Abstract
Despite increased morbidity associated with secondary respiratory viral infections in cystic fibrosis (CF) patients with chronic Pseudomonas aeruginosa infection, the underlying mechanisms are not well understood. Here, we investigated the effect of P. aeruginosa infection on the innate immune responses of bronchial epithelial cells to rhinovirus (RV) infection. CF cells sequentially infected with mucoid P. aeruginosa (MPA) and RV showed lower levels of interferons (IFNs) and higher viral loads than those of RV-infected cells. Unlike results for CF cells, normal bronchial epithelial cells coinfected with MPA/RV showed higher IFN expression than RV-infected cells. In both CF and normal cells, the RV-stimulated IFN response requires phosphorylation of Akt and interferon response factor 3 (IRF3). Preinfection with MPA inhibited RV-stimulated Akt phosphorylation and decreased IRF3 phosphorylation in CF cells but not in normal cells. Compared to normal, unstimulated CF cells or normal cells treated with CFTR inhibitor showed increased reactive oxygen species (ROS) production. Treatment of CF cells with antioxidants prior to MPA infection partially reversed the suppressive effect of MPA on the RV-stimulated IFN response. Together, these results suggest that MPA preinfection inhibits viral clearance by suppressing the antiviral response particularly in CF cells but not in normal cells. Further, increased oxidative stress in CF cells appears to modulate the innate immune responses to coinfection.
INTRODUCTION
The significance of secondary bacterial infection following a viral infection has been known for a long time. However, the effects of bacterial infection on host responses to secondary viral infections are poorly understood. It is possible that bacterial infection-induced changes in host mucosa may modulate the innate immune responses to viral infection. For example, previously, we have shown that the preinfection of airway epithelial cells with nontypeable Haemophilus influenzae increases expression of intercellular adhesion molecule 1 (ICAM-1) (30), which is a cellular receptor for major group rhinovirus (RV) (17, 23). This in turn increases RV binding to airway epithelial cells, leading to an exaggerated chemokine response (30). Nontypeable H. influenzae infection also increases the expression of toll-like receptor 3 (TLR3), which recognizes double-stranded RNA (dsRNA) and elicits an interleukin-8 (IL-8) and/or interferon (IFN) response (30, 38). Pseudomonas aeruginosa infection also increases ICAM-1 expression in airway epithelial cells (12). Further, treatment with lipopolysaccharide has been demonstrated to prevent antiviral responses in macrophages (27, 34), indicating that prior infection with bacteria may enhance viral binding and decrease viral clearance.
Secondary viral infections may increase the severity of lung disease in patients with chronic bacterial infections, such as those with cystic fibrosis (CF). Although CF is an inherited genetic disorder, pulmonary manifestations due to chronic bacterial lung infection is the leading cause of morbidity and mortality in these patients. The majority of CF patients show a slow progressive loss of pulmonary function because of smoldering chronic infection with P. aeruginosa and inflammation. This is punctuated by episodes of acute exacerbations due to infection or acquisition of new infectious agents. Respiratory viruses are detected approximately in 28 to 48% of CF patients with pulmonary exacerbations; hence, viruses may be important triggers of exacerbation in CF (11, 37, 40, 41).
RV is a single-stranded RNA virus and is responsible for majority of the common colds and >50% of virus-associated exacerbations in patients with asthma or chronic obstructive pulmonary disease (reviewed in reference 9). Similarly, RV was also detected in 22 to 58% of virus-associated CF exacerbations (8, 11, 35, 41). Other respiratory viruses detected in CF patients include respiratory syncytial virus, influenza A/B virus, parainfluenza virus, and adenovirus (1, 7, 8, 11, 26, 35, 41). RV infection in CF patients was associated with increased lower respiratory symptoms and required prolonged use of intravenous antibiotics and hospitalization (8, 25), suggesting that RV may synergize with existing bacterial flora in exacerbating the disease. Recently, we showed that secondary RV infection increases chemokine responses of bronchial epithelial cells preinfected with mucoid P. aeruginosa (MPA) by liberating planktonic bacteria from biofilm (5).
The airway mucosal epithelium is the primary target for respiratory viruses and plays a pivotal role in mounting appropriate early innate immune responses to clear infecting virus. In CF, airway epithelial cells are constantly exposed to an inflammatory milieu, and this may alter the innate immune responses to infection. There is evidence suggesting that CF airway epithelial cells are attenuated in viral clearance (42, 44, 45); however, what is not known is whether this deficiency is due to changes caused by persistent bacterial infection or due to dysfunction of CF transmembrane conductance regulator (CFTR). Therefore, in the present study, we examined the antiviral responses to rhinovirus infection in CF bronchial epithelial cells preinfected with P. aeruginosa, a principal pathogen in CF.
MATERIALS AND METHODS
Bacteria and growth conditions.
The MPA isolate used in this study was isolated from the sputum of a CF patient and has been described previously (15, 36). MPA was prepared for infection assays as described previously (5, 36). Briefly, MPA was subcultured on brain heart infusion agar (BD diagnostics, Franklin Lakes, NJ) and grown for 24 h at 37°C. A single colony was transferred to 10 ml tryptic soy broth (BD Diagnostics, Sparks, MD) and grown overnight in a shaking incubator. Bacteria were harvested by centrifugation, washed with phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO), and finally suspended in PBS or cell culture medium to a required concentration based on an optical density at 600 nm (OD600; 1 OD unit is equivalent to 1 × 109 CFU/ml). The actual concentration of bacteria in a suspension was determined by plating.
Rhinovirus.
Rhinovirus RV39 was purchased from the American Type Culture Collection (ATCC; Manassas, VA). Viral stocks were generated by infecting H1-HeLa cells (ATCC CRL-1958) as described previously (29, 30) and 50% tissue culture infectivity doses (TCID50) of viral stock were determined by the Spearman-Karber method (16). Supernatant from uninfected HeLa cells and RV exposed to UV light for 15 min on ice at 100 mJ/cm2 (UV-RV; replication-deficient virus) were used as negative controls. In selected experiments, a partially purified RV preparation was subjected to ultrafiltration and a filtrate containing molecules of <100 kDa was used as an additional negative control.
Bronchial epithelial cell cultures and infection.
Primary bronchial epithelial cells were obtained from three CF patients (two males, aged 16 and 33 years, and one 23-year-old female) who were homozygous for the ΔF508 mutation and undergoing double lung transplantation. Non-CF bronchial epithelial cells were isolated from lung donors (two males, aged 21 and 41 years, and one 40-year-old female) with no known lung disease. The epithelial cells isolated from bronchial segments of healthy and CF donors were grown at air-liquid interface to promote mucociliary differentiation, as described previously (5, 28). Use of these tissue samples for isolation of cells was approved by the University of Michigan Institutional Review Board. Well-differentiated CF cells were treated apically with 10 μl of PBS alone (sham infection) or infected with 10 μl of PBS containing MPA at a multiplicity of infection (MOI) of 0.01 and incubated for 24 h at 37°C. Both sham- and MPA-infected cells were then shifted to medium containing 50 μg/ml gentamicin to prevent cell death due to excessive growth of bacteria and superinfected with RV at an MOI of 1 (TCID50 of 3 × 106), UV-killed RV (UV-RV), or an equal volume of HeLa cell supernatant (sham infection) and incubated at 33°C for another 24 h. Total RNA or protein was isolated from cells to assess the expression of genes by quantitative PCR (qPCR) or phosphorylation of proteins by Western blot analysis, respectively. Basolateral medium was used for determination of protein levels of cytokines. In some experiments, cells were treated with diphenylene iodonium (DPI; Sigma) prior to P. aeruginosa infection.
