Abstract
Two human-specific neisserial pathogens, Neisseria gonorrhoeae and Neisseria meningitidis, require the expression of type IV pili (tfp) for initial attachment to the host during infection. However, the mechanisms controlling the assembly and functionality of tfp are poorly understood. It is known that the gonococcal pilE gene, encoding the major subunit, is positively regulated by IHF, a multifunctional DNA binding protein. A neisserial specific repetitive DNA sequence, termed the Correia repeat-enclosed element (CREE) is situated upstream of three pil loci: pilHIJKX (pilH-X), pilGD, and pilF. CREEs have been shown to contain strong promoters, and some CREE variants contain a functional IHF binding site. CREEs might therefore be involved in the regulation of tfp biogenesis in pathogenic Neisseria. Site-directed and deletion mutagenesis on promoter::cat reporter constructs demonstrated that transcription of pilH-X and pilGD is from a σ70 promoter and is independent of the CREE. The insertion of a CREE in the pilF promoter region in N. meningitidis generated a functional σ70 promoter. However, there is also a functional promoter at this position in N. gonorrhoeae, where there is no CREE. These results suggest CREE insertion in these three pil loci does not influence transcription and that IHF does not coordinately regulate tfp biogenesis.
INTRODUCTION
Both Neisseria gonorrhoeae and Neisseria meningitidis are obligate human pathogens. N. gonorrhoeae colonizes nonciliated columnar epithelial cells of the urogenital tract and causes the sexually transmitted disease gonorrhoea (55). N. meningitidis colonizes the human upper respiratory tract and exists in a carriage state in 10 to 20% of the human population (10, 11). N. meningitidis is also a major bacterial agent of cerebrospinal meningitis and septicemia. Both N. gonorrhoeae and N. meningitidis initiate infection via type IV pili (tfp) (56), an inner membrane-anchored organelle that participates in a diverse range of cellular functions, such as formation of bacterial aggregates (28, 30, 58), DNA uptake during natural transformation (1, 53, 54), and twitching motility (41, 62, 63). Nonpiliated N. gonorrhoeae is known to be avirulent (34).
The main component of a tfp filament is pilin protein encoded by the pilE gene (43). Although the overall structure of the tfp filament is relatively simple, the tfp biogenesis apparatus consists of as many as 23 ancillary Pil proteins involved in the assembly and functionality of the organelle (5, 8, 33). The tfp biogenesis process, in brief, involves the maturation of prepilin through the cleavage of the signal peptide by a prepilin peptidase encoded by pilD (22, 57). The assembled pilus filament is translocated through a membrane secretin, PilQ (20), which itself is stabilized by PilW (9). The mechanical energy required for pilus elongation and retraction is generated by two ATPases, known as PilF (22) and PilT (4). The products of pilG, pilH, pilI, pilJ, pilK, and pilW are known to be important for tfp homeostasis by counteracting PilT-mediated pilus retraction (8, 60). Furthermore, PilX (28, 29) and PilC1 and PilC2 (35, 44, 49) are important for the fine tuning of tfp adhesiveness and functionality. Many of these Pil proteins show homology to components of type II protein secretion systems (36).
Despite extensive research into the roles of these Pil proteins, little is known about the transcriptional control of the genes encoding the proteins, except for the transcription of the gonococcal pilE gene. The pilE gene is transcribed from a σ70 promoter during growth in vitro (24). A DNA binding protein, known as the integration host factor (IHF) (23), binds to the pilE promoter region and enhances pilE transcription (31). Deletion of this IHF binding site (BS) resulted in a 10-fold reduction of pilE transcription (31). IHF, a small heterodimeric protein encoded by ihfA and ihfB binds to the target DNA in a sequence-specific manner and causes the DNA molecule to bend (21, 23). Fyfe and Davies demonstrated that the AT-rich tract positioned between the −35 box of the pilE promoter and the IHF BS is required for maximal pilE transcription (25). It appears that the binding of IHF to the pilE promoter bends the DNA and allows the DNA to wrap around RNA polymerase bound to the promoter, and a second AT-rich region upstream of the IHF BS seems to facilitate this event (C. S. Ryan and J. K. Davies, unpublished data).
The genomes of pathogenic Neisseria isolates contain large amounts of repetitive DNA (2, 15, 46, 59). Some of these repetitive sequences have been shown to affect gene expression (39, 51). One of the common repetitive sequences is the Correia repeat-enclosed element (CREE), which occupies 1 to 2% of the genome (17, 37). The CREE consists of two terminal inverted repeats, termed the Correia repeats (CR), which are 25 to 27 bp long and of which there are two types, α and β. The CR flank a central core sequence (6, 14, 37, 40). The two types of CR can be further categorized depending on whether they are present on the left- or right-hand side of the core sequence. This is of particular importance, as both types of terminal CR can generate functional promoters, but the conferred promoter strengths can be different, as demonstrated by Siddique et al. (51). In general, the first 6 nucleotides on the left-end CR resemble a −10 box of a σ70 promoter, and the −35 box is contributed by the native sequence upstream of the CREE. This type of promoter was first described by Snyder et al. for the transcription of dcw in Neisseria lactamica (52). The right-end CR can contain a −35 box, and the last 4 nucleotides are usually TATA, which forms part of a −10 motif and can be completed by the native sequence to generate a fully functional promoter. The functionality of this promoter was first demonstrated by Black et al. upstream of the uvrB gene (3). Later studies have shown that the transcription of drg (7), lst (45), and mtrCDE (48) are also dependent on this type of promoter.
The CREE can also regulate gene expression posttranscriptionally. When the CREE is transcribed as part of the mRNA, the two inverted CR can form a stem-loop, which may be targeted by RNase III, potentially altering the level of translation (16, 18, 40). Moreover, some CREE variants contain an IHF BS (6). It was shown by Rouquette-Loughlin et al. that IHF can bind to the IHF BS contained within the CREE to negatively regulate the expression of the mtrCDE operon (48).
In this study, we found the CREE upstream of 3 pil loci in N. gonorrhoeae and N. meningitidis, namely, pilHIJKX (pilH-X), encoding minor pilins that are known to be essential for wild-type (WT) level tfp expression and tfp dynamics (28, 61); pilF, encoding an ATPase belonging to the GspE family, which provides mechanical energy to power pilus assembly (8, 22); and pilGD, encoding a tfp biogenesis protein that can bind DNA (13, 60) and a prepilin peptidase (22, 57), respectively.
