp, respectively) were purified using the Qiagen PCR cleanup kit, mixed 1:1 (vol/vol), diluted appropriately, and used in an overlapping PCR using primers oSRB108 and oSRB111. The resulting ∼900-bp fragment containing the ΔflgB1 allele was digested with KpnI and SacII and ligated to the same sites of pBB544. The resulting plasmid of ∼5.2 kb was named pSRB28.
(iv) pSRB30.
Plasmid pSRB28 was cut with BamHI and SalI, to which a 1.45-kb BamHI/SalI fragment of pJPM14 containing the erm cassette conferring Emr was ligated, generating allele ΔflgB2::erm. This ∼6.6-kb plasmid was named pSRB30.
(v) pSRB32.
Regions upstream and downstream of the bcd coding sequence were amplified from strain SMY DNA using primers oSRB121 (5′-TCG TCA ATG AAT TCT GCT CCT T-3′) and oSRB122 (5′-CAT GTT CGT TCC TCC TAA TGT GTG TA-3′) as well as oSRB123 (5′-ACA TTA GGA GGA ACG AAC ATG TAA GAA ATT GAT CTG GAG GTT-3′) and oSRB124 (5′-ATA TAC GAA GGG ATC CTC AGG CCG TCA-3′). Underlined bases denote the EcoRI and BamHI sites engineered into the primers, respectively. Boldfaced bases denote a region of complementarity for overlapping PCR. The 503-bp and 437-bp fragments were purified, mixed 1:1 (vol/vol), and diluted appropriately for use in an overlapping PCR using outer primers oSRB121 and oSRB124. The resultant 940-bp fragment carrying allele Δbcd1 was cut with EcoRI and BamHI, gel extracted, and ligated to the same sites of pBB544, generating pSRB32 (∼5.2 kb).
B. subtilis strain constructions.
B. subtilis ΔguaA, ΔflgB, ΔybgE, and Δbcd strains were constructed by introducing E. coli strain JM107-propagated (recA+), plasmid-borne mutant alleles into strain SMY by transformation. For marked guaA and flgB alleles, we selected for double-crossover recombinants using resistance conferred by erm (Emr) and then scored for loss of the plasmid-encoded neo gene. We generated markerless, in-frame deletions of ybgE and bcd as previously described (12). Briefly, we selected for the inheritance of the plasmid as single-crossover recombinants (Nmr). Recombinants were PCR screened for merodiploids with two copies of the mutant allele (due to homogenotization). Strains were then serially passaged in rich medium lacking neomycin and plated on L medium. Colonies were replica printed on DS medium with and without Nm and screened for loss of Nmr to obtain clones that had undergone a second homologous recombination event. To construct strain SRB316 (triple mutant strain derivative with ilvBp4 and ilvDpΔCBS alleles), we began with strain SRB159 (ybgE bcd), transformed it to Nmr using chromosomal DNA from SRB150 (ilvDpΔCBS Ω pSRB36 [ilvDpΔCBS neo]), and followed the method described above for generating markerless mutations. We then moved allele liv-1::Tn917 (erm) into this strain by transformation using chromosomal DNA from strain 1A619 as the donor. The resulting strain was an ILV auxotroph and Emr. Using chromosomal DNA from strain SRB94 as the donor (ilvBp4; linked to liv-1), we transformed the auxotroph to ILV+ by selecting for prototrophy on TSS plates and scored for Ems. We then moved ΔywaA2::spc into this recipient strain by selecting Spcr on TSS-plus-ILV plates with Spc. We constructed strain SRB315 (ybgE bcd ywaA ilvBp4) similarly.
codY allele sequencing.
The codY coding sequences were amplified using primers oKK30 (5′-TGT CGA AGA AAA GCT CGG-3′) and oKK31 (5′-CAT AGA AAG ACT TTC AAC-3′). PCR products (∼1 kb) were purified and sequenced as described above.
RNA sampling, preparation, and cDNA synthesis.
Samples were collected and processed as previously described (12), carefully ensuring that shake flask cultures were in steady state. Cells were collected after at least three generations and within approximately one generation of one another. RNA was prepared as described previously (12), except that we used the High Pure RNA isolation kit (Roche Applied Science). RNaseOUT recombinant RNase inhibitor (200 units; Invitrogen) was added after purification to prevent RNA degradation. RNA preparations were treated with the Turbo DNA-free DNase treatment and removal kit (Ambion) to reduce contaminating DNA to insignificant levels. Treated RNAs were assessed for quality and integrity using agarose gel electrophoresis and ethidium bromide staining. cDNAs were prepared from each 250-ng sample of total RNA using SuperScript II reverse transcriptase (Invitrogen) with random primers (Promega) per the manufacturer's instructions.
Real-time qPCR.
We performed quantitative PCR (qPCR) using the LightCycler 480 system and associated SYBR green I chemistry (Roche Applied Science) to analyze transcript abundance from prepared cDNA samples. Each 25-μl reaction mixture contained either 600 nM ilvB-, ilvD-, or bcaP-specific primers or 300 nM rpoC-specific primers (Table 2). Thermal cycling proceeded as per the LightCycler 480 SYBR green I template protocol, except that we used annealing temperatures of 50°C and 55°C as the minimum temperatures for melting curve analysis. Standard curves were generated for each target using gel-purified, PCR-amplified fragments. Serial dilutions spanning at least 6 orders of magnitude were analyzed, allowing for absolute quantification of 125 to 1.25 × 107 copies of the bcaP transcript, 273 to 2.73 × 107 copies of the ilvB transcript, 146 to 1.46 × 107 copies of the ilvD transcript, and 203 to 2.03 × 108 copies of the rpoC transcript. Standard curves as well as standard PCR controls (including no-template and no-reverse transcriptase reactions) were run on each plate along with test reactions. All qPCRs proceeded with at least 85% efficiency, and initial transcript concentrations were calculated using the second-derivative maximum analysis algorithm. Points lay within polynomial (nonlinear) regression. Single amplification products were verified by melting curve analysis (melting temperature (Tm)-calling algorithm). Data are presented as copies of target transcript per copy of rpoC transcript multiplied by 100. The rpoC transcript, which codes for the β′ subunit of the DNA-dependent RNA polymerase, was used for normalization because its abundance was not expected to change significantly under the conditions tested.
Table 2.
Primers used for real-time quantitative RT-PCR
| Primer | Sequence | Target | Referencea |
|---|---|---|---|
| oSRB83 | 5′-ATT TAC GAT AAG CTA TAC AAT TCA-3′ | ilvB | 12 |
| oSRB94 | 5′-AAT CAA TCA TGG CAT CAG CAA-3′ | ilvB | 12 |
| oSRB116 | 5′-AAT GCA GGA AGA TTA AAC AGG GTA-3′ | bcaP | |
| oSRB117 | 5′-TGG GAT TTA ATT TTA GAA TAC ATG CT-3′ | bcaP | |
| oSRB170 | 5′-TCT TGA AGC TCG TTT TCG TTG A-3′ | ilvD | 12 |
| oSRB171 | 5′-TCG CAA TGG GGC ATA TCG GTA T-3′ | ilvD | 12 |
| oSRB194 | 5′-CCG TTC ATG GTC TTT TGG TG-3′ | rpoC | |
| oSRB195 | 5′-TTT AGC CCG TGT TAC TTC GAC-3′ | rpoC |
Unless specified, primers were designed during this investigation.
