Abstract
Biofilms are communities of cells held together by a self-produced extracellular matrix typically consisting of protein, exopolysaccharide, and often DNA. A natural signal for biofilm disassembly in Bacillus subtilis is certain d-amino acids, which are incorporated into the peptidoglycan and trigger the release of the protein component of the matrix. d-Amino acids also prevent biofilm formation by the related Gram-positive bacterium Staphylococcus aureus. Here we employed fluorescence microscopy and confocal laser scanning microscopy to investigate how d-amino acids prevent biofilm formation by S. aureus. We report that biofilm formation takes place in two stages, initial attachment to surfaces, resulting in small foci, and the subsequent growth of the foci into large aggregates. d-Amino acids did not prevent the initial surface attachment of cells but blocked the subsequent growth of the foci into larger assemblies of cells. Using protein- and polysaccharide-specific stains, we have shown that d-amino acids inhibited the accumulation of the protein component of the matrix but had little effect on exopolysaccharide production and localization within the biofilm. We conclude that d-amino acids act in an analogous manner to prevent biofilm development in B. subtilis and S. aureus. Finally, to investigate the potential utility of d-amino acids in preventing device-related infections, we have shown that surfaces impregnated with d-amino acids were effective in preventing biofilm growth.
INTRODUCTION
Most bacteria form matrix-enclosed communities, or biofilms, when growing on surfaces. In clinical settings, biofilms are particularly problematic since they tend to form on indwelling devices and cause persistent infections and sepsis. Biofilm-associated bacteria are much less sensitive to antibiotics, making biofilm-related infections especially difficult to cure (7, 13). Often the only solution for a biofilm-infected catheter is complete replacement, a procedure that can range from uncomfortable and inconvenient to painful, expensive, and life threatening. Consequently, the development of methods to prevent biofilm formation may be just as important for treating hospital-acquired infections as the development of new antibiotics.
Bacterial signaling molecules that trigger the dispersal of old biofilms hold promise as possible therapeutic agents. Recent work has demonstrated that d-amino acids may be an exemplary class of such compounds (10, 19). Certain d-amino acids isolated from the supernatants of disassembled Bacillus subtilis biofilms were shown to prevent biofilm formation in fresh cultures by disrupting the connection between an extracellular matrix protein and the cell. Similar inhibitory effects for Staphylococcus aureus and Pseudomonas aeruginosa biofilms suggested that d-amino acids might constitute a general strategy for inhibiting biofilm formation in opportunistic pathogens (10). Genes whose products are involved in biofilm formation are not orthologous across these species, however, and the mechanism of action of d-amino acids against biofilms formed by these dissimilar pathogens remains unknown.
Here we describe investigations of the mechanism by which d-amino acids inhibit biofilm formation by S. aureus using fluorescence and confocal scanning laser microscopy. These techniques provide a more detailed picture of biofilm development on surfaces than visual inspection and bulk staining alone. Using dyes for specific components of the biofilm, such as cells, proteins, and polysaccharides, we have found that d-amino acids inhibit biofilm formation in S. aureus in much the same way as in B. subtilis: by preventing protein localization at the cell surface. Since S. aureus employs cell surface-associated proteins to connect neighboring cells in large aggregates (9), d-amino acids could prove effective at preventing mature biofilm development.
MATERIALS AND METHODS
Bacterial growth.
Staphylococcus aureus wild-type (WT) strain SC01 (2) was obtained from the Kolter lab collection. Tryptic soy broth (TSB) medium and d and l isomers of proline, tyrosine, phenylalanine, tryptophan, and leucine were obtained from Sigma-Aldrich (Atlanta). Cells were cultured in a shaking LB medium overnight and diluted 1:100 in TSB medium supplemented with NaCl (3%), glucose (0.5%), and the appropriate concentrations of l- or d-amino acids (or lack thereof). Cells were grown for the specified period of time in the bottom of 6- or 12-well polystyrene plates or in 6-well plates with submerged substrates without shaking at 37°C. Planktonic cells were removed by gentle rinsing with phosphate-buffered saline (PBS) before further treatment.
Crystal violet staining.