Given that the CREE may generate promoters and that some of these CREE variants contain an IHF BS, the aim of this study was to investigate the effect of the CREE on the transcription of these pil loci. Here, we provide evidence suggesting the CREE does not affect the transcription of pil genes.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Escherichia coli DH5α (24) was used for propagating plasmids carrying pil promoter::cat reporter constructs (Ppil::cat) constructed in this study. Standard E. coli competent cell preparation and transformation methods were used (27). Transformed E. coli cells were selected on LB agar containing 100 μg ml−1 ampicillin and 10 μg ml−1 chloramphenicol. Plasmids containing Ppil::cat were extracted from 50 to 100 ml of overnight E. coli broth cultures using the High Pure Plasmid Isolation Kit (Roche) according to the manufacturer's instructions. Genomic DNA needed for subsequent recombinant DNA manipulation was extracted from N. gonorrhoeae strains FA1090 (accession number AE004969; http://www.genome.ou.edu/gono.html) and MS11-A (50) and N. meningitidis strain FAM18 (2) using the GenElute Bacterial Genomic DNA Kit (Sigma) according to the manufacturer's instructions. All Ppil::cat reporter plasmids were transformed into N. gonorrhoeae strain MS11-A (50) to allow recombination into the recipient chromosome. Neisserial transformations were performed using the spot transformation method on GC agar plates (19). Ten microliters of uncut plasmid DNA (approximately 1 μg to 10 μg) was used, and selection was on GC agar containing spectinomycin (60 μg ml−1). All Ppil::cat reporter plasmids and neisserial reporter strains used in this study are listed in Table S1 in the supplemental material and Table 1, respectively. The broth and plate growth conditions used for E. coli, N. gonorrhoeae, and N. meningitidis have been described previously (24).
Table 1.
NG MS11-A derived reporter strains used in this study
| Reporter strain | Description |
|---|---|
| JKD5222 | Contains an empty vectora |
| JKD5243 | Contains PpilG::cat cassette with CREE and IHF BS |
| JKD5244 | Contains PpilH::cat cassette with CREE and IHF BS |
| JKD5245 | Contains PpilF::cat cassette without CREE |
| JKD5250 | Contains PpilH::cat cassette with CREE and IHF BS; sequence upstream of DAP1751 deleted (Fig. 1b) |
| JKD5253 | Contains PpilG::cat cassette with sequence upstream of DAP1752 deleted (Fig. 3b) |
| JKD5256 | Contains PpilH::cat cassette with sequence downstream of DAP1763 deleted (Fig. 1b) |
| JKD5259 | Contains PpilG::cat cassette with sequence upstream of DAP1753 deleted (Fig. 3b) |
| JKD5261 | Contains PpilH::cat cassette with P1 −10 box mutated to CCCCCC (Fig. 1b) |
| JKD5262 | Contains PpilF::cat cassette with P1 −10 box mutated to CCCCCC (Fig. 2b) |
| JKD5264 | Contains PpilH::cat cassette with P2 −10 box mutated to GGGGGG (Fig. 1b) |
| JKD5265 | Contains PpilF::cat cassette with P2 −10 box mutated to GGGGGG (Fig. 2b) |
| JKD5269 | Contains PpilH::cat cassette without CREE (amplified from N. gonorrhoeae strain MS11-A) |
| JKD5271 | Contains PpilF::cat cassette with CREE and IHF BS (amplified from N. meningitidis strain FAM18) |
| JKD5275 | Contains PpilG::cat cassette with P1 −10 box mutated to ATCTTG (Fig. 3b) |
C. S. Ryan, unpublished data.
Total RNA extraction.
N. gonorrhoeae cells were grown to mid-exponential phase (optical density at 600 nm [OD600], 0.6 after approximately 3.5 h) in GC broth (24). For RNA protection, 0.5 volume of RNAlater RNA stabilization reagent (Qiagen) was added immediately to the liquid culture and allowed to incubate with the bacterial suspension for 15 min at room temperature. The cells were pelleted at 8,500 × g for 5 min, followed by the TRIzol-chloroform RNA extraction method described by Rio et al. (47). After the addition of TRIzol (Invitrogen), the samples were heated to 65°C for 15 min and allowed to cool to room temperature for 5 min before the addition of chloroform. RNA was treated with DNase twice using the Turbo DNA-free kit (Ambion) according to the manufacturer's instructions. RNA was immediately purified using the RNeasy Mini Kit (Qiagen) according to the manufacturer's instructions. DNA-free RNA was confirmed by the absence of signals in PCR. For RNA storage at −80°C, 2 volumes of 100% ethanol and 0.1 volume of 1 M NaCl (prepared in diethyl pyrocarbonate [DEPC]-treated water) were added. For use, the appropriate volume was removed, and the RNA was pelleted by centrifugation at 13,000 × g for 30 min at 4°C. The RNA pellet was washed with 75% ethanol, air dried, and suspended in an appropriate volume of DEPC-treated water.
Fluorescent primer extension analysis.
Ten micrograms of total RNA was used in the fluorescent primer extension protocol described by Lloyd et al. (38), except for the following modifications: 100 units of SuperScript II reverse transcriptase (RT) (Invitrogen) was used in both the initial primer extension reaction and the enrichment step, and the final concentration of high-performance liquid chromatography (HPLC)-purified 5′-6-carboxyfluorescein (FAM)-labeled oligonucleotides (see Table S2 in the supplemental material) was 10 μM. Gel electrophoresis and data analysis of the dried-down FAM-labeled cDNA was performed by the Australian Genome Research Facility Ltd. (Melbourne, Australia).
cDNA synthesis, RT-PCR, and qRT-PCR.
RNA was extracted from N. gonorrhoeae strains FA1090 and MS11-A and N. meningitidis strain FAM18. Five micrograms of total RNA was used for the first-strand cDNA synthesis using SuperScript II reverse transcriptase as instructed by the manufacturer (Invitrogen). For quantitative real-time PCR (qRT-PCR), the reverse transcription step was extended to 2.5 h at 42°C.
The standard RT-PCRs were performed with Taq DNA polymerase (Roche) using the annealing and extension conditions recommended by the supplier.
In qRT-PCR, a 20-μl reaction mixture contained 10 μl Power SYBR green (Applied Biosystems), 1.6 μl of both the forward and reverse primers (see Table S2 in the supplemental material) at 625 nM, 2.5 μl of cDNA, and 4.3 μl of water. All qRT-PCRs were performed in triplicate. The standards for the qRT-PCR were genomic DNAs of N. gonorrhoeae strains FA1090 and MS11-A and N. meningitidis strain FAM18. The PCR conditions were as follows: 50°C for 2 min, 95°C for 10 min, and 35 cycles of 95°C for 15 s and 60°C for 1 min, followed by melting-curve analysis. qRT-PCR was performed on 3 biological replicates. Quantification of transcripts was done using the Realplex real-time PCR system (Eppendorf).
Construction of promoter::cat reporter constructs.