IB3 cells are immortalized CF bronchial epithelial cells and were kindly provided by P. Zeitlin (Johns Hopkins University, Baltimore, MD) and grown in LHC-8 medium containing 5% fetal calf serum and 5 mM glutamine (all from Invitrogen, Carlsbad, CA). BEAS-2B cells are immortalized normal bronchial epithelial cells (ATCC) cultured in bronchial epithelial cell growth medium (BEGM; Lonza, Walkersville, MD). For coinfection assays, cells grown in 6-well plates were serum starved for 6 h, treated with medium (control), or infected with MPA at an MOI of 0.01 and incubated for 16 h. Both control and MPA-infected cells were shifted to serum-free medium amended with 50 μg/ml gentamicin and superinfected with RV at an MOI of 1 (TCID50 of 1 × 106), UV-killed RV (UV-RV), or an equal volume of HeLa cell supernatant (sham infection) and incubated at 33°C up to 24 h, and medium and cells were harvested at the time points indicated in Results. In some experiments, cells were incubated with DPI, LY294002 (Cayman Chemical, Ann Arbor, MI), N-acetylcysteine (Sigma), or CFTR inhibitor 172 prior to and during infection (EMD Chemicals, Gibbstown, NJ) as indicated in the text.
Viral load.
Infectious viral load in RV-infected cells was determined as described previously (33). Briefly, cells along with medium were subjected to two freeze-thaw cycles and centrifuged. A 10-fold serial dilution of supernatants was mixed with HeLa cells and plated in 96-well plates. After incubation at 33°C for 3 days, the number of wells with viral cytopathic effect (CPE) were counted to calculate the viral titer, TCID50/ml.
ELISA.
IL-8/CXCL8, IFN-β, IL-29/IFN-λ1, and 1IL-28A/IFN-λ2 protein levels in the condition medium from cells after the relevant infections were detected by DuoSet enzyme-linked immunosorbent assay (ELISA) kits or individual ELISA kits (R&D Systems, Minneapolis, MN).
Real-time PCR.
Total RNA was extracted from the cells (RNeasy kit; Qiagen) and reverse transcribed using random hexamers and TaqMan reverse transcription reagents (Applied Biosystems). The cDNA was used to assess the expression of IL-8/CXCL8, IFN-β, IL-29/IFN-λ1, 1IL-28A/IFN-λ2, and IFN-stimulated genes by real-time PCR using gene-specific PCR primer pairs (33). All PCRs were performed with an Eppendorf Mastercycler (Westbury, NY) using the comparative threshold cycle (CT) method. Specificity of the PCR products was confirmed by melting curve analysis.
Western blot analysis.
After appropriate treatment, cells were washed with PBS and lysed in cold radioimmunoprecipitation assay (RIPA) buffer (Santa Cruz Biotechnology) containing 1× complete protease inhibitor cocktail (Roche), 1 mM sodium fluoride, and 1 mM sodium orthovanadate. Aliquots of cell lysates corresponding to equal amount of total protein were resolved by SDS-PAGE, transferred to nitrocellulose membrane, and blocked with 5% skim milk or bovine serum albumin (BSA; Fraction V; Sigma). Membranes were then incubated with p-IRF3 (Cell Signaling Technology, Danvers, MA), total interferon response factor 3 (IRF3; Santa Cruz Biotechnology, Santa Cruz, CA), p-Akt (Cell Signaling Technology), total Akt (Cell Signaling Technology) or β-actin (Sigma). Bound antibody was detected by appropriate second antibody conjugated with horseradish peroxidase (HRP) and chemiluminescence substrate (Pierce Biotechnology, Rockford, IL).
Expression of ICAM-1 and TLR3.
Expression of ICAM-1 and TLR3 was determined as previously described (30). After the relevant treatment, cells were harvested using cell-dissociating buffer (Invitrogen), and washed with PBS-BSA (PBS containing 0.5% BSA). Cells were then incubated with monoclonal antibody (MAb) to ICAM-1 (Serotec, Raleigh, NC) or permeabilized with 0.1% saponin on ice for 30 min, washed once with PBS, and incubated with TLR3 (Abcam, Cambridge, MA) for 1 h on ice. Cells were washed and incubated for 30 min on ice with the second antibody conjugated to Alexa Fluor 488 (Invitrogen). Cells were washed, suspended in PBS-BSA, and analyzed with a Becton Dickinson FACSCalibur using CellQuest software. Cells incubated with matched IgG isotype controls instead of primary antibody served as controls.
RV binding to and endocytosis by IB3 cells.
Binding of RV to IB3 cells was determined as described previously (23, 30). Briefly, after the relevant treatment, IB3 cells were prechilled for 30 min at 4°C and then incubated with cold PBS-BSA (PBS containing 1% BSA) containing RV or equal volumes of sham control in the presence or absence of 10 mM EDTA for 1 h at 4°C. Cells were washed with PBS-BSA or PBS-BSA containing 10 mM EDTA to remove unbound RV. Cells were then incubated with MAb to RV16 (MAb R16-7) (21, 22), which recognizes VP2, a capsid protein of not only RV16 but also RV39, or isotype IgG control for 1 h on ice. Cells were washed, incubated with antimouse IgG-conjugated with Alexa Fluor 488 for 30 min, and analyzed by flow cytometry. To obtain an estimate of specific binding of RV to epithelial cells, we subtracted the mean fluorescence index (MFI) obtained in the presence of EDTA from the MFI in the absence of EDTA. We then subtracted the MFI associated with cells incubated with sham-infected cells. To measure endocytosis of RV, IB3 cells were incubated with RV or equal volumes of sham at 4°C for 30 min, washed, and then incubated at 37°C for 30 min to facilitate endocytosis of bound RV. Cells were permeabilized, and endocytosis of RV was determined by flow cytometry and corrected for controls as described for binding.
Measurement of reactive oxygen species (ROS).
After the relevant treatment, cells were incubated with 2 μM carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Invitrogen) in Hanks balanced salt solution (HBSS) for 30 min. Cells were then shifted to serum-free medium and incubated for 1 h. Cells were collected and suspended in PBS, fluorescent emission was quantified by flow cytometry, and the results were analyzed using CellQuest software.
Statistical analysis.
Results are expressed as means ± standard deviations (SD) from at least two or three independent experiments done in duplicate or triplicate. Data were analyzed by using SigmaStat statistical software (SigmaStat Software, Inc., San Jose, CA). To compare two groups, Student's t test was used. To compare more than two groups, one-way analysis of variance (ANOVA) with Tukey-Kramer post hoc analysis was performed. When data were not normally distributed, results were expressed as medians with ranges and compared by either ANOVA on ranks or Mann-Whitney test. A P value of ≤0.05 was considered significant.
RESULTS
CF bronchial epithelial cells preinfected with MPA show a deficient IFN response and viral clearance.
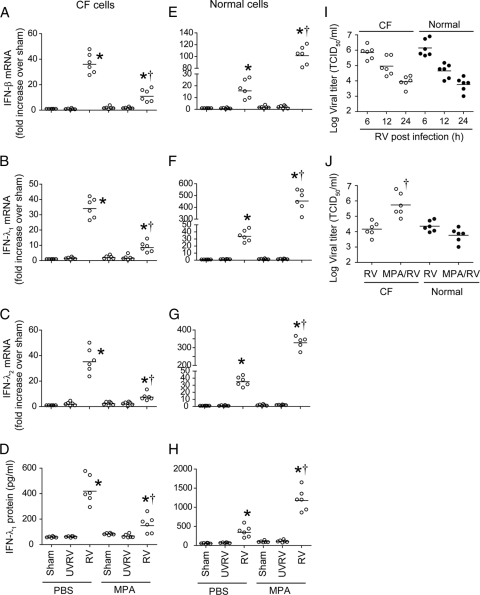
Primary CF or non-CF bronchial epithelial cells differentiated into a mucociliary phenotype were sequentially infected with MPA followed by RV, and mRNA levels of beta IFN (IFN-β), IFN-λ1, IFN-λ2 (Fig. 1), and IFN-stimulated genes (ISGs) (Table 1) were determined by qPCR. While UV-RV or sham (negative-control) infections did not stimulate IFN responses, RV infection stimulated expression of type I and type III IFNs and ISGs, irrespective of preinfection with MPA, in both CF and normal cells. Treatment of cells with an RV preparation devoid of RV did not stimulate IFN expression, and results were similar to those for sham-infected cells, suggesting that the observed IFN response was specifically due to RV and not other contaminants that may be present in the RV preparation (data not shown). CF cells infected with MPA followed by RV showed significantly suppressed expression of both IFNs and ISGs compared to that of cells infected with RV alone (Fig. 1A to D) (Table 1). In contrast, normal cells infected with MPA and RV showed 2- to 3-fold increases in IFN and IFN-stimulated genes compared to results for cells infected with RV alone (Fig. 1E to H). Expression of ICAM-1, NADPH oxidase 1, matrix metalloprotease 12, and IL-8 was increased in both normal and CF cells coinfected with MPA and RV compared to that for cells infected with RV alone (Table 1).
