Abstract
Some hypervirulent strains of Clostridium difficile produce the binary actin-ADP-ribosylating toxin C. difficile transferase (CDT) in addition to Rho-glucosylating toxins A and B. It has been suggested that the presence of CDT increases the severity of C. difficile-associated diseases, including pseudomembranous colitis. CDT contains a binding and translocation component, CDTb, that mediates the transport of the separate enzyme component CDTa into the cytosol of target cells, where CDTa modifies actin. Here we investigated the mechanism of cellular CDT uptake and found that bafilomycin A1 protects cultured epithelial cells from intoxication with CDT, implying that CDTa is translocated from acidified endosomal vesicles into the cytosol. Consistently, CDTa is translocated across the cytoplasmic membranes into the cytosol when cell-bound CDT is exposed to acidic medium. Radicicol and cyclosporine A, inhibitors of the heat shock protein Hsp90 and cyclophilins, respectively, protected cells from intoxication with CDT but not from intoxication with toxins A and B. Moreover, both inhibitors blocked the pH-dependent membrane translocation of CDTa, strongly suggesting that Hsp90 and cyclophilin are crucial for this process. In contrast, the inhibitors did not interfere with the ADP-ribosyltransferase activity, receptor binding, or endocytosis of the toxin. We obtained comparable results with the closely related iota-toxin from Clostridium perfringens. Moreover, CDTa and Ia, the enzyme component of iota-toxin, specifically bound to immobilized Hsp90 and cyclophilin A in vitro. In combination with our recently obtained data on the C2 toxin from C. botulinum, these results imply a common Hsp90/cyclophilin A-dependent translocation mechanism for the family of binary actin-ADP-ribosylating toxins.
INTRODUCTION
Clostridium difficile infection causes human diseases ranging from mild diarrhea to severe and potentially life-threatening pseudomembranous colitis. C. difficile-associated diseases occur in patients treated with broad-spectrum antibiotics. Under these conditions, the disturbed gut flora allows germination of C. difficile spores and colonization of the gut by this pathogen. C. difficile produces two exotoxins, toxin A (308 kDa) and toxin B (270 kDa), which are the causative agents of pseudomembranous colitis. The toxin-catalyzed glucosylation of Rho, Rac, and Cdc42 results in the inhibition of GTPase-mediated cell signaling, destruction of the actin cytoskeleton, and cell rounding that are the reasons for loss of integrity of the intestinal wall (for a review, see references 20 and 21).
During the last 10 years, hypervirulent C. difficile strains were identified that produce, in addition to toxins A and B, a third exotoxin, the binary C. difficile transferase (CDT). Up to 35% of the strains tested produce CDT, and it has been suggested that the presence of CDT correlates with the severity of C. difficile-associated diseases (12, 14, 25, 27, 52). CDT belongs to the family of binary actin-ADP-ribosylating toxins and consists of two nonlinked proteins, the binding/translocation component CDTb and the separate enzyme component CDTa, which harbors the ADP-ribosyltransferase activity. Like the other members of this toxin family, CDT exerts its toxic effects on mammalian cells by mono-ADP-ribosylation of G-actin at arginine 177 (1), thereby inhibiting actin polymerization (54). This results in cell rounding. Recently, it was reported that CDT interferes with the organization of microtubule structures too, resulting in the formation of long, microtubule-based protrusions around the cell body. Importantly, C. difficile can bind to these microtubule-based protrusions, resulting in increased bacterial adherence and colonization in infection models (46).
The family of binary actin-ADP-ribosylating toxins comprises the C2 toxin from C. botulinum and the iota-toxin-like toxins. The latter include iota-toxin, which is produced by C. perfringens type E strains and causes sporadic diarrheic outbreaks in farm animals (47, 50, 51); CDT from C. difficile (37); and C. spiroforme transferase (CST) (36). Iota-toxin, CDT, and CST are distinguished from C2 toxin (30) because they are related more closely to each other than to C2 toxin in terms of sequence homology, functional and immunological aspects (34), and the different modification of individual actin isoforms (31, 43).
The sophisticated mechanism by which the binding/translocation component mediates the transport of the enzyme components into the cytosol of mammalian target cells was discovered for C2 toxin and iota-toxin (3, 5). The binding/translocation component of iota-toxin, Ib (98 kDa), becomes proteolytically activated, binds to an unknown protein receptor, and then forms heptamers, which act as a docking platform for the enzyme component Ia (47 kDa) (5, 16, 49). After receptor-mediated endocytosis of the Ib/Ia complex, Ib mediates the translocation of Ia from acidified endosomal vesicles into the cytosol (29, 48). Under acidic conditions, Ib heptamers adopt a pore conformation and form pores in endosomal membranes (5), which serve as translocation channels for the enzyme component. A broadly comparable uptake mechanism was reported for C2 toxin. For both toxins, pore formation by the binding/translocation components is an essential prerequisite for the translocation of the enzyme components C2I and Ia, respectively, into the cytosol (6, 23). It was shown earlier that C2I unfolds to be translocated cross endosomal membranes (19). Most likely, the enzyme components are translocated through the pore lumen, driven by the pH gradient between the endosomal lumen and the cytosol (23). Although the two toxins show mechanisms of uptake via acidified endosomes that are comparable overall, the enzyme components are translocated from endosomal vesicles to the cytosol differently. While Ia appears to escape from endocytotic carrier vesicles, which are in a state between early and late endosomes (13), C2I is translocated from early endosomes to the cytosol (3), suggesting that Ia requires more acidic conditions to cross membranes. Moreover, the translocation of Ia seems to require a membrane potential gradient in addition to the pH gradient (13). We have reported earlier that the membrane translocation of C2, and iota-toxin, is facilitated by the chaperone heat shock protein 90 (Hsp90) (17, 18) and more recently that cyclophilin A (CypA), a peptidyl-prolyl cis/trans isomerase (PPIase) (22), is crucial for the translocation of C2 toxin. PPIases catalyze cis-trans isomerization of proline-peptide bonds, often a rate-limiting step during protein refolding (2, 9, 44, 45). It is not clear whether PPIases are also involved in the membrane translocation of iota-toxin.