Statistical analyses.
We used unpaired, two-tailed Student's t tests with 95% confidence intervals to test for statistical significance. The comparisons were made using Prism version 4.0a software (GraphPad). Outliers were detected using Dixon's Q-test and associated critical Q values (18, 47).
RESULTS
The ybgE bcd ywaA strain is not viable on rich medium.
As part of a project seeking to control intracellular ILV pools, we sought B. subtilis strains defective in BCAA biosynthesis. Since ybgE and ywaA had previously been identified as redundant genes coding for BCAA aminotransferases (10, 53), we constructed a ybgE ywaA double mutant strain. Unexpectedly, the ybgE ywaA strain was not fully auxotrophic for the BCAAs. The double mutant strain required isoleucine, but not valine or leucine, for growth in a minimal glucose-ammonium medium. The growth rate of the double mutant strain was as high as the growth rate of the wild-type (WT) strain in isoleucine-containing medium (Fig. 2A and B, compare the wild type and ybgE ywaA). In contrast, we saw no growth of a strain that harbors a Tn917 transposon insertion in the ilvB operon unless we added all three BCAAs to the medium (data not shown). We hypothesized that leucine dehydrogenase, the product of the bcd gene and an enzyme known to be involved in synthesis of BCFAs from BCAAs (13), might contribute to the final, transamination step in valine and leucine biosynthesis.
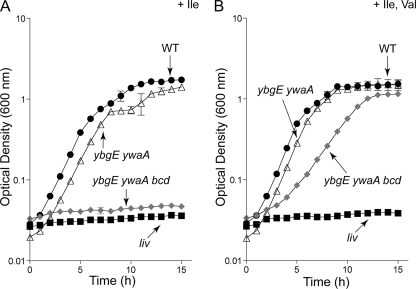
Fig. 2.
Growth behavior of B. subtilis strains lacking BCAA aminotransferases. Growth behavior was performed in microtiter plate format as described in Materials and Methods. Although readings were taken every 15 min, only data points corresponding to hourly readings are displayed to enhance clarity. Error bars denote standard errors of the mean optical density (OD600) at each time point for at least two technical replicates. When error bars are not visible, they are smaller than the edge of the symbol. Curves are representative of at least two independent experiments. Wild type (WT; SRB4), black circles; liv, transposon insertion mutant carried by the ilvB operon (SRB102), black squares; ybgE ywaA strain (SRB146), open triangles; ybgE ywaA bcd strain (SRB163), gray diamonds. (A) Growth in minimal glucose-ammonium medium supplemented with 1.5 mM isoleucine (Ile). (B) Growth in minimal glucose-ammonium medium supplemented with 1.5 mM (each) isoleucine (Ile) and valine (Val).
We attempted to construct the ybgE ywaA bcd triple mutant strain but, to our surprise, in multiple trials, we failed to obtain transformants when plating on rich (DS) medium. We did, however, obtain transformants on minimal glucose-ammonium-Spc medium supplemented with ILV to fulfill the expected nutritional requirement for the triple mutant strain. This strain was still not a total BCAA auxotroph and required both isoleucine and valine (but not leucine) for growth in a minimal glucose-ammonium medium. The growth rate for the triple mutant strain in the absence of leucine was lower, however, compared to that of the wild-type or ybgE ywaA strain (Fig. 2A and B, compare the wild type or ybgE ywaA and ybgE ywaA bcd), indicating that inactivation of bcd reduces but does not abolish the synthesis of leucine.
We speculated that the repeated failure of our selection on rich medium revealed a synthetic lethal genetic combination. In fact, upon prolonged incubation, two types of colonies arose on DS medium—large, rough colonies typical of B. subtilis and small, smooth colonies. These apparent suppressor mutants were purified on DS medium and maintained their characteristic appearance. Each mutant was identified with the allele designation sld (suppressor of lethality on DS medium) (Fig. 3).
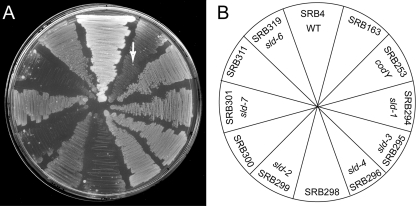
Fig. 3.
Growth behavior of B. subtilis strains on DS medium. (A) Suppression of the lethality of strain SRB163 on DS medium by mutations in codY (sld-1, sld-3, sld-4, and sld-6), guaA (sld-7), and guaB (sld-2). SRB4 (wild type) serves as the positive control, and SRB253 is the CodY-deficient triple mutant strain. Strains SRB298, -300, and -311 are each isogenic control strains harboring markers linked to sld. Strains were streaked and incubated at 37°C. The arrow indicates an exemplary extragenic suppressor mutant. (B) Plate schematic depicting the arrangement of strains on DS medium.
Large-colony suppressor mutations map to codY.
The involvement of the mutated genes in BCAA biosynthesis and the amino acid-responsive phenotype of the triple mutant strain raised the possibility that the activity of the pleiotropic global transcriptional regulator CodY might be involved in the mutant phenotype. We therefore tested the hypothesis that the suppressor mutant strains harbored lesions in the codY gene by introducing chromosomal DNA carrying the closely linked ΔflgB2::erm allele by transformation (as shown below, the mutation in flgB, which encodes a component of the flagellar hook-basal body complex, homologous to the Salmonella enterica protein [60], did not affect CodY-dependent regulation). In fact, most Emr derivatives (selected on minimal glucose-ammonium ILV medium) of four independent large-colony suppressor mutants (sld-1, sld-3, sld-4, and sld-6) lost the ability to form colonies when streaked on DS medium, indicating that the lesions necessary for suppression are tightly linked to flgB. Plasmid pSRB30 (which carries the ΔflgB2::erm allele but not the codY gene) was introduced by transformation into the sld strains SRB166, -189, -190, and -192 to place the Emr marker near the sld alleles without affecting codY. Chromosomal DNA from the resulting strains was used as the donor in transformation crosses, using the ybgE bcd ywaA strain as the recipient. In a separate experiment, we knocked out codY in the triple mutant strain. These reconstructed strains and the triple mutant strain with an authentic null allele of codY (strain SRB253) grew on DS medium, whereas a control strain harboring the ybgE, ywaA, and bcd mutations, but not the sld mutation, did not (Fig. 3), suggesting that sld alleles were necessary and sufficient for suppressing lethality. We amplified the codY gene from the chromosome of the sld-1, sld-3, sld-4, and sld-6 strains and identified point mutations in the coding sequence for CodY, creating the variants CodY(L126P), CodY(A227V), CodY(E101K), and CodY(S12P), respectively.
sld-2 and sld-7 alleles encode defective enzymes for purine synthesis.