Cells were grown in 12-well plates for 24 h, and after rinsing in PBS, adhered cells were stained with crystal violet (CV) as described previously (16). Wells were stained with 500 μl of 0.1% crystal violet dye, rinsed twice with 2 ml double-distilled water, and thoroughly dried. Images were taken with an AMT 2k charge-coupled-device (CCD) camera. For the quantification of biofilm growth, 0.5 ml of ethanol (95%) was added to the CV-stained wells and incubated with shaking for 2 h. The resulting solutions of dissolved CV were diluted by a factor of 20, and their optical density was measured at 595 nm. Each data point is composed of three independent samples.
Polymer substrate fabrication.
Polymer substrates used for these studies were fabricated by UV curing an epoxy (UVO-114; Epoxy Technology) or a polyurethane (Norland optical adhesive 61) in a polydimethylsiloxane mold as previously reported (1).
Assessment of d-amino acid effects on biofilms adhered to different surfaces.
Cells were grown as described above for 24 h with an either d or l mixture of equal ratios of Pro, Phe, and Tyr in a final concentration of 100 μM. Cells were grown either on 6-well polystyrene plates or on 18- by 18-mm glass cover slides (VWR) or on epoxy surfaces submerged in a 6-well plate. Glass and epoxy surfaces were submerged in 6 ml of the growth medium. After rinsing, adhered cells were fixed using a 5% glutaraldehyde solution for 1 h with 0.05 M surfactin (Sigma-Aldrich). Cells were washed twice in PBS. Images were taken with an AMT 2k CCD camera against a black background.
Fluorescence microscopy.
All dyes (Sytox green, fluorescein isothiocyanate [FITC], Syto 63, and Texas Red-concanavalin A [ConA]) were obtained from Invitrogen-Molecular Probes (Oregon). Fluorescence microscopy images were obtained on a Leica DMRX compound microscope fitted with a 63× water immersion lens. Cells were grown in 6-well polystyrene plates in duplicate, as described above. Cells were fixed as described above after 2, 12, and 24 h for 1 h at 50°C. Cells were then rinsed with PBS, and their membranes were made permeable by soaking the cell growth substrates in Triton X-100 (VWR), 0.1% (vol/vol) in PBS (PBST), for 15 min. The cells were labeled by replacing the PBST with Sytox green nucleic acid stain, 0.5 μM in PBST, for 15 min to an hour. The substrates were rinsed again with PBS and then imaged with the water immersion lens using a Leica FITC (K3) filter cube.
Confocal microscopy.
Confocal microscopy images were obtained on a Leica TCS SP5 spectral confocal inverted microscope. S. aureus cells were grown without shaking in 37°C in TSB medium with 3% NaCl and 0.5% glucose for 24 h, applied with either a d or an l mixture of equal ratios of Pro, Phe, and Tyr in a final concentration of 100 μM. Cells were grown on a glass cover slide of 18 by 18 mm submerged in 6 ml of TSB with NaCl and glucose. Planktonic cells were removed. Adhered cells were fixed using a 5% gluteraldehyde solution for 1 h in 50°C, applied with surfactin (0.05 M). Cells were washed in 2 ml PBS. FITC (0.001%) and Syto 63 (100 μM) were added to the well, and the plate was incubated with shaking for 5 min and for an additional hour without shaking at room temperature. Cells were washed twice in PBS. Samples were then removed from the PBS rinse solution and inverted onto a cover glass (no. 1; VWR). Biofilms or adhered cells were imaged through the cover glass using the following excitation and emission wavelengths (11): 488 nm excitation and 505 to 530 nm emission detection range for FITC, 543 nm and 560 to 800 nm for Texas Red-ConA, and 633 nm and 650 to 800 nm for Syto 63.
Controlled release of d-amino acids from polymer substrates.
Epoxy and polyurethane substrates were soaked overnight in 1 mM solution of either l or d isomers of Pro, Phe, and Tyr in equal molar ratios. The substrates were rinsed thoroughly with PBS, and then cells were grown on them in 6-well plates for 24 h as described above. Fluorescence microscopy images of the adhered cells were obtained as described above.