The oligonucleotide primers used to generate WT, deletant, and mutant promoter-reporter constructs are listed in Table S2 in the supplemental material. The WT promoter sequence was PCR amplified from the genomic DNA of N. gonorrhoeae strain FA1090, unless otherwise specified. The promoterless cat gene was amplified from pJKD699 (26) using the appropriate promoter-specific forward Ppil::cat primers and oligonucleotide primer M13RP (42). The PCR products were gel purified using the QIAquick Gel Purification Kit (Qiagen) according to the manufacturer's instructions. Approximately equal amounts of the promoter fragment and cat fragment were fused using site-overlapping extension (SOE) PCR (25 cycles of 94°C for 1 min, 50°C for 1 min, and 72°C for 2 min, followed by 94°C for 1 min and 72°C for 10 min) (32). The fusion PCR product was gel purified (QIAquick Gel Purification Kit; Qiagen) into 40 μl water. The DNA in this 40 μl was blunt ended (24) in a 50-μl reaction mixture containing a final concentration of 1× T4 polynucleotide kinase buffer (NEB), 20 units T4 polynucleotide kinase (NEB), 30 units T4 DNA polymerase (NEB), 0.2 mM ATP (NEB), and 0.04 mM PCR deoxynucleoside triphosphates (dNTPs) (Promega). The blunt-ending reaction mixture was incubated at 37°C for 1 h and cleaned with a QIAquick PCR Purification Kit (Qiagen).
All Ppil::cat constructs were cloned into pJKD3172, which contains a spectinomycin resistance cassette inserted into a copy of the gonococcal iga gene (Ryan and Davies, unpublished; see Table S1 in the supplemental material). pJKD3172 was linearized by digestion with BglII, blunt ended as described above, and alkaline phosphatase (Boehringer Mannheim) treated. The blunt-ended Ppil::cat SOE products were cloned into the BglII site in the iga gene in pJKD3172 (in opposite orientation to the iga gene). Transformation of N. gonorrhoeae strain MS11-A resulted in the integration of the Ppil::cat constructs into the chromosomal iga gene via homologous recombination (25). The potential transformants carrying Ppil::cat constructs were selected by spectinomycin resistance and screened by PCR and sequencing to ensure the promoter and reporter sequences were correct. Each of the respective WT Ppil::cat constructs was used as a template for the construction of deletant and mutant Ppil::cat constructs.
Determination of CAT production.
Cell extracts were prepared from liquid cultures of N. gonorrhoeae and N. meningitidis Ppil::cat reporter strains grown to mid-exponential phase as described previously (25). The total protein concentration was determined using the bicinchoninic acid (BCA) Protein Assay Kit (Pierce) according to the manufacturer's instructions. At least three cell extracts were prepared on separate days for each reporter strain. A minimum of one chloramphenicol acetyltransferase (CAT) assay was performed for each cell extract.
RESULTS
The complete genome sequences of N. gonorrhoeae strains FA1090 (accession number AE004969; http://www.genome.ou.edu/gono.html) and NCCP (12) and N. meningitidis strains Z2491 (46), MC58 (59), and FAM18 (2) are available. As many as 23 genes have been annotated as being involved in pilus biogenesis in N. gonorrhoeae and N. meningitidis. All sequenced strains of N. gonorrhoeae and N. meningitidis share similar pil gene arrangements. An alignment of DNA sequences of each N. gonorrhoeae and N. meningitidis pil gene revealed that these pil loci are very similar at the nucleotide level (data not shown).
Sequences upstream of each of the pil genes were also aligned. CREEs were found to be present upstream of 3 pil genes, pilH, pilG, and pilF, in some but not all backgrounds. For example, the CREE is found upstream of pilH in all the N. gonorrhoeae and N. meningitidis strains examined, except in N. gonorrhoeae strain MS11-A (Fig. 1b), whereas the CREE is found upstream of pilF only in N. meningitidis strains, but not in N. gonorrhoeae strains (Fig. 2b). Furthermore, the CREE is found upstream of pilG in all N. gonorrhoeae strains but in only one N. meningitidis strain, FAM18 (Fig. 3b). It was therefore tempting to speculate that the transcriptional control of these pil genes may be different because of the presence or absence of the CREE and IHF BS.
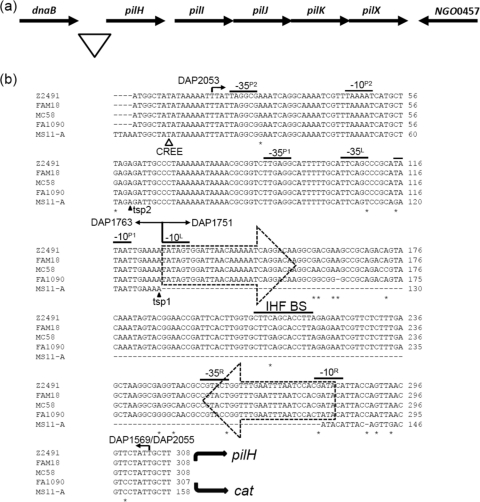
Fig. 1.
The pilHIJKX locus. (a) Schematic representation of pilHIJKX in N. gonorrhoeae strain FA1090. The triangle marks the region of DNA sequence examined. (b) Sequence alignment of the promoter region for pilH-X in N. gonorrhoeae strains FA1090 and MS11-A and N. meningitidis strains Z2491, MC58, and FAM18. The triangle indicates the position where the CREE was inserted into N. gonorrhoeae strain NCCP. The dashed arrows enclose the two terminal CR. The −10 and −35 boxes of the four putative σ70 promoters, designated P1, P2, L, and R, are overlined. The transcriptional start points (tsps 1 and 2) are marked by solid arrowheads. The IHF BS contained within the CREE is overlined. Base differences among the five sequences are marked with asterisks. The curved arrows indicate the directions of pilH and cat transcription. The numbered arrows indicate the oligonucleotide primers used (see Table S2 in the supplemental material). DAP1569 is specific for N. gonorrhoeae strain FA1090, and DAP2055 is specific for N. gonorrhoeae strain MS11-A.
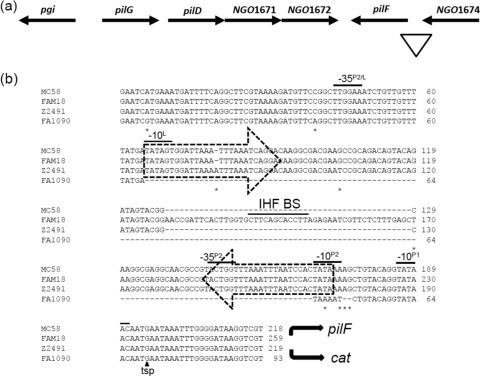
Fig. 2.