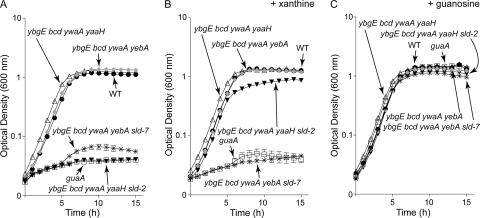
Two small-colony variant suppressor mutants isolated on DS medium failed to grow on minimal glucose-ammonium-ILV medium. We inferred that the allele(s) conferring the ability to grow on rich medium created a secondary auxotrophy. Knowing that guanine auxotrophs are among a limited number of auxotrophs that form small colonies on DS medium, we tested whether the GMP precursor xanthine or guanosine would stimulate growth of our mutants on minimal medium. On minimal glucose-ammonium-ILV plates, the strain carrying the sld-2 allele grew when the medium was supplemented with xanthine or guanosine. The strain carrying the sld-7 allele grew only when the medium was supplemented with guanosine. These results suggested that the lesions were in guaB and guaA for sld-2 and sld-7, respectively (Fig. 1B). Using available strains in the B. subtilis mutant collection (National BioResource Project [NIG, Japan]), we obtained markers linked to guaB (Ω pMUTIN4 [yaaH′ lacI lacZ erm]) or guaA (Ω pMUTIN4 [yebA′ lacI lacZ erm]) and used those linked mutations to transform the original triple mutant strain (ybgE bcd ywaA strain) to Emr. The sld mutations were separable from but linked to the erm markers, resulting in isogenic pairs of strains. We analyzed the growth behavior of these strains. Derivatives of the triple mutant strain lacking sld alleles grew in the absence of added purine, with a doubling time of 57.0 min, similar to that of the wild-type parent strain (Fig. 4A, compare the WT with the ybgE bcd ywaA yaaH strain and the WT with the ybgE bcd ywaA yebA strain in TSS-plus-ILV medium). In contrast, addition of xanthine or guanosine stimulated growth of the isogenic sld-2 strain, but only guanosine permitted growth of the isogenic sld-7 strain. An authentic guaA null allele (strain SRB50, ΔguaA4::erm) yielded similar results (Fig. 4A to C). All strains derived from SRB163 failed to grow when ILV was omitted from the medium (data not shown). The growth phenotypes of the sld-2 and sld-7 strains as well as their genetic linkages suggest that they have mutations in guaB and guaA, respectively. Indeed, sequencing revealed single point mutations coding for the variant proteins GuaB(T310K) and GuaA(Q325K).
Fig. 4.
sld-2 and sld-7 alleles encode defective Gua enzymes. Growth behavior was performed in microtiter plate format as described in Materials and Methods. Although readings were taken every 15 min, only data points corresponding to hourly readings are displayed to enhance clarity. Error bars denote standard errors of the mean optical density at each time point for at least two technical replicates. When error bars are not visible, they are smaller than the edge of the symbol. Curves are representative of at least two independent experiments. Wild type (WT; SRB4), black circles; guaA (SRB50), open squares; ybgE bcd ywaA yaaH strain (SRB298), open triangles; ybgE bcd ywaA yaaH sld-2 strain (SRB299), black inverted triangles; ybgE bcd ywaA yebA strain (SRB300), gray diamonds; ybgE bcd ywaA yebA sld-7 strain (SRB301), asterisks. (A) Growth in minimal glucose-ammonium-ILV medium. (B) Growth in minimal glucose-ammonium-ILV medium with 200 μM xanthine. (C) Growth in minimal glucose-ammonium-ILV medium with 250 μM guanosine.
Derepressing ilv synthesis or supplying short, branched-chain fatty acids bypasses the conditions of the original lethality.
We reasoned that under the original conditions that caused lethality, CodY was either activating a gene that prevents growth on rich medium or repressing the expression of a gene that is needed for growth. Furthermore, since DS medium contains ILV, the inability of the triple mutant strain to form colonies on DS medium was not caused by ILV auxotrophy per se. Rather, since the biosynthesis of BCFAs is essential for growth in B. subtilis (59) and the cells could not efficiently convert exogenously supplied ILV to their α-keto acid precursors due to lack of YbgE, Bcd, and YwaA, we hypothesized that cells were dependent on de novo BCFA precursor synthesis by the Ilv enzymes. We suspected that insufficient enzymes were being made to support the demand, due to CodY-dependent repression of the ilvB operon and the ilvD and ilvA genes in the presence of excess amino acids (35, 49, 56). To see if relieving CodY-mediated repression of the ilvB operon or the ilvD gene would overcome lethality, we combined ilvBp4 (an allele containing two point mutations in the major CodY binding site [CBS] in the ilvB promoter region [12]) or ilvBp4 and ilvDpΔCBS (a deletion of the CodY binding site in the ilvD promoter region [12]) alleles (12) with the ybgE, bcd, and ywaA alleles and assessed the ability of the resulting strains to grow on rich medium. While ilvBp4 alone did not suppress the lethality of the triple mutant strain, introducing both the ilvBp4 and ilvDpΔCBS alleles (each of which remove CodY-dependent regulation) rescued the strain on DS medium (Fig. 5A and B, compare ybgE bcd ywaA ilvBp4 and ybgE bcd ywaA ilvBp4 ilvDpΔCBS strains). The wild-type and the triple mutant strain were included as positive and negative controls for growth, respectively. Moreover, in contrast to the wild-type strain, the triple mutant strain failed to grow on DS medium unless we included in the medium 0.1 mM (each) short, branched-chain fatty acids (sBCFAs; isovaleric, isobutyric, and 2-methyl butyric) (Fig. 6A and B). Interestingly, we observed that the triple mutant strain appeared to be cross-fed by a diffusible factor secreted by growing strains on DS medium (Fig. 3, 5, and 6). This factor is likely to be a precursor of the branched-chain fatty acids or the fatty acids themselves.
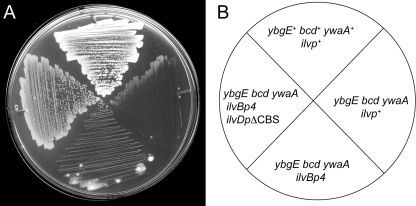
Fig. 5.
Derepression of the ILV pathway restores growth of strain SRB163 on DS medium. Strain SRB4 (wild type), ybgE+ bcd+ ywaA+ ilvp+; SRB163, ybgE bcd ywaA ilvp+; SRB315, ybgE bcd ywaA ilvp4; SRB316, ybgE bcd ywaA ilvp4 ilvDpΔCBS. (A) Strains were streaked to DS medium and incubated overnight at 37°C. (B) Plate schematic depicting the orientation of strains on DS medium.