Fig. 1.
MPA preinfection alters IFN response to RV infection in primary CF and normal bronchial epithelial cells. Mucociliary-differentiated CF bronchial epithelial cells were infected apically with MPA or treated with PBS and incubated for 24 h. Cells were then infected with RV39 and incubated for another 24 h. Sham- or UV-RV-infected cells were used as controls. Total RNA was isolated from CF (A to C) or normal (D to F) cells, and mRNA levels of IFNs were measured by qPCR. IFN-λ1 protein levels in the basolateral medium from CF (G) and normal (H) cells were determined by ELISA. Cells infected with RV were harvested at 6, 12, or 24 h postinfection, and the viral titer was determined (I). Cells infected with RV or coinfected with MPA/RV and incubated for 24 h. Cells along with medium were harvested, and the viral titer was determined (J). Data represent the ranges and medians (horizontal lines) calculated from experiments performed with cells obtained from three CF and three healthy individuals in duplicate. *, different from sham-treated group (P ≤ 0.05); †, different from cells infected with RV alone (P ≤ 0.05) (ANOVA on ranks [A to H] or Mann-Whitney test [I]).
Table 1.
mRNA expression in primary CF bronchial epithelial cells in response to RV infection
| Gene name | Fold increase in mRNA levela |
|||||
|---|---|---|---|---|---|---|
| CF cells |
Normal cells |
|||||
| PBS/RV | MPA/PBS | MPA/RV | PBS/RV | MPA/PBS | MPA/RV | |
| IFN-stimulated antiviral genes | ||||||
| ISG15 | 20.26 | 1.58 | 2.75* | 22.62 | 1.48 | 93.51* |
| IFIT1 | 37.26 | 1.02 | 5.81* | 35.12 | 1.82 | 104.59* |
| IFIT2 | 46.09 | 4.62 | 1.87* | 39.85 | 1.59 | 89.43* |
| IFIT3 | 17.58 | 1.86 | 1.40* | 15.92 | 1.37 | 32.56* |
| IFI16 | 2.94 | 1.32 | 1.34 | 1.61 | 1.27 | 2.30 |
| IFI35 | 6.25 | 1.71 | 1.34* | 5.55 | 1.33 | 15.83* |
| IFI44 | 3.21 | 0.97 | 1.17* | 4.47 | 1.16 | 10.17* |
| MX1 | 10.42 | 1.04 | 1.95* | 15.70 | 1.93 | 53.44* |
| MX2 | 10.92 | 1.15 | 2.03 | 12.65 | 2.02 | 43.87* |
| RSAD2 | 33.89 | 2.36 | 19.25* | 39.23 | 6.98 | 68.99* |
| OAS1 | 5.74 | 1.24 | 1.14* | 4.47 | 1.07 | 7.10 |
| OASL | 22.16 | 1.94 | 8.34* | 15.06 | 1.07 | 44.75* |
| Other genes | ||||||
| NOX1 | 1.98 | 1.71 | 2.72 | 5.84 | 3.21 | 8.16 |
| ICAM1 | 1.91 | 2.64 | 4.34* | 2.02 | 2.35 | 5.14 |
| MMP12 | 2.80 | 1.48 | 4.16* | 3.96 | 2.01 | 3.79 |
| IL-8 | 1.99 | 9.28 | 15.16* | 2.57 | 4.14 | 8.13* |
mRNA expression was calculated as the fold increase over results for the appropriate sham control. Data are the medians calculated from experiments performed in triplicate with cells obtained from 3 CF and 3 healthy individuals. *, different from respective RV-infected cultures (P ≤ 0.05) (Mann-Whitney test). PBS/RV, infected with PBS instead of MPA and then infected with RV; MPA/PBS, infected with MPA and then sham infected with PBS instead of RV; MPA/RV, coinfection with MPA and RV.
Next, we examined the capacity of CF and normal cells to clear virus. CF or normal cells were infected with RV alone, and viral load was determined 6, 12, and 24 h postinfection. CF and normal cells showed similar viral loads at all the time points examined, suggesting that both cell types clear virus equally well and with similar kinetics (Fig. 1I). However, CF and normal cells showed a significant difference in the viral load following coinfection with MPA and RV, which correlated with IFN expression (Fig. 1J). CF cells coinfected with MPA followed by RV showed 2-log-higher infectious virus than that for cells infected with RV alone. In contrast, normal cells coinfected with MPA and RV showed a decreasing trend in viral load compared to results for cells infected with RV alone. In both CF and normal cells, the viral load inversely correlated with levels of IFN. The decreased IFN response and increased viral load in MPA/RV-infected CF cells was not due to cell death, as there was no difference in lactate dehydrogenase (LDH) levels or numbers of apoptotic cells between RV- and MPA/RV-infected cells (data not shown). Together, these results indicate that MPA suppresses the IFN response to subsequent RV infection and interferes with viral clearance in CF cells but not in normal cells.
CF and normal bronchial epithelial cell lines respond to MPA and RV coinfection in a manner similar to that of primary cells.
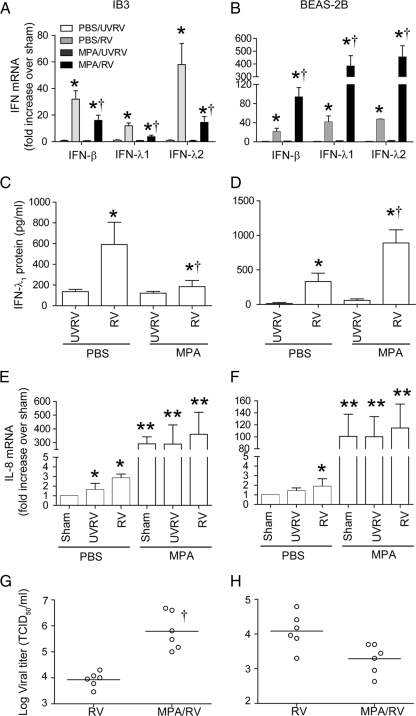
To delineate the mechanisms by which MPA modulates RV-induced IFN responses, we used immortalized CF (IB3) and normal (BEAS-2B) bronchial epithelial cells. These cell lines have been previously used extensively to elucidate mechanisms of innate immune responses to bacterial or viral infection. In our preliminary studies, we also examined the IB3 cells corrected for CFTR expression (C38 and S9), but they failed to respond to RV infection (data not shown). We found that CFTR-overexpressing cells produce large amounts of soluble ICAM-1, which serves as a cellular receptor for RV39, and this can potentially inhibit RV binding and endocytosis, thereby blocking IFN expression. Therefore, in the present study, we used BEAS-2B cells instead of isogenic C38 or S9 cells. Both IB3 and BEAS-2B cells infected with RV alone showed significant increases in mRNA levels of IFN-β, IFN-λ1, and IFN-λ2 compared to those of cells treated with medium or UV-RV-infected cells (Fig. 2A and B) and also showed similar kinetics of RV clearance (see Fig. S1 in the supplemental material). As observed with primary cells, while IB3 cells infected with MPA followed by RV showed decreased mRNA expression of all three IFNs, BEAS-2B cells showed significantly increased IFN mRNA levels compared to those for the respective cells infected with RV alone. Similar differences in IFN-λ1 protein levels were observed for MPA- and RV-coinfected IB3 and BEAS-2B cells (Fig. 2C and D). We also measured the protein levels of IFN-β and IFN-λ2, but they were below the detection limit in all the samples. Similar to our observations, IFN-β protein was undetectable in RV-infected normal bronchial epithelial cells despite significant increases at the mRNA level (31, 38). This may be due to the sequestration of IFN-β protein by its cellular receptors, IFNAR1 and IFNAR2, following its secretion into medium, leading to depletion of IFN in cell-free supernatants.
Fig. 2.