RESULTS
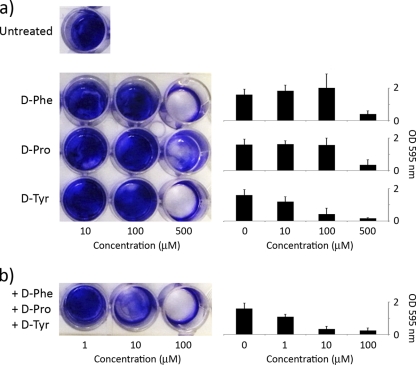
S. aureus forms biofilms on submerged surfaces (4, 17). These biofilms can be detected by staining the adhered cells with crystal violet. We screened all d-amino acids for those that were effective in inhibiting biofilm formation by strain SC01. d-Tyr, d-Pro, and d-Phe were the most effective, whereas other d-amino acids had little or no effect (Fig. 1 and data not shown; see also Fig. S1 in the supplemental material). An equimolar mixture of d-Tyr, d-Pro, and d-Phe was more effective in preventing biofilm formation than any of the individual amino acids (Fig. 1). In contrast, l-Tyr, l-Pro, l-Phe, and a mixture of the three had little or no inhibitory effect (data not shown). The d-Tyr, d-Pro, and d-Phe mixture was also more effective than the previously described mixture of d-Trp, d-Met, d-Leu, and d-Tyr that was optimal for inhibiting biofilm formation by B. subtilis (10) (see Fig. S2). It should be noted that d-amino acids did not significantly impair the growth of S. aureus cells, even at millimolar concentrations (see Fig. S3), and therefore, as observed for B. subtilis, the inhibitory effect on biofilm formation was not a result of growth inhibition. The inhibitory effect of the d-amino acid mixture was not restricted to strain SC01 and was also seen with three other S. aureus strains (see Fig. S4 and S5). Finally, the d-amino acid mixture could also disassemble an existing biofilm, but this effect required much higher concentrations (10 mM) than that needed for inhibition (<100 μM) (see Fig. S6).
Fig. 1.
d-Pro, d-Tyr, and d-Phe inhibit biofilm formation in S. aureus. Cells were cultured overnight in 12-well plates and stained with crystal violet to assess biofilm formation (a). Cells were significantly impaired in biofilm formation at concentrations of individual d-amino acids of 500 μM. Cells exposed to an equimolar mixture of all three d-amino acids were significantly impaired in biofilm formation at a concentration as low as 10 μM (b). Shown on the right is the quantification of biofilm formation as determined by crystal violet staining.
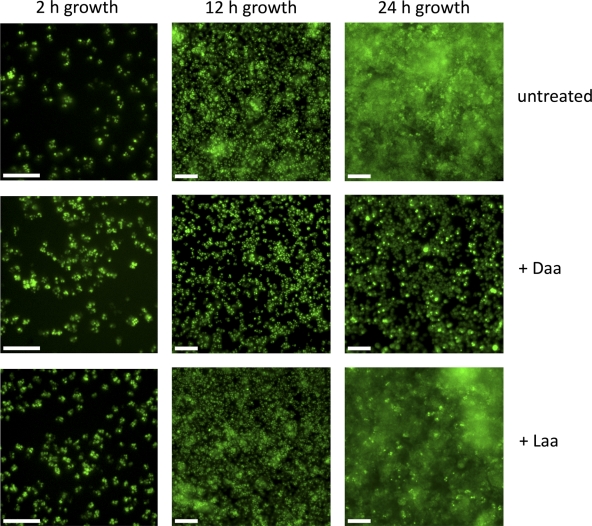
The adhesion of cells to a surface is an essential first step of biofilm formation and can occur by specific and nonspecific cell-surface interactions. To study whether d-amino acids disrupted the attachment stage of biofilm growth, we screened a representative sample of substrates. As shown in Fig. 2, d-amino acids but not l-amino acids inhibited biofilm formation equivalently on polystyrene, epoxy, and glass surfaces, suggesting that the surface properties of these different substrates were irrelevant to the mode of action of d-amino acids. This result led us to use fluorescence microscopy to investigate the effect of d-amino acids on biofilm development and morphology. As shown in Fig. 3, S. aureus cells adhered to epoxy surfaces and continued to attach and divide up to 12 h of growth. This initial attachment step (seen at 2 and 12 h), which appears as foci in the figure, was not affected by exposure to d-amino acids. Under our growth conditions, S. aureus subsequently formed large aggregates characteristic of mature biofilms on submerged surfaces within 24 h. The formation of these aggregates was blocked when cells were grown in the presence of d- but not l-amino acids (Fig. 3). We conclude that d-amino acids prevent biofilm development at a stage subsequent to the initial attachment to surfaces and continue to inhibit biofilm growth for at least 48 h.
Fig. 2.