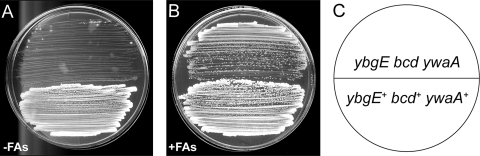
Fig. 6.
Supplying short, branched-chain fatty acids bypasses the original lethality of strain SRB163 on DS medium. Strains were streaked to DS media and incubated at 37°C. SRB4 (wild type), ybgE+ bcd+ ywaA+; SRB163, ybgE bcd ywaA. (A) DS medium lacking branched-chain fatty acids (−FAs). (B) DS medium supplemented with 0.1 mM isovaleric, isobutyric, and 2-methylbutyric sBCFAs (+FAs). (C) Schematic depicting strain orientation on media.
Reducing CodY activity bypasses lethality by derepressing the BCAA biosynthetic pathway.
To quantify the effects of sld mutations in codY on the BCAA biosynthesis pathway, we performed quantitative, real-time reverse transcription-PCR (RT-PCR) to measure the abundance of ilvB and ilvD transcripts in the triple mutant and suppressor strains. The bcaP gene (formerly yhdG [5]), another target of CodY, was also included. The bcaP, ilvB, and ilvD promoters are directly repressed by CodY (5, 39, 49). We cultivated each of the reconstructed strains containing the codY sld alleles in DS medium supplemented with 0.1 mM sBCFAs to bypass lethality. In this medium, the wild-type strain grew with a doubling time of 36.8 min, and we observed relatively low transcript abundance for bcaP, ilvB, and ilvD (0.96, 0.59, and 0.53 copies of transcript relative to the rpoC transcript, respectively) (Table 3). Transcript abundances in the triple mutant strain (and in the same strain with flgB knocked out) were essentially equivalent, though growth was slower. We observed 53-, 45-, and 7-fold derepression of bcaP, ilvB, and ilvD, respectively, when the codY gene was knocked out (Table 3, compare the WT and codY). Similarly, when we knocked out codY in the triple mutant strain, we observed a 44- to 53-fold increase in the bcaP transcript, a 19- to 30-fold increase in the ilvB transcript, and an approximately 5- to 6-fold increase in the ilvD transcript (Table 3, compare ybgE bcd ywaA or ybgE bcd ywaA flgB and ybgE bcd ywaA codY).
Table 3.
Transcript analysis of CodY-regulated targets by real-time quantitative RT-PCR
| Genotype | Mediuma | CodY protein | GuaA/GuaB protein | Doubling time (min) | No. of copies of transcriptb |
||
|---|---|---|---|---|---|---|---|
| bcaP | ilvB | ilvD | |||||
| WT | DSM | WT | WT/WT | 36.8 ± 1.8 | 0.96 ± 0.23 | 0.59 ± 0.08 | 0.53 ± 0.09 |
| codY | DSM | —-d | WT/WT | 41.9 ± 1.8 | 51.29 ± 1.86 | 26.71 ± 1.08 | 3.58 ± 0.01 |
| guaA | DSM | WT | —/WT | 54.2 ± 3.3 | 1.23 ± 0.34 | 4.16 ± 0.10 | 0.70 ± 0.07 |
| guaA codY | DSM | —d | —/WT | 74.2 ± 0.3 | 90.88 ± 12.74 | 77.86 ± 5.53 | 4.36 ± 1.16 |
| ybgE bcd ywaA | DSM | WT | WT/WT | 48.7 ± 4.7 | 1.41 ± 0.13 | 1.81 ± 0.68 | 0.95 ± 0.26 |
| ybgE bcd ywaA ilvBp4 | DSM | WT | WT/WT | 40.3 ± 2.4 | 0.73 ± 0.05 | 8.51 ± 1.28 | 0.47 ± 0.05 |
| ybgE bcd ywaA ilvBp4 ilvDpΔCBS | DSM | WT | WT/WT | 43.0 ± 3.2 | 0.34 ± 0.07 | 5.74 ± 0.29 | 2.80 ± 0.04 |
| ybgE bcd ywaA flgB | DSM | WT | WT/WT | 45.8 ± 1.2 | 1.16 ± 0.16 | 1.11 ± 0.23 | 0.70 ± 0.12 |
| ybgE bcd ywaA flgB sld-1 | DSM | L126P | WT/WT | 41.0 ± 1.0 | 64.15 ± 4.24 | 37.23 ± 4.10 | 4.72 ± 0.68 |
| ybgE bcd ywaA flgB sld-3 | DSM | A227V | WT/WT | 40.8 ± 1.2 | 71.90 ± 12.94 | 42.87 ± 4.76 | 6.14 ± 1.11 |
| ybgE bcd ywaA flgB sld-4 | DSM | E101K | WT/WT | 40.0 ± 0.6 | 48.35 ± 1.42 | 24.59 ± 2.82 | 3.95 ± 0.52 |
| ybgE bcd ywaA flgB sld-6 | DSM | S12P | WT/WT | 36.5 ± 0.2 | 33.63 ± 1.75 | 18.62 ± 2.44 | 4.00 ± 0.74 |
| ybgE bcd ywaA flgB codY | DSM | F71A | WT/WT | 46.1 ± 3.4 | 7.77 ± 1.61 | 3.16 ± 0.06 | 1.96 ± 0.28 |
| ybgE bcd ywaA flgB codY | DSM | R61K | WT/WT | 49.6 ± 6.2 | 17.17 ± 0.32 | 7.20 ± 0.05 | 2.88 ± 0.10 |
| ybgE bcd ywaA codY | DSM | — | WT/WT | 43.8 ± 3.3 | 61.79 ± 3.78 | 33.82 ± 4.00 | 4.52 ± 0.64 |
| ybgE bcd ywaA yaaH | DSM | WT | WT/WT | 45.6 ± 3.6 | 0.77 ± 0.10 | 0.87 ± 0.11 | 0.54 ± 0.14 |
| ybgE bcd ywaA yaaH sld-2 | DSM | WT | WT/GuaB(T310K) | 71.5 ± 8.0 | 3.71 ± 0.68 | 15.22 ± 1.57 | 1.65 ± 0.18 |
| ybgE bcd ywaA yebA | DSM | WT | WT/WT | 47.7 ± 2.3 | 0.65 ± 0.05 | 0.77 ± 0.05 | 0.44 ± 0.05 |
| ybgE bcd ywaA yebA sld-7 | DSM | WT | GuaA(Q325K)/WT | 57.4 ± 5.8c | 0.81 ± 0.19c | 4.02 ± 1.03c | 0.52 ± 0.08c |
| ybgE bcd ywaA yaaH | DSM+Guo | WT | WT/WT | 48.8 ± 0.4 | 0.67 ± 0.19 | 0.92 ± 0.20 | 0.56 ± 0.12 |
| ybgE bcd ywaA yaaH sld-2 | DSM+Guo | WT | WT/GuaB(T310K) | 48.8 ± 2.8 | 0.42 ± 0.10 | 0.36 ± 0.10 | 0.24 ± 0.08 |
| ybgE bcd ywaA yebA | DSM+Guo | WT | WT/WT | 48.2 ± 1.7 | 0.56 ± 0.14 | 0.61 ± 0.01 | 0.34 ± 0.04 |
| ybgE bcd ywaA yebA sld-7 | DSM+Guo | WT | GuaA(Q325K)/WT | 47.5 ± 0.4 | 0.62 ± 0.16 | 0.68 ± 0.06 | 0.35 ± 0.01 |
In all cases, the growth medium DS was supplemented with 0.1 mM (each) short, branched-chain fatty acids (isovaleric, isobutyric, and 2-methylbutyric). In some cases, guanosine (Guo) was added to a final concentration of 1 mM.