MPA suppresses the IFN response to RV infection in IB3 cells but not in BEAS-2B cells. Confluent monolayer of immortalized CF airway epithelial (IB3) or normal (BEAS-2B) cells were infected with MPA or treated with medium and incubated for 16 h. Cells were then infected with RV, UV-RV, or an equal volume of sham and incubated for a further 24 h. Total RNA isolated from cells was used for determination of mRNA levels of IFNs and IL-8 (A, B, E, and F). Conditioned medium was used to determine IFN-λ1 protein by ELISA (C and D). Cells along with the medium were harvested, and the viral titer was determined (G and H). Data represent averages ± SD calculated from four experiments performed in duplicate or the ranges and medians. *, different from sham-treated group (P ≤ 0.05); †, significantly different from cells infected with RV alone (P ≤ 0.05) (ANOVA [A to F] or Mann-Whitney test [G and H]).
In contrast to IFN expression, IL-8 mRNA levels in MPA/RV-coinfected IB3 cells showed 2- to 3-fold increases compared to those of similarly infected normal cells (Fig. 2E and F). Also, there was no difference in the levels of LDH or number of apoptotic cells between the RV- or MPA/RV-infected cells (data not shown).
We examined the viral titer to determine whether the IFN response corresponds to viral load as observed for primary cells. IB3 cells coinfected with MPA and RV showed a significantly increased viral load compared to that for cells infected with RV alone. In contrast, BEAS-2B cells coinfected with MPA and RV showed a viral load slightly lower than that for the cells infected with RV alone (Fig. 2G and H). Together, these results suggest that IB3 and BEAS-2B cells show responses to infection with RV alone or MPA/RV coinfection that are similar to those of primary CF and normal cells, respectively. Therefore, we used these cells in our subsequent experiments.
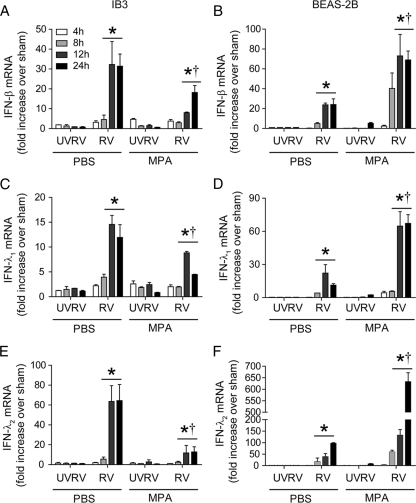
Kinetics of IFN expression is not altered in MPA/RV-infected cells.
Next, we examined the kinetics of the IFN response to RV infection to rule out the possibility that the observed IFN suppression is due to altered kinetics in IB3 cells coinfected with MPA and RV. We determined IFN-β, IFN-λ1, and IFN-λ2 mRNA expression at 4, 8, 12, and 24 h after RV infection. In both RV- and MPA/RV-infected IB3 cells, maximum expression of IFN was observed at 12 h postinfection and persisted up to 24 h, but MPA/RV-infected cells showed decreased expression at all the time points examined (Fig. 3A, C, and E). Similar kinetics of IFN expression in response to RV infection was observed in BEAS-2B cells (Fig. 3B, D, and E). However, compared to BEAS-2B cells infected with RV alone, cells infected with MPA/RV showed increased IFN expression at all the time points examined. Since UV-RV infection was similar to sham infection with regard to stimulating IFN expression, we used UV-RV-infected cells instead of sham-infected cells as negative controls in the following experiments.
Fig. 3.
RV- or MPA/RV-infected cells show similar kinetics of IFN production in IB3 and BEAS-2B cells. Confluent monolayers of IB3 (A, C, and E) or BEAS-2B (B, D, and F) cells were infected with MPA or treated with medium and incubated for 16 h. Cells were then infected with RV or UV-RV, incubated for 4, 8, 12, or 24 h, and mRNA levels of IFNs were determined. Data represent averages ± SD calculated from four independent experiments performed in duplicate. *, different from sham-treated group (P ≤ 0.05); †, significantly different from cells infected with RV alone (P ≤ 0.05) (ANOVA).
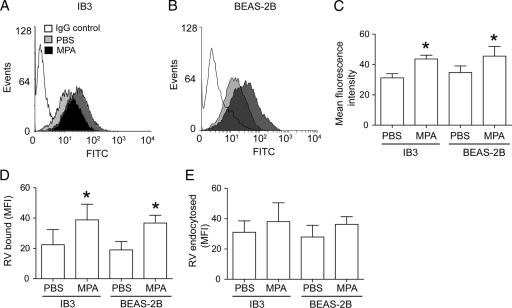
Altered IFN expression is not due to a difference in viral binding or endocytosis.
Viral binding and endocytosis are essential primary steps for subsequent IFN response. RV39 binds to ICAM-1; therefore, we determined the expression of ICAM-1. Following MPA infection, both IB3 and BEAS-2B cells showed increased expression of ICAM-1 compared to that of the respective uninfected cells (Fig. 4A to C). Further, there was no significant difference between IB3 and BEAS-2B cells in the expression of ICAM-1. Next, we examined the binding of RV to uninfected and MPA-infected cells. There was a small but significant increase in the amount of RV binding to MPA-infected cells compared to that binding to uninfected cells, irrespective of cell type (Fig. 4D), a result which was consistent with increased expression of ICAM-1. Determination of endocytosis of RV39 by IB3 and BEAS-2B cells revealed that although there was an increasing trend of endocytosis of RV39 in MPA-preinfected cells, it was not significantly different from that in uninfected cells (Fig. 4E). Together, these results suggest that the altered IFN response in MPA-preinfected cells is not due to differences in viral binding or endocytosis.
Fig. 4.
MPA infection increases ICAM-1 expression and binding of RV to bronchial epithelial cells. IB3 (A) or BEAS-2B (B) cells were infected with MPA and immunostained with antibody to ICAM-1 and analyzed by flow cytometry. Representative histogram (A and B) and mean fluorescence intensity (C) from three independent experiments. MPA-infected cells were incubated with RV39 or the same volume of sham in the presence or absence of 10 mM EDTA for 1 h at 4°C. Cells were washed and immunolabeled with antibody to RV and subjected to flow cytometry. Specific binding was calculated by subtracting the MFI associated with cells incubated with sham, as well as the MFI obtained in the presence of EDTA, from the MFI obtained in the absence of EDTA (D). To determine endocytosis of RV, cells were incubated with virus, unbound virus was removed, and cells were shifted to 33°C and incubated for another 30 min. Cells were washed and immunolabeled, and the MFI determined as described above (E). Data in panels C, D, and E represent averages ± SD calculated from four independent experiments performed in duplicate. *, different from sham-treated group (P ≤ 0.05) (Student's t test). FITC, fluorescein isothiocyanate.
Altered IFN expression is not due to difference in the expression of dsRNA receptors.
Previously, we have shown that RV-stimulated IFN expression requires recognition of dsRNA generated during RV replication by TLR3 and melanoma differentiation-associated protein 5 (MDA5), but not the retinoic acid inducible gene I (RIG-I) product (38). Therefore, we determined the expression of TLR3 and MDA5 after MPA infection for both IB3 and BEAS-2B cells. Compared to uninfected cells, MPA-infected IB3 and BEAS-2B cells showed a significant increase in TLR3 expression (see Fig. S2A in the supplemental material). However, there was no difference in TLR3 expression between IB3 and BEAS-2B cells. Expression of MDA5 was not affected by MPA infection in either IB3 or BEAS-2B cells (see Fig. S2B in the supplemental material). Together, these results suggest that the altered IFN response in MPA/RV-coinfected cells is not due to differences in the expression of TLR3 or MDA5.
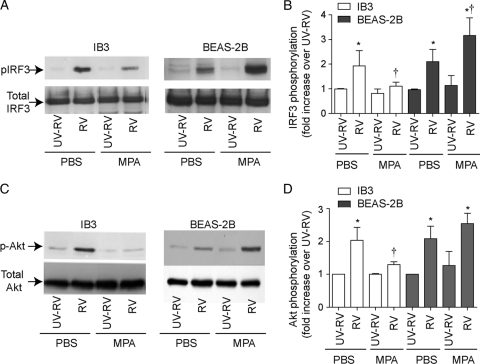
MPA preinfection inhibits IRF3 and Akt phosphorylation in response to RV infection in IB3, but not in BEAS-2B cells.