The inhibitory effect of d-amino acids on biofilm growth is similar on different substrates. Biofilms formed equally on polystyrene, epoxy, and glass substrates after 24 h of growth and did not form in the presence of d-amino acids (+ D-aa), suggesting the mechanism of action of d-amino acids is unrelated to initial surface attachment. Biofilm attached to the surface appears white in these unstained samples. For the glass and epoxy substrates, cover glass and molded epoxy were submerged in medium in 6-well plates. For growth on polystyrene, the bottom of the well was used as the substrate.
Fig. 3.
Mature biofilm development is inhibited by d-amino acids. Biofilm growth was arrested at initial (2 h), intermediate (12 h), and advanced (24 h) developmental stages on epoxy substrates, and the cells were fixed and stained for fluorescence microscopy imaging. The dye used is a nucleic acid stain, effectively labeling the entire cytoplasm. At 12 h there is little difference between the morphology of adhered cells in untreated cultures, those treated with l-amino acids (+ Laa), and those treated with d-amino acids (+ Daa). After 24 h, however, the inhibition of biofilm growth by d-amino acids is apparent. The scale bars are 10 μm.
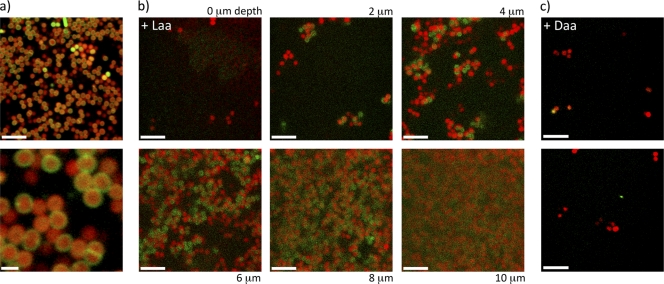
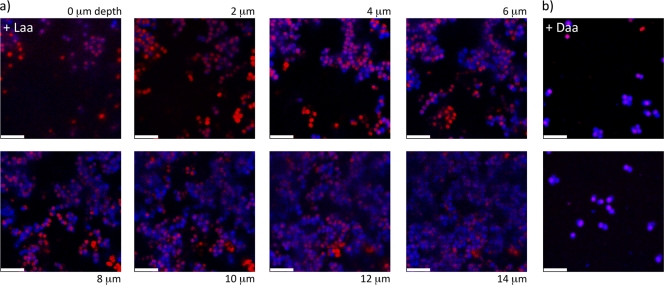
We hypothesized that aggregate formation during S. aureus biofilm development is blocked by d-amino acids as a result of a change in the properties of the extracellular matrix, as was shown in the case of d-amino acid inhibition of B. subtilis biofilms (10). To investigate this possibility, we characterized biofilms formed on glass coverslips with and without d-amino acids, focusing on the protein and exopolysaccharide (EPS) components of the extracellular matrix. We stained the EPS with a fluorescent concanavalin A (ConA) conjugate, and proteins were stained with the amine-reactive fluorescein isothiocyanate (FITC) (14). These dyes may also bind to proteins and carbohydrates inside the cells if the cell membrane has been compromised. Therefore, we also stained with Syto 63, a DNA-reactive dye, to distinguish binding sites in the extracellular matrix from those inside cells. Figures 4 and 5 show stacked sections of S. aureus biofilms imaged using confocal laser scanning microscopy (CLSM). Image slices were obtained at 2-μm increments moving from the top of the biofilm toward the substrate. In this way we determined the minimum thickness of the biofilm. This thickness determination was limited by the diffusion of dye into the biofilm and therefore is given as a minimum value for mature biofilms. As can be seen in Fig. 4a and 4b, cells in mature biofilms displayed large amounts of proteins at their surface. These cell surface-associated proteins were also present when biofilms were grown in the presence of l-amino acids. However, such proteins were largely absent when cells were grown in the presence of d-amino acids (Fig. 4c; see also Fig. S7A in the supplemental material). In contrast, the localization of cell wall sugars and EPS exhibited little difference between cells grown in the presence of d-amino acids and those grown with l-amino acids (Fig. 5; see also Fig. S7B and S8). It should be noted that biofilms grown in the presence of d-amino acids were significantly thinner (2 to 4 μm) than the biofilms grown in the presence of l-amino acids (10 to 24 μm). Also, the large cell aggregates found in biofilms grown with l-amino acids were absent in surface-associated cells grown in the presence of d-amino acids.