Data are the means ± standard errors of the means from at least two independent biological replicates. Target transcript abundances are relative to the rpoC transcript abundance.
Data are from four independent biological replicates. A fifth biological replicate was deemed an outlier by Dixon's Q-test (18, 47) and was discarded. If all five biological replicates had been included, the doubling time would have been 57.6 ± 4.5 min, and transcript levels would have been 2.03 ± 1.22, 12.04 ± 8.06, and 1.64 ± 1.12 copies for bcaP, ilvB, and ilvD, respectively.
—, no Cody protein in null mutant.
Strains harboring sld alleles encoding CodY(L126P) and CodY(A227V) exhibited derepression essentially equivalent to that exhibited by the codY null mutant strains. However, strains producing CodY(E101K) and CodY(S12P) were derepressed only about 42- and 29-fold for bcaP, respectively (Table 3, compare ybgE bcd ywaA flgB and ybgE bcd ywaA flgB sld-4 or ybgE bcd ywaA flgB sld-6 and compare ybgE bcd ywaA flgB sld-4 or ybgE bcd ywaA flgB sld-6 and ybgE bcd ywaA codY). A Student's t test suggested that these transcript abundances are significantly different from those of fully derepressed bcaP (P < 0.05). For the less highly regulated targets ilvB and ilvD, the level of derepression caused by these codY alleles was indistinguishable from full derepression (Table 3, compare ybgE bcd ywaA flgB sld-4 or ybgE bcd ywaA flgB sld-6 with ybgE bcd ywaA codY). To confirm that full derepression was not necessary for growth on DS medium lacking sBCFAs, we tested the ability of two bona fide partial activity variants of CodY to suppress lethality on DS medium (5). Variant protein CodY(F71A) retained sufficient activity to keep the ilv promoters repressed and did not suppress lethality on DS medium. Analysis of transcript abundances showed 7-, 3-, and 3-fold derepression for bcaP, ilvB, and ilvD, respectively (Table 3, compare ybgE bcd ywaA flgB and ybgE bcd ywaA flgB codY [F71A]). In contrast, the strain producing CodY(R61K) showed 15-, 6-, and 4-fold derepression of bcaP, ilvB, and ilvD. The corresponding reduction in CodY activity was apparently sufficient to promote enough sBCFA synthesis to make cells producing this variant viable on DS medium. For both variants, derepression of bcaP and ilvB was apparently significantly different from that observed in cells lacking CodY. ilvD derepression was statistically indistinguishable from that observed in codY null mutant strains (Table 3).
Elimination of endogenous guanine nucleotide synthesis results in partial CodY activity in DS medium.
We also assayed bcaP, ilvB, and ilvD transcript abundance in strains carrying lesions in guaB and guaA (sld-2 and sld-7, respectively) during growth in DS medium. DS medium was the sole source of guanine nucleotides for these guanine/guanosine auxotrophs, and differences in doubling times between gua and gua+ isogenic strains suggest that guanine nucleotides may be limiting (Table 3, compare doubling times of DSM and DSM plus Guo). Therefore, we hypothesized that lowering the pools of guanine nucleotides in these strains reduces CodY activity. Isogenic sld+ derivatives had transcript abundances similar to the wild-type and triple mutant strains (Table 3). We observed approximately a 5-fold increase in the bcaP transcript, an 18-fold increase in the ilvB transcript, and a 3-fold increase in the ilvD transcript in the strain producing the variant GuaB(T310K) (Table 3, compare ybgE bcd ywaA yaaH and ybgE bcd ywaA yaaH sld-2). These transcript abundances suggest that CodY still retains some activity compared to abundances measured in the absence of CodY (a Student's t test yields a P value of <0.05). Surprisingly, we saw no effect on the bcaP or ilvD transcript in the strain producing the GuaA(Q325K) protein (Table 3, compare ybgE bcd ywaA yebA and ybgE bcd ywaA yebA sld-7) (P < 0.05) or in a guaA null mutant (Table 3, compare the WT and guaA). In fact, only a ∼5- to 7-fold increase in the ilvB transcript was observed and was sufficient to suppress lethality. While the triple mutant strain requires sBCFAs for growth in rich medium, guaA and guaB derivatives lacking functional IMP dehydrogenase and GMP synthetase do not. In accord with our hypothesis that CodY activity is lower because the pool of guanine nucleotides is reduced, supplementing the medium with 1 mM guanosine restored full CodY-dependent regulation and lethality (sBCFA requirement) on DS medium (Table 3 and data not shown).
DISCUSSION
The global transcriptional regulator CodY is activated as a DNA-binding protein in vitro by ILV and GTP. The structure of CodY in complex with the amino acids revealed a conformational change induced by binding of the amino acids that presumably enhances CodY activity (32). In contrast, no crystal structure of CodY in complex with GTP has been obtained to date. Similarly, changes in the rate of ILV synthesis in vivo alter CodY activity (12), but proof that the GTP pool is an intracellular signal for CodY has been difficult to obtain. New evidence for the intracellular role of guanine nucleotides in activating CodY, as described here, came unexpectedly from analysis of the growth defect caused by inactivation of three enzymes critical for interconverting BCAAs and BCKAs. Since the only nucleotides that activate CodY in vitro are GTP and dGTP (24), it is very likely that one of these molecules is the true intracellular activator of CodY. Since the pool of dGTP is too low to be effective (11), GTP remains the prime candidate.
The ybgE bcd ywaA strain depends on de novo BCFA synthesis for viability.
Branched-chain fatty acids are derived from branched-chain α-keto acids produced de novo or by degradation of ILV (13). The fact that elimination of three enzymes that interconvert ILV and their α-keto acids led to a growth defect in rich medium suggested that either branched-chain fatty acids or another metabolite synthesized from α-keto acids (i.e., coenzyme A or pantothenate) was limiting for growth. Consistent with this hypothesis, derepression of the biosynthetic pathway for BCKAs (Fig. 5) or supplying short, branched-chain fatty acids (Fig. 6) restored growth of the triple mutant strain, revealing both the identity of the missing factor(s) (BCFAs or BCKAs; they likely cross-feed the triple mutant strain [Fig. 3]) and the relevant target of CodY-dependent regulation (the ILV biosynthetic pathway).
codY and gua suppressor mutations are loss-of-function mutations that decrease CodY activity.