In contrast to the mechanisms of cellular C2 toxin and iota-toxin uptake, that of CDT uptake is not known. Therefore, we have investigated the uptake of CDT into cultured African green monkey kidney epithelial (Vero) cells and in particular studied the membrane translocation of the toxin. We focused on the role of the host cell factors Hsp90 and cyclophilin A in the membrane translocation of CDT in comparison with that of iota-toxin. The specific pharmacological inhibition of Hsp90 by radicicol (Rad) and the inhibition of cyclophilin by cyclosporine A (CsA) protected Vero cells from intoxication by CDT and iota-toxin and inhibited the pH-dependent membrane translocation of both toxins.
MATERIALS AND METHODS
Materials.
Cell culture medium (minimum essential medium [MEM]) and fetal calf serum were purchased from Invitrogen (Karlsruhe, Germany), and cell culture materials were from TPP (Trasadingen, Switzerland). Complete protease inhibitor and streptavidin-peroxidase were from Roche (Mannheim, Germany). The protein molecular weight markers PageRuler Prestained Protein Ladder and PageRuler Stained Protein Ladder were obtained from Fermentas (St. Leon-Rot, Germany). Biotinylated NAD+ was supplied by R&D Systems GmbH (Wiesbaden-Nordenstadt, Germany). Bafilomycin A1 (BafA1) was obtained from Calbiochem (Bad Soden, Germany), CsA was obtained from Fluka (Munich, Germany), and Rad was obtained from Sigma-Aldrich (Munich, Germany). The enhanced chemiluminescence (ECL) system was purchased from Millipore (Schwalbach, Germany). Alexa 568-maleimide was from Invitrogen (Karlsruhe, Germany).
Protein expression, purification, and biotinylation.
Ia and Ib were purified as described earlier (33). Recombinant CDTa and CDTb (from C. difficile strain 196) were produced and purified as His-tagged proteins in the B. megaterium expression system as described by others for the large clostridial glycosylating toxins (32, 55). Labeling of CDTa with Alexa 568-maleimide was performed according to the manufacturer's protocol (Invitrogen, Karlsruhe, Germany). The purified proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and stained with Coomassie blue, and protein concentrations were determined via densitometry by using Photoshop 7.0 software (Adobe Systems Inc.). CypA was purified as described earlier (8), and human Hsp90 was purified as described previously (41). Biotinylation of C2I, Ia, and CDTa was performed with sulfo-NHS-biotin (Pierce, Rockford, IL) according to the manufacturer's instructions.
Cell culture and cytotoxicity assays.
African green monkey kidney (Vero) cells and human intestinal Caco-2 cells were cultivated at 37°C and 5% CO2 in MEM containing 10% heat-inactivated fetal calf serum, 1.5 g/liter sodium bicarbonate, 1 mM sodium pyruvate, 2 mM l-glutamine, 0.1 mM nonessential amino acids, and 10 mg/ml penicillin-streptomycin. Cells were trypsinized and reseeded, at most, 15 to 20 times. For cytotoxicity experiments, cells were seeded into culture dishes and incubated in serum-free medium with CDT or iota-toxin. To inhibit the PPIase activity of Cyp proteins or the activity of Hsp90, the cells were incubated for 30 min with the indicated concentrations of CsA or Rad, respectively. Subsequently, toxin was added and cells were further incubated at 37°C with toxin plus inhibitor. After the incubation periods indicated, the cells were visualized by using a Zeiss (Oberkochen, Germany) Axiovert 40CFl microscope with a Jenoptik ProgRes C10 charge-coupled device camera (Carl Zeiss GmbH, Jena, Germany). The cytopathic effects caused by the toxins were analyzed in terms of morphological changes.
Fluorescence microscopy to detect internalized CDTa.
Caco-2 cells were preincubated with 10 μM CsA-10 μM Rad for 30 min at 37°C. Subsequently, the cells were cooled to 4°C and 1 μg/ml CDTa labeled with Alexa 568 plus 2 μg/ml CDTb was added. Cells were incubated at 4°C for 30 min to allow toxin binding. Cells were transferred to 37°C for 20 min to induce endocytosis and fixed. Actin was stained by fluorescein isothiocyanate (FITC)-phalloidin. Fixed samples were analyzed with an inverted Axiovert 200M microscope (Carl Zeiss GmbH, Jena, Germany) equipped with Plan-Apochromat objectives and driven by Metamorph imaging software (Universal Imaging, Downingtown, PA). Confocal images were collected with a Yokogawa (Tokyo, Japan) CSU-X1 spinning-disc confocal head with an emission filter wheel, a Coolsnap HQ II digital camera (Roper Scientific, Tucson, AZ), and 488- and 561-nm laser lines.
Preparation of cell extracts, SDS-PAGE, and immunoblot analysis.
Following incubation with the toxin, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and lysed in 20 mM Tris-HCl (pH 7.5) containing 1 mM EDTA, 1 mM dithiothreitol (DTT), 5 mM MgCl2, and Complete protease inhibitor. Following lysis of the cells and centrifugation (20,800 × g, 7 min, 4°C), the supernatant was stored at −20°C. For immunoblot analysis, equal amounts of lysate protein were subjected to SDS-PAGE according to the method of Laemmli (24). Subsequently, the proteins were transferred to a nitrocellulose membrane (Whatman, Dassel, Germany). The membrane was blocked for 30 min with 5% nonfat dry milk in PBS containing 0.1% Tween 20 (PBS-T). For the detection of actin, the samples were probed with a mouse monoclonal anti-β-actin antibody (clone AC-15; Sigma-Aldrich, Seelze, Germany). After washing with PBS-T, the membrane was incubated for 1 h with an anti-mouse antibody coupled to horseradish peroxidase (Santa Cruz Biotechnology, Heidelberg, Germany). The membrane was washed, and the proteins were visualized using an ECL system according to the manufacturer's instructions.
Sequential ADP-ribosylation of actin in lysates from toxin-treated cells.
For ADP-ribosylation of actin in a cell-free system, 20 μg of whole-cell lysate protein was incubated for 30 min at 37°C in a buffer containing 20 mM Tris-HCl (pH 7.5), 1 mM EDTA, 1 mM DTT, 5 mM MgCl2, and Complete protease inhibitor together with biotin-labeled NAD+ (10 μM) and 300 ng of C2I protein. The reaction was stopped with 5× SDS sample buffer (625 mM Tris-HCl [pH 6.8], 20% SDS, 8.5% glycerol, 0.2% bromphenol blue, 100 mM DTT) and heating of the samples for 5 min at 95°C. The samples were subjected to SDS-PAGE and transferred to a nitrocellulose membrane, and biotin-labeled, ADP-ribosylated actin was detected with peroxidase-coupled streptavidin and a subsequent chemiluminescence reaction.