The pilF locus. (a) Schematic representation of pilF in N. gonorrhoeae strain FA1090. The triangle marks the region of DNA sequence examined. (b) Sequence alignment of the promoter regions for pilF in N. gonorrhoeae strain FA1090 and N. meningitidis strains Z2491, MC58, and FAM18. The dashed arrows enclose the two terminal CR. The −10 and −35 boxes of the three putative σ70 promoters, designated P1, P2, and L, are overlined. The −35 box for P2 in N. gonorrhoeae strain FA1090 is shown further upstream, due to the absence of the CREE in this strain. The mapped tsp is marked by a solid arrowhead. The IHF BS contained within the CREE in strain FAM18 is overlined. Base differences between the four sequences are marked with asterisks. The curved solid arrows indicate the directions of pilF and cat transcription.
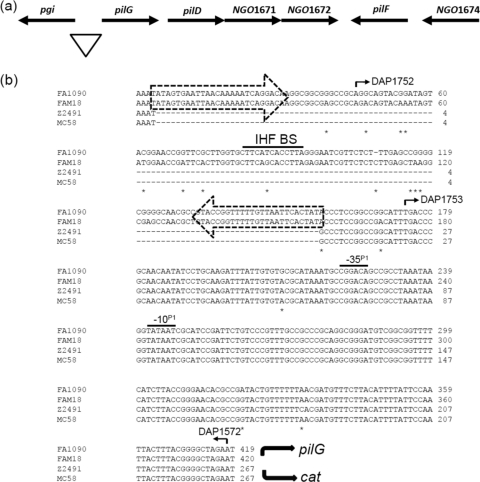
Fig. 3.
The pilGD locus. (a) Schematic representation of pilGD in N. gonorrhoeae strain FA1090. The triangle marks the region of DNA sequence examined. (b) Sequence alignment of the promoter regions for pilG in N. gonorrhoeae strains FA1090 and N. meningitidis strains Z2491, MC58, and FAM18. The dashed arrows enclose the two terminal CR. A −10 box and the corresponding −35 box of a σ70 promoter, termed P1, are shared by all strains and are overlined. The IHF BS contained within the CREE is overlined. Base differences among the four sequences are marked with asterisks. The curved solid arrows indicate the directions of pilG and cat transcription. The numbered arrows indicate the oligonucleotide primers used (see Table S2 in the supplemental material).
pilHIJKX transcription does not appear to be affected by the CREE.
pilH-X are closely spaced and oriented in the same direction, suggesting they may be transcribed as an operon in both N. gonorrhoeae and N. meningitidis (Fig. 1a). RT-PCR was able to demonstrate the cotranscription of pilH-X (data not shown), indicating there is a promoter upstream of pilH. In N. gonorrhoeae strain NCCP, the CREE was found to be inserted 143 bp upstream of the ATG start codon (Fig. 1b). The inserted CREE does not have an IHF BS and is only 108 bp in length. Both the left and right ends of the CREE belong to the α type. Given that this CREE was inserted away from the start of the coding sequence, it is unlikely that it is involved in the transcription of pilH-X in N. gonorrhoeae strain NCCP. In N. gonorrhoeae strain FA1090 and N. meningitidis strains Z2491, MC58, and FAM18, the CREE was found to be much closer to the ATG start codon (22 bp upstream) and is the longer 155- to 156-bp variant, which contains an IHF BS (Fig. 1b). In all 4 strains, the left end of the CREE belongs to the α type and contains a −10 box sequence (−10L) that has 5 out of 6 bases identical to the σ70 promoter sequence consensus and a corresponding −35 box sequence (−35L) located 17 bp further upstream that is contributed by the native sequence (Fig. 1b). The right end of the CREE belongs to the β class, with the first 4 bases at the terminus (−10R) being GATA in all 3 N. meningitidis strains and TATA in N. gonorrhoeae strain FA1090. The CREE insertion has not generated a −10 box in N. meningitidis, and the −10 box in N. gonorrhoeae has only 4 out of 6 bases identical to the consensus (Fig. 1b). The corresponding −35 box (−35R) located 17 bp further upstream is found in all 4 strains. Lastly, the IHF BS contained within the CREE in N. gonorrhoeae strain FA1090 and N. meningitidis strains Z2491 and FAM18 is identical to the IHF BS contained within the CREE found upstream of the mtrCDE genes (48).
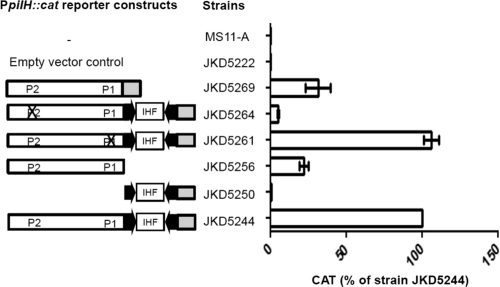
Fluorescence-based primer extension (FPE) analysis was performed on total RNA extracted from N. gonorrhoeae strain MS11-A, the strain that does not have the CREE in the pilH-X promoter region, and two FPE products were detected (Fig. 1b). Based on the two transcriptional start point (tsps) suggested by the FPE results, two putative −10 motifs, termed P1 and P2, could be deduced. In order to determine which, if any, of these putative promoters was functional, each −10 motif was separately mutated in strains JKD5261 (P1 mutated) and JKD5264 (P2 mutated) (Table 1), and the amount of CAT produced by each strain was measured and compared to the WT strain, JKD5244 (Table 1).
The two negative-control strains, MS11-A and JKD5222, which contain the vector alone, did not produce CAT, as shown by CAT enzyme-linked immunosorbent assay (ELISA) (Fig. 4). The CAT ELISA data suggested P2 is the functional promoter, because altering this sequence resulted in substantially reduced CAT levels in JKD5264, whereas when the P1 sequence was mutated, the CAT levels in JKD5261 remained the same as in the WT strain, JKD5244 (Fig. 4). A −35 box (−35P2) was found to be located 18 bp upstream of P2 and had 3 out 6 bases matching the consensus (Fig. 1b).
Fig. 4.
Transcriptional analysis of the pilH promoter region in an N. gonorrhoeae strain MS11-A background. PpilH::cat reporter constructs are shown schematically. The white boxes indicate sequence upstream of the CREE, and the gray boxes indicate sequence downstream of the CREE. The arrows are the terminal CR. The Xs indicate promoter mutations. The amount of CAT protein produced by each N. gonorrhoeae reporter strain was quantified by CAT ELISAs, and at least 3 independent biological samples were prepared. The CAT level is expressed as the percentage of the wild-type (N. gonorrhoeae strain JKD5244) level. Strains MS11-A and JKD5222 were included as negative controls. The error bars represent standard deviations of the mean.