Relatively large pseudorevertant colonies harbored mutations that mapped to the codY gene, creating variant proteins lacking full regulatory activity. The mutations fell within or near known functional domains of CodY (Fig. 7). Interestingly, one allele (sld-8) obtained but not analyzed in detail during this investigation encodes variant protein CodY(A207V), which was independently isolated 6 years ago (29). An ilvB-lacZ transcriptional fusion was 44-fold derepressed in a strain producing CodY(A207V) (29). An additional allele, sld-11, is a 93-bp deletion near the 5′ end of the codY gene, resulting in a frameshift and production of a truncated protein containing only 22 N-terminal amino acids (data not shown).
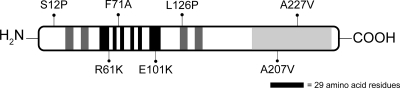
Fig. 7.
Single-amino acid substitutions reduce CodY activity. Variant residues are mapped onto a schematic representation of the CodY polypeptide. The regions involved in binding ILV (black), in dimerization (dark gray), and in binding DNA (winged helix-turn-helix [wHTH]; light gray) are depicted as rectangles. The representation is approximate.
The variants CodY(L126P) and CodY(A227V) appear to lack any significant regulatory activity, but the variants CodY(S12P) and CodY(E101K) appear to retain partial regulatory activity (Table 3). This partial activity of CodY(S12P) and CodY(E101K) is visible only when in vivo CodY activity is maximized (i.e., in cells grown in amino acid-containing medium).
Substitutions in GMP synthetase and IMP dehydrogenase [GuaA(Q325K) and GuaB(T310K) variants, respectively] are in conserved regions predicted to be in or near the active sites of these enzymes. We have not biochemically characterized these proteins in any detail, but we presume them to have, at most, low residual activity based on the inability to support the growth of B. subtilis in a defined medium in the absence of added guanosine.
We were surprised to observe differences between the guaA and guaB alleles with respect to transcript abundances of CodY targets. For example, in the guaB mutant, we saw ∼5-, ∼18-, and ∼3-fold increases in abundances of the bcaP, ilvB, and ilvD transcripts, respectively. These numbers represent ∼6%, ∼45%, and ∼36% of the level seen in a codY null mutant (Table 3 and Fig. 8). In contrast, mutations in guaA caused an up to ∼7-fold increase in ilvB transcript abundance but no significant change in expression of bcaP or ilvD (Table 3). The simplest explanation for relatively mild derepression in a guaA mutant is that reduced IMP dehydrogenase activity has a greater effect on the guanine nucleotide pool than does reduced GMP synthetase activity. Whereas the effect of a guaB mutation appears to be mediated through CodY, we conclude from comparing transcript abundances in the codY strain and the codY guaA strain that there is a positive, CodY-independent guaA effect on ilvB transcription due to guanine/guanosine limitation (Table 3) (54, 55).
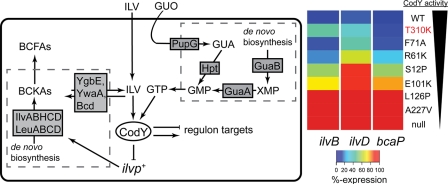
Fig. 8.
CodY activity is intimately connected to intracellular BCAA and GTP pools to control major metabolic pathways. (Left) De novo precursors provide sources of carbon skeletons for synthesis of ILV and GTP by the Ilv and Gua enzymes. The aminotransferases YbgE and YwaA along with leucine dehydrogenase (Bcd) interconvert ILV and their α-keto acid precursors, which serve as building blocks for branched-chain fatty acids (BCFAs). CodY activity (defined as the ratio of active CodY molecules to total CodY molecules) is modulated by internal pools of ILV and a guanine nucleotide (presumably GTP). CodY-repressed targets (e.g., ilvBp+, ilvDp+, and bcaPp+) are differentially regulated depending on the extent of CodY activity and the strength of CodY binding site. (Right) Heat map depicting transcriptional output for each promoter over the full dynamic range for sld alleles, some of which appear to partially deactivate CodY. Minimum expression is governed by maximally activated wild-type CodY (DS medium, purple), and maximum expression occurs in CodY-deficient cells (DS medium, red; 100%). Values are represented as a percentage of the total possible expression in DS medium. CodY proteins are indicated to the right of the heat map, and the GuaB(T310K) variant is indicated by red text. The black wedge depicts the apparent trend in CodY activity. GUA, guanine; GUO, guanosine; Hpt, hypoxanthine-guanine phosphoribosyltransferase; PupG, guanosine/deoxyguanosine-inosine/deoxyinosine phosphorylase.
The apparent lack of derepression of ilvD in the guaA mutant strains seems at odds with our finding that lethality of the triple mutant strain can be bypassed (though growth was apparently not fully restored) by inactivating the CodY binding sites in the ilvB and ilvD promoters but not by inactivation of the ilvB site alone (Fig. 5). Perhaps ilvD is slightly overexpressed in guaA cells at a level that cannot be detected by our qPCR assay. We know that at least 3-fold derepression is sufficient to suppress lethality (Table 3, compare ybgE bcd ywaA and ybgE bcd ywaA ilvBp4 ilvDpΔCBS). Alternatively, in the absence of substantial CodY activity, IlvD enzyme activity may be stabilized by a factor whose gene is normally repressed by CodY. Interestingly, the yfm locus, which was shown to be a direct target of CodY in chromatin immunoprecipitation with microarray technology (ChIP-to-chip) experiments (39), encodes proteins that facilitate the uptake of iron and is a target of the Fur (ferric uptake regulator) global regulatory protein (41). IlvD is an iron-sulfur cluster-containing dehydratase whose activity may increase with increased iron uptake.
Distinct regulatory activities afforded by decreases in guanine nucleotides or CodY variants demonstrate hierarchical organization of selected CodY regulon members.
Deducing the true in vivo effectors of a regulatory protein is one of the greatest challenges in cell physiology. We have presented genetic evidence that a guanine nucleotide controls CodY activity, consistent with previous results based on induction of stringency and treatment of cells with decoyinine (8, 9, 22, 26–28, 30, 37, 46, 54, 55). Figure 8 presents a summary of our results and their interpretation. When the triple mutant strain, which cannot derive BCFAs from ILV, is cultivated in DS medium, the concentration of ILV and other amino acids in the medium must be high enough to activate CodY-dependent repression of the ilv and leu genes, whose products synthesize the BCKAs that are the precursors of the BCFAs. As a result, the cells are unable to synthesize membrane fatty acids at a rate high enough to support growth. By reducing CodY activity either directly or indirectly (by altering the guanine nucleotide pool), suppressor strains bypass the original lethality by derepressing the ilv promoters. The heat map shown in Fig. 8 reflects transcript abundances of the ilvB, ilvD, and bcaP promoters as a function of CodY activity and illustrates that these CodY targets are differentially sensitive to CodY activity. That is, the bcaP promoter requires substantial reduction in CodY activity to be highly expressed. In contrast, the ilv promoters—especially ilvD—are more sensitive to CodY activity and are maximally expressed with only modest reductions in CodY activity. This graded response to various effector levels is undoubtedly dependent on the various affinities of CodY for its many targets (5). We are currently determining the hierarchy of CodY-regulated gene expression at different levels of nutrient sufficiency. Since CodY controls expression of both metabolic genes and some of the most important virulence genes carried by pathogenic low-G+C Gram-positive bacteria, a comprehensive understanding of the interplay between metabolite pools and global gene expression will give an unprecedented view of how metabolic and virulence gene expression programs are interrelated and interdependent.