Previously, we have shown that expression of IFN response to RV infection in BEAS-2B cells requires activation of IRF3. Also genetic inhibition of IRF3 blocked the IFN response stimulated by RV infection (38). To examine whether MPA suppresses the expression of IFN response to RV by inhibiting IRF3 activation in IB3 cells, we determined the phosphorylation of IRF3. CF cells infected with RV alone showed IRF3 activation starting from 8 h postinfection, which increased further up to 24 h (see Fig. S3 in the supplemental material) (Fig. 5A and B). In contrast, in MPA preinfected CF cells, RV infection did not induce phosphorylation of IRF3 until 12 h. Although, the IRF3 phosphorylation increased with time of incubation, it was lower than that observed in cells infected with RV alone at all the time points examined. In contrast, BEAS-2B cells coinfected with MPA/RV showed increased IRF3 phosphorylation compared to cells infected with RV alone. Together, these results suggest that MPA suppresses IFN expression in response to subsequent RV infection by reducing and delaying the IRF3 activation in IB3, but not in BEAS-2B cells.
Fig. 5.
MPA suppresses the RV-induced IFN response by inhibiting IRF3 and Akt phosphorylation in IB3 cells. IB3 or BEAS-2B cells were infected with MPA and RV as described above. Cells were harvested 4 h (for Akt) or 12 h (for IRF3) after RV infection and subjected to Western blot analysis with antibodies to p-IRF3 or total IRF3 (A) or p-Akt or total Akt (C). A representative blot from three or four independent experiments is presented in panels A and C. (B and D) Blots from replicate experiments were quantified by densitometry, and the density of p-IRF3 and p-Akt was normalized to that of total IRF3 and Akt, respectively, and expressed as a fold increase over results for the respective UV-RV-infected cells. *, different from sham-treated group (P ≤ 0.05); †, significantly different from cells infected with RV alone (P ≤ 0.05) (ANOVA).
PI-3 kinase-induced Akt phosphorylation is required for maximal IRF3 activation upon recognition of dsRNA by TLR3, RIG-I, and MDA5 (13). Therefore, we examined Akt phosphorylation in cells infected with RV or MPA/RV. Cells infected with RV alone showed increased Akt phosphorylation at 4 h in IB3 cells (Fig. 5C and D) (see Fig. S4 in the supplemental material), which returned to basal levels by 8 h, whereas in MPA-preinfected cells, RV did not increase Akt phosphorylation even 12 h after infection. Similar to IB3 cells, BEAS-2B cells also showed maximum Akt phosphorylation 4 h after RV infection, which further increased in cells preinfected with MPA. These results imply that MPA may suppress IRF3 activation to subsequent RV infection by inhibiting Akt phosphorylation in IB3 cells but not in BEAS-2B cells.
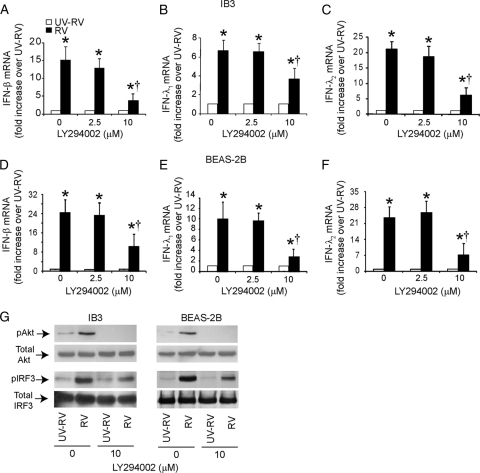
To assess whether PI-3 kinase activity is required for the RV-stimulated IFN response, IB3 or BEAS-2B cells were infected with RV and incubated for 90 min to allow binding to and endocytosis of RV by the cells. Infection medium was replaced with medium containing LY294002, a pharmacological inhibitor of PI-3 kinase and incubated for 24 h, and IFN expression was determined. LY294002 at 10 μM caused a partial but significant reduction in expression of all three IFNs in both IB3 and BEAS-2B cells (Fig. 6A to F). In addition, we observed that in both cell types, LY294002 completely abrogated Akt phosphorylation as anticipated and partially inhibited IRF3 phosphorylation (Fig. 6G). These results indicate that PI-3 kinase-induced Akt phosphorylation is required for maximal IRF3 phosphorylation and the subsequent expression of IFNs in RV-infected CF and normal cells.
Fig. 6.
RV-induced activation of IRF3 and IFN expression requires PI-3 kinase activity in CF cells. IB3 (A, C, and E) or BEAS-2B (B, D, and F) cells were infected with RV and incubated for 90 min. The infection medium was replaced with fresh medium containing DMSO or LY294002 and further incubated for 4 or 12 h (for determination of phosphorylation of Akt or IRF3, respectively) or 24 h (for determination of the IFN response) as described above. mRNA expression of IFN was measured by qPCR (A to F). Data represent averages ± SD calculated from three independent experiments. *, different from UV-RV-treated group (P ≤ 0.05) (ANOVA); †, different from cells not treated with Ly294002 (P ≤ 0.05; ANOVA). Phosphorylation of Akt and IRF3 was determined by Western blot analyses. Representative blot from three independent experiments (G).
Antioxidants block the suppressive effect of MPA on IFN expression in MPA/RV-coinfected IB3 cells.
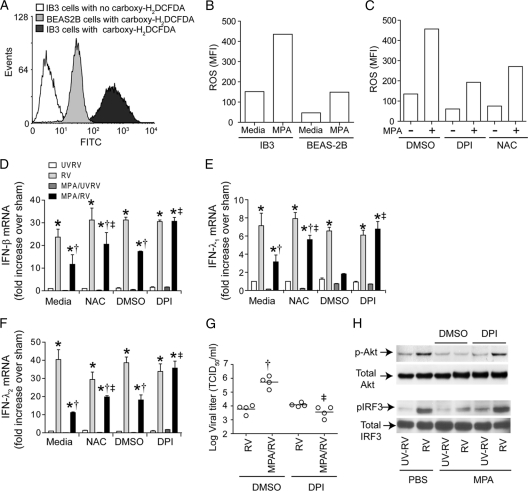
Loss of CFTR function or exposure to persistent inflammatory milieu in vivo may alter the redox state of CF airway epithelial cells, and this may modulate innate immune responses to infection (2, 18). To examine whether CF cells are under increased oxidative stress, we determined the levels of cellular ROS. Compared to BEAS-2B cells, IB3 cells showed increased levels of ROS (Fig. 7A and B), which further increased with MPA infection (Fig. 7B). Although MPA infection increased ROS generation in BEAS-2B cells, this increase was significantly less than in similarly infected IB3 cells. Pretreatment with 10 μM DPI or 25 mM N-acetylcysteine (Fig. 7C) decreased ROS levels in both uninfected and MPA-infected IB3 cells. To test whether this increased oxidative stress plays a role in the observed differences between CF and normal cells, IB3 cells were pretreated with DPI or NAC for 6 h. Dimethyl sulfoxide (DMSO)- or medium-treated cells were used as controls, respectively, for DPI- and NAC-treated cells. Cells were then infected with MPA and RV as described above in the absence of antioxidants. As observed earlier, CF cells pretreated with medium or DMSO and infected with MPA/RV showed suppressed expression of IFNs compared to levels for cells infected with RV alone (Fig. 7D to F). In contrast, cells pretreated with DPI or NAC and then infected with MPA/RV showed IFN expression similar to that of RV-infected cells. However, IFN expression in antioxidant-treated IB3 cells infected with MPA/RV was still significantly lower than that of the normal cells infected with MPA/RV. In addition, DPI treatment also decreased viral load in MPA/RV-coinfected cells, correlating with increased levels of IFN expression (Fig. 7G). DPI- but not DMSO-treated cells coinfected with MPA/RV also showed phosphorylation of Akt and IRF3, similar to results for cells infected with RV alone (Fig. 7H), implying that DPI restores IRF3 activation and subsequent IFN expression by increasing Akt phosphorylation. In BEAS-2B cells, however, DPI pretreatment showed a trend toward increased IFN response in both RV- and MPA/RV-infected cells; the IFN response was not significantly different from that of similarly infected DMSO-pretreated cells (Fig. 8). Together, these results indicate that CF and normal cells differ with respect to their antiviral response to coinfection with MPA and RV, and this difference is due at least in part to persistent oxidative stress in CF cells.
Fig. 7.