Deletional analysis of the PpilH::cat strain JKD5244 was performed to assess the role of the CREE in the transcription of pilH-X. Two deletion mutants, JKD5250 and JKD5256 (Table 1), were made. Deleting the sequence upstream of the CREE (primer DAP1751) (Fig. 1b) significantly affected transcription, as CAT produced by JKD5250 is negligible compared to that of the WT strain JKD5244 (Fig. 4), despite the fact that it still contains an intact −10 box (−10L) at the left-end CR (Fig. 1b). This result implies that either −10L is nonfunctional or the activity of the promoter is abolished because the corresponding −35 box (−35L) (Fig. 1b) is absent. A significant amount of CAT was detected for JKD5256, which contains the sequence upstream of the CREE but not the CREE itself (the sequence between primers DAP1751 and DAP1569 is deleted) (Fig. 1b). However, compared to the WT strain, JKD5256 exhibited a 75% reduction in the CAT level (Fig. 4). In addition, CAT produced by strain JKD5269 carrying the MS11-A version of the PpilH::cat construct, that is, the pilH promoter sequence without the CREE, also showed more than 50% reduction compared to the WT strain, JKD5244 (Fig. 4). In summary, it seems that P2 is the only functional promoter present upstream of pilH-X.
As the CAT ELISA data implied different levels of pilH transcription in different reporter strains, we directly compared the relative expression levels, using qRT-PCR, of pilH in N. gonorrhoeae strains FA1090 (PpilH-X containing the CREE) and MS11-A (PpilH-X without the CREE) (Fig. 1b). To ascertain the reliability of the data, two housekeeping genes, recA and rpoD, were included as internal controls. It was shown that the average ratios of pilH to recA transcript levels in FA1090 and MS11-A were 0.41 and 0.39, respectively (Table 2). The average ratios of pilH to rpoD transcript levels in FA1090 and MS11-A were 0.87 and 0.83, respectively (Table 2). This indicates that the level of pilH transcripts present in strains FA1090 and MS11-A are very similar and that the CREE found in the pilH promoter region in N. gonorrhoeae strain FA1090 and the 3 N. meningitidis strains have no effect on transcription.
Table 2.
qRT-PCR performed on total RNA extracted from N. gonorrhoeae strains FA1090 and MS11-A
| Strain | Samplea | RNA fold change |
||
|---|---|---|---|---|
| pilH-recA | pilH-rpoD | recA-rpoD | ||
| MS11-A | 1 | 0.45 | 0.88 | 1.98 |
| 2 | 0.44 | 0.87 | 1.99 | |
| 3 | 0.29 | 0.73 | 2.53 | |
| Avg | 0.39 | 0.83 | 2.17 | |
| FA1090 | 1 | 0.61 | 1.0 | 1.64 |
| 2 | 0.34 | 0.75 | 2.18 | |
| 3 | 0.28 | 0.87 | 3.10 | |
| Avg | 0.41 | 0.87 | 2.31 | |
Three biological RNA samples were obtained from each strain for qRT-PCR.
A CREE insertion in N. meningitidis has generated a functional promoter for the transcription of pilF.
In Neisseria, pilF is located downstream of and in the opposite orientation to the pilGD locus (Fig. 2a). Detailed analysis of the sequence upstream of pilF revealed the insertion of a CREE, with a β left and β right CR, 47 bp upstream of the ATG start codon in the N. meningitidis strains examined in this study (Fig. 2b). The CREE insertion generated two putative promoters at either end of the CREE (L and P2) (Fig. 2b). In N. meningitidis strains Z2491 and MC58, the inserted CREE is 108 bp in length and does not contain an IHF BS. The CREE inserted in the same location in N. meningitidis strain FAM18 is 157 bp in length and contains an IHF BS at its center. The DNA sequence of the pilF promoter regions in N. gonorrhoeae strains FA1090, NCCP, and MS11-A is the same as in N. meningitidis, except that it does not contain the CREE. FPE analysis on total RNA extracted from N. gonorrhoeae strain FA1090 and N. meningitidis strain FAM18 gave rise to the same extension product (data not shown). The mapped tsp and a putative −10 box, designated P1, are indicated in Fig. 2b.
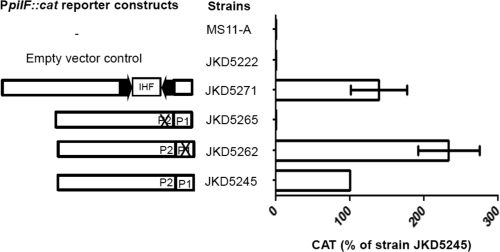
In order to determine which of the putative promoter sequences was functional, mutational analysis of the N. gonorrhoeae strain FA1090 pilF promoter region was performed. JKD5245 (WT PpilF::cat) and mutant strains JKD5262 (P1 mutated) and JKD5265 (P2 mutated) were assayed by CAT ELISA. The CAT ELISA results suggested P2 is the functional promoter for pilF transcription, because JKD5265 produced negligible amounts of CAT (Fig. 5). This was not surprising, because P2 has a corresponding −35 box (−35P2) (Fig. 2b) while P1 does not. Given that the CREE insertion generated a −10 sequence slightly different from that in N. gonorrhoeae and the CREE upstream of PpilF in N. meningitidis strain FAM18 contained an IHF BS, we constructed a reporter strain, JKD5271 (FAM18 PpilF::cat with the CREE plus IHF BS), to determine if the CREE and the IHF BS have an effect on pilF transcription. There was no significant difference in promoter strength conferred by JKD5245 and JKD5271, as indicated by the CAT ELISA results (Fig. 5). This suggests that pilF transcription does not seem to be affected by the presence or absence of the CREE.
Fig. 5.
Transcriptional analysis of the pilF promoter (PpilF) region in an N. gonorrhoeae MS11-A background. The arrows are the terminal CR. An X indicates a particular promoter that was mutated. The amount of CAT protein produced by each N. gonorrhoeae reporter strain was quantified by CAT ELISAs, and at least 3 independent biological samples were prepared. The CAT level is expressed as the percentage of the wild-type level produced by JKD5245. The error bars represent standard deviations of the mean.
To provide direct evidence that the CREE has no effect on pilF transcription, qRT-PCR was performed on total RNA extracted from N. gonorrhoeae strain FA1090 and N. meningitidis strain FAM18 to quantify the levels of pilF transcripts (Table 3). The average ratios of pilF to recA transcripts were 0.99 and 0.28 in N. gonorrhoeae strain FA1090 and N. meningitidis strain FAM18, respectively. The average ratios of pilF to rpoD transcripts were 2.38 and 0.32, respectively. This result suggests N. meningitidis strain FAM18 produced significantly lower levels of pilF transcripts than N. gonorrhoeae strain FA1090.
Table 3.
qRT-PCR performed on total RNA extracted from N. gonorrhoeae strain FA1090 and N. meningitidis strain FAM18
| Strain | Samplea | RNA fold change |
||
|---|---|---|---|---|
| pilF-recA | pilF-rpoD | recA-rpoD | ||
| FAM18 | 1 | 0.24 | 0.37 | 1.57 |
| 2 | 0.20 | 0.20 | 1.00 | |
| 3 | 0.39 | 0.40 | 1.02 | |
| Avg | 0.28 | 0.32 | 1.20 | |
| FA1090 | 1 | 1.02 | 2.10 | 2.06 |
| 2 | 1.16 | 2.82 | 2.43 | |
| 3 | 0.78 | 2.23 | 2.85 | |
| Avg | 0.99 | 2.38 | 2.45 | |
Three biological RNA samples were obtained from each strain for qRT-PCR.