ADP-ribosylation of actin by CDTa in a cell-free system.
Vero cell lysate (50 μg of protein) was incubated for 2, 5, or 15 min at 37°C together with 50 ng/ml CDTa, 10 μM biotin-labeled NAD+, and 10 μM CsA. Samples were subjected to SDS-PAGE and blotted onto a nitrocellulose membrane, and ADP-ribosylated actin was detected with streptavidin-peroxidase. The intensity of biotin-labeled actin was determined by densitometry using the Adobe Photoshop 7.0 software.
Toxin translocation assay with intact cells.
The pH-dependent translocation of CDTa and Ia through their corresponding pores across endosomal membranes was experimentally mimicked on the intact cell surface as described for iota-toxin earlier (5). In brief, Vero cells were exposed to an acidic pulse (pH 4.0) after the binding of either CDTb/CDTa or Ib/Ia to the cell surface. Under acidic conditions, CDTa and Ia are translocated across the cytoplasmic membrane into the cytosol. Cell rounding was monitored and documented by photography.
Dot blot analysis of the interaction of immobilized CypA and Hsp90 with CDTa, Ia, and C2I.
Different amounts of CypA and Hsp90 were vacuum aspirated onto a nitrocellulose membrane using a dot blot system (Bio-Rad, Munich, Germany) according to the manufacturer's instructions. Subsequently, the membrane was blocked for 1 h with PBS-T containing 5% nonfat dry milk and incubated with biotin-labeled C2I, Ia, or CDTa (200 ng/ml) for 1 h. The membrane was washed three times with PBS-T, and the bound biotinylated proteins were detected with streptavidin-peroxidase using the ECL system.
Reproducibility of the experiments and statistics.
All experiments were performed independently at least 2 times. Results from representative experiments are shown in the figures. In each individual immunoblot analysis panel shown in the figures, the protein bands were originally detected on the same membrane and only cut out and recombined for presentation in the figures. Values (n = ≥3) are calculated as means ± standard deviations (SD) using the Prism4 software (GraphPad Software, Inc.).
RESULTS
CDT is translocated from acidified endosomal vesicles into the host cell cytosol.
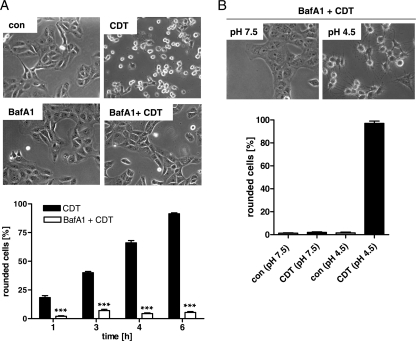
The binary actin-ADP-ribosylating C2 toxin and iota-toxin deliver their A components from endosomal vesicles into the cytosol. Importantly, acidification of the endosomal lumen is an essential prerequisite for this process. Therefore, we used BafA1, which inhibits the vesicular ATPase and thereby prevents acidification of the endosomes, to test whether CDT is translocated from acidified endosomes to the cytosol. Pretreatment of Vero cells with BafA1 very efficiently protected Vero cells from intoxication with CDT. While there was a time-dependent increase in the amount of round cells after the application of CDT, toxin-induced cell rounding was inhibited by the presence of 100 nM BafA1 (Fig. 1A). This concentration of BafA1 exhibited its protective effect even when the cells were incubated with CDT for 24 h in the presence of BafA1 but had no effect on the morphology of the cells (not shown). Moreover, a 0.1% final concentration of dimethyl sulfoxide (used as a solvent control) had no inhibitory effect on CDT-induced cell rounding (not shown). This result suggests that the A component CDTa is translocated from the acidified endosomal vesicle to the cytosol.
Fig. 1.
Effect of BafA1 on the intoxication of Vero cells with CDT. (A) Vero cells were preincubated with 100 nM BafA1 or left untreated (control [con]). After 30 min, CDT (40 ng/ml CDTa plus 80 ng/ml CDTb) was added and pictures were taken after 6 h. A quantitative analysis of rounded Vero cells at the indicated time points of toxin treatment is shown. Values are means ± SD (n = 3). The significance of differences from CDT-treated cells was tested by using the Student t test (***, P < 0.0001). (B) An acidic pH triggers the membrane translocation of CDTa. Vero cells were preincubated with 100 nM BafA1 for 30 min at 37°C and then at 4°C with CDT (100 ng/ml CDTa plus 200 ng/ml CDTb; control, without toxin). Subsequently, the medium was adjusted to pH 4.5 with HCl and the cells were incubated at 37°C in this acidic medium (control, in neutral medium). Pictures were taken after 1.5 h. The percentages of round cells were determined from these pictures. Values are means ± SD (n = 3).
We performed an alternative assay to verify that the membrane translocation of CDTa essentially requires acidic conditions. Therefore, we experimentally mimicked the acidic conditions of the endosomal lumen on the intact cell surface. Vero cells were pretreated with BafA1 to block the “normal” uptake of CDTa into the cytosol, and then the cells were incubated at 4°C with CDTb plus CDTa to enable toxin binding to the cell surface receptors. Subsequently, the pH of the culture medium was adjusted to 4.5 (control, pH 7.5) and the cells were incubated at 37°C to trigger the membrane translocation of CDTa. Cell rounding was monitored to verify that CDTa was delivered into the cytosol and modified actin. Cell rounding was observed only when CDT-treated cells were exposed to low pH (Fig. 1B), indicating that a pH gradient is essential for the translocation of cell-bound CDTa across the cytoplasmic membrane into the cytosol.
Taken together, the results imply that CDTa is translocated from acidified endosomal vesicles to the host cell cytosol, which is in agreement with other members of this toxin family (3, 5).
Pharmacological inhibition of host cell cyclophilin and Hsp90 protects Vero cells from intoxication with CDT.