Antioxidant treatment decreases ROS in IB3 cells and reverses the suppressive effect of MPA on RV-induced IFN responses in IB3 cells. IB3 or BEAS-2B cells grown as monolayers were incubated with medium or MPA for 6 h in the presence or absence of DPI or NAC. Cells were then incubated with carboxy-H2DCFDA for 30 min in HBSS, washed, and analyzed by flow cytometry. Representative histogram (A) and mean fluorescence intensity (B and C) from three independent experiments showing increased levels of ROS in IB3 cells, both basal and postinfection with MPA. ROS generation in uninfected and MPA-infected IB3 cells in the presence or absence of DPI or NAC (C). IB3 cells treated with medium, DMSO, DPI, or NAC for 6 h were infected with MPA and RV as described above in the absence of antioxidants, and IFN expression (D, E, and F) and viral load (G) were determined as described above. Data represent averages ± SD or ranges with medians calculated from two or three independent experiments performed in duplicate. *, different from UVRV-infected group (P ≤ 0.05); †, significantly different from cells infected with RV alone (P ≤ 0.05); ‡, different from cells not pretreated with DPI or NAC (P ≤ 0.05) (ANOVA). Representative blot showing increased phosphorylation of Akt and IRF3 in IB3 cells coinfected with MPA/RV in the presence of DPI (H).
Fig. 8.
DPI does not affect IFN expression in MPA/RV-infected BEAS-2B cells. BEAS-2B cells treated with medium, DMSO, or DPI for 6 h were infected with MPA and RV as above in the absence of antioxidants and IFN expression was determined. Data represent average ± SD calculated from 3 independent experiments performed in duplicate. *, different from sham-treated group (P ≤ 0.05); †, significantly different from cells infected with RV alone (P ≤ 0.05); #, different from cells not pretreated with DPI or NAC (P ≤ 0.05) (ANOVA).
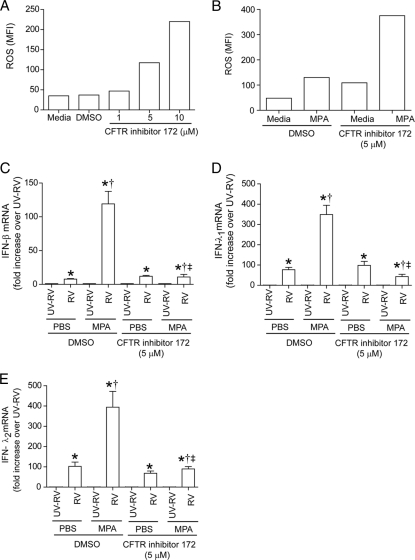
To assess whether CFTR contributes to the observed differences between CF and normal cells, we treated BEAS-2B cells with CFTR inhibitor 172 and determined the ROS generation. CFTR inhibitor increased ROS generation in BEAS-2B cells in a concentration-dependent manner, showing ROS levels similar to those for IB3 cells when cells were treated with 5 μM CFTR inhibitor (Fig. 9A). Therefore, we used 5 μM CFTR inhibitor in the subsequent experiments examining the effect of loss of CFTR function on virus-induced IFN responses. The BEAS-2B cells infected with MPA in the presence of CFTR inhibitor showed a further increase in ROS generation (Fig. 9B), similar to that observed for IB3 cells (Fig. 7B). In addition, BEAS-2B cells treated with CFTR also showed a significantly decreased IFN response following MPA/RV coinfection compared to that of vehicle-treated controls. These observations suggest that loss of CFTR function may contribute to the observed differences between CF and normal cells with regard to ROS generation and IFN response to coinfection with MPA/RV.
Fig. 9.
BEAS-2B cells treated with CFTR inhibitor respond to coinfection in a manner similar to that of IB3 cells. ROS levels were measured for BEAS-2B cells pretreated with CFTR inhibitor 172 overnight (A) or infected with MPA in the presence or absence of 5 μM CFTR inhibitor (B). BEAS-2B cells were pretreated with 5 μM CFTR inhibitor or infected with RV alone or MPA/RV, and mRNA levels of IFNs were determined (C to E). Data represent averages ± SD calculated from three independent experiments performed in duplicate. *, different from UV-RV-treated group (P ≤ 0.05); †, significantly different from cells infected with RV alone (P ≤ 0.05); ‡, different from cells not treated with CFTR inhibitor (P ≤ 0.05) (ANOVA).
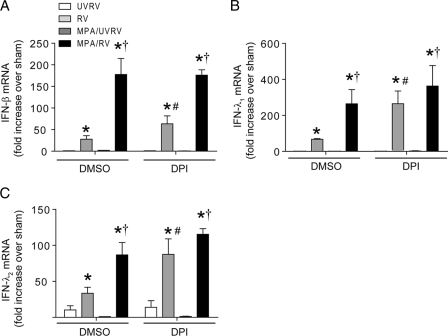
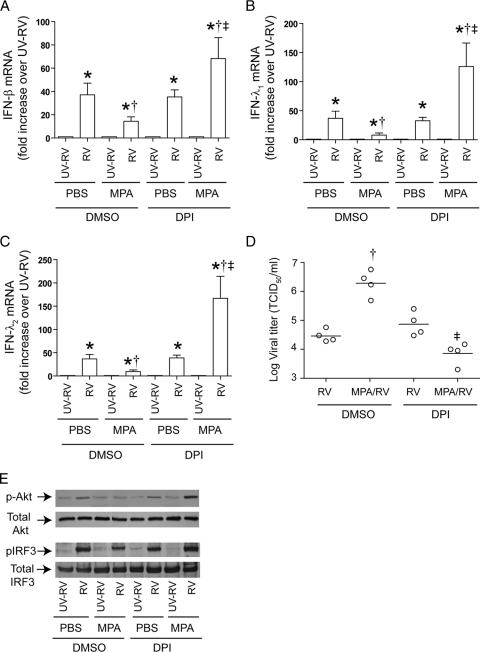
DPI reverses the suppressive effects of MPA on the RV-induced IFN response in primary CF airway epithelial cells.
To confirm that the results obtained with CF cell lines holds true for primary cells, CF primary cells differentiated into a mucociliary phenotype were pretreated with DPI or DMSO (vehicle control) and then infected with RV alone or with MPA followed by RV, and mRNA levels of IFNs were examined (Fig. 10). Upon coinfection with MPA/RV, DMSO-pretreated cells showed less IFN expression and phosphorylation of Akt and IRF3 than the cells infected with RV alone, as anticipated. In contrast, DPI-treated cells coinfected with MPA/RV showed similar or slightly increased IFN levels compared to those of DPI-treated cells infected with RV alone. In addition, DPI treatment also increased the Akt and IRF3 phosphorylation in MPA/RV-infected cells, similar to that observed for cells infected with RV alone. Together, these results suggest that antioxidant treatment reverses the suppressive effects of MPA on the RV-induced IFN response in primary CF airway epithelial cells.
Fig. 10.
DPI treatment reverses the suppressive effect of MPA on RV-induced IFN responses in primary CF airway epithelial cells. Mucociliary differentiated primary CF airway epithelial cells were pretreated with 10 μM DPI for 12 h. Cells were then infected with MPA or treated with PBS in the presence of DPI, incubated for 24 h, and then infected with RV and further incubated for 24 h. Total RNA was used for determination of IFN mRNA expression (A to C). Cells along with medium were harvested, and the viral titer was determined (D). Data represent averages ± SD or ranges with medians calculated from two or three independent experiments performed in duplicate. *, different from UVRV-infected group (P ≤ 0.05); †, significantly different from cells infected with RV alone (P ≤ 0.05); ‡, different from cells not treated with DPI (P ≤ 0.05) (ANOVA). In some experiments, cells were harvested 6 h (for Akt) or 18 h (for IRF3) after RV infection, and phosphorylation of Akt and IRF3 was determined (E). Representative blot showing phosphorylation of Akt and IRF3 in the presence or absence of DPI.