The upstream CREE does not influence transcription of the pilG gene.
RT-PCR showed that pilG is cotranscribed with pilD and the two genes (NGO1671 and NGO1672) (Fig. 3a) downstream of pilD encoding hypothetical proteins (data not shown). A CREE, with α right and α left CR types and a putative IHF BS, was found to be inserted 220 bp upstream of the start of the pilG coding sequence in N. gonorrhoeae strains FA1090, MS11-A, and NCCP and N. meningitidis strain FAM18 (Fig. 3b). N. meningitidis strains Z2491 and MC58 have identical upstream sequences, except that they do not have CREE insertions (Fig. 3b).
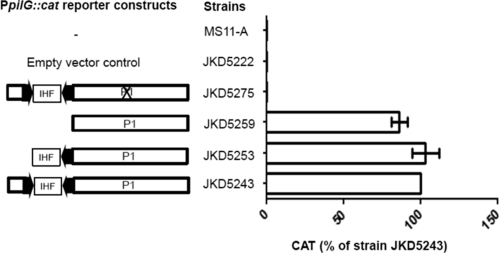
To assess if the CREE is needed for pilG transcription, the promoter region was PCR amplified from N. gonorrhoeae strain FA1090 and used as the WT template in strain JKD5243. From this, two deletion mutant reporter strains were made by deleting part of the CREE (the sequence upstream of primer DAP1752 was deleted) (Fig. 3b) and the entire CREE (the sequence upstream of primer DAP1753 was deleted) (Fig. 3b) in strains JKD5253 and JKD5259, respectively. The CAT levels produced by the two N. gonorrhoeae deletant strains were similar to those of the WT strain, JKD5243, confirming that the CREE is not involved in pilG transcription (Fig. 6).
Fig. 6.
Transcriptional analysis of the pilG promoter (PpilG) region in an N. gonorrhoeae MS11-A background. The arrows are the terminal CR. The X indicates the mutation of a putative −10 box termed P1. The amount of CAT protein produced by each N. gonorrhoeae reporter strain was quantified by CAT ELISAs, and at least 3 independent biological samples were prepared. The CAT level is expressed as the percentage of the wild-type level produced by strain JKD5243. The error bars represent standard deviations of the mean.
FPE was performed on total RNA extracted from N. gonorrhoeae strain FA1090 and N. meningitidis strain MC58, but it was unable to show a tsp (data not shown). A potential promoter, based on its sequence similarity to the promoter consensus sequence, was found to be downstream of the CREE in all strains examined (Fig. 3b). It consists of a perfect −10 box (−10P1) and a corresponding −35 box (−35P1) located 15 bp further upstream (Fig. 3b). The −10 box was mutated in strain JKD5275, and CAT activity was completely abolished (Fig. 6).
DISCUSSION
CREEs have been proposed to be mobile elements, although the associated mobilization mechanism(s) remains unknown (6). The insertion of the CREE upstream of genes can generate promoters (51), further modulate gene expression by IHF binding (48), and affect transcript stability by forming secondary structures that can either be targeted by or protected from RNase III (16, 18). In contrast to these reports, we have evidence suggesting the CREEs present upstream of pilH-X, pilGD, and pilF in some pathogenic Neisseria strains are not involved in the regulation of pil gene expression. To support this, we have mapped the σ70-dependent promoters for the relevant pil loci and have shown that these promoters are not part of the CREE sequence for PpilH-X and PpilGD.
It remains unclear to us why both PpilH::cat reporter strains JKD5244 (FA1090 PpilH with the CREE) and JKD5261 (P1 mutant) produced substantially more CAT protein than strains JKD5269 (MS11-A PpilH without the CREE) and JKD5256 (FA1090 PpilH without the CREE) (Fig. 4). It should be noted that the IHF BS present within the CREE is positioned downstream of the functional P2 sequence, which is unlike the situation in the N. gonorrhoeae pilE promoter region, where it is located 55 bp upstream of the promoter motif (31). The presence of an IHF BS downstream of a promoter has been shown to have a negative impact on gene expression, and this is exemplified by the transcription of the mtrCDE operon in N. meningitidis (48). Therefore, the larger amount of CAT protein produced by the WT strain, JKD5244, does not appear to be due to the presence of the IHF BS.
It was also possible that the higher levels of CAT observed with strains JKD5244 and JKD5261 was due to the presence of the CREE, which might help stabilize the transcripts when the CREE is transcribed as part of the message (18). However, the qRT-PCR results showed that the pilH transcript levels are similar in N. gonorrhoeae strains FA1090 and MS11-A, suggesting the CREE has no effect on transcription. We therefore suggest that in this specific instance the presence of the CREE may somehow positively influence translation of the mRNA.
Although the PpilF promoter is partly contained within the CREE in N. meningitidis, the strength of the promoter is similar to that of the N. gonorrhoeae version, which does not have the CREE (Fig. 5). The difference in the pilF transcript levels in N. gonorrhoeae strain FA1090 and N. meningitidis strain FAM18 may be attributable to the difference in the mRNA turnover rates in these strains. Additionally, it was noted that the level of CAT produced by JKD5262 (P1 mutant) was 2-fold higher than that of the WT strain, JKD5245 (Fig. 5). As P2 was shown to be the functional promoter, the actual tsp is therefore likely to be located upstream of P1 (Fig. 2b), and as a result, the P1 sequence would be transcribed. It may be that the P1 mutation has resulted in relaxation of a secondary structure in the mRNA, which would allow more efficient translation. We have searched for DNA sequences that might contribute to the formation of a secondary mRNA structure, but none were identified. An alternative explanation could be that the P1 −10 motif is part of an IHF BS, as it has 10 out of 13 bases matching the IHF consensus. The binding of IHF to this site may partially repress pilF transcription, and the mutation introduced into the P1 −10 motif could relieve that repression.
Since the CREE insertions upstream of the three pil loci are neither strain nor species specific, it seems that they are the result of random transposition. The data generated in this study suggest that in these specific cases, CREEs have no effect on gene expression. Nonetheless, given its abundance and the differential distribution across the neisserial genomes, there is no doubt that the CREE has a significant role in controlling neisserial gene expression on a global scale.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a project grant from the Australian National Health and Medical Research Council.
We are grateful to Chenai Khoo for her assistance with the quantitative real-time PCR experiments.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 19 August 2011.