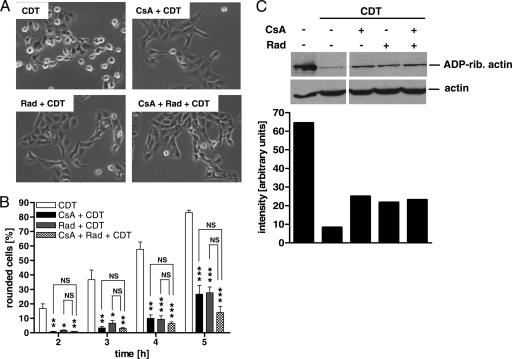
To investigate whether the cellular uptake of CDT depends on the host cell cyclophilin and/or Hsp90, we first tested whether pharmacological inhibition of these factors by CsA and Rad, respectively, has any effect on the intoxication of cells with CDT. Pretreatment of Vero cells with each inhibitor alone and with the combination of CsA and Rad protected Vero cells from CDT-induced cell rounding within 4 h after toxin application (Fig. 2A). The inhibitors alone had no effects on cell morphology under these conditions (not shown). A quantitative analysis revealed that CsA and Rad caused a significant and time-dependent delay in intoxication rather than complete inhibition (Fig. 2B). The combination of CsA and Rad had a slightly stronger protective effect than the individual inhibitors; however, this effect was not statistically significant (Fig. 2B). Because higher concentrations of CsA had some morphological effects on Vero cells, a concentration of 10 μM was used in this study.
Fig. 2.
CsA and Rad inhibit the intoxication of Vero cells by CDT. (A) Vero cells were preincubated with 10 μM CsA, 10 μM Rad, or a combination of the two for 30 min at 37°C. Subsequently, toxin (40 ng/ml CDTa plus 80 ng/ml CDTb) was added. The pictures shown were taken after 4 h. (B) Time course of Vero cell intoxication with CDT in the presence of CsA and Rad. Vero cells were preincubated with 10 μM CsA, 10 μM Rad, or a combination of the two inhibitors. Afterwards, CDT (40 ng/ml CDTa plus 80 ng/ml CDTb) was applied. At the indicated time points, the percentage of rounded cells was determined. Values are means ± SD (n = 3). For each time point, the significance of differences from cells treated with CDT alone (if not indicated otherwise by lines) was tested by using the Student t test (*, P < 0.05; **, P < 0.001; ***, P < 0.001; NS, not significant). (C) Effects of CsA and Rad on the ADP-ribosylation status of actin in CDT-treated Vero cells. After 1.5 h of incubation with CDT (40 ng/ml CDTa plus 80 ng/ml CDTb), cells were lysed and the ADP-ribosylation status of actin from these cells was analyzed by incubation with C2I and biotin-labeled NAD+. Biotin-labeled (i.e., ADP-ribosylated) actin is shown at the top (ADP-rib actin, ADP-ribosylated actin). Equal protein loading was confirmed with an anti-β-actin antibody (bottom). The intensities of the ADP-ribosylated (rib.) actin bands were measured by using Photoshop software and are shown at the bottom. The bars correspond to the bands of ADP-ribosylated actin at the top.
The morphology-based analysis of the protective effect was confirmed by analyzing the ADP-ribosylation status of actin from cells. Cells were treated with CDT in the absence or presence of the inhibitors. After 1.5 h, cells were lysed and lysates were incubated with fresh C2I and biotin-NAD+ as a cosubstrate to enable ADP-ribosylation of actin in vitro and thereby its biotin labeling. Biotin-labeled, i.e., ADP-ribosylated, actin was detected by Western blot analysis with streptavidin-peroxidase (Fig. 2C, top), and the intensity of the bands was quantified (Fig. 2C; the bars correspond to the bands of ADP-ribosylated actin at the top). In this assay, actin from control cells gives a strong signal because it was not ADP-ribosylated in the intact cells before lysis. In contrast, actin from CDT-treated cells gives a weaker signal because most of the actin was already modified in the intact cells by the toxin and is therefore no longer a substrate in vitro. Most importantly, cells incubated with CDT in the presence of CsA, Rad, or a combination of the two inhibitors gave a stronger biotin-labeled actin signal than did cells treated with CDT alone. This result indicates that less actin was modified by the toxin in intact cells when CsA or Rad was present. An anti-β-actin immunoblot analysis of the identical lysates confirmed comparable protein loading (Fig. 2C).
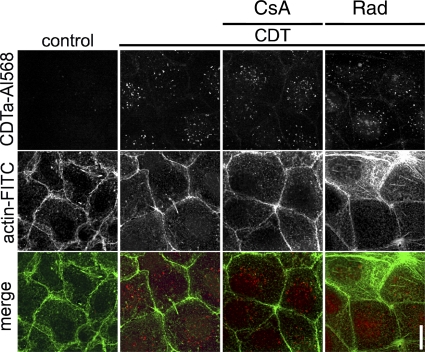
Taken together, the results clearly indicate that there was less CDTa activity in the cytosol of cells in the presence of CsA or Rad, strongly suggesting that cyclophilin and Hsp90 are crucial for the intoxication of cells with CDT. However, from this result, it is not clear whether the inhibitors interfere with the enzyme activity of CDTa and/or the uptake of CDTa into the host cell cytosol. Therefore, we first excluded the possibility that CsA and Rad inhibit the CDTa-catalyzed ADP-ribosylation of actin in vitro (data not shown). This finding implies that the inhibitors interfere with the uptake of CDTa into the cytosol, and therefore we investigated which step during toxin uptake into the cytosol is affected. First, we investigated whether the inhibitors interfere with binding of CDTa/CDTb to the cell surface and subsequent internalization of the toxin into endosomal vesicles. Caco-2 cells pretreated with either CsA or Rad were incubated for 30 min at 4°C with CDTb and Alexa 568-labeled CDTa to allow binding and for 20 min at 37°C for internalization of the toxin complex. The internalized CDTa-Alexa 568 protein was visualized by fluorescence microscopy. As shown in Fig. 3, there was a comparable amount of CDTa detectable in the cells, independently of whether the cells had been treated with inhibitors. Thus, neither CsA nor Rad inhibited toxin binding to the receptor or internalization by receptor-mediated endocytosis. CsA and Rad did not inhibit the uptake of C. difficile toxins A and B into Vero cells under comparable experimental conditions (data not shown). These toxins are internalized via receptor-mediated endocytosis and translocated from acidified endosomal vesicles into the cytosol, where they modify Rho proteins, leading to cell rounding. In our experiments, the combination of CsA and Rad did not cause a significant delay in toxin A-induced rounding of Vero cells (data not shown), indicating endocytosis in the presence of these inhibitors.
Fig. 3.