Fig. 4.
Confocal microscopy images of S. aureus biofilms grown with or without d-amino acids reveal a difference in the presence of extracellular protein. Biofilms were stained with protein-specific (green) and DNA-specific (red) dyes to image the localization of the peptide component of the biofilm matrix. (a) CLSM imaging of wild-type biofilms shows a clear localization of extracellular proteins to the cell wall in an image slice taken from the interior of a large cell aggregate. The higher-magnification image on the bottom shows this localization clearly, with even the septa between dividing cells stained. (b) Images were taken as lateral cross sections of the biofilm, with the height of each section indicated from the top of the biofilm. The presence of protein at the cell surface is clearly seen throughout the aggregates. (c) In d-amino acid-treated cultures, there was no biofilm present on the substrate surface and there was a distinct lack of protein localization on the cell surfaces, in contrast to the images in panel b. The dispersed cells and clusters seen in panel c were adhered to the substrate surface, and the two images shown are typical of the morphology and staining across the entire substrate as seen in multiple additional images. The scale bars are 5 μm except for that in the lower frame of panel a, which is 1 μm.
Fig. 5.
Confocal laser scanning microscopy images show no difference in the localization of EPS in S. aureus aggregates from cultures grown with d- or l-amino acids. These cells were stained with EPS-specific (blue) and DNA-specific (red) dyes, and the imaging sections were obtained in the same manner as those in Fig. 4. (a) Cultures treated with l-amino acids exhibited robust biofilms with localization of EPS around the cells in the aggregates. (b) Those treated with d-amino acids retained the EPS surrounding the cells but adhered as a submonolayer film, lacking the thickness and structure of a mature biofilm. As in Fig. 4c, the cells shown in panel b were attached only at the surface of the substrate. The scale bars are 5 μm.
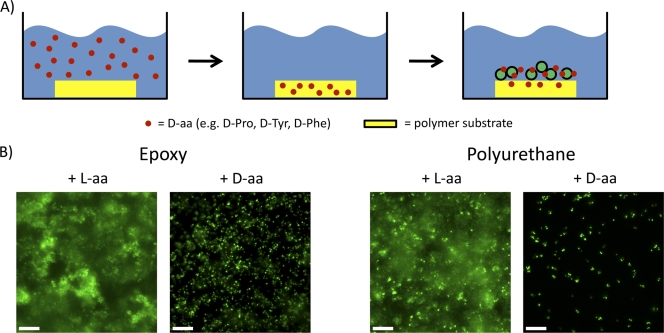
The ability of d-amino acids to disrupt biofilm formation makes them potentially useful for addressing the problems associated with device-related infections. To investigate this possibility, we asked whether the slow release of d-amino acids from the substrate, rather than supplying them in the growth medium, would be sufficient to impede biofilm formation. Accordingly, we soaked substrates, including epoxy- and polyurethane-based polymers, in medium containing d-amino acids or l-amino acids. The substrates were washed and placed in fresh growth medium lacking supplemented d-amino acids or l-amino acids. The results shown in Fig. 6 demonstrate that S. aureus formed robust biofilms when cultured on immersed surfaces impregnated with l-amino acids. In contrast, biofilm formation was inhibited on surfaces impregnated with d-amino acids. As a negative control, glass surfaces, which do not absorb the d-amino acid mixture, were used (see Fig. S9 in the supplemental material). On these surfaces, S. aureus biofilm growth was unaffected. Therefore, diffusion of d-amino acids was as effective as d-amino acids in the medium for inhibiting biofilm formation.
Fig. 6.
The diffusive release of d-amino acids from polymer surfaces inhibits biofilm growth. (A) Polymer substrates were soaked in l- or d-amino acids overnight and rinsed before use as growth substrates in medium lacking supplemented amino acids of either kind. (B) Both epoxy- and polyurethane-based growth substrates resisted biofilm accumulation when soaked with d-amino acids but not l-amino acids, recapitulating the results of cultures grown in the presence of amino acids of either chirality. The images were obtained by fluorescence microscopy using Sytox green to label the cytoplasm. The scale bars are 10 μm.
