Abstract
The creation of subunit vaccines to prevent malaria infection has been hampered by the intrinsically weak immunogenicity of the recombinant antigens. We have developed a novel strategy to increase immune responses by creating genetic fusion proteins to target specific antigen-presenting cells (APCs). The fusion complex was composed of three physically linked molecular entities: (i) a vaccine antigen, (ii) a multimeric α-helical coiled-coil core, and (iii) an APC-targeting ligand linked to the core via a flexible linker. The vaccine efficacy of the tricomponent complex was evaluated using an ookinete surface protein of Plasmodium vivax, Pvs25, and merozoite surface protein-1 of Plasmodium yoelii. Immunization of mice with the tricomponent complex induced a robust antibody response and conferred substantial levels of P. vivax transmission blockade as evaluated by a membrane feed assay, as well as protection from lethal P. yoelii infection. The observed effect was strongly dependent on the presence of all three components physically integrated as a fusion complex. This system, designated the tricomponent immunopotentiating system (TIPS), onto which any recombinant protein antigens or nonproteinaceous substances could be loaded, may be a promising strategy for devising subunit vaccines or adjuvants against various infectious diseases, including malaria.
INTRODUCTION
Adjuvants are defined as any substances that enhance immune responses to vaccine antigens; however, they can be categorized into targeting molecules or systems that facilitate the delivery of antigens to antigen-presenting cells (APCs) and substances that help elicit their activation (2, 11, 14, 25, 27). Adjuvants are essential components of recombinant protein-based subunit vaccines, because nonreplicating inert antigens are often weakly immunogenic when administered in the absence of extraneous adjuvants (25). Aluminum hydroxide (Alum) is the only adjuvant that has been used clinically over the past several decades (11), but recently a number of APC-activating adjuvants have been developed, and some of them have already been released to the international market (14, 27). However, no targeting molecules or systems are licensed for clinical use yet.
Dendritic cells (DCs) are considered the most effective APCs. They have highly efficient and specialized functions in the uptake and presentation of foreign antigens to T and B lymphocytes, allowing them to mount appropriate immune responses (5, 8). DCs are important initiators and modulators of immune responses, and hence, in the field of vaccine research, much attention has been focused on the APC function of DCs. However, B lymphocytes also take up foreign antigens via surface immunoglobulin (Ig) (B cell receptors [BCRs]) and present protein epitopes through the major histocompatibility complex (MHC) class II–T cell receptor interaction for antibody production (9, 10, 17, 23, 24, 33, 34). Thus, B lymphocytes are unique in that they are APCs as well as effector cells. An additional unique feature of B lymphocytes, which distinguishes them from DCs, is that they can recognize conformational epitopes (for example, those present on the surfaces of protein antigens) in addition to linear epitopes. They capture cognate antigens via specific BCRs, present linear epitopes within the captured antigen on the MHC class II molecules, and subsequently receive cognate help from T lymphocytes in the secondary lymphoid organs, such as local draining lymph nodes (10). This T-B lymphocyte interaction does not require DC involvement (26, 33). Therefore, it is theoretically plausible that targeting of the BCRs present on the surfaces of the B lymphocytes in the follicles of the draining lymph nodes by exploiting the Ig binding ligands would increase the chance that antigens would encounter cognate B lymphocytes in the follicles and would be captured and presented to T lymphocytes for efficient antibody production. By exploiting this immunological mechanism, it may be possible to augment immune responses to otherwise weakly immunogenic recombinant antigens (2).
It should be noted that certain anti-infectious vaccines need to rely on recombinant subunit proteins, because some infectious diseases, including malaria and other parasitic diseases, defy conventional methods of pathogen inactivation or attenuation for vaccine production; therefore, antigens derived from these pathogens need to be transformed into efficacious vaccines with the help of adjuvants (2).
In this study, we devised a new immune enhancing system for the development of malaria vaccines, and to demonstrate its efficacy, we exploited two malaria parasite antigens, the Plasmodium vivax ookinete surface protein (OSP) Pvs25 and Plasmodium yoelii merozoite surface protein-1 (MSP1), which are known to require native conformational epitopes in order to function as effective vaccines (7, 16, 31, 32). We also exploited the Z domain, a derivative of the B domain of the Ig-binding domains (IBDs) of Staphylococcus aureus protein A (SpA), as a targeting ligand for B lymphocytes (19). The Z domain was genetically conjugated to an α-helical coiled-coil multimer-forming domain (20) of tetrabrachion (TB) (29) or cartilage oligomeric matrix protein (COMP) (12) both to increase its structural stability and binding avidity and to facilitate receptor cross-linking. We demonstrated that antigens loaded onto these multimeric delivery complexes targeted B lymphocytes and robustly enhanced antiparasitic immunity when administered to mice through the subcutaneous (s.c.) or intranasal (i.n.) route. Furthermore, not only are all three of these components (i.e., the antigen, the core motif, and the ligand) essential, but they must also be integrated into the fusion complex for the efficient induction of an immune response.
MATERIALS AND METHODS
Construction of the TB- and COMP-based delivery molecule expression plasmids.
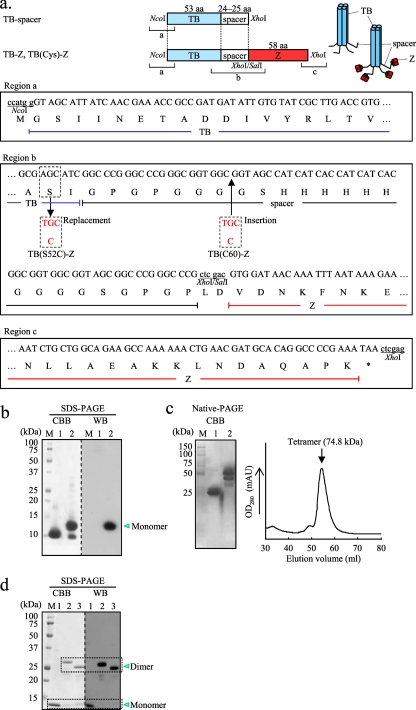
An Escherichia coli codon-optimized synthetic gene encoding the TB coiled-coil domain (Gly1 to Ile52; Protein Data Bank [PDB] accession no. 1YBK) fused to a spacer sequence (see Fig. 1a, region b) was constructed by annealing two overlapping oligonucleotides (oligonucleotides #1 [sense] and #2 [antisense], containing an NcoI and an XhoI site, respectively), followed by PCR amplification using Vent DNA polymerase (New England BioLabs, Beverly, MA). The amplified fragment was cloned into pCR2.1 (Life Technologies, Carlsbad, CA), and then, after digestion with NcoI and XhoI, the fragment generated was subcloned into the corresponding sites in pET-21d (Merck KGaA, Darmstadt, Germany) to construct the TB-spacer (TB coiled-coil domain fused to a spacer) expression plasmid (see Fig. 1a). Similarly, a synthetic gene encoding the Z domain (Val1 to Lys58; PDB accession no. 2SPZ), a derivative of the B domain of SpA, was PCR amplified by annealing two overlapping oligonucleotides (oligonucleotides #3 [sense] and #4 [antisense], containing a SalI and an XhoI site, respectively). The amplified fragment was cloned into pCR2.1, which was then digested with SalI and XhoI, and the fragment generated was subcloned into the XhoI site on the pET-21d-TB-spacer expression plasmid to construct the TB-Z expression plasmid (Fig. 1a).
Fig. 1.
Expression of the TB-based delivery molecules. (a) Schematic drawing of the tetrabrachion (TB)-derived coiled-coil domain-based constructs: TB-spacer, the TB coiled-coil domain fused to a spacer sequence; TB-Z, TB-spacer fused to the Z domain; TB(Cys)-Z, TB-Z into which a cysteine (Cys) residue has been introduced. The nucleotide and predicted amino acid sequences of the 5′-terminal, junction, and the 3′-terminal regions are shown as regions a, b, and c, respectively. All constructs were cloned between the NcoI and XhoI sites of pET-21d. TB(S52C)-Z is a TB(Cys)-Z in which a Cys was substituted for Ser52 within the coiled-coil domain to introduce a sulfhydryl group for chemical conjugation (as indicated in region b). TB(C60)-Z is another TB(Cys)-Z with a Cys insertion at amino acid position 60 within the hinge region (as indicated in region b). (b and c) SDS-PAGE (b) and native PAGE (c) (left) of the affinity-purified TB-spacer (lanes 1) and TB-Z (lanes 2) and size exclusion chromatography of the TB-Z (c) (right). The protein bands were either stained with Coomassie brilliant blue (CBB) or subjected to Western blotting (WB). (d) SDS-PAGE of the affinity-purified TB-Z (lanes 1), TB(S52C)-Z (lanes 2), and TB(C60)-Z (lanes 3). M, molecular mass marker. The protein bands were either stained with CBB or subjected to WB. For WB (panels b and d), HRP-conjugated goat IgG was applied directly to the blotted membrane for detection of the Z domain-containing proteins.
Next, to introduce a cysteine (Cys) residue into the TB-Z to construct TB(Cys)-Z, site-directed mutagenesis was performed (QuikChange II site-directed mutagenesis kit; Agilent Technologies Inc., Wilmington, DE). TB(S52C)-Z was constructed by substituting Cys for serine 52 (Ser52) within the TB coding region (see Fig. 1a, region b) by PCR using primer set #5 (sense)–#6 (antisense) with the TB-Z expression plasmid as template DNA. Similarly, TB(C60)-Z was constructed by PCR using primer set #7 (sense)–#8 (antisense) to introduce a Cys residue immediately after Gly59 (see Fig. 1a, region b).