DISCUSSION
Innate immune responses of the airway epithelium play a critical role in clearing infectious agents during the early stages of infection. Exposure to secondary infection, while the airway epithelium is responding to ongoing primary infection, may affect the early innate defense mechanisms of the airway epithelium in multiple ways, depending on the types of infection. Alteration in the redox state of airway epithelial cells as observed for CF (2, 4, 10) may further modulate innate immune responses to coinfections. In this study, we demonstrate that CF and normal bronchial epithelial cells respond differently to coinfection with MPA/RV due to differences in ROS levels. Compared to normal cells, CF cells generate increased basal levels of ROS, which further increase upon MPA infection. Increased ROS levels correlated with the suppression of IFN responses induced by subsequent RV infection and persistent viral load. This appears to be due to abrogation of Akt phosphorylation which is required for maximal activation of IRF3 and IFN expression. Treatment with the antioxidant DPI reversed the suppressive effects of MPA infection and decreased viral load by restoring the Akt phosphorylation. In contrast, normal cells, which show low levels of ROS after MPA infection, show neither inhibition of Akt phosphorylation nor suppressed IFN expression in response to subsequent RV infection. Further, DPI had no effect on the expression of IFNs in normal cells coinfected with MPA and RV. Finally, normal cells treated with CFTR inhibitor generated ROS and responded to coinfection with MPA and RV in a manner similar to that of CF cells. Together, these results suggest that innate immune responses to infection are modulated not only by preinfection with bacteria but also by oxidative stress due to loss of CFTR function in CF cells.
Type I IFNs play an essential role in mounting antiviral responses (13, 43). Previously, we and others have demonstrated that normal airway epithelial cells express IFN-α and IFN-β in response to RV infection, and this requires activation of IRF3 (31, 38). Subsequently, type I IFNs bind to IFNAR1 and IFNAR2 receptors and stimulate activation of the JAK/STAT signaling pathway, leading to amplification of the type I interferon response and expression of type III interferons and other antiviral IFN-stimulated genes (ISGs), including those encoding viperin, OASL, and MX proteins (3). Previously, CF bronchial epithelial cells were demonstrated to be more susceptible to viral infection due to impairment of STAT1 activation and loss of its downstream target, NOS2 (45). In addition, primary CF cells were also shown to be blunted in production of IFN-β and subsequent ISGs in response to influenza A virus infection (42). Although we observed impaired NO production in CF cells (data not shown), we found that both primary and immortalized CF airway epithelial cells were capable of mounting IFN-β responses at the mRNA level, and we observed expression of subsequent type III IFNs and ISGs in response to RV infection and cleared virus similar to that in normal airway epithelial cells. In addition, inhibition of CFTR function in normal cells by a pharmacological inhibitor also did not suppress RV-induced type I or type III IFNs. Therefore, the observed discrepancy between the present and previous studies may be due to different types of viruses used. It may also be due to the use of passage zero cells in the previous report (42) as opposed to passage one cells; passage zero cells may still be under the influence of effects of bacteria or bacterial products, and this can potentially inhibit antiviral responses. Indeed, in the present study, we found that prior infection with MPA inhibits expression of IFN and ISGs in response to subsequent RV infection in both primary and immortalized CF airway epithelial cells.
IFN production occurs following the recognition of double-stranded RNA generated during the replication of virus by pattern recognition molecules, TLR3, RIG-I, or the MDA5 gene. In RV-infected normal airway epithelial cells, MDA5 and TLR3, but not RIG-I, play a role in recognition of dsRNA (38). Uninfected and MPA-infected CF cells did not show a decrease in the expression of TLR3 or MDA5, suggesting that the observed suppression of the IFN response is not due to the expression levels of these receptors in MPA-infected cells. Further, it was also not due to decreased binding or endocytosis of RV by MPA-infected cells, as we observed increased binding and endocytosis of RV in MPA-infected cells. These observations suggest that MPA may inhibit IFN expression by blocking events that occurs after double-stranded RNA ligation to TLR3 or MDA5 and upstream of IFN signaling that leads to amplification of IFN response and expression of ISGs.
Activation of PI-3 kinase and downstream Akt phosphorylation is required for maximal activation of IRF3 and subsequent IFN production following the recognition of double-stranded RNA by TLR3, MDA5, and RIG-I (13, 32). In the present study, we found that inhibition of PI-3 kinase abrogated RV-induced Akt phosphorylation and partially inhibited IRF3 phosphorylation and subsequent IFN responses in both CF and normal cells, suggesting that Akt phosphorylation is also required for maximal IRF3 activation induced by RV infection. MPA infection abrogated RV-induced Akt phosphorylation in CF but not in normal cells. Therefore, it is conceivable that MPA infection may suppress IFN responses to subsequent RV infection by abrogating Akt phosphorylation in CF cells but not in normal cells.
Previously, it was demonstrated that LPS treatment suppresses IFN responses to subsequent treatment with LPS or double-stranded RNA in vivo and in cultured macrophages by increasing SH2-containing inositol-5′-phosphatase (SHIP), which inhibits PI-3 kinase (6, 34). Signal regulatory protein α (SIRP-α) was also shown to inhibit the synthetic double-stranded RNA-induced IFN response by sequestering PI-3 kinase (13). However, we did not observe differences in the expression of either SHIP or SIRP-α in both CF and normal cells (S. S. Chattoraj and U. S. Sajjan, unpublished results).
Induction of oxidative stress by treatment with hydrogen peroxide or increased ROS generation was shown to inhibit Akt phosphorylation and induce autophagy in human glioma cells and in monocytic cell lines (14, 43). Since CF and normal cells showed significant differences in ROS levels after MPA infection, it is plausible that oxidative stress may contribute to inhibition of RV-induced Akt phosphorylation in MPA-infected CF cells. Consistent with this notion, we observed higher basal levels of ROS in CF cells than in normal cells; these levels further increased with MPA infection. Second, pretreatment with antioxidants inhibited the ROS generation and restored RV-induced Akt phosphorylation, IRF3 phosphorylation, and IFN expression in MPA-infected cells. In addition, normal cells treated with CFTR inhibitor showed increased ROS generation, which further increased upon MPA infection and suppressed IFN responses to coinfection with MPA/RV, similar to the case with CF cells. Together, these observations indicate that the oxidative stress in CF cells may modulate the IFN response to coinfection with bacteria and RV. However, the oxidative stress in CF cells does not appear to affect gene expression globally after coinfection with MPA and RV, because the expression of IL-8 and ICAM-1 (19, 20, 24), each of which are regulated by NF-κB, was increased in MPA/RV-coinfected cells compared to that in cells infected with RV or MPA alone.
In addition to increased ROS generation, decreased expression of antioxidants may also contribute to increased oxidative stress. Compared to normal mice, CF mice failed to show significant increase in epithelial lining fluid glutathione (10). Similarly, bronchoalveolar lavage fluid from CF patients showed diminished levels of glutathione (4). In our preliminary studies, we observed reduced expression of antioxidant gene products, such as glutathione reductase, hemoxygenase 1, and NF-E2-related factor 2 (Nrf-2), a key antioxidant transcription factor in MPA-infected CF cells, compared to levels in similarly infected normal cells (S. Ganesan and U. S. Sajjan, unpublished results). Such decreased antioxidant levels in the airways may increase oxidative stress, and this in turn may affect the innate immune responses to infection.
In summary, we have shown that bacterial infection may suppress innate immune responses to subsequent viral infection, particularly in CF cells, which show increased oxidative stress. This may lead to viral persistence and prolong the inflammatory responses, thereby increasing the length and the severity of lung disease. In fact, in patients with asthma, decreased viral clearance and prolonged increased lower respiratory symptoms correlated with impaired IFN responses (39). Therefore, these data provide an important insight into the mechanisms by which bacterial infection suppresses antiviral responses in CF patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank S. Keshavjee, University Health Network, Toronto, Canada, for providing bronchial segments from CF and healthy donors.
This work was supported by Cystic Fibrosis Foundation grant SAJJAN08G0 and National Institutes of Health grant HL 897720 (to U.S.S.).
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 8 August 2011.