DISCUSSION
Many bacteria, including S. aureus, produce d-amino acids upon entry into the stationary phase of growth (11). d-Amino acids incorporate into the peptide cross bridge of the peptidoglycan component of the cell wall (5, 6, 11). It was previously shown that d-tyrosine, d-leucine, d-tryptophan, and d-methionine were active in inhibiting biofilm formation by B. subtilis, whereas d isomers of other amino acids, such as d-phenylalanine, were inert in inhibiting biofilm formation (10). In contrast, in S. aureus the d isomers that were found to be active in inhibiting biofilm formation were d-phenylalanine, d-proline, and d-tyrosine (Fig. 1). Under the conditions described here, a mixture of d-Tyr, d-Pro, and d-Phe was more effective in preventing biofilm formation than the previously described mixture of d-Trp, d-Met, d-Leu, and d-Tyr (see Fig. S2 in the supplemental material). These differences suggest S. aureus and B. subtilis differ either in the mechanism by which d-amino acids are integrated in the cell wall or, alternatively, in the way they sense and respond to the incorporation of unusual d-amino acids.
Exposure to d-amino acids did not inhibit the growth of S. aureus in shaking cultures (see Fig. S3 in the supplemental material). Rather, the effect of d-amino acids appeared to be specific to biofilm formation. The first step of biofilm formation is the attachment of bacteria to a surface. The fact that S. aureus adhered to and formed biofilms equally well on various substrates and that d-amino acids prevented biofilm growth in an identical manner across all of the substrates tested (Fig. 2) suggested that the inhibition step was unrelated to the mechanism of initial attachment of bacteria to surfaces. Indeed, fluorescence microscopy (Fig. 3) revealed that initial surface attachment was unaffected by treatment with d-amino acids. Instead, these images showed that bacteria adhered to the submerged surface but did not proceed to form fully developed biofilms. The initial surface attachment of bacteria was similar irrespective of treatment with l- or d-amino acids. By 24 h of growth, however, the untreated and l-amino acid-treated cultures had developed mature biofilms with large cell aggregates. In contrast, biofilm development in cultures exposed to d-amino acids was arrested at the attachment stage. It is likely, therefore, that the effects of d-amino acids are restricted to the accumulation step in biofilm development.
Confocal microscopy gave a more detailed picture of biofilm development during exposure to d-amino acids. Using FITC and Syto 63 as a cell body counterstain, protein could be seen to localize at or around the cell wall of the constituent cells of S. aureus biofilms (Fig. 4a). Whereas cells in biofilm aggregates that had formed in l-amino acid-treated cultures were clearly decorated with protein (Fig. 4b), the lack of these large supracellular structures and of protein surrounding the cells in the d-amino acid cultures (Fig. 4c; see also Fig. S7A in the supplemental material) suggests a functional relationship between the effect of d-amino acids and a protein component of the matrix. Several surface proteins play an important role in biofilm aggregates for S. aureus (8, 9, 15, 20). Thus, an appealing hypothesis is that d-amino acids prevent the localization of cell-cell adhesion proteins, thereby inhibiting the aggregate formation necessary for biofilm development. If so, then the role of d-amino acids in S. aureus would be analogous to that observed in B. subtilis, for which d-amino acid treatments have been shown to trigger the release from cells of the matrix protein TasA (3, 18). In B. subtilis, a recently characterized accessory protein, TapA (TasA anchoring/assembly protein), serves to anchor the TasA amyloid fibers to the cell wall and is mislocalized in response to d-amino acids (10, 19). Interestingly, however, TapA has no apparent ortholog in S. aureus. The fact that d-amino acids are effective at blocking these different and unrelated adhesion proteins from localizing at the cell wall suggests that they may comprise a general biofilm dispersal strategy.
The polysaccharide component of the S. aureus biofilms, on the other hand, was present in attached cells irrespective of amino acid exposure. These polysaccharides were seen to be concentrated in the large aggregates along with protein. Whereas biofilms did not form in d-amino acid-treated cultures, polysaccharides were still seen associated with the cell surface, confirming that the principal effect of the d-amino acids is to disrupt cell surface protein localization. These results suggest that the mechanism of action of d-amino acids may be similar across dissimilar bacterial species.