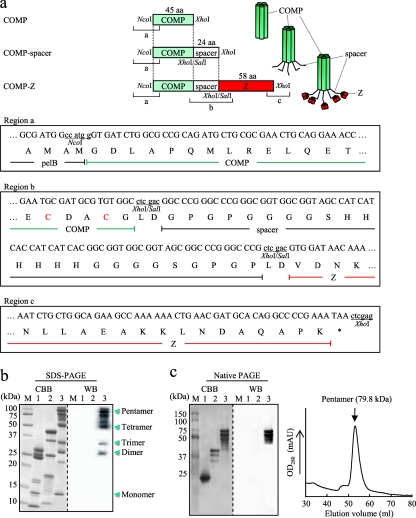
A gene encoding the COMP coiled-coil domain (Gly27 to Gly72; PDB accession no. 1VDF) was PCR amplified by using primer set #9 (sense)–#10 (antisense), containing an NcoI and an XhoI site, respectively, and plasmid DNA containing an E. coli codon-optimized synthetic gene encoding COMP(Gly26–Gly80) as the template (12). The amplified fragment was digested with NcoI and XhoI and was subcloned into the corresponding sites in pET-22b (Merck KGaA) to construct the COMP expression plasmid (see Fig. 2a). To fuse a spacer sequence to the COMP, two oligonucleotides (oligonucleotides #11 [sense] and #12 [antisense]) were annealed and inserted into a unique XhoI site in the COMP expression plasmid, constructing the COMP-spacer expression plasmid. For construction of the COMP-spacer fused to the Z domain (COMP-Z), oligonucleotides #3 and #4 were used as described above for the construction of the TB-Z expression plasmid (Fig. 2a). Table 1 lists the sequences of all the oligonucleotides and of a synthetic gene encoding the COMP(Gly26–Gly80) coiled-coil domain (12) used in this study.
Fig. 2.
Expression of the COMP-based delivery molecules. (a) Schematic drawing of the rat cartilage oligomeric matrix protein (COMP)-derived coiled-coil domain-based constructs: COMP, COMP coiled-coil domain; COMP-spacer, COMP fused to a spacer sequence; COMP-Z, COMP-spacer fused to the Z domain. All constructs were cloned between the NcoI and XhoI sites of pET-22b and were expressed as pelB fusion proteins. The nucleotide and predicted amino acid sequences of the 5′-terminal, junction, and the 3′-terminal regions are shown as regions a, b, and c, respectively. Two inherent Cys residues within the COMP coiled-coil domain (as indicated in region b) are specific sites used for chemical conjugation. (b and c) SDS-PAGE (b) and native PAGE (c) (left) of the affinity-purified COMP (lanes 1), COMP-spacer (lanes 2), and COMP-Z (lanes 3) and size exclusion chromatography of the COMP-Z (c) (right). M, molecular mass marker. The protein bands were either stained with Coomassie brilliant blue (CBB) or subjected to Western blotting (WB). For WB, HRP-conjugated goat IgG was applied directly to the blotted membrane for detection of the Z domain-containing proteins.
Table 1.
Sequences of oligonucleotides and a synthetic gene encoding the COMP(Gly26–Gly80) coiled-coil domain used in this study
| Oligonucleotide | Sequencea |
|---|---|
| #1 | 5′-CCATGGGTAGCATTATCAACGAAACCGCCGATGATATTGTGTATCGCTTGACCGTGATCATTGATGATCGCTATGAAAGCCTGAAAAATCTGATTACCTTACGTGCCGACCGCCTGGAAATGATTATTAATG-3′ |
| #2 | 5′-CTCGAGCGGGCCCGGGCCGCTACCGCCACCGCCGTGATGATGGTGATGATGGCTACCGCCACCGCCCGGGCCCGGGCCGATGCTCGCCAAGATGGTCGAAACATTGTCATTAATAATCATTTCCAGGCGGTC-3′ |
| #3 | 5′-GTCGACGTGGATAACAAATTTAATAAAGAACAGCAGAACGCCTTCTATGAAATTCTGCATCTGCCGAACCTGAACGAAGAACAGCGTAACGCCTTTATTCAGAGCCT-3′ |
| #4 | 5′-CTCGAGTTATTTCGGGGCCTGTGCATCGTTCAGTTTTTTGGCTTCTGCCAGCAGATTGGCGCTCTGGCTCGGATCATCTTTCAGGCTCTGAATAAAGGCGTTACGCT-3′ |
| #5 | 5′-TCGACCATCTTGGCGTGCATCGGCCCGGGCCCG-3′ |
| #6 | 5′-CGGGCCCGGGCCGATGCACGCCAAGATGGTCG-3′ |
| #7 | 5′-CCGGGCCCGGGCGGTTGCGGCGGTAGCCATCATCAC-3′ |
| #8 | 5′-ATGATGGCTACCGCCGCAACCGCCCGGGCCCGG-3′ |
| #9 | 5′-GCGCCATGGGTGATCTGGCGCCGCAGATG-3′ |
| #10 | 5′-GGCTCGAGGCCACACGCATCGCATTCCATAAC-3′ |
| #11 | 5′-TCGACGGCCCGGGCCCGGGCGGTGGCGGTAGCCATCATCACCATCATCACGGCGGTGGCGGTAGCGGCCCGGGCCCGC-3′ |
| #12 | 5′-TCGAGCGGGCCCGGGCCGCTACCGCCACCGCCGTGATGATGGTGATGATGGCTACCGCCACCGCCCGGGCCCGGGCCG-3′ |
| COMP(Gly26–Gly80) | 5′-GGCGGTGATCTGGCGCCGCAGATGCTGCGCGAACTGCAGGAAACCAACGCGGCCCTGCAAGATGTGCGTGAACTGCTGCGCCAGCAAGTGAAAGAAATTACCTTTCTGAAAAATACCGTTATGGAATGCGATGCGTGTGGCATGCAGCCGGCCCGTACCCCGGGC-3′ |
Underlined sequences represent restriction enzyme recognition sites.
Expression and purification analysis of the delivery molecules.
E. coli BL21(DE3) was transformed with each engineered expression plasmid and was cultured in LB broth with ampicillin; then protein expression was induced by the addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After a 16-h induction, the supernatant was collected by centrifugation (9,600 × g, 20 min), followed by filtration (FastCap filter; pore size, 0.2 mm; Nalgene Nunc International Inc., Rochester, NY). The supernatant was subjected to Ni-nitrilotriacetic acid (NTA) affinity chromatography (HisTrap FF [Fast Flow] columns prepacked with Ni Sepharose 6; GE Healthcare, Little Chalfont, United Kingdom).
Affinity-purified proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) or native PAGE. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane, blocked with 10% skim milk in phosphate-buffered saline (PBS), and washed with PBS-T (PBS with 0.05% Tween 20). Then the membrane was incubated with horseradish peroxidase (HRP)-conjugated goat IgG (1:4,000; Santa Cruz Biotechnology Inc., Santa Cruz, CA). Chemiluminescence was detected using the Western Lightning kit (Perkin-Elmer, Inc., Waltham, MA). Purified proteins were also analyzed by size exclusion chromatography (flow rate, 0.8 ml/min; HiLoad 16/60 Superdex 75 pg column; GE Healthcare).
Chemical conjugation of antigens to delivery molecules.
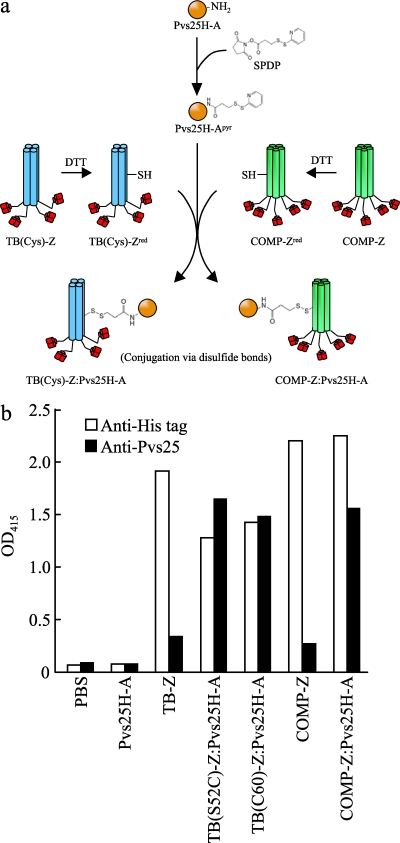
Recombinant Pvs25H-A was expressed and purified as described previously (22) and was chemically conjugated to the affinity-purified TB(Cys)-Z [TB(S52C)-Z or TB(C60)-Z] or COMP-Z using the heterobifunctional cross-linker N-succinimidyl-3-(2-pyridyldithio)propionate (SPDP; Thermo Scientific, Inc., Rockford, IL). Six milligrams of Pvs25H-A (2 mg/ml in PBS-EDTA) was incubated with SPDP (final concentration, 0.6 mM) for 1 h at room temperature (designated Pvs25H-Apyr in Fig. 3a). The reaction mixture was buffer-exchanged with PBS (Amicon Ultra-15 centrifugal filter unit; molecular weight cutoff [MWCO], 10,000; Millipore, Billerica, MA) to remove excess reagents and by-products. Concomitantly, 5 mg of the TB(Cys)-Z or COMP-Z (2 mg/ml in PBS-EDTA) was treated with dithiothreitol (DTT; 50 mM) for 30 min at 37°C and was buffer-exchanged with PBS [designated TB(Cys)-Zred or COMP-Zred in Fig. 3a]. Three milligrams of Pvs25H-Apyr and 1 mg of TB(Cys)-Zred or COMP-Zred were mixed and incubated at room temperature overnight for conjugation. The conjugated sample was buffer-exchanged with PBS as before.