ACKNOWLEDGMENTS
We thank Boris Belitsky and James Baleja for helpful comments and suggestions and Andrew Camilli for the use of his laboratory's Bio-Tek plate reader. We thank Kathy O'Day Kerstein for her gift of primers used for codY allele sequencing. We also thank the National BioResource Project (NIG, Japan): B. subtilis for providing us with their B. subtilis mutant strain collection.
This work was supported in part by an NIH National Research Service Award (F32 GM085911) to S.R.B. and an NIH research grant (R01 GM042219) to A.L.S.
Footnotes
Published ahead of print on 19 August 2011.
REFERENCES
- 1. Aguilar P. S., Lopez P., de Mendoza D. 1999. Transcriptional control of the low-temperature-inducible des gene, encoding the delta-5 desaturase of Bacillus subtilis. J. Bacteriol. 181:7028–7033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atkinson M. R., Fisher S. H. 1991. Identification of genes and gene products whose expression is activated during nitrogen-limited growth in Bacillus subtilis. J. Bacteriol. 173:23–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Atkinson M. R., Wray L. V., Jr., Fisher S. H. 1990. Regulation of histidine and proline degradation enzymes by amino acid availability in Bacillus subtilis. J. Bacteriol. 172:4758–4765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Belitsky B. R., Gustafsson M. C. U., Sonenshein A. L., von Wachenfeldt C. 1997. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J. Bacteriol. 179:5448–5457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Belitsky B. R., Sonenshein A. L. 2011. Contributions of multiple binding sites and effector-independent binding to CodY-mediated regulation in Bacillus subtilis. J. Bacteriol. 193:473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Belitsky B. R., Sonenshein A. L. 2008. Genetic and biochemical analysis of CodY-binding sites in Bacillus subtilis. J. Bacteriol. 190:1224–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Belitsky B. R., Sonenshein A. L. 1998. Role and regulation of Bacillus subtilis glutamate dehydrogenase genes. J. Bacteriol. 180:6298–6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bennett H. J., et al. 2007. Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol. Microbiol. 63:1453–1467 [DOI] [PubMed] [Google Scholar]
- 9. Bergara F., et al. 2003. CodY is a nutritional repressor of flagellar gene expression in Bacillus subtilis. J. Bacteriol. 185:3118–3126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Berger B. J., English S., Chan G., Knodel M. H. 2003. Methionine regeneration and aminotransferases in Bacillus subtilis, Bacillus cereus, and Bacillus anthrasis. J. Bacteriol. 185:2418–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bochner B. R., Ames B. N. 1982. Complete analysis of cellular nucleotides by two-dimensional thin layer chromatography. J. Biol. Chem. 257:9759–9769 [PubMed] [Google Scholar]
- 12. Brinsmade S. R., Kleijn R. J., Sauer U., Sonenshein A. L. 2010. Regulation of CodY activity through modulation of intracellular branched-chain amino acid pools. J. Bacteriol. 192:6357–6368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Débarbouillé M., Gardan R., Arnaud M., Rapoport G. 1999. Role of BkdR, a transcriptional activator of the SigL-dependent isoleucine and valine degradation pathway in Bacillus subtilis. J. Bacteriol. 181:2059–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Mendoza D., Grau R., Cronan J. E., Jr 1993. Biosynthesis and function of membrane lipids, p. 411–421 In Sonenshein A. L., Hoch J. A., Losick R., (ed.), Bacillus subtilis and other Gram-positive bacteria. ASM Press, Washington, DC [Google Scholar]
- 15. den Hengst C. D., et al. 2005. The Lactococcus lactis CodY regulon: identification of a conserved cis-regulatory element. J. Biol. Chem. 280:34332–34342 [DOI] [PubMed] [Google Scholar]
- 16. Dineen S. S., McBride S. M., Sonenshein A. L. 2010. Integration of metabolism and virulence by Clostridium difficile CodY. J. Bacteriol. 192:5350–5362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dineen S. S., Villapakkam A. C., Nordman J. T., Sonenshein A. L. 2007. Repression of Clostridium difficile toxin gene expression by CodY. Mol. Microbiol. 66:206–219 [DOI] [PubMed] [Google Scholar]
- 18. Dixon W. J. 1950. Analysis of extreme values. Ann. Math. Stat. 21:488–506 [Google Scholar]
- 19. Fink P. S. 1993. Biosynthesis of the branched-chain amino acids, p. 307–317 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and other Gram-positive bacteria. American Society for Microbiology, Washington, DC [Google Scholar]
- 20. Fisher S. H., Débarbouillé M. 2002. Nitrogen source utilization and its regulation, p. 181–192 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 21. Fouet A., Sonenshein A. L. 1990. A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis. J. Bacteriol. 172:835–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Geiger T., et al. 2010. Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect. Immun. 78:1873–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guédon E., Sperandio B., Pons N., Ehrlich S. D., Renault P. 2005. Overall control of nitrogen metabolism in Lactococcus lactis by CodY, and possible models for CodY regulation in Firmicutes. Microbiology 151:3895–3909 [DOI] [PubMed] [Google Scholar]
- 24. Handke L. D., Shivers R. P., Sonenshein A. L. 2008. Interaction of Bacillus subtilis CodY with GTP. J. Bacteriol. 190:798–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hendriksen W. T., et al. 2008. CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization. J. Bacteriol. 190:590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hsueh Y. H., Somers E. B., Wong A. C. 2008. Characterization of the codY gene and its influence on biofilm formation in Bacillus cereus. Arch. Microbiol. 189:557–568 [DOI] [PubMed] [Google Scholar]
- 27. Inaoka T., Ochi K. 2002. RelA protein is involved in induction of genetic competence in certain Bacillus subtilis strains by moderating the level of intracellular GTP. J. Bacteriol. 184:3923–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Inaoka T., Takahashi K., Ohnishi-Kameyama M., Yoshida M., Ochi K. 2003. Guanine nucleotides guanosine 5′-diphosphate 3′-diphosphate and GTP co-operatively regulate the product of an antibiotic bacilysin in Bacillus subtilis. J. Biol. Chem. 278:2169–2176 [DOI] [PubMed] [Google Scholar]