Influence of CsA and Rad on CDT binding to and endocytosis in Caco-2 cells. Caco-2 cells were preincubated with 10 μM CsA and 10 μM Rad for 30 min at 37°C. Subsequently, the cells were cooled to 4°C and toxin was added (1 μg/ml CDTa labeled with Alexa 568 plus 2 μg/ml CDTb). The control consisted of cells in medium alone. Cells were incubated at 4°C for 30 min to allow toxin binding. Cells were transferred to 37°C for 20 min to induce endocytosis and fixed. Actin was stained with FITC-phalloidin. Pictures were acquired with a confocal microscope.
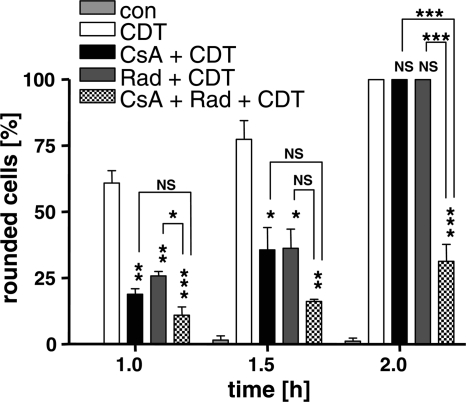
CsA and Rad inhibit the pH-dependent membrane translocation of CDTa.
Having excluded the possibility that CsA and Rad interfere with the early steps of toxin uptake, we focused on the membrane translocation of CDTa. To test for an effect of the inhibitors on this process, we performed a well-established assay that mimics endosomal conditions on the intact cell surface. In brief, Vero cells were pretreated with BafA1 to block the “normal” uptake of CDT. The cells were incubated at 4°C with CDTb plus CDTa and then exposed to warm acidified medium (37°C, pH 4.5) as described before to trigger the translocation of cell-bound CDTa across the cytoplasmic membrane into the cytosol. During this step, CsA, Rad, or a combination of both inhibitors was present in the culture medium. Successful translocation of CDTa into the cytosol was determined by the amount of round cells (Fig. 4). In the presence of CsA or Rad, there was a significant decrease in the amount of round cells after 1, 1.5, and 2 h, indicating that both CsA and Rad inhibit the membrane translocation of CDTa. The combination of CsA and Rad, however, exhibited a synergistic inhibitory effect which caused a delay in the intoxication of cells longer than that caused by the individual substances. In conclusion, these results imply that cyclophilin and Hsp90 are crucial for the pH-dependent membrane translocation of CDTa and suggest that the two factors might act in a synergistic manner during this process.
Fig. 4.
CsA and Rad inhibit the pH-dependent membrane translocation of CDTa. Vero cells were incubated with 100 nM BafA1 in combination with 10 μM CsA, 10 μM Rad, or a combination of the two inhibitors at 37°C. Subsequently, CDT was added (80 ng/ml CDTa plus 160 ng/ml CDTb; control, without toxin) and the cells were incubated on ice for 30 min. The medium was adjusted to pH 4.5, and the cells were incubated at 37°C for 2 h. After 1, 1.5, and 2 h, pictures were taken and the percentages of round cells were determined from these pictures. Values are means ± SD (n = 3). The significance of differences from CDT-treated cells was tested (if not indicated otherwise by lines) by using the Student t test (***, P < 0.0005; **, P < 0.005; *, P < 0.05; NS, not significant). con, control.
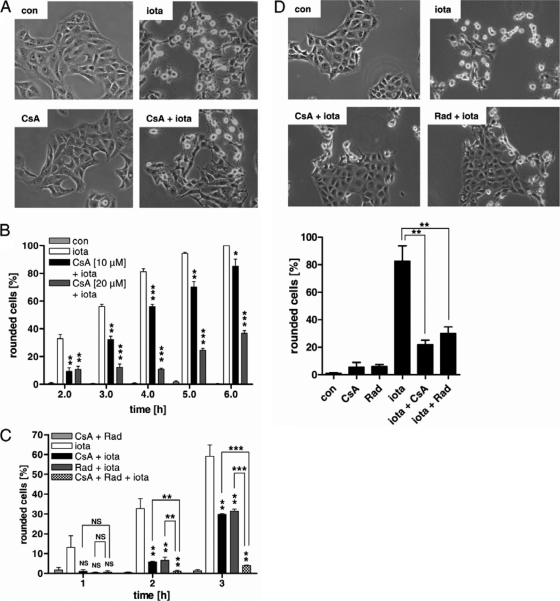
CsA and Rad inhibit the membrane translocation of the C. perfringens iota-toxin and thereby protect cells from intoxication.
We have observed earlier that pharmacological inhibition of Hsp90 protected Vero cells from intoxication with iota-toxin; however, the underlying molecular mechanism was not investigated. Prompted by the results obtained with CDT, we finally tested whether Hsp90 is crucial for the membrane translocation of enzyme component Ia and whether cyclophilin is also involved in this process. To determine whether cyclophilins are involved in the uptake of iota-toxin, Vero cells were incubated with iota-toxin in the presence or absence of CsA. Toxin-induced cell rounding was analyzed after 4 h (Fig. 5A). Most of the toxin-treated cells were round, while the presence of CsA prevented cell rounding. The observed iota-toxin-induced cell rounding correlated with the ADP-ribosylation status of actin in these cells (not shown). CsA inhibited this iota-toxin-induced cell rounding in a time- and concentration-dependent manner (Fig. 5B), and as observed before for CDT, the combination of CsA and Rad showed a synergistic protective effect compared to that of the individual inhibitors (Fig. 5C). Most importantly, CsA and Rad inhibited the pH-dependent translocation of cell-bound iota-toxin across the cytoplasmic membrane into the cytosol. This becomes evident in a significantly decreased amount of round cells in the presence of the inhibitors (Fig. 5D). In conclusion, the data imply that Hsp90 and cyclophilin are crucial for the membrane translocation of iota-toxin, which is consistent with the results obtained for the closely related binary toxin CDT. Moreover, in this aspect, the iota-toxin-like toxins behave comparably to the binary actin-ADP-ribosylating C2 toxin from C. botulinum.
Fig. 5.