S. aureus is a leading cause of hospital-acquired infections (12, 17). Many of the problems associated with this pathogen stem from its capacity to form biofilms. The apparently ubiquitous nature of the effects of d-amino acid exposure is promising for applications in preventing biofilms in medical and industrial settings. Specifically, the ability to control the release of d-amino acids from device surfaces, such as implant coatings or catheter walls, to inhibit the formation of biofilms would significantly reduce the difficulty and cost associated with keeping these devices in place. The experimental procedure to evaluate the effectiveness of surfaces impregnated with d-amino acids is illustrated in Fig. 6A. The effects of d-amino acids on biofilm growth from impregnated substrates were identical to those on biofilms grown from cultures exposed to d-amino acids in the growth medium. Biofilms formed normally on glass surfaces, which should not absorb d-amino acids (see Fig. S9 in the supplemental material), demonstrating that the effect of the d-amino acids was a result of controlled release from the polymer substrate. Lacking the protective properties of a mature biofilm, these adhered cells are likely more susceptible to antimicrobial agents such as antibiotics, which can be used to treat bacterial infection more easily.
Supplementary Material
ACKNOWLEDGMENTS
We thank D. A. Weitz and the Harvard Materials Research and Engineering Center (DMR-0213805) for the use of their confocal imaging facilities and M. Gilmore for strains MN8, NCTC 10833, and RN4220.
I.K.-G. is a postdoctoral fellow of the Human Frontier Science Program. This work was supported by NIH grants to R.K. (GM58213) and R.L. (GM18546), as well as grants from the BASF Advanced Research Initiative at Harvard University to J.A., R.K., and R.L.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
Published ahead of print on 19 August 2011.
REFERENCES
- 1. Aizenberg J., Pokroy B., Epstein A. K., Persson-Gulda M. C. M. 2009. Fabrication of bioinspired actuated nanostructures with arbitrary geometry and stiffness. Adv. Mater. 21:463–469 [Google Scholar]
- 2. Blevins J. S., et al. 2003. Role of sarA in the pathogenesis of Staphylococcus aureus musculoskeletal infection. Infect. Immun. 71:516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Branda S. S., Chu F., Kearns D. B., Losick R., Kolter R. 2006. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 59:1229–1238 [DOI] [PubMed] [Google Scholar]
- 4. Bryers J. D. 2008. Medical biofilms. Biotechnol. Bioeng. 100:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cava F., de Pedro M. A., Lam H., Davis B. M., Waldor M. K. 2011. Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids. EMBO J. 30:3442–3453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cava F., Lam H., de Pedro M. A., Waldor M. K. 2011. Emerging knowledge of regulatory roles of D-amino acids in bacteria. Cell Mol. Life Sci. 68:817–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Costerton J. W., Stewart P. S., Greenberg E. P. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318–1322 [DOI] [PubMed] [Google Scholar]
- 8. Cucarella C., et al. 2001. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 183:2888–2896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Geoghegan J. A., et al. 2010. Role of surface protein SasG in biofilm formation by Staphylococcus aureus. J. Bacteriol. 192:5663–5673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kolodkin-Gal I., et al. 2010. D-Amino acids trigger biofilm disassembly. Science 328:627–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lam H., et al. 2009. D-Amino acids govern stationary phase cell wall remodeling in bacteria. Science 325:1552–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez D., Vlamakis H., Kolter R. 2010. Biofilms. Cold Spring Harb. Perspect. Biol. 2:a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mah T. F., O'Toole G. A. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34–39 [DOI] [PubMed] [Google Scholar]
- 14. McSwain B. S., Irvine R. L., Hausner M., Wilderer P. A. 2005. Composition and distribution of extracellular polymeric substances in aerobic flocs and granular sludge. Appl. Environ. Microbiol. 71:1051–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Neill E., et al. 2008. A novel Staphylococcus aureus biofilm phenotype mediated by the fibronectin-binding proteins, FnBPA and FnBPB. J. Bacteriol. 190:3835–3850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. O'Toole G. A., Kolter R. 1998. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 30:295–304 [DOI] [PubMed] [Google Scholar]
- 17. Otto M. 2008. Staphylococcal biofilms. Curr. Top. Microbiol. Immunol. 322:207–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romero D., Aguilar C., Losick R., Kolter R. 2010. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. U. S. A. 107:2230–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Romero D., Vlamakis H., Losick R., Kolter R. 2011. An accessory protein required for anchoring and assembly of amyloid fibers in B. subtilis biofilms. Mol. Microbiol. 80:1155–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schroeder K., et al. 2009. Molecular characterization of a novel Staphylococcus aureus surface protein (SasC) involved in cell aggregation and biofilm accumulation. PLoS One 4:e7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.