REFERENCES
- 1. Aas F. E., et al. 2002. Competence for natural transformation in Neisseria gonorrhoeae: components of DNA binding and uptake linked to type IV pilus expression. Mol. Microbiol. 46:749–760 [DOI] [PubMed] [Google Scholar]
- 2. Bentley S. D., et al. 2007. Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18. PLoS Genet. 3:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Black C. G., Fyfe J. A. M., Davies J. K. 1995. A promoter associated with the neisserial repeat can be used to transcribe the uvrB gene from Neisseria gonorrhoeae. J. Bacteriol. 177:1952–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brossay L., Paradis G., Fox R., Koomey M., Hebert J. 1994. Identification, localization and distribution of the PilT protein in Neisseria gonorrhoeae. Infect. Immun. 62:2302–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brown D. R., Helaine S., Carbonnelle E., Pelicic V. 2010. Systematic functional analysis reveals that a set of seven genes is involved in fine-tuning of the multiple functions mediated by type IV pili in Neisseria meningitidis. Infect. Immun. 78:3053–3063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buisine N., Tang C. M., Chalmers R. 2002. Transposon-like Correia elements: structure, distribution and genetic exchange between pathogenic Neisseria sp. FEBS Lett. 522:52–58 [DOI] [PubMed] [Google Scholar]
- 7. Cantalupo G., et al. 2001. Evolution and function of the neisserial dam-replacing gene. FEBS Lett. 495:178–183 [DOI] [PubMed] [Google Scholar]
- 8. Carbonnelle E., Helaine S., Nassif X., Pelicic V. 2006. A systematic genetic analysis in Neisseria meningitidis defines the Pil proteins required for assembly, functionality, stabilization and export of type IV pili. Mol. Microbiol. 61:1510–1522 [DOI] [PubMed] [Google Scholar]
- 9. Carbonnelle E., Helaine S., Nassif X., Pelicic V. 2005. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occuring after pilus assembly, essential for fibre stability and function. Mol. Microbiol. 55:54–64 [DOI] [PubMed] [Google Scholar]
- 10. Cartwright K. A., Stuart J. M., Jones D. M., Noah N. D. 1987. The stonehouse survey: nasopharyngeal carriage of meningcocci and Neisseria lactamica. Epidemiol. Infect. 99:591–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caugant D. A., Tzanakaki G., Kriz P. 2007. Lessons from meningococcal carriage studies. FEMS Microbiol. Rev. 31:52–63 [DOI] [PubMed] [Google Scholar]
- 12. Chung G. T., et al. 2008. Complete genome sequence of Neisseria gonorrhoeae NCCP11945. J. Bacteriol. 190:6035–6036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Collins R. F., Saleem M., Derrick J. P. 2007. Purification and three-dimensional electron microscopy structure of the Neisseria meningitidis type IV pilus biogenesis protein PilG. J. Bacteriol. 189:6389–6396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Correia F. F., Inouye S., Inouye M. 1988. A family of small repeated elements with some transposon-like properties in the genome of Neisseria gonorrhoeae. J. Biol. Chem. 263:12194–12198 [PubMed] [Google Scholar]
- 15. Davidsen T., Tonjum T. 2006. Meningococcal genome dynamics. Nat. Rev. Microbiol. 4:11–22 [DOI] [PubMed] [Google Scholar]
- 16. De Gregorio E., Abrescia C., Carlomagno M. S., Di Nocera P. P. 2002. The abundant class of nemis repeats provides RNA substrates for ribonuclease III in Neisseria. Biochim. Biophys. Acta 1576:39–44 [DOI] [PubMed] [Google Scholar]
- 17. De Gregorio E., Abrescia C., Carlomagno M. S., Di Nocera P. P. 2003. Asymmetrical distribution of Neisseria miniature insertion sequence DNA repeats among pathogenic and nonpathogenic Neisseria strains. Infect. Immun. 71:4217–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Gregorio E., Abrescia C., Carlomagno M. S., Di Nocera P. P. 2003. Ribonuclease III-mediated processing of specific Neisseria meningitidis mRNAs. Biochem. J. 374:799–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillard J. P. 2006. Genetic manipulation of Neisseria gonorrhoeae. Curr. Protoc. Microbiol. 4:4A 2. [DOI] [PubMed] [Google Scholar]
- 20. Drake S. L., Koomey M. 1995. The product of the pilQ gene is essential for the biogenesis of type IV pili in Neisseria gonorrhoeae. Mol. Microbiol. 18:975–986 [DOI] [PubMed] [Google Scholar]
- 21. Ellenberger E., Landy A. 1997. A good turn for DNA: the structure of integration host factor bound to DNA. Structure 5:153–157 [DOI] [PubMed] [Google Scholar]
- 22. Freitag N. E., Seifert H. S., Koomey M. 1995. Characterization of the pilF-pilD pilus-assembly locus of Neisseria gonorrhoeae. Mol. Microbiol. 16:575–586 [DOI] [PubMed] [Google Scholar]
- 23. Friedman D. I. 1988. Integration host factor: a protein for all reasons. Cell 55:545–554 [DOI] [PubMed] [Google Scholar]
- 24. Fyfe J. A. M., Carrick C. S., Davies J. K. 1995. The pilE gene of Neisseria gonorrhoeae MS11 is transcribed from a σ70 promoter during growth in vitro. J. Bacteriol. 177:3781–3787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fyfe J. A. M., Davies J. K. 1998. An AT-rich tract containing an integration host factor-binding domain and two UP-like elements enhances transcription from the pilEP1 promoter of Neisseria gonorrhoeae. J. Bacteriol. 180:2152–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fyfe J. A. M., Strugell R. A., Davies J. K. 1993. Control of gonococcal pilin-encoding gene expression in Escherichia coli. Gene 123:45–50 [DOI] [PubMed] [Google Scholar]
- 27. Hannahan D. 1985. Techniques for transformation of E. coli, p. 109–135 In Glover G. M. (ed.), DNA cloning: a practical approach, vol. 1. IRL Press, Oxford, United Kingdom [Google Scholar]
- 28. Hélaine S., et al. 2005. PilX, a pilus-associated protein essential for bacterial aggregation, is a key to pilus-facilitated attachment of Neisseria meningitidis to human cells. Mol. Microbiol. 55:65–77 [DOI] [PubMed] [Google Scholar]
- 29. Helaine S., Dyer D. H., Nassif X., Pelicic V., Forest K. T. 2007. 3D structure/function analysis of PilX reveals how minor pilins can modulate the virulence properties of type IV pili. Proc. Natl. Acad. Sci. U. S. A. 104:15888–15893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Higashi D. L., et al. 2007. Dynamics of Neisseria gonorrhoeae attachment: Microcolony development, cortical plaque formation, and cytoprotection. Infect. Immun. 75:4743–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hill S. A., et al. 1997. Integration host factor is a transcriptional cofactor of pilE in Neisseria gonorrhoeae. Mol. Microbiol. 23:649–656 [DOI] [PubMed] [Google Scholar]