Fig. 3.
Chemical conjugation of Pvs25H-A to TB(Cys)-Z [TB(S52C)-Z or TB(C60)-Z] or COMP-Z. (a) Chemical conjugation scheme for the construction of the TB(Cys)-Z:Pvs25H-A and COMP-Z:Pvs25H-A tricomponent complexes. The heterobifunctional cross-linker N-succinimidyl-3-(2-pyridyldithio)propionate (SPDP) was used to link the Pvs25H-A antigen (22) to the TB(Cys)-Z or COMP-Z delivery molecule via disulfide bonds. The delivery molecules were first treated with dithiothreitol (DTT) to expose free sulfhydryls [designated TB(Cys)-Zred or COMP-Zred], and then pyridyldithiol-activated Pvs25H-A (Pvs25H-Apyr) was reacted with the delivery molecules to generate the tricomponent complexes. (b) The complexes generated were analyzed by a human IgG-ELISA using an anti-His (open bars) or anti-Pvs25 (filled bars) antiserum.
A 19-kDa C-terminal fragment of MSP1 (MSP1-19) of the rodent malaria parasite P. yoelii was loaded onto the delivery molecules using essentially the same conjugation method as that described above for the Pvs25H-A antigen.
The endotoxin levels of all of the conjugated proteins were measured (Pyrogen Single Test Vials; Cambrex, East Rutherford, NJ) prior to administration to mice, and we found that they were less than 15 pg endotoxin/μg of protein.
Ig-ELISA.
A human IgG–enzyme-linked immunosorbent assay (ELISA) was conducted to analyze the tricomponent complexes. Briefly, 5 μg/ml of human IgG (Sigma-Aldrich, St. Louis, MO) diluted with bicarbonate buffer (15 mM Na2CO3, 35 mM NaHCO3 [pH 9.6]) (50 μl/well) was used as a capture antigen for the Z domain-containing proteins; it was applied to a 96-well microtiter plate (Sumilon; Sumitomo Bakelite Co., Ltd., Tokyo, Japan) and was incubated at 4°C overnight. The plate was blocked with PBS containing 1% bovine serum albumin (BSA) for 2 h at 37°C. Samples (2 μg of total protein/well) were applied and incubated for 2 h at 37°C, followed by incubation with 5 μg/ml of human IgG for 2 h at 37°C to mask unbound free Z domains. A mouse anti-His tag antibody (1:4,000; GE Healthcare), a mouse anti-Pvs25 antiserum (1:500), or a mouse anti-MSP1-19 antiserum (1:100) was applied and incubated for 2 h at 37°C. Anti-mouse IgG conjugated to alkaline phosphatase (AP) (1:4,000; Sigma-Aldrich), followed by p-nitrophenylphosphate (Bio-Rad Laboratories Inc., Redmond, WA), was added and incubated for 20 min at 37°C. The optical density at 415 nm (OD415) was measured using a microplate reader (Bio-Rad).
Using essentially the same human IgG-ELISA protocol as that described above, the affinity of the COMP-Z delivery molecule for various human or mouse Igs was evaluated. The Igs used as capture antigens were 5 μg/ml of human or mouse IgG (Sigma-Aldrich), 10 μg/ml of human (Sigma-Aldrich) or mouse (Bethyl Laboratories Inc., Montgomery, TX) IgM, 10 μg/ml of human or mouse IgA (Sigma-Aldrich), 10 μg/ml of human IgG1 Fab lambda or kappa (Bethyl Laboratories Inc.), or 10 μg/ml of mouse IgG Fab (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). After the plate was blocked with 1% BSA in PBS, the COMP-Z or COMP (2 μg/well each) was applied and incubated for 2 h at 37°C, followed by incubation with 5 μg/ml of human IgG for 2 h at 37°C to mask unbound free Z domains. A mouse (1:4,000; GE Healthcare) or rabbit (1:4,000; Rockland Immunochemicals, Inc., Boyertown, PA) anti-His tag antibody was applied for detection of the human or mouse Ig-bound delivery molecules, respectively. Then anti-mouse or anti-rabbit IgG conjugated to AP (1:4,000; Sigma-Aldrich), followed by its substrate, was applied for analysis.
Analysis of the target immune cells of the COMP-Z by flow cytometry.
The COMP (2 mg; 62.5 nmol) or COMP-Z (2 mg; 27.6 nmol) was treated with DTT (50 mM) for 30 min at 37°C, and the buffer was replaced with PBS (Amicon Ultra-15 centrifugal filter unit; MWCO, 10,000; Millipore). The DTT-treated COMP or COMP-Z (2 mg/ml in PBS) was incubated with maleimide-polyethylene oxide (PEO2)-biotin (final concentration, 20 mM; Thermo Scientific Inc.) for 16 h at room temperature, and the buffer was replaced with PBS as before.
Splenocytes isolated from naïve BALB/c mice were suspended in RPMI medium (Gibco Inc., Grand Island, NY) and were collected by centrifugation (377 × g, 10 min). Cells were incubated in red blood cell (RBC) lysis buffer (17 mM Tris buffer [pH 7.6], 140 mM NH4Cl) for 5 min at room temperature, washed, and resuspended in RPMI medium. Cells were counted (KOVA Glasstic slide 10; Hycor Biomedical Inc., Garden Grove, CA), and the concentration was adjusted to 5 × 106 cells/ml with cell suspension buffer (PBS containing 2% newborn calf serum and 0.03% NaN3). Fluorescein isothiocyanate (FITC)-conjugated antibodies to CD19 (clone 1D3), CD3e (clone 145-2C11), CD11b (clone M1/70), or CD11c (clone HL3), along with R-phycoerythrin (PE)-conjugated antibodies to Ly-6G and Ly-6C (Gr-1, clone RB6-8C5) (BD Biosciences, Sparks, MD), allophycocyanin-conjugated anti-mouse MHC class II (I-A/I-E) (eBioscience, San Diego, CA), and isotype control antibodies (BD Biosciences), were used for analysis. All antibodies were used at the concentrations recommended by the manufacturers.
Spleen cells (5 × 105) were first treated with a monoclonal antibody (clone 2.4G2) to block Fc receptors and were then washed, and biotinylated COMP or COMP-Z (0.31 pmol) was added to the cells and incubated. Then PE-conjugated streptavidin (BD Biosciences) was added to the cells and incubated. Cells were washed and adjusted to 1 ml with cell suspension buffer. The incubation conditions for each step were 20 min at 4°C. Data were acquired using a FACSCalibur flow cytometer and were analyzed with CellQuest software (BD Biosciences).
Immunization of mice.
Seven-week-old female BALB/c or C57BL/6 mice (Japan SLC, Shizuoka, Japan), 4 to 10 per group, were immunized at weeks 0, 2, and 4 via the s.c. or i.n. route with various immunization samples. For all mouse immunization studies, 30 μg of the vaccine antigen (i.e., Pvs25H-A or MSP1-19) was administered as a conjugated or unconjugated protein. Incomplete Freund's adjuvant (IFA; Difco Laboratories, Detroit, MI), Alum (Imject Alum adjuvant; Thermo Scientific, Inc.), or cholera toxin (CT; List Biological Laboratories, Campbell, CA) was used as an s.c. or i.n. adjuvant where indicated. The endotoxin levels of all of the immunization samples were measured (Cambrex) prior to administration to mice, and we found that they were less than 15 pg endotoxin/μg of protein.
Animal experimental protocols were approved by the University of the Ryukyus Animal Care and Use Committee, and the experiments were conducted according to the institutional ethical guidelines for animal experiments.
Determination of antigen-specific serum IgG titers by ELISA.
Mice were anesthetized 2 weeks after the third immunization (week 6) by intraperitoneal (i.p.) injection of pentobarbital sodium salt (Nacalai Tesque Inc., Kyoto, Japan) and were euthanized by exsanguination for the collection of antisera. For analysis of the long-term serum IgG response to the Pvs25H-A antigen, blood was collected at days 0, 14, 42, 91, 126, 196, and 273.
Antigen-specific serum antibodies were analyzed by ELISA as described previously (3, 4, 21, 22). Briefly, the ELISA plate was coated with the Pvs25H-A (5 μg/ml) or MSP1-19 (5 μg/ml) protein in bicarbonate buffer at 4°C overnight and was then blocked with 1% BSA in PBS for 2 h at 37°C. Twofold serial dilutions of the antisera, starting with a 50-fold dilution in PBS with 0.5% BSA, were applied and incubated for 2 h at 37°C. AP-conjugated anti-mouse IgG (1:4,000; Sigma-Aldrich), IgG1 (1:4,000; MP Biomedicals, Solon, OH), or IgG2a (1:4,000; MP Biomedicals) was applied and incubated for 2 h at 37°C. Then p-nitrophenylphosphate (Bio-Rad) was applied, and the OD415 was measured after 20 min of incubation at 37°C by using a microplate reader (Bio-Rad). The antibody titer was defined either as the serum dilution that gave an OD415 value equal to 0.1 or as the serum dilution for which a 1-magnitude-higher dilution gave an OD415 value less than 0.1.
Mosquito membrane feed assay and detection of native Pvs25 protein by antisera derived from immunized mice.
The mosquito membrane feed assay was conducted by a method described previously (3, 4, 21, 22). Single-species infection with P. vivax was confirmed by Giemsa staining of thick and thin blood smears. The levels of parasitemia and gametocytemia were 0.03% and 0.002% for donor 1, 0.23% and 0.01% for donor 2, and 0.21% and 0.01% for donor 3, respectively. Native Pvs25 protein on the surfaces of P. vivax ookinetes was detected by immunofluorescence (21, 22) using antisera obtained from mice immunized with the tricomponent complex.