- 29. Joseph P., Ratnayake-Lecamwasam M., Sonenshein A. L. 2005. A region of Bacillus subtilis CodY protein required for interaction with DNA. J. Bacteriol. 187:4127–4139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lemos J. A., Nascimento M. M., Lin V. K., Abranches J., Burne R. A. 2008. Global regulation by (p)ppGpp and CodY in Streptococcus mutans. J. Bacteriol. 190:5291–5299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lennox E. S. 1955. Transduction of linked genetic characters of the host by bacteriophage P1. Virology 1:190–206 [DOI] [PubMed] [Google Scholar]
- 32. Levdikov V. M., et al. 2009. Structural rearrangement accompanying ligand binding in the GAF domain of CodY from Bacillus subtilis. J. Mol. Biol. 390:1007–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levdikov V. M., Blagova E., Joseph P., Sonenshein A. L., Wilkinson A. J. 2006. The structure of CodY, a GTP- and isoleucine-responsive regulator of stationary phase and virulence in Gram-positive bacteria. J. Biol. Chem. 281:11366–11373 [DOI] [PubMed] [Google Scholar]
- 34. Lopez J. M., Marks C. L., Freese E. 1979. The decrease of guanine nucleotides initiates sporulation of Bacillus subtilis. Biochim. Biophys. Acta 587:238–252 [DOI] [PubMed] [Google Scholar]
- 35. Mäder U., Hennig S., Hecker M., Homuth G. 2004. Transcriptional organization and posttranscriptional regulation of the Bacillus subtilis branched-chain amino acid biosynthesis genes. J. Bacteriol. 186:2240–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Majerczyk C. D., et al. 2010. Direct targets of CodY in Staphylococcus aureus. J. Bacteriol. 192:2861–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Malke H., Steiner K., McShan W. M., Ferretti J. J. 2006. Linking the nutritional status of Streptococcus pyogenes to alteration of transcriptional gene expression: the action of CodY and RelA. Int. J. Med. Microbiol. 296:259–275 [DOI] [PubMed] [Google Scholar]
- 38. Martin N., Lombardía E., Altabe S. G., de Mendoza D., Mansilla M. C. 2009. A lipA (yutB) mutant, encoding lipoic acid synthase, provides insight into the interplay between branched-chain and unsaturated fatty acid biosynthesis in Bacillus subtilis. J. Bacteriol. 191:7447–7455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Molle V., et al. 2003. Additional targets of the Bacillus subtilis global regulator CodY identified by chromatin immunoprecipitation and genome-wide transcript analysis. J. Bacteriol. 185:1911–1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ochi K., Kandala J., Freese E. 1982. Evidence that Bacillus subtilis sporulation induced by the stringent reponse is caused by the decrease in GTP or GDP. J. Bacteriol. 151:1062–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ollinger J., Song K. B., Antelmann H., Hecker M., Helmann J. D. 2006. Role of the Fur regulon in iron transport in Bacillus subtilis. J. Bacteriol. 188:3664–3673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pao C. C., Dyess B. T. 1981. Effect of unusual guanosine nucleotides on the activities of some Escherichia coli cellular enzymes. Biochim. Biophys. Acta 77:358–362 [DOI] [PubMed] [Google Scholar]
- 43. Petranovic D., et al. 2004. Intracellular effectors regulating the activity of the Lactococcus lactis CodY pleiotropic transcription regulator. Mol. Microbiol. 53:613–621 [DOI] [PubMed] [Google Scholar]
- 44. Phadtare S. 2004. Recent developments in bacterial cold-shock response. Curr. Issues Mol. Biol. 6:125–136 [PubMed] [Google Scholar]
- 45. Pohl K., et al. 2009. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J. Bacteriol. 191:2953–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ratnayake-Lecamwasam M., Serror P., Wong K.-W., Sonenshein A. L. 2001. Bacillus subtilis CodY represses early-stationary-phase genes by sensing GTP levels. Genes Dev. 15:1093–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rorabacher D. B. 1991. Statistical treatment for rejection of deviant values: critical values of Dixon Q. parameter and related subrange ratios at the 95 percent confidence level. Anal. Chem. 83:139–146 [Google Scholar]
- 48. Sambrook J., Russell D. W. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- 49. Shivers R. P., Sonenshein A. L. 2004. Activation of the Bacillus subtilis global regulator CodY by direct interaction with branched-chain amino acids. Mol. Microbiol. 53:599–611 [DOI] [PubMed] [Google Scholar]
- 50. Sinensky M. 1974. Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 71:522–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Smith I., Paress P., Cabane K., Dubnau E. 1980. Genetics and physiology of the rel system of Bacillus subtilis. Mol. Gen. Genet. 178:271–279 [DOI] [PubMed] [Google Scholar]
- 52. Switzer R. L., Zalkin H., Saxild H. H. 2002. Purine, pyrimidine, and pyridine nucleotide metabolism, p. 255–269 In Sonenshein A. L., Hoch J. A., Losick R. (ed.), Bacillus subtilis and its closest relatives: from genes to cells. ASM Press, Washington, DC [Google Scholar]
- 53. Thomaides H. B., et al. 2007. Essential bacterial functions encoded by gene pairs. J. Bacteriol. 189:591–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tojo S., Kumamoto K., Hirooka K., Fujita Y. 2010. Heavy involvement of stringent transcription control depending on the adenine or guanine species of the transcription initiation site in glucose and pyruvate metabolism in Bacillus subtilis. J. Bacteriol. 192:1573–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tojo S., Satomura T., Kumamoto K., Hirooka K., Fujita Y. 2008. Molecular mechanisms underlying the positive stringent response of the Bacillus subtilis ilv-leu operon, involved in the biosynthesis of branched-chain amino acids. J. Bacteriol. 190:6134–6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tojo S., et al. 2005. Elaborate transcription regulation of the Bacillus subtilis ilv-leu operon involved in the biosynthesis of branched-chain amino acids through global regulators of CcpA, CodY, and TnrA. Mol. Microbiol. 56:1560–1573 [DOI] [PubMed] [Google Scholar]
- 57. van Schaik W., et al. 2009. The global regulator CodY regulates toxin gene expression in Bacillus anthracis and is required for full virulence. Infect. Immun. 77:4437–4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wach A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259–265 [DOI] [PubMed] [Google Scholar]
- 59. Willecke K., Pardee A. B. 1971. Fatty acid-requiring mutant of Bacillus subtilis defective in branched chain alpha-keto acid dehydrogenase. J. Biol. Chem. 246:5264–5272 [PubMed] [Google Scholar]
- 60. Zuberi A. R., Ying C., Bischoff D. S., Ordal G. W. 1991. Gene-protein relationships in the flagellar hook-basal body complex of Bacillus subtilis: sequences of the flgB, flgC, flgG, fliE, and fliF genes. Gene 101:23–31 [DOI] [PubMed] [Google Scholar]