CsA and Rad protect Vero cells from intoxication with the actin-ADP-ribosylating iota-toxin from C. perfringens. (A) Vero cells were preincubated with 10 μM CsA or left untreated (control [con]). After 30 min, iota-toxin (20 ng/ml Ia plus 40 ng/ml Ib) was added and pictures were taken after 4 h. (B) A quantitative analysis of rounded Vero cells after 4 h of toxin treatment is shown. Values are means ± SD (n = 3). The significance of differences from iota-toxin-treated cells was tested by using the Student t test (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). (C) Vero cells were preincubated with 10 μM CsA, 10 μM Rad, or a combination of the two or left untreated. After 30 min, iota-toxin (20 ng/ml Ia plus 40 ng/ml Ib) was added and pictures were taken at the indicated time points. The percentages of round cells were determined from the pictures. Values are means ± SD (n = 3). The significance of differences from cells treated with iota-toxin alone (if not indicated otherwise by lines) was tested by using the Student t test (***, P < 0.0001; **, P < 0.005, NS, not significant). (D) CsA and Rad inhibit the pH-dependent membrane translocation of Ia. Vero cells were incubated with 100 nM BafA1 in combination with either 10 μM CsA or 10 μM Rad at 37°C. Subsequently, cells were exposed to acidic medium (pH 4.0, 37°C) containing iota-toxin (50 ng/ml Ia plus 100 ng/ml Ib; control, without toxin) and incubated for 15 min at 37°C, still in the presence of BafA1. The medium was removed, neutral medium was added, and after a further 30 min at 37°C, pictures of the cells were taken. The percentages of round cells were determined from the pictures. Values are means ± SD (n = 3). The significance of differences from iota-toxin-treated cells was tested by using the Student t test (**, P < 0.005).
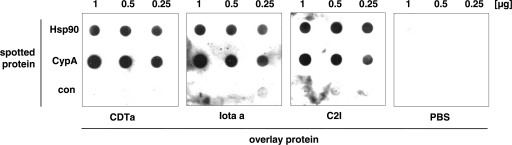
The enzyme components of CDT and iota-toxin directly interact with Hsp90 and CypA in vitro.
From these results, we were not able to conclude which particular cyclophilin is involved in the uptake of CDT and iota-toxin. We hypothesized, however, that cyclophilin A might interact with the enzyme components of both toxins. This hypothesis is plausible because cyclophilin A is the prominent cyclophilin in the cytosol of mammalian cells and the major molecular target of CsA. Moreover, we have reported earlier that it interacts with C2I, the enzyme component of the C2 toxin. Therefore, we finally investigated whether the purified cyclophilin A and Hsp90 proteins interact with CDTa and Ia by dot blot analysis in vitro and included C2I as a positive control (Fig. 6). Starting with 1 μg of protein, decreasing amounts of the Hsp90 and CypA proteins were spotted onto a nitrocellulose membrane and the membranes (for a control, PBS was used) were incubated in an overlay assay with biotin-labeled CDTa, Ia, or C2I protein in solution (200 ng/ml [final concentration]). After extensive washing, the membrane-bound enzyme components of the toxins were detected. Most importantly, CDTa, Ia, and C2I bound to Hsp90 and CypA and this binding was specific because there was no toxin bound to the membrane in the absence of Hsp90 or CypA. Moreover, there was no signal when the immobilized Hsp90 and CypA proteins were mock incubated with PBS instead of toxin or incubated with the nonbinding lethal factor (LF) from Bacillus anthracis, as demonstrated recently (7).
Fig. 6.
CDTa, Ia, and C2I directly interact with Hsp90 and CypA in vitro. The proteins Hsp90 and CypA (1, 0.5, or 0.25 μg of each) were vacuum aspirated onto a nitrocellulose membrane using a dot blot system. As a control (con), PBS was aspirated instead of protein. The membrane was blocked and subsequently incubated with the biotinylated proteins CDTa, Ia, and C2I at 200 ng/ml. As a control, PBS was used as an overlay. After washing, the membrane was incubated with streptavidin-peroxidase to detect the bound biotinylated proteins by the ECL reaction.
In conclusion, this result indicates that the enzyme components of the binary CDT and iota-toxin directly interact with Hsp90 and CypA in vitro. This is in line with our results recently obtained with the C2 toxin, implying that this interaction with Hsp90 and CypA might be a feature common to the members of the binary actin-ADP-ribosylating toxin family.
DISCUSSION
In the present study, we performed a series of experiments to analyze the cellular uptake of the binary actin-ADP-ribosylating toxin CDT from C. difficile, in particular, the membrane translocation of its enzyme component CDTa. We demonstrate that CDTa is translocated from acidified endosomal vesicles into the cytosol and that this translocation depends on a pH gradient across the membrane. This is in agreement with earlier findings on the translocation of the binary toxins iota-toxin and C2 toxin (3, 5). Recently, we reported that the membrane translocation of some binary toxins but not of others is facilitated by host cell chaperones and PPIases (7, 17, 18, 22). Therefore, we tested here whether CDT and the closely related iota-toxin require such factors for translocation. Indeed, the membrane translocation of the enzyme components CDTa and Ia was blocked by the pharmacological inhibitors Rad and CsA, implying that Hsp90 and cyclophilin facilitate this step. Consequently, both inhibitors protected cultured cells from intoxication by CDT and iota-toxin and the relative effects of CsA and Rad on CDT and iota-toxin actions were comparable overall. The inhibitory effect on the intoxication of Vero cells with iota-toxin was significantly stronger when CsA and Rad were combined than when CsA or Rad alone was used, while this synergistic inhibitory effect was less pronounced and not statistically significant for the intoxication of Vero cells with CDT. Importantly, we ruled out that the inhibitors did influence the enzyme activities of CDTa and Ia or other steps in toxin internalization, such as binding of the toxin complex to the cell surface or endocytosis. Thus, we conclude that the inhibitors exclusively interfere with toxin translocation and thereby inhibit the uptake of CDTa and Ia into the cytosol. To investigate the membrane translocation of CDTa and Ia, we mimicked the endosomal conditions on the surface of intact Vero cells. Only when cells were exposed to an acidic pulse was membrane translocation of the cell-bound toxin triggered. CsA and Rad, however, blocked the pH-driven translocation of CDTa and Ia. This assay was originally established to investigate the pH-dependent membrane translocation of diphtheria toxin (42) and successfully used for a variety of toxins that are translocated from acidified endosomes into the cytosol (10, 15, 28), including the binary actin-ADP-ribosylating C2 toxin and iota-toxin (3, 5, 6). Interestingly, the inhibitory effect of CsA and Rad on CDT-induced cell rounding appeared less efficient when CDTa was introduced into the cytosol by an acidic shift in comparison to the “normal” uptake of the toxin via receptor-mediated endocytosis and subsequent translocation from acidified endosomes. One possible explanation for this observation might be the synchronous translocation of a comparatively large amount of CDTa across the cytoplasmic membrane under these artificial conditions while less CDTa might be translocated into the cytosol when the toxin is taken up via acidified endosomes. In agreement with this hypothesis, cell rounding was faster when CDT was introduced into cells by an acidic pulse than during “normal” uptake. On the other hand, there might be differences regarding the recruitment of chaperones/PPIases which are crucial for the translocation of CDT to the endosomal membrane compared to the cytoplasmic membrane. From our results obtained by this method, we conclude that Hsp90 and cyclophilins facilitate the translocation of CDTa and Ia across the membranes of acidified endosomes during the uptake of the toxin into mammalian cells. Moreover, this is most likely the explanation for an earlier finding that Rad prevents the intoxication of cells with iota-toxin although it did not inhibit the ADP-ribosyltransferase activity of Ia (17).