All human subject research conducted in this study was reviewed and approved by the Ethics Committee of the Thai Ministry of Public Health and the Institutional Review Board of the Walter Reed Army Institute of Research.
Rodent malaria parasite infection and parasitemia determination.
C57BL/6 mouse erythrocytes parasitized by the lethal P. yoelii 17XL strain were inoculated i.p. into female C57BL/6 mice (1 × 104 parasitized RBCs/mouse). At various times after inoculation, parasitemia in peripheral blood was monitored by microscopy on Giemsa-stained thin blood smears.
Statistical analysis.
The Wilcoxon-Mann-Whitney test was performed to compare antibody titers, or the number of oocysts per mosquito, between the nonimmune control group and a particular immunization group, or between two immunization groups. The Kruskal-Wallis test was performed to compare antibody titers, or the number of oocysts per mosquito, among particular groups. The chi-square test was performed to analyze the difference in the proportion of parasite-free mosquitoes among the total number of mosquitoes examined between the nonimmune control group and a particular immunization group, or between two immunization groups. Kaplan-Meier analyses with the log rank test were performed to compare the survival rates for P. yoelii-infected mice between the PBS control group and a particular immunization group. All statistical analyses were conducted with JMP software, version 8.0 (SAS Institute Inc., Cary, NC).
RESULTS
Expression of delivery molecules.
The expression of the TB-based constructs, i.e., the TB-spacer and the TB-Z (Fig. 1a), was analyzed (Fig. 1b to d). E. coli cultures, including culture supernatants and cell extracts, were subjected to SDS-PAGE. The proteins were detected mainly in the culture supernatants, even though they lacked the leader peptide. Interestingly, the TB constructs fused to the pelB signal sequence were not expressed, for unknown reasons. The secreted proteins were conveniently purified by Ni-NTA chromatography. The affinity-purified TB-spacer and the TB-Z appeared on SDS-PAGE as protein bands with molecular masses of approximately 10 kDa and 12 kDa, respectively (Fig. 1b, CBB). Since the molecular masses predicted from the primary structures of the TB-spacer and TB-Z were 9.2 kDa and 14.9 kDa, respectively, the bands we observed presumably represented their monomers. In contrast, protein bands that were predicted tetramers of the TB-spacer and TB-Z were observed by native PAGE (Fig. 1c, left). In addition, size exclusion chromatography of the TB-Z showed a single chromatographic peak with an estimated molecular mass of 74.8 kDa (Fig. 1c, right), which appeared substantially higher than its calculated mass (i.e., 59.6 kDa for the tetramer). This discrepancy may be attributed to the long rod-like structure of the TB coiled-coil domain, because such molecules generally appear higher in molecular mass than globular proteins of the same mass. These results indicated that the TB-based constructs were secreted from recombinant E. coli predominantly as tetramers but were disassembled into monomers in the presence of SDS. The TB-Z, but not the TB-spacer, was shown to bind to the Ig molecule (Fig. 1b, WB).
Next, Cys residues were introduced into the wild-type sequence of the TB-Z for use as a specific antigen-coupling site, because no Cys residues existed within the TB and Z protein moieties. Three candidate sites were selected for Cys insertion. Two of these sites represented Ser substitutions (Ser3 and Ser52), which were chosen out of the four possible Ser residues (Ser3, Ser26, Ser47, and Ser52) because of their presumed degree of side chain exposure to the surface of the coiled-coil domain, based on the 3-dimensional (3D) crystal structure (PDB accession no. 1YBK), and one site represented de novo Cys insertion based on the presumed molecular flexibility within the spacer region. Thus, three TB-Z constructs containing Cys residues [TB(Cys)-Z] were engineered: TB(S3C)-Z, TB(S52C)-Z, and TB(C60)-Z. The TB(S3C)-Z construct failed to be expressed in any cellular compartment or in the culture supernatant, but the TB(S52C)-Z and TB(C60)-Z constructs (Fig. 1a, region b) were fully expressed in the culture supernatant, as observed for parental TB-Z, and were purified by Ni-NTA chromatography. For the affinity-purified TB(Cys)-Z [TB(S52C)-Z and TB(C60)-Z], but not for the TB-Z, dimers were observed as predominant molecular species by SDS-PAGE analysis (Fig. 1d, compare lanes 1 with lanes 2 and 3), indicating that disulfide bonds formed between the two subunit pairs within the tetramer. The TB(Cys)-Z could also bind to the Ig molecule, as seen for the TB-Z (Fig. 1d, WB).
COMP-based constructs, i.e., the COMP, COMP-spacer, and COMP-Z, were also engineered (Fig. 2a) and analyzed for their expression in E. coli. All three constructs were expressed in culture supernatants similarly to the TB-based constructs. They were purified by Ni-NTA chromatography and were subjected to SDS-PAGE and native PAGE (Fig. 2b and c). Unlike the TB-based proteins, the COMP-based proteins appeared as several bands of various molecular masses under denaturing conditions (Fig. 2b). However, by native PAGE, they appeared predominantly as pentameric forms (Fig. 2c, left). Only the COMP-Z bound to the Ig molecule (Fig. 2b and c, WB). A single chromatographic peak was observed for the COMP-Z by size exclusion chromatography (Fig. 2c, right); its estimated molecular mass was 79.8 kDa, higher than its calculated mass (i.e., 72.5 kDa for the pentamer). This difference may be attributed to the rod-like structure of the COMP coiled-coil domain, as observed for the TB-Z.
Taking these findings together, we concluded that the TB-Z and COMP-Z were secreted as tetrameric and pentameric forms, respectively, retaining their binding affinities for the Ig molecule. Their expression levels reached 30 mg/liter of bacterial culture. Multimerization of the Z domain mediated by the coiled-coil domain assembly significantly enhanced the avidity of the delivery molecules for the Ig molecule, as evidenced by the fact that the disassembled monomeric Z domain exhibited reduced affinity for the Ig molecule (Fig. 1d and 2b). This was also true when the Z domain was expressed as a single independent monomeric protein (data not shown).
Chemical conjugation of the Pvs25H-A antigen to the TB(Cys)-Z or COMP-Z delivery molecule to generate tricomponent complexes.
The TB(Cys)-Z [TB(S52C)-Z and TB(C60)-Z] or COMP-Z constructs contained one artificially introduced (Fig. 1a, region b) or two inherent (Fig. 2a, region b) Cys residues per subunit, respectively. The recombinant Pvs25H-A protein expressed in the yeast Pichia pastoris (22) was chemically conjugated to the delivery molecules via the sulfhydryl groups of the Cys residues by a heterobifunctional cross-linker, SPDP (Fig. 3a). Since disulfide bonds in the Pvs25 protein are known to be important for vaccine function (16, 31, 32), the delivery molecules, but not the Pvs25H-A antigen, were treated with a reducing agent to expose free sulfhydryls, making them reactive with the pyridyldithiol groups added to Pvs25H-A (Fig. 3a). The human IgG-ELISA indicated that all complexes, but not the delivery molecules alone, reacted strongly with an anti-Pvs25 antiserum (Fig. 3b). In contrast, all proteins, except for Pvs25H-A, which could not be captured by the human IgG, reacted to the anti-His antibody, since each of them contained a hexahistidine (6×His) tag (Fig. 3b). These results indicated that delivery molecules that retained affinity for the Ig molecule were loaded with the Pvs25H-A antigen to generate the tricomponent complexes.
Immunogenicity of the tricomponent complexes.
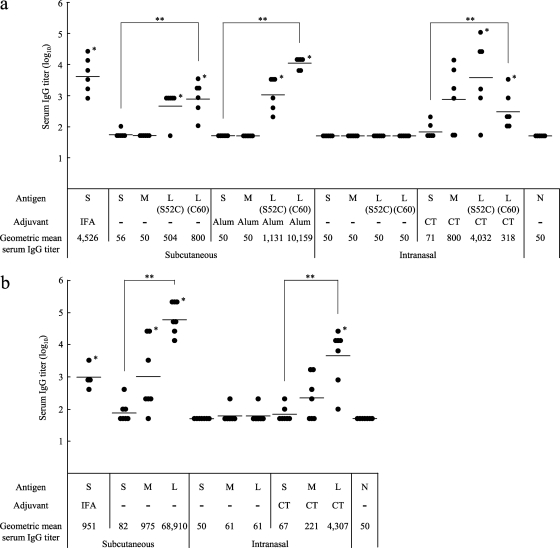
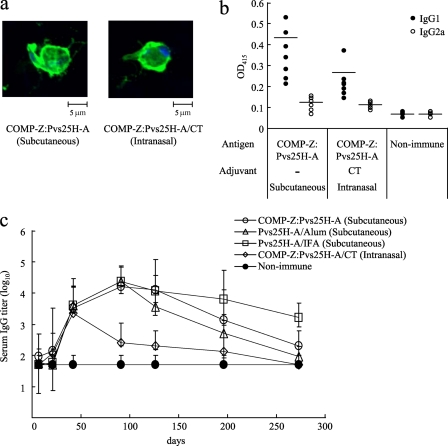
Female BALB/c mice (4 to 7 per group) were immunized with the Pvs25H-A antigen alone (designated S), a mixture of the antigen and the delivery molecules (designated M), or the antigen ligated to the delivery molecules (the tricomponent complexes, designated L), by the s.c. or i.n. route, with or without the indicated adjuvants, at weeks 0, 2, and 4, and antisera collected at week 6 were analyzed for the antigen-specific IgG (Fig. 4a and b [TB-based and COMP-based proteins, respectively]). We found that for s.c. immunization, (i) the tricomponent complexes consistently induced higher IgG responses than the antigen alone or the mixture of proteins, regardless of the adjuvant present; (ii) the mixture of the antigen with the COMP-Z, but not with the TB-Z, augmented the response; (iii) the COMP-based tricomponent complex induced higher responses than the TB-based tricomponent complexes; (iv) the COMP-based tricomponent complex without the addition of an extraneous adjuvant induced a greater response than that induced by the antigen emulsified with IFA. We also found that for i.n. immunization, the general trends were similar to those observed for s.c. immunization, but supplementation with CT was essential for induction of the response. We tested the i.p. and intravenous immunization routes and found no immune-enhancing effects (data not shown).