REFERENCES
- 1. Abman S. H., Ogle J. W., Butler-Simon N., Rumack C. M., Accurso F. J. 1988. Role of respiratory syncytial virus in early hospitalizations for respiratory distress of young infants with cystic fibrosis. J. Pediatr. 113:826–830 [DOI] [PubMed] [Google Scholar]
- 2. Bartling T. R., Drumm M. L. 2009. Oxidative stress causes IL8 promoter hyperacetylation in cystic fibrosis airway cell models. Am. J. Respir. Cell Mol. Biol. 40:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bonjardim C. A., Ferreira P. C., Kroon E. G. 2009. Interferons: signaling, antiviral and viral evasion. Immunol. Lett. 122:1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brown R. K., Wyatt H., Price J. F., Kelly F. J. 1996. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur. Respir. J. 9:334–339 [DOI] [PubMed] [Google Scholar]
- 5. Chattoraj S. S., et al. 2011. Rhinovirus infection liberates planktonic bacteria from biofilm and increases chemokine responses in cystic fibrosis airway epithelial cells. Thorax 66:333–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chattoraj S. S., et al. 2010. Pseudomonas aeruginosa alginate promotes Burkholderia cenocepacia persistence in cystic fibrosis transmembrane conductance regulator knockout mice. Infect. Immun. 78:984–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clifton I. J., et al. 2008. Ten years of viral and non-bacterial serology in adults with cystic fibrosis. Epidemiol. Infect. 136:128–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Collinson J., et al. 1996. Effects of upper respiratory tract infections in patients with cystic fibrosis. Thorax 51:1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Contoli M., et al. 2009. Viral infections in exacerbations of asthma and chronic obstructive pulmonary disease. Minerva Med. 100:467–478 [PubMed] [Google Scholar]
- 10. Day B. J., van Heeckeren A. M., Min E., Velsor L. W. 2004. Role for cystic fibrosis transmembrane conductance regulator protein in a glutathione response to bronchopulmonary Pseudomonas infection. Infect. Immun. 72:2045–2051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Almeida M. B., et al. 2010. Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. Emerg. Infect. Dis. 16:996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dechecchi M. C., et al. 2007. MPB-07 reduces the inflammatory response to Pseudomonas aeruginosa in cystic fibrosis bronchial cells. Am. J. Respir. Cell Mol. Biol. 36:615–624 [DOI] [PubMed] [Google Scholar]
- 13. Dong L. W., et al. 2008. Signal regulatory protein alpha negatively regulates both TLR3 and cytoplasmic pathways in type I interferon induction. Mol. Immunol. 45:3025–3035 [DOI] [PubMed] [Google Scholar]
- 14. Gao N., Rahmani M., Dent P., Grant S. 2005. 2-Methoxyestradiol-induced apoptosis in human leukemia cells proceeds through a reactive oxygen species and Akt-dependent process. Oncogene 24:3797–3809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hoffmann N., et al. 2005. Novel mouse model of chronic Pseudomonas aeruginosa lung infection mimicking cystic fibrosis. Infect. Immun. 73:2504–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnston S. L., Tyrrell D. A. J. 1997. Rhinoviruses, p. 533–563 In Lennette E. H., Schmidt N. J. (ed.), Diagnostic procedures for viral, rickettsial, and chlamydial infections. American Public Health Association, Washington, DC [Google Scholar]
- 17. Kim J., Sanders S. P., Siekierski E. S., Casolaro V., Proud D. 2000. Role of NF-kappa B in cytokine production induced from human airway epithelial cells by rhinovirus infection. J. Immunol. 165:3384–3392 [DOI] [PubMed] [Google Scholar]
- 18. Koarai A., et al. 2010. Oxidative stress enhances Toll-like receptor 3 response to double-stranded RNA in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 42:651–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krunkosky T. M., Martin L. D., Fischer B. M., Voynow J. A., Adler K. B. 2003. Effects of TNFalpha on expression of ICAM-1 in human airway epithelial cells in vitro: oxidant-mediated pathways and transcription factors. Free Radic. Biol. Med. 35:1158–1167 [DOI] [PubMed] [Google Scholar]
- 20. Matsukura S., et al. 2006. Synthetic double-stranded RNA induces multiple genes related to inflammation through Toll-like receptor 3 depending on NF-kappaB and/or IRF-3 in airway epithelial cells. Clin. Exp. Allergy 36:1049–1062 [DOI] [PubMed] [Google Scholar]
- 21. Mosser A. G., et al. 2002. Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J. Infect. Dis. 185:734–743 [DOI] [PubMed] [Google Scholar]
- 22. Mosser A. G., et al. 2005. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am. J. Respir. Crit. Care Med. 171:645–651 [DOI] [PubMed] [Google Scholar]
- 23. Newcomb D. C., et al. 2005. Phosphatidylinositol 3-kinase is required for rhinovirus-induced airway epithelial cell interleukin-8 expression. J. Biol. Chem. 280:36952–36961 [DOI] [PubMed] [Google Scholar]
- 24. Newcomb D. C., et al. 2007. Cooperative effects of rhinovirus and TNF-α on airway epithelial cell chemokine expression. Am. J. Physiol. Lung Cell. Mol. Physiol. 293:L1021–L1028 [DOI] [PubMed] [Google Scholar]
- 25. Ong E. L., et al. 1989. Infective respiratory exacerbations in young adults with cystic fibrosis: role of viruses and atypical microorganisms. Thorax 44:739–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ramsey B. W., et al. 1989. The effect of respiratory viral infections on patients with cystic fibrosis. Am. J. Dis. Child. 143:662–668 [DOI] [PubMed] [Google Scholar]
- 27. Sahay B., et al. 2009. CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance. PLoS Pathog. 5:e1000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sajjan U., Keshavjee S., Forstner J. 2004. Responses of well-differentiated airway epithelial cell cultures from healthy donors and patients with cystic fibrosis to Burkholderia cenocepacia infection. Infect. Immun. 72:4188–4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sajjan U., Wang Q., Zhao Y., Gruenert D. C., Hershenson M. B. 2008. Rhinovirus disrupts the barrier function of polarized airway epithelial cells. Am. J. Respir. Crit. Care Med. 178:1271–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sajjan U. S., et al. 2006. H. influenzae potentiates airway epithelial cell responses to rhinovirus by increasing ICAM-1 and TLR3 expression. FASEB J. 20:2121–2123 [DOI] [PubMed] [Google Scholar]
- 31. Sapey E., Stockley R. A. 2006. COPD exacerbations. 2: aetiology. Thorax 61:250–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sarkar S. N., et al. 2004. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 11:1060–1067 [DOI] [PubMed] [Google Scholar]
- 33. Schneider D., et al. 2010. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 182:332–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sly L. M., et al. 2009. SHIP prevents lipopolysaccharide from triggering an antiviral response in mice. Blood 113:2945–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smyth A. R., Smyth R. L., Tong C. Y., Hart C. A., Heaf D. P. 1995. Effect of respiratory virus infections including rhinovirus on clinical status in cystic fibrosis. Arch. Dis. Child. 73:117–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsai W. C., Hershenson M. B., Zhou Y., Sajjan U. 2009. Azithromycin increases survival and reduces lung inflammation in cystic fibrosis mice. Inflamm. Res. 58:491–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van Ewijk B. E., et al. 2008. Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics 122:1171–1176 [DOI] [PubMed] [Google Scholar]
- 38. Wang Q., et al. 2009. Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. J. Immunol. 183:6989–6997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wark P. A., et al. 2005. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J. Exp. Med. 201:937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wat D., Doull I. 2003. Respiratory virus infections in cystic fibrosis. Paediatr. Respir. Rev. 4:172–177 [DOI] [PubMed] [Google Scholar]
- 41. Wat D., et al. 2008. The role of respiratory viruses in cystic fibrosis. J. Cyst. Fibros. 7:320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu W., et al. 2006. Cystic fibrosis and normal human airway epithelial cell response to influenza a viral infection. J. Interferon Cytokine Res. 26:609–627 [DOI] [PubMed] [Google Scholar]
- 43. Zhang H., et al. 2009. Oxidative stress induces parallel autophagy and mitochondria dysfunction in human glioma U251 cells. Toxicol. Sci. 110:376–388 [DOI] [PubMed] [Google Scholar]
- 44. Zheng S., et al. 2003. Impaired innate host defense causes susceptibility to respiratory virus infections in cystic fibrosis. Immunity 18:619–630 [DOI] [PubMed] [Google Scholar]
- 45. Zheng S., et al. 2004. Impaired nitric oxide synthase-2 signaling pathway in cystic fibrosis airway epithelium. Am. J. Physiol. Lung Cell. Mol. Physiol. 287:L374–L381 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.