- 32. Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68 [DOI] [PubMed] [Google Scholar]
- 33. Jain S., et al. 2011. Structural characterization of outer membrane components of the type IV pili system in pathogenic Neisseria. PLoS One 6:e16624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kellogg D. S., Jr., Peacock W. L., Jr., Deacon W. E., Brown L., Pirkle D. I. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 85:1274–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kirchner M., Meyer T. F. 2005. The PilC adhesion of the Neisseria type IV pilus-binding specificities and new insight into the nature of the host cell receptor. Mol. Microbiol. 56:945–957 [DOI] [PubMed] [Google Scholar]
- 36. Lauer P., Albertson N. H., Koomey M. 1993. Conservation of genes encoding components of a type IV pilus assembly/two-step protein export pathway in Neisseria gonorrhoeae. Mol. Microbiol. 8:357–368 [DOI] [PubMed] [Google Scholar]
- 37. Liu S. V., Saunders J. R., Jefferies A. C., Rest R. F. 2002. Genome analysis and strain comparison of correia repeats and correia repeat-enclosed elements in pathogenic Neisseria. J. Bacteriol. 184:6163–6173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lloyd A. L., Marshall B. J., Mee M. J. 2005. Identifying cloned Helicobacter pylori promoters by primer extension using a FAM-labeled primer and GeneScan analysis. J. Microbiol. Methods 60:291–298 [DOI] [PubMed] [Google Scholar]
- 39. Lovett S. T. 2004. Encoded errors: mutations and rearrangements mediated by misalignment at repetitive DNA sequences. Mol. Microbiol. 52:1243–1253 [DOI] [PubMed] [Google Scholar]
- 40. Mazzone M., et al. 2001. Whole-genome organization and functional properties of miniature DNA insertion sequences conserved in pathogenic Neisseriae. Gene 278:211–222 [DOI] [PubMed] [Google Scholar]
- 41. Merz A. J., So M., Sheetz M. P. 2000. Pilus retraction powers bacterial twitching motility. Nature 407:98–102 [DOI] [PubMed] [Google Scholar]
- 42. Messing J. 1983. New M13 vectors for cloning. Methods Enzymol. 101:20–78 [DOI] [PubMed] [Google Scholar]
- 43. Meyer T. S., Billyard E., Haas R., Storzbach S., So M. 1984. Pilus genes of Neisseria gonorrhoeae: chromosomal organization and DNA sequence. Proc. Natl. Acad. Sci. U. S. A. 81:6110–6114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nassif X., et al. 1994. Roles of pilin and PilC in adhesion of Neisseria meningitidis to human epithelial and endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 91:3769–3773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Packiam M., et al. 2006. Differential expression and transcriptional analysis of the alpha-2,3-sialyltransferase gene in pathogenic Neisseria spp. Infect. Immun. 74:2637–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Parkhill J., et al. 2000. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature 404:502–506 [DOI] [PubMed] [Google Scholar]
- 47. Rio D. C., Ares M., Jr., Hannon G. J., Nilsen T. W. 2010. Purification of RNA using TRIzol (TRI reagent). Cold Spring Harb. Protoc. 2010:pdb.prot5439. [DOI] [PubMed] [Google Scholar]
- 48. Rouquette-Loughlin C. E., Balthazar J. T., Hill S. A., Shafer W. M. 2004. Modulation of the mtrCDE-encoded efflux pump gene complex of Neisseria meningitidis due to a Correia element insertion sequence. Mol. Microbiol. 54:731–741 [DOI] [PubMed] [Google Scholar]
- 49. Scheuerpflug I., Rudel T., Ryll R., Pandit J., Meyer T. F. 1999. Roles of PilC and PilE proteins in pilus-mediated adherence of Neisseria gonorrhoeae and Neisseria meningitidis to human erythrocytes and endothelial epithelial cells. Infect. Immun. 67:834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Segal E., Billyard E., So M., Storzbach S., Meyer T. F. 1985. Role of chromosomal rearrangement in N. gonorrhoeae pilus phase variation. Cell 40:293–300 [DOI] [PubMed] [Google Scholar]
- 51. Siddique A., Buisine N., Chalmers R. 2011. The transposon-like corriea elements encode numerous strong promoters and provide a potential new mechanism for phase variation in the meningococcus. PLoS Genet. 7:e1001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Snyder L. A., Shafer W. M., Saunders N. J. 2003. Divergence and transcriptional analysis of the division cell wall (dcw gene) cluster in Neisseria spp. Mol. Microbiol. 47:431–442 [DOI] [PubMed] [Google Scholar]
- 53. Sparling P. F. 1966. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J. Bacteriol. 92:1364–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stein D. C. 1991. Transformation of Neisseria gonorrhoeae: physical requirements of the transforming DNA. Can. J. Microbiol. 37:345–349 [DOI] [PubMed] [Google Scholar]
- 55. Stephens D. S. 1989. Gonococcal and meningococcal pathogenesis as defined by human cell, cell culture, and organ culture assays. Clin. Microbiol. Rev. 2:S104–S111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stephens D. S., McGee Z. A. 1981. Attachment of Neisseria meningitidis to human mucosal surfaces: influence of pili and type of receptor cell. J. Infect. Dis. 143:525–532 [DOI] [PubMed] [Google Scholar]
- 57. Strom M. S., Nunn D., Lory S. 1993. A single bifunctional enzyme, PilD, catalyses cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc. Natl. Acad. Sci. U. S. A. 90:2404–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Swanson J. 1973. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J. Exp. Med. 137:571–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tettelin H., et al. 2000. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science 287:1809–1815 [DOI] [PubMed] [Google Scholar]
- 60. Tønjum T., Freitag N. E., Namork E., Koomey M. 1995. Identification and characterization of pilG, a highly conserved pilus-assembly gene in pathogenic Neisseria. Mol. Microbiol. 16:451–464 [DOI] [PubMed] [Google Scholar]
- 61. Winther-Larsen H. C., et al. 2005. A conserved set of pilin-like molecules controls type IV pilus dynamics and organelle-associated functions in Neisseria gonorrhoeae. Mol. Microbiol. 56:903–917 [DOI] [PubMed] [Google Scholar]
- 62. Wolfgang M., et al. 1998. PilT mutations lead to simultaneous defects in competence for natural transformation and twitching motility in piliated Neisseria gonorrhoeae. Mol. Microbiol. 29:321–330 [DOI] [PubMed] [Google Scholar]
- 63. Wolfgang M., Park H. S., Hayes S. F., van Putten J. P., Koomey M. 1998. Suppression of an absolute defect in type IV pilus biogenesis by loss-of-function mutations in pilT, a twitching motility gene in Neisseria gonorrhoeae. Proc. Natl. Acad. Sci. U. S. A. 95:14973–14978 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.