Fig. 4.
Immunogenicity of the tricomponent complex. Mice were immunized by the subcutaneous or intranasal route three times, at weeks 0, 2, and 4, and antisera were collected 2 weeks after the third immunization to evaluate the Pvs25-specific IgG titers. All mice received 30 μg of the Pvs25H-A antigen as a conjugated or unconjugated protein. Incomplete Freund's adjuvant (IFA), aluminum hydroxide (Alum), or cholera toxin (CT) (1 μg) was used as the subcutaneous or intranasal adjuvant, as indicated. N, nonimmune sera from unimmunized control mice. Antibody titers were defined either as the serum dilution that resulted in an OD415 of 0.1 or as the serum dilution for which a 1-point-higher dilution (2-fold) resulted in an OD415 of <0.1. (a) Female BALB/c mice (six per group) were immunized with either the Pvs25H-A antigen alone (30 μg) (S), a mixture of the antigen (30 μg) and the TB-Z (21.4 μg) (M), the TB(S52C)-Z:Pvs25H-A tricomponent complex (51.4 μg) [L(S52C)], or the TB(C60)-Z:Pvs25H-A tricomponent complex (51.4 μg) [L(C60)]. (b) Female BALB/c mice (four or seven per group) were immunized with either the Pvs25H-A antigen alone (30 μg) (S), a mixture of the antigen (30 μg) and the COMP-Z (10.8 μg) (M), or the COMP-Z:Pvs25H-A tricomponent complex (40.8 μg) (L). Asterisks indicate significant differences from the unimmunized control group by the Wilcoxon-Mann-Whitney test (*, P < 0.05) or among the three groups indicated [S, M, and L(S52C) or L(C60) for the TB-based constructs, or S, M, and L for the COMP-based constructs] by the Kruskal-Wallis test (**, P < 0.001).
Parasite recognition, IgG subclasses, and the maintenance of induced serum IgG levels by the COMP-Z-based tricomponent complex.
The antisera obtained from the immunized mice as described for Fig. 4 were analyzed for parasite recognition and IgG subclasses. The antisera specifically recognized the surfaces of immature P. vivax ookinetes as determined by immunofluorescence (Fig. 5a). The antisera predominantly contained the IgG1 subclass, indicative of a Th2 response (Fig. 5b). In a separate experiment, mice were immunized as described for Fig. 4, and the Pvs25H-A-specific serum IgG responses over a prolonged period were evaluated (Fig. 5c). The IgG titers attained by s.c. immunization with the tricomponent complex were comparable to those attained by the IFA- or Alum-assisted immunization regimen up to day 100. However, after this time point, the tricomponent complex-induced response decreased below the level of the IFA-assisted response but remained higher than the Alum-assisted response. In contrast, the period of tricomponent complex-induced serum IgG maintenance was significantly shorter via the i.n. immunization route than via the s.c. route.
Fig. 5.
Parasite recognition, IgG subclasses, and maintenance of the antisera induced by the COMP-Z-based tricomponent complex. The antisera obtained from the immunized mice in the experiments described in the legend to Fig. 4 were analyzed for parasite recognition (a) and IgG subclasses (b). (a) The ookinete-specific reactivities of the antisera induced by subcutaneous or intranasal immunization with the COMP-Z:Pvs25H-A tricomponent complex were determined by immunofluorescence analysis. The antisera specifically recognized native Pvs25 protein expressed on the surfaces of immature Plasmodium vivax ookinetes. (b) Pvs25H-A-specific IgG1 and IgG2a analysis of the antisera induced by the COMP-Z:Pvs25H-A tricomponent complex. (c) Mice were immunized as described in the legend to Fig. 4, and the Pvs25H-A-specific serum IgG responses over a prolonged period were evaluated. Antibody titers were defined as described in the legend to Fig. 4.
TBV efficacies of the tricomponent complexes.
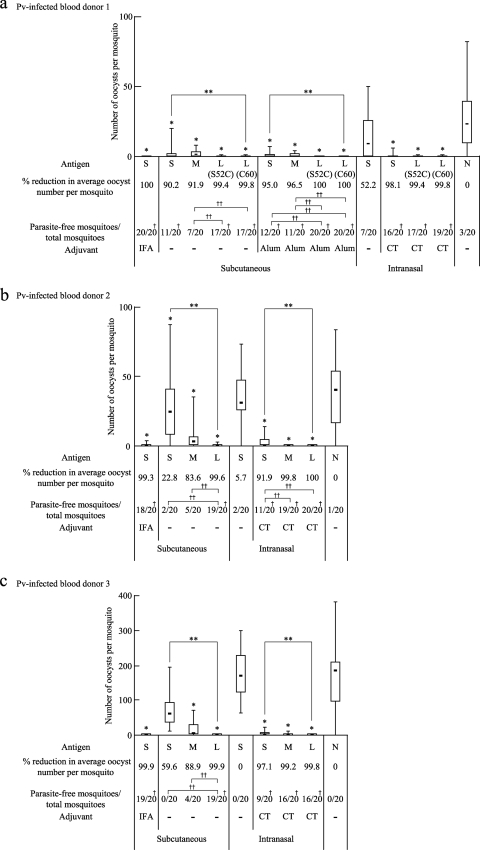
The antisera obtained from the immunized mice as described for Fig. 4 were evaluated for their transmission-blocking vaccine (TBV) efficacies against P. vivax parasites by a membrane feed assay using P. vivax-infected blood samples obtained from P. vivax patients in Thailand. The experiments were performed in triplicate, once with the TB-based (Fig. 6a) and twice with the COMP-based (Fig. 6b and c) tricomponent complex-induced mouse antisera, using blood samples from three volunteer donors. The experiments with blood samples from P. vivax-infected donors 1 and 2 demonstrated similar levels of infection, whereas the experiment with a blood sample from donor 3 showed a much greater level of infection.
Fig. 6.
TBV efficacies of the tricomponent complexes. The antisera (i.e., 1/2 dilution of the pooled antisera) obtained from the immunized mice in the experiments described in the legend to Fig. 4 were analyzed for TBV efficacy. The data are expressed as median numbers of oocysts per mosquito (bars within boxes), with 25% and 75% quartiles (the boxes) and ranges (whiskers above and below boxes). The percentage of reduction was defined as the reduction in the average number of oocysts for each group from that for the unimmunized control group. The number of parasite-free mosquitoes compared with the total number of mosquitoes (20 mosquitoes) is also provided. Experiments were performed three times using different blood samples from three donors. A Plasmodium vivax-infected blood sample from donor 1 (a) was used to evaluate the TBV efficacies of the TB-based constructs, and P. vivax-infected blood samples from donors 2 and 3 (b and c) were used for the COMP-based constructs. Asterisks and daggers indicate significant differences from the unimmunized control group by the Wilcoxon-Mann-Whitney test (*, P < 0.001), among the three groups indicated [S, M, and L(S52C) or L(C60) for the TB-based constructs, or S, M, and L for the COMP-based constructs] by the Kruskal-Wallis test (**, P < 0.001), from the unimmunized control group by the χ2 test (†, P < 0.005), and between the two groups indicated by the χ2 test (††, P < 0.005). The data for 10 immunization groups (8 S groups and 2 N groups) analyzed using blood samples from donors 2 and 3 were the same as those derived from a previously published study (22).
In all of the blood samples, the average number of oocysts per mosquito was reduced by more than 99% from that with nonimmune control serum (N) when the mouse antisera induced by s.c. immunization with the Pvs25H-A antigen alone (S) emulsified with IFA were mixed with the patient's blood samples. Omission of the adjuvant significantly abated the effect (20 to 90% reduction) (Fig. 6a to c). However, loading of the antigen onto the delivery molecules (L) resulted in a dramatic restoration of vaccine efficacy, increasing it to close to 100% (Fig. 6a to c). Loading of the antigen onto the TB-based delivery molecules resulted in a higher vaccine efficacy than that for the antigen alone mixed with Alum (Fig. 6a), and the use of Alum further increased the efficacy of the tricomponent complexes, conferring complete parasite transmission blockade [Fig. 6a, L(S52C) with Alum and L(C60) with Alum]. The two TB-based tricomponent complexes were equally effective (Fig. 6a). For the i.n. immunization, the vaccine efficacy of the antigen alone mixed with CT was high, conferring a >90% reduction, and loading of the antigen onto the delivery molecules further increased the efficacy (Fig. 6a to c). The mixture of the antigen and delivery molecules (M) enhanced vaccine efficacy over that of the antigen alone, and this was particularly notable for the COMP-Z (Fig. 6b and c). These results were consistent with those obtained for the Pvs25H-A antigen-specific serum IgG titers (Fig. 4).
In the blood sample from donor 3 (Fig. 6c), the level of P. vivax infection was much higher than that in the other two samples (Fig. 6a and b). Despite this, the COMP-Z-based tricomponent complex conferred a robust transmission blockade even without Alum, and this efficacy was as high as the efficacy achieved by administering the antigen with IFA (Fig. 6c). Taking these findings together, we concluded that the Pvs25H-A antigen, when loaded onto a TB(Cys)-Z or COMP-Z delivery molecule, was transformed into a robustly efficacious TBV.