The results corroborate our recent finding that cyclophilins and Hsp90 facilitate the membrane translocation of C2I, the enzyme component of the binary actin-ADP-ribosylating C2 toxin (22). Immunoprecipitation experiments revealed that CypA, the most abundant cyclophilin in the cytosol of mammalian cells and the major target of CsA, interacts with C2I (22). In the present study, we found that CDTa and Ia bound to the immobilized Hsp90 and CypA proteins in vitro, a hint that CypA might be the relevant cyclophilin that interacts with CDT and iota-toxin during cellular uptake too. As observed for CDT and iota-toxin in the present study, the inhibitor Rad or CsA, respectively, prevented the membrane translocation of C2I. Thus, in the presence of Rad or CsA, less C2I, if any, reached the cytosol and therefore cells were protected from intoxication by C2 toxin (22). The finding that the membrane translocation of CDTa, Ia, and C2I is facilitated by Hsp90 and cyclophilins is interesting, because differences between C2 toxin and iota-toxin during the uptake of their enzyme components into the target cell cytosol have been reported. First, C2I is translocated from early endosomes into the cytosol while Ia is released at a later stage in vesicle transport between early and late endosomes, implying that the translocation of Ia is triggered by more acidic conditions (13). Second, the translocation of Ia, but not that of C2I, requires a membrane potential gradient in addition to the pH gradient (13). Finally, there are different regions within the Ia and C2I proteins that, respectively, mediate their interaction with Ib and C2IIa and their membrane translocation (4, 26).
The observation that the membrane translocation of C2I and that of CDTa and Ia are facilitated by the same host cell factors is a strong hint for a common role for Hsp90 and cyclophilins during the translocation of binary actin-ADP-ribosylating toxins. Interestingly, the intoxication of cells with the binary lethal toxin from B. anthracis was not influenced by Rad and CsA (7, 18, 57), although that toxin shares significant sequence and structural homology and an overall common cellular uptake mechanism with binary actin-ADP-ribosylating toxins (for a review, see references 38 and 56. Just like Ib and C2IIa, the activated binding/translocation component protective antigen (PA63) forms heptameric pores in membranes of acidified endosomes that facilitate the pH-dependent membrane translocation of the enzyme component LF (56). However, when the enzyme domain of LF, a protease, was replaced with the enzyme domain of diphtheria toxin (DTA), an ADP-ribosyltransferase, the PA63-dependent uptake of the LFn-DTA fusion toxin was inhibited by Rad and CsA (7). Moreover, we demonstrated that the inhibitors blocked the pH-dependent membrane translocation of this fusion toxin across endosomal membranes, as found for the binary actin-ADP-ribosylating toxins (7). This unexpected finding strongly suggests that the interaction with Hsp90 and cyclophilin during membrane translocation might be specific for bacterial ADP-ribosyltransferases. This hypothesis is confirmed by earlier reports that Hsp90 is crucial for the membrane translocation of the enzyme moieties of diphtheria toxin (40) and cholera toxin (53).
These findings are in agreement with an earlier report by Ratts and coworkers that the translocation of diphtheria toxin from early acidified endosomes is facilitated by a multiprotein translocation complex containing Hsp90 and thioredoxin reductase (40). The composition of such complexes and the contributions of individual PPIases might differ, depending on the type of toxin. However, PPIases such as Cyp-40, FKBP51, and FKBP52 have been identified as functional cochaperones in Hsp90-containing protein complexes (35, 39). Therefore, we cannot exclude the possibility that other cyclophilins besides CypA are involved in the translocation of CDTa and/or Ia because CsA inhibits the PPIase activity of most human cyclophilins. Moreover, it will be important to investigate whether further PPIases besides the cyclophilins, for instance, FK506 binding proteins (11), are also involved in the translocation of the binary actin-ADP-ribosylating toxins.
In conclusion, our study provides new information on the interaction of binary clostridial toxins with target cell cyclophilins and Hsp90. However, further investigation is required to unravel the precise molecular mechanisms by which these host cell factors facilitate the membrane translocation of these toxins in mammalian cells.
ACKNOWLEDGMENTS
This work was financially supported by the Deutsche Forschungsgemeinschaft (grant BA 2087/2-1 to H.B. and grant AK6/20-1 to K.A.).
We thank Ulrike Binder for excellent technical assistance.
Footnotes
Published ahead of print on 18 July 2011.