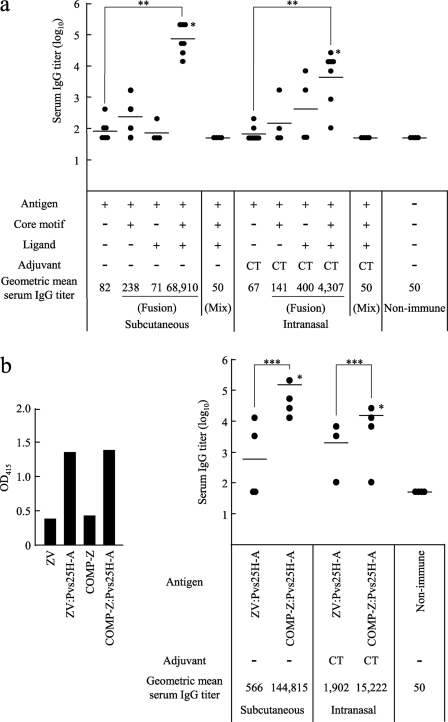
Essentiality of each component of the tricomponent complex and effects of the ligand arrangement on the immune response.
To determine if there were any dispensable components of the tricomponent complex, we compared the immunogenicities of various combinations of the three components. We found, for both the s.c. and i.n. immunization routes, that all three components were essential and were required to be concomitantly integrated into the fusion complex for efficient induction of the immune response (Fig. 7a). As expected, a dicomponent molecule (i.e., a mixture of two physically separate components, such as the antigen plus the core motif or the antigen plus the ligand) failed to induce any response (data not shown). These results indicated that not only were all three components indispensable; they also needed to be integrated into the fusion complex.
Fig. 7.
Essentiality of each component of the tricomponent complex and effects of the ligand arrangement on the immune response. Mice were immunized by the subcutaneous or intranasal route three times, at weeks 0, 2, and 4, and antisera were collected 2 weeks after the third immunization in order to evaluate the Pvs25-specific IgG titers. All mice received 30 μg of the Pvs25H-A antigen as a conjugated or unconjugated protein. Cholera toxin (CT) (1 μg) was used as the intranasal adjuvant. Antibody titers were defined as described in the legend to Fig. 4. Asterisks indicate significant differences from the unimmunized control group by the Wilcoxon-Mann-Whitney test (*, P < 0.05), among the four groups indicated by the Kruskal-Wallis test (**, P < 0.001), or between the two groups indicated by the Wilcoxon-Mann-Whitney test (***, P < 0.01). (a) Female BALB/c mice (seven or four per group) were immunized with one of the following, from left to right: the Pvs25H-A antigen alone (30 μg), the COMP-spacer:Pvs25H-A fusion complex (36.3 μg), the antigen fused directly to the monomeric Z domain (34.5 μg), the COMP-Z:Pvs25H-A tricomponent complex (40.8 μg), or a mixture of the antigen (30 μg), the COMP-spacer (6.3 μg), and the Z domain (4.5 μg). The data for four groups (the antigen alone, the antigen mixed with CT, and the tricomponent complex administered by the s.c. or i.n. route) are duplicates of the data presented in Fig. 4b. (b) Female BALB/c mice (seven or four per group) were immunized with the ZV:Pvs25H-A fusion complex (43.2 μg) or the COMP-Z:Pvs25H-A tricomponent complex (40.8 μg). The ZV:Pvs25H-A fusion complex was generated by reacting a free sulfhydryl group of the C-terminally introduced Cys residue in the ZV delivery molecule, which consists of five tandemly repeated Z domains, with the SPDP-modified Pvs25H-A antigen. Chemical conjugation between the antigen and the ZV delivery molecule was confirmed by a human IgG-ELISA using an anti-Pvs25 antiserum (left) prior to immunization experiments (right).
Next, to evaluate whether a unique molecular configuration of the tricomponent complex is important for its immunopotentiating activity, we compared the immunogenicity of the COMP-Z:Pvs25H-A tricomponent complex with that of a fusion complex in which five tandemly repeated Z domains were chemically fused to the antigen (ZV:Pvs25H-A). For the construction of the ZV:Pvs25H-A fusion complex, chemical conjugation was conducted by reacting a free sulfhydryl group of the C-terminally introduced Cys residue in the ZV delivery molecule with the SPDP-modified Pvs25H-A antigen. Chemical conjugation between the antigen and ZV was found to be as efficient as conjugation between the antigen and COMP-Z, as determined by a human IgG-ELISA (Fig. 7b, left). Although the ZV-based fusion complex induced a higher serum IgG response than the antigen alone or the antigen fused to a single or two tandemly repeated Z domains (data not shown), it was much less efficacious than the tricomponent complex (Fig. b, right). This suggested that multiple ligands in a parallel arrangement, such as that found in the tricomponent complex, represent a better molecular configuration than the same ligands with the same valence, arranged in a tandemly repeated fashion, like those found in the ZV fusion complex.
Analysis of the target cells of the tricomponent complex.
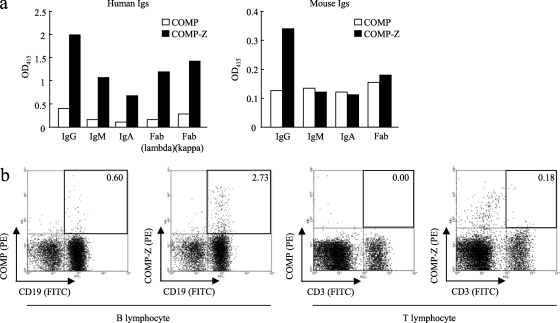
In our first attempt to elucidate the mechanism behind the immune-enhancing effect of the tricomponent complex, the profiles of the binding of COMP-Z to various human or mouse Ig isotypes were analyzed (Fig. 8a). The affinity for human IgG was the highest, but the molecule also bound to human IgM, IgA, and even Fab molecules. In addition, the COMP-Z bound to mouse IgG but not to mouse IgM, IgA, or Fab. We found that the COMP-Z exhibited higher affinity for human IgG than for mouse IgG.
Fig. 8.
Analysis of the target cells of the tricomponent complex. (a) Affinity of the COMP-Z (filled bars) for various human or mouse immunoglobulin (Ig) isotypes. The COMP coiled-coil domain devoid of the Z domain ligand (open bars) was used as a negative control. (b) Flow cytometry of the COMP-Z. Freshly isolated splenocytes were first double stained with an FITC-conjugated anti-CD19 or anti-CD3 antibody and with PE-conjugated COMP or COMP-Z and were then analyzed on a FACSCalibur flow cytometer.
We hypothesized that the most likely target of the COMP-Z in vivo is B lymphocytes, since they harbor surface Ig receptors of various isotypes. Fluorescence-activated cell sorter (FACS) analysis of a fluorescein-conjugated COMP-Z indicated that it bound to CD19+ B lymphocytes but not to CD3+ T lymphocytes in vitro (Fig. 8b). However, the COMP-Z did not bind to other immune cell types, including CD11c+ MHC class II+ DCs, CD11b+ macrophages, and CD11b+ Gr-1+ neutrophils, in this assay (data not shown). These data suggested that the immune-enhancing effect of the tricomponent complex is based partially, if not exclusively, on its B lymphocyte-targeting capability. The most likely reasons why only a small fraction of CD19+ mouse B lymphocytes bound to the COMP-Z (Fig. 8b, second panel from the left) were that the delivery molecule exhibited a lower affinity for mouse IgG than for human IgG and that the molecule did not efficiently bind to other mouse Ig isotypes.
Protective efficacy of the tricomponent complex against a lethal malaria parasite infection in mice.
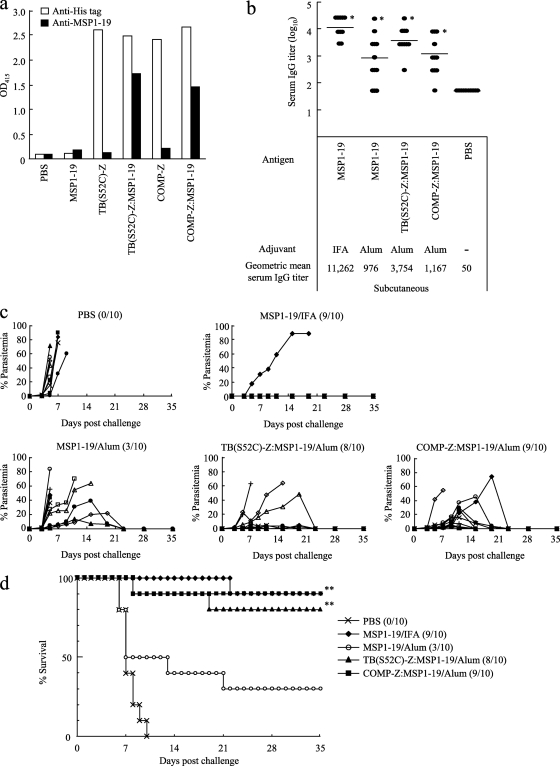
Finally, we evaluated whether the tricomponent complex is effective at inducing protective immunity against a lethal malaria parasite infection. The MSP1-19 fragment of the rodent malaria P. yoelii was expressed and purified from P. pastoris by Ni-NTA chromatography, followed by size exclusion chromatography to obtain a properly folded antigen (T. Harakuni et al., submitted for publication). Then the purified MSP1-19 antigen was loaded onto the TB(S52C)-Z or COMP-Z delivery molecule by the same chemical coupling method used for the Pvs25H-A antigen, as schematized in Fig. 3a. Successful coupling of MSP1-19 to the delivery molecules was confirmed by the human IgG-ELISA (Fig. 9a).
Fig. 9.
Protective efficacy of the tricomponent complex against a lethal malaria parasite infection in mice. (a) The TB(S52C)-Z:MSP1-19 or COMP-Z:MSP1-19 tricomponent complex was generated by the same chemical coupling method used for the Pvs25H-A antigen, as schematized in Fig. 3a. The complexes generated were analyzed by a human IgG-ELISA using an anti-His (open bars) or anti-MSP1-19 (filled bars) antiserum. (b to d) Female C57BL/6 mice (10 per group) were immunized with the MSP1-19 antigen alone (30 μg), the TB(S52C)-Z:MSP1-19 tricomponent complex (51.4 μg), or the COMP-Z:MSP1-19 tricomponent complex (40.8 μg) by the subcutaneous route, three times, at weeks 0, 2, and 4. All mice received 30 μg of the MSP1-19 antigen as a conjugated or unconjugated protein. Incomplete Freund's adjuvant (IFA) or aluminum hydroxide (Alum) was used as the adjuvant. Antibody titers were defined as described in the legend to Fig. 4. Immunized mice were challenged 2 weeks after the third immunization with a lethal number of Plasmodium yoelii 17XL-parasitized erythrocytes (1 × 104 infected red blood cells/mouse) by the intraperitoneal route. Serum IgG titers immediately before parasite challenge (b), levels of parasitemia (c), and survival rates (d) are shown. In panels c and d, the number of mice who survived among the 10 mice in each group is given in parentheses. Asterisks indicate significant differences (P < 0.001) from the PBS control group by the Wilcoxon-Mann-Whitney test (*) or the log rank test (**).
Female C57BL/6 mice (10 per group) were s.c. administered either the MSP1-19 antigen mixed with IFA or Alum or the TB(S52C)-Z:MSP1-19 or COMP-Z:MSP1-19 tricomponent complex mixed with Alum, three times, at weeks 0, 2, and 4. A strong serum IgG response was observed for MSP1-19 with IFA, followed by the TB- and COMP-based tricomponent complexes; the weakest response was observed with the MSP1-19–Alum immunization regimen (Fig. 9b). At week 6, mice were challenged i.p. with a lethal number of parasitized erythrocytes (1 × 104 P. yoelii 17XL-parasitized RBCs/mouse), and then parasitemia was monitored for 5 weeks (Fig. 9c). All mice administered PBS died within 10 days postchallenge (Fig. 9d). In contrast, mice immunized with the tricomponent complex showed an 80 to 90% survival rate, and mice immunized with MSP1-19–IFA or MSP1-19–Alum showed a 90% or 30% survival rate, respectively, indicating that loading of the antigen onto the delivery molecules significantly augmented protective efficacy against lethal parasite infection.
Taken together, the results obtained from the transmission-blocking experiments (Fig. 6) and the rodent malaria infection experiments (Fig. 9) demonstrated that the tricomponent complexes not only induce antibodies that possess strong parasite-killing activity in the mosquito midgut but also provide substantial protective immunity against parasite replication in the infected mammalian host.
DISCUSSION
Recombinant protein-based anti-infectious subunit vaccines are attractive alternatives to conventional vaccines produced by inactivation or attenuation of pathogenic organisms, because they are likely to be safer to produce and administer. Furthermore, vaccines against some pathogens, such as malaria parasites and other parasitic microbes, can be produced only by recombinant techniques, because they defy conventional methods of vaccine production. However, nonreplicating, inert recombinant antigens are often weakly immunogenic, and therefore, adjuvants (i.e., immune-enhancing materials that physiologically activate immune cells and/or delivery systems that increase the concentration of antigens near or at APCs in lymphoid organs, such as lymph nodes) are indispensable components of such vaccines (2, 25, 27). Therefore, the fact that recombinant protein antigens are often weak immunogens does not nullify their potential as good vaccines, with adjuvants playing an essential role in enhancing the immunogenicity of such weakly immunogenic antigens.
In this study, we reported a novel antigen delivery system that was able to target B lymphocytes by exploiting the α-helical coiled-coil domain-mediated multimerized IBDs as target ligands. Of the various immune cells, DCs are generally considered the most efficient APCs, but the antigen-presenting ability of B lymphocytes has recently attracted renewed interest (25, 30), because B lymphocytes are known to serve as efficient APCs for stimulating memory T cells and for priming naïve CD4+ T cells (17). Therefore, the activation of B lymphocytes constitutes an important aspect of vaccine design.
BCRs have been shown to play important roles in the activation of B cells (24). This occurs by cross-linking of the BCRs for signal transduction, followed by the uptake of antigens and the accelerated expression of costimulatory molecules, leading to an enhanced immune response (23, 24). The Z domain has the ability to bind to a wide variety of Ig isotypes, including membrane-bound Ig (19). Thus, the IBDs bind to the B lymphocyte surface, and the tricomponent complex takes advantage of this unique feature. However, the utilization of a monomeric IBD may not activate B lymphocytes, because cross-linking of the BCRs does not occur in this situation. It may be possible, however, to cross-link the BCRs by using tandemly repeated multimers of IBDs (19). Agren et al. reported that tandem repeats of the D domain of SpA (DD) could be used to target and activate B lymphocytes (1). To test whether tandemly arranged IBDs exhibit an affinity for the IgG molecule equal to that of IBDs fused to the multimeric coiled-coil domains, we constructed the ZV delivery molecule and evaluated its binding affinity for human IgG. The dissociation constant (Kd) was determined according to a method described by Friguet et al. (13), and we found that the Kd values were 8.97 × 10−10 M and 1.56 × 10−9 M for the COMP-Z and ZV, respectively, suggesting that coiled-coil domain-mediated IBDs in a parallel arrangement have much higher avidity than multiple IBDs in a tandem arrangement with the same valence. Presumably as a consequence of this, the immunogenicity of the antigen loaded onto the COMP-Z became much higher than that of the antigen loaded onto the ZV as the delivery molecule (Fig. 7b). Besides the difference in their avidities for IgG, another explanation for the observation that the COMP-Z was more efficacious at inducing antibody responses to the loaded antigen than the ZV is that the COMP-Z, which contains multiple (i.e., 10) Cys residues per pentamer, could form cross-linked high-molecular-mass complexes when chemically coupled to antigens, whereas the ZV, which contains only 1 Cys residue, could not. Furthermore, the COMP-Z also exhibited immune-enhancing activity; when it was mixed with the antigen, it augmented serum IgG responses and vaccine efficacy (Fig. 4b and 6b and c).
B lymphocytes in the draining lymph nodes near the injection sites are presumed to be the in vivo target immune cells of the tricomponent complex. A large portion of the locally administered proteinaceous complex may move quickly through the afferent lymphatics into the follicles of the lymph nodes via the subcapsular sinuses, where they first encounter repertoires of B lymphocyte clones (26). We hypothesized that in the follicles, the IBD-bearing complex binds to a larger number of B lymphocyte repertoires than do antigens lacking the IBD function, which would increase the chances of the loaded antigen encountering its cognate B lymphocytes. This may facilitate the uptake of loaded antigen by the cognate B lymphocytes and the presentation of this antigen to T lymphocytes, aiding in the transition of B lymphocytes to antibody-secreting cells after their move into the T lymphocyte area (6).
The TB and the COMP contain a multimeric coiled-coil domain (12, 20, 29) with self-assembling activity in vivo and in vitro. These domains have high thermal stability; the TB and COMP coiled-coil domains are resistant to 131°C and 100°C, respectively (15, 28). Thus, the core motifs composed of such domains may contribute to the overall molecular stability of the delivery molecules. In addition, they function as a scaffold for the vaccine antigen. This scaffold is potentially useful for the chemical coupling of various substances to the delivery molecules; these substances are not necessarily confined to proteinaceous materials but include, e.g., nonproteinaceous substances with innate immunity-inducing pathogen-associated molecular patterns (18). In this study, we selected a site-specific chemical conjugation scheme using the sulfhydryl group of the Cys residues within the core motifs to prevent masking of the ligand surface, which would likely interfere with binding to the receptors. According to our calculations, 5 mol of antigen was linked to 1 mol of the COMP-Z pentamer (data not shown). In addition, the core motifs also provided a convenient handle for affinity purification.
No significant differences in vaccine efficacy were detected between the tetravalent TB-based and pentavalent COMP-based tricomponent complexes. In addition, these two delivery molecules were produced with equal efficiency as secreted multimeric proteins from E. coli. The only distinctive difference between the two core motifs was that the former was exogenous and the latter was endogenous in mammals; thus, a relatively strong antibody response against the TB was raised in immunized mice, but almost no response against the COMP was observed (data not shown).
The genetic fusion method is generally superior to the chemical conjugation method for the construction of homogenous molecules; however, the efficiency of expression of soluble forms or of refolding, e.g., from E. coli inclusion bodies, can sometimes become problematic, depending on the type of antigen being fused to the delivery molecules. However, the production of all genetically conjugated tricomponent complexes is technically feasible, and we have already been successful in this approach with some other constructs (unpublished results). Besides the fact that both chemical and genetic fusion methods can be employed to construct the tricomponent complex, all three components can, in theory, be changed depending on the purpose. For example, any antigens, as we proved in part in this study, can be chemically loaded or genetically fused; different core motifs can be selected based on differences, for example, in their endogenous or exogenous origin or valence; other cell-targeting ligands, including other IBDs, can be employed (unpublished results); and DC-targeting motifs could also be integrated into the system in the future.
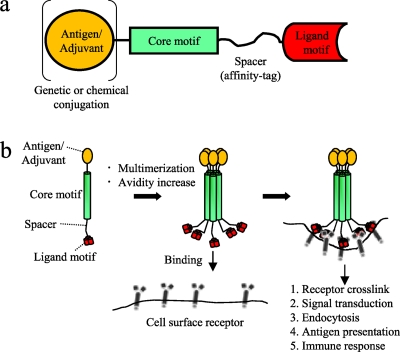
The results of the present study suggest that the tricomponent immunopotentiating system (TIPS) may become an efficacious antigen delivery system for the design of subunit vaccines against various infectious diseases (Fig. 10), where the use of weakly immunogenic recombinant proteins is desirable or unavoidable. TIPS, as a novel vaccine platform technology, therefore has the potential to be used in the development of various subunit vaccines against infectious diseases in the future.
Fig. 10.
Proposed mechanism of action of the tricomponent immunopotentiating system. (a) Design concept of the TIPS. Adjuvants or vaccine antigens, which may be proteins or other substances, are loaded onto the core motif by using genetic or chemical conjugation techniques. The core motif is connected to the ligand motif with a spacer arm, including an affinity tag. (b) Assembly of a monomeric tricomponent complex into a multimeric form, mediated by the coiled-coil core motifs, presumably increasing the avidity of the complex to facilitate the targeting of antigen-presenting cells mediated by the specific ligand motif used.
ACKNOWLEDGMENTS
We thank Charles J. Arntzen of Arizona State University for valuable comments on our manuscript.
This work was supported by the following grants: Grants-in-Aid for Scientific Research (20590425) and Scientific Research on Priority Areas (21022034) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; the Program for Promotion of Basic Research Activities for Innovative Biosciences from the Bio-oriented Technology Research Advancement Institution; the Cooperative Research Grant from the Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan; and a research grant from the Okinawa Industry Promotion Public Corp. (Naha, Okinawa, Japan).
Footnotes
Published ahead of print on 1 August 2011.
REFERENCES
- 1. Agren L. C., Ekman L., Lowenadler B., Lycke N. Y. 1997. Genetically engineered nontoxic vaccine adjuvant that combines B cell targeting with immunomodulation by cholera toxin A1 subunit. J. Immunol. 158:3936–3946 [PubMed] [Google Scholar]
- 2. Arakawa T. 2011. Adjuvants: no longer a ‘dirty little secret', but essential key players in vaccines of the future. Expert Rev. Vaccines 10:1–5 [DOI] [PubMed] [Google Scholar]
- 3. Arakawa T., et al. 2005. Nasal immunization with a malaria transmission-blocking vaccine candidate, Pfs25, induces complete protective immunity in mice against field isolates of Plasmodium falciparum. Infect. Immun. 73:7375–7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arakawa T., et al. 2003. Serum antibodies induced by intranasal immunization of mice with Plasmodium vivax Pvs25 co-administered with cholera toxin completely block parasite transmission to mosquitoes. Vaccine 21:3143–3148 [DOI] [PubMed] [Google Scholar]
- 5. Banchereau J., Steinman R. M. 1998. Dendritic cells and the control of immunity. Nature 392:245–252 [DOI] [PubMed] [Google Scholar]
- 6. Batista F. D., Harwood N. E. 2009. The who, how and where of antigen presentation to B cells. Nat. Rev. Immunol. 9:15–27 [DOI] [PubMed] [Google Scholar]
- 7. Burns J. M., Jr., Majarian W. R., Young J. F., Daly T. M., Long C. A. 1989. A protective monoclonal antibody recognizes an epitope in the carboxyl-terminal cysteine-rich domain in the precursor of the major merozoite surface antigen of the rodent malarial parasite, Plasmodium yoelii. J. Immunol. 143:2670–2676 [PubMed] [Google Scholar]
- 8. Cella M., Sallusto F., Lanzavecchia A. 1997. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 9:10–16 [DOI] [PubMed] [Google Scholar]
- 9. Cheng P. C., Dykstra M. L., Mitchell R. N., Pierce S. K. 1999. A role for lipid rafts in B cell antigen receptor signaling and antigen targeting. J. Exp. Med. 190:1549–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Clark M. R., Massenburg D., Zhang M., Siemasko K. 2003. Molecular mechanisms of B cell antigen receptor trafficking. Ann. N. Y. Acad. Sci. 987:26–37 [DOI] [PubMed] [Google Scholar]
- 11. De Gregorio E., Tritto E., Rappuoli R. 2008. Alum adjuvanticity: unraveling a century old mystery. Eur. J. Immunol. 38:2068–2071 [DOI] [PubMed] [Google Scholar]
- 12. Efimov V. P., Lustig A., Engel J. 1994. The thrombospondin-like chains of cartilage oligomeric matrix protein are assembled by a five-stranded alpha-helical bundle between residues 20 and 83. FEBS Lett. 341:54–58 [DOI] [PubMed] [Google Scholar]
- 13. Friguet B., Chaffotte A. F., Djavadi-Ohaniance L., Goldberg M. E. 1985. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 77:305–319 [DOI] [PubMed] [Google Scholar]
- 14. Garcon N., Chomez P., Van Mechelen M. 2007. GlaxoSmithKline adjuvant systems in vaccines: concepts, achievements and perspectives. Expert Rev. Vaccines 6:723–739 [DOI] [PubMed] [Google Scholar]
- 15. Guo Y., Kammerer R. A., Engel J. 2000. The unusually stable coiled-coil domain of COMP exhibits cold and heat denaturation in 4-6 M guanidinium chloride. Biophys. Chem. 85:179–186 [DOI] [PubMed] [Google Scholar]
- 16. Hisaeda H., Collins W. E., Saul A., Stowers A. W. 2001. Antibodies to Plasmodium vivax transmission-blocking vaccine candidate antigens Pvs25 and Pvs28 do not show synergism. Vaccine 20:763–770 [DOI] [PubMed] [Google Scholar]
- 17. Kakiuchi T., Chesnut R. W., Grey H. M. 1983. B cells as antigen-presenting cells: the requirement for B cell activation. J. Immunol. 131:109–114 [PubMed] [Google Scholar]
- 18. Kawai T., Akira S. 2006. TLR signaling. Cell Death Differ. 13:816–825 [DOI] [PubMed] [Google Scholar]
- 19. Ljungberg U. K., et al. 1993. The interaction between different domains of staphylococcal protein A and human polyclonal IgG, IgA, IgM and F(ab′)2: separation of affinity from specificity. Mol. Immunol. 30:1279–1285 [DOI] [PubMed] [Google Scholar]
- 20. Lupas A. N., Gruber M. 2005. The structure of alpha-helical coiled coils. Adv. Protein Chem. 70:37–78 [DOI] [PubMed] [Google Scholar]
- 21. Miyata T., et al. 2011. Adenovirus-vectored Plasmodium vivax ookinete surface protein, Pvs25, as a potential transmission-blocking vaccine. Vaccine 29:2720–2726 [DOI] [PubMed] [Google Scholar]
- 22. Miyata T., et al. 2010. Plasmodium vivax ookinete surface protein Pvs25 linked to cholera toxin B subunit induces potent transmission-blocking immunity by intranasal as well as subcutaneous immunization. Infect. Immun. 78:3773–3782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mond J. J., Seghal E., Kung J., Finkelman F. D. 1981. Increased expression of I-region-associated antigen (Ia) on B cells after cross-linking of surface immunoglobulin. J. Immunol. 127:881–888 [PubMed] [Google Scholar]
- 24. Monroe J. G., Cambier J. C. 1983. B cell activation. II. Receptor cross-linking by thymus-independent and thymus-dependent antigens induces a rapid decrease in the plasma membrane potential of antigen-binding B lymphocytes. J. Immunol. 131:2641–2644 [PubMed] [Google Scholar]
- 25. O'Hagan D. T., Valiante N. M. 2003. Recent advances in the discovery and delivery of vaccine adjuvants. Nat. Rev. Drug Discov. 2:727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pape K. A., Catron D. M., Itano A. A., Jenkins M. K. 2007. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 26:491–502 [DOI] [PubMed] [Google Scholar]
- 27. Peek L. J., Middaugh C. R., Berkland C. 2008. Nanotechnology in vaccine delivery. Adv. Drug Deliv. Rev. 60:915–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Peters J., Baumeister W., Lupas A. 1996. Hyperthermostable surface layer protein tetrabrachion from the archaebacterium Staphylothermus marinus: evidence for the presence of a right-handed coiled coil derived from the primary structure. J. Mol. Biol. 257:1031–1041 [DOI] [PubMed] [Google Scholar]
- 29. Peters J., et al. 1995. Tetrabrachion: a filamentous archaebacterial surface protein assembly of unusual structure and extreme stability. J. Mol. Biol. 245:385–401 [DOI] [PubMed] [Google Scholar]
- 30. Rodriguez-Pinto D. 2005. B cells as antigen presenting cells. Cell. Immunol. 238:67–75 [DOI] [PubMed] [Google Scholar]
- 31. Saxena A. K., et al. 2006. The essential mosquito-stage P25 and P28 proteins from Plasmodium form tile-like triangular prisms. Nat. Struct. Mol. Biol. 13:90–91 [DOI] [PubMed] [Google Scholar]
- 32. Saxena A. K., Wu Y., Garboczi D. N. 2007. Plasmodium p25 and p28 surface proteins: potential transmission-blocking vaccines. Eukaryot. Cell 6:1260–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scandella E., et al. 2007. Dendritic cell-independent B cell activation during acute virus infection: a role for early CCR7-driven B-T helper cell collaboration. J. Immunol. 178:1468–1476 [DOI] [PubMed] [Google Scholar]
- 34. Wang L. D., Clark M. R. 2003. B-cell antigen-receptor signalling in lymphocyte development. Immunology 110:411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]