REFERENCES
- 1. Aktories K., et al. 1986. Botulinum C2 toxin ADP-ribosylates actin. Nature 322:390–392 [DOI] [PubMed] [Google Scholar]
- 2. Bang H., Fischer G. 1991. Slow conformational changes in protein folding can be accelerated by enzymes. Biomed. Biochim. Acta 50:S137–S142 [PubMed] [Google Scholar]
- 3. Barth H., et al. 2000. Cellular uptake of Clostridium botulinum C2 toxin requires oligomerization and acidification. J. Biol. Chem. 275:18704–18711 [DOI] [PubMed] [Google Scholar]
- 4. Barth H., Roebling R., Fritz M., Aktories K. 2002. The binary Clostridium botulinum C2 toxin as a protein delivery system: identification of the minimal protein region necessary for interaction of toxin components. J. Biol. Chem. 277:5074–5081 [DOI] [PubMed] [Google Scholar]
- 5. Blöcker D., Behlke J., Aktories K., Barth H. 2001. Cellular uptake of the binary Clostridium perfringens iota-toxin. Infect. Immun. 69:2980–2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blöcker D., et al. 2003. Clostridium botulinum C2 toxin: low pH-induced pore formation is required for translocation of the enzyme component C2I into the cytosol of host cells. J. Biol. Chem. 278:37360–37367 [DOI] [PubMed] [Google Scholar]
- 7. Dmochewitz L., et al. 2011. Role of CypA and Hsp90 in membrane translocation mediated by anthrax protective antigen. Cell. Microbiol. 13:359–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fanghänel J., Fischer G. 2003. Thermodynamic characterization of the interaction of human cyclophilin 18 with cyclosporin A. Biophys. Chem. 100:351–366 [DOI] [PubMed] [Google Scholar]
- 9. Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. 1989. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature 337:476–478 [DOI] [PubMed] [Google Scholar]
- 10. Friedlander A. M. 1986. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 261:7123–7126 [PubMed] [Google Scholar]
- 11. Galat A. 2003. Peptidylprolyl cis/trans isomerases (immunophilins): biological diversity—targets—functions. Curr. Top. Med. Chem. 3:1315–1347 [DOI] [PubMed] [Google Scholar]
- 12. Geric B., Rupnik M., Gerding D. N., Grabnar M., Johnson S. 2004. Distribution of Clostridium difficile variant toxinotypes and strains with binary toxin genes among clinical isolates in an American hospital. J. Med. Microbiol. 53:887–894 [DOI] [PubMed] [Google Scholar]
- 13. Gibert M., et al. 2007. Differential requirement for the translocation of clostridial binary toxins: iota toxin requires a membrane potential gradient. FEBS Lett. 581:1287–1296 [DOI] [PubMed] [Google Scholar]
- 14. Gonçalves C., Decre D., Barbut F., Burghoffer B., Petit J. C. 2004. Prevalence and characterization of a binary toxin (actin-specific ADP-ribosyltransferase) from Clostridium difficile. J. Clin. Microbiol. 42:1933–1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gordon V. M., Leppla S. H., Hewlett E. L. 1988. Inhibitors of receptor-mediated endocytosis block the entry of Bacillus anthracis adenylate cyclase toxin but not that of Bordetella pertussis adenylate cyclase toxin. Infect. Immun. 56:1066–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hale M. L., Marvaud J. C., Popoff M. R., Stiles B. G. 2004. Detergent-resistant membrane microdomains facilitate Ib oligomer formation and biological activity of Clostridium perfringens iota-toxin. Infect. Immun. 72:2186–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Haug G., Aktories K., Barth H. 2004. The host cell chaperone Hsp90 is necessary for cytotoxic action of the binary iota-like toxins. Infect. Immun. 72:3066–3068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haug G., et al. 2003. The host cell chaperone Hsp90 is essential for translocation of the binary Clostridium botulinum C2 toxin into the cytosol. J. Biol. Chem. 278:32266–32274 [DOI] [PubMed] [Google Scholar]
- 19. Haug G., et al. 2003. Cellular uptake of Clostridium botulinum C2 toxin: membrane translocation of a fusion toxin requires unfolding of its dihydrofolate reductase domain. Biochemistry 42:15284–15291 [DOI] [PubMed] [Google Scholar]
- 20. Heinlen L., Ballard J. D. 2010. Clostridium difficile infection. Am. J. Med. Sci. 340:247–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jank T., Aktories K. 2008. Structure and mode of action of clostridial glucosylating toxins: the ABCD model. Trends Microbiol. 16:222–229 [DOI] [PubMed] [Google Scholar]
- 22. Kaiser E., Pust S., Kroll C., Barth H. 2009. Cyclophilin A facilitates translocation of the Clostridium botulinum C2 toxin across membranes of acidified endosomes into the cytosol of mammalian cells. Cell. Microbiol. 11:780–795 [DOI] [PubMed] [Google Scholar]
- 23. Knapp O., Benz R., Gibert M., Marvaud J. C., Popoff M. R. 2002. Interaction of the binding component of Clostridium perfringens iota-toxin with lipid bilayer membranes: demonstration of channel formation by the activated binding component Ib and channel block by the enzyme component Ia. J. Biol. Chem. 277:6143–6152 [DOI] [PubMed] [Google Scholar]
- 24. Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 25. Martin H., et al. 2008. Characterization of Clostridium difficile strains isolated from patients in Ontario, Canada, from 2004 to 2006. J. Clin. Microbiol. 46:2999–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marvaud J. C., et al. 2002. Clostridium perfringens iota toxin. Mapping of the Ia domain involved in docking with Ib and cellular internalization. J. Biol. Chem. 277:43659–43666 [DOI] [PubMed] [Google Scholar]
- 27. McDonald L. C., et al. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433–2441 [DOI] [PubMed] [Google Scholar]
- 28. Miller C. J., Elliott J. L., Collier R. J. 1999. Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 38:10432–10441 [DOI] [PubMed] [Google Scholar]
- 29. Nagahama M., Nagayasu K., Kobayashi K., Sakurai J. 2002. Binding component of Clostridium perfringens iota-toxin induces endocytosis in Vero cells. Infect. Immun. 70:1909–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ohishi I., Iwasaki M., Sakaguchi G. 1980. Purification and characterization of two components of botulinum C2 toxin. Infect. Immun. 30:668–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ohishi I., Tsuyama S. 1986. ADP-ribosylation of nonmuscle actin with component I of C2 toxin. Biochem. Biophys. Res. Commun. 136:802–806 [DOI] [PubMed] [Google Scholar]
- 32. Papatheodorou P., Zamboglou C., Genisyuerek S., Guttenberg G., Aktories K. 2010. Clostridial glucosylating toxins enter cells via clathrin-mediated endocytosis. PLoS One 5:e10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Perelle S., Domenighini M., Popoff M. R. 1996. Evidence that Arg-295, Glu-378, and Glu-380 are active-site residues of the ADP-ribosyltransferase activity of iota toxin. FEBS Lett. 395:191–194 [DOI] [PubMed] [Google Scholar]
- 34. Perelle S., Scalzo S., Kochi S., Mock M., Popoff M. R. 1997. Immunological and functional comparison between Clostridium perfringens iota toxin, C. spiroforme toxin, and anthrax toxins. FEMS Microbiol. Lett. 146:117–121 [DOI] [PubMed] [Google Scholar]
- 35. Pirkl F., Buchner J. 2001. Functional analysis of the Hsp90-associated human peptidyl prolyl cis/trans isomerases FKBP51, FKBP52 and Cyp40. J. Mol. Biol. 308:795–806 [DOI] [PubMed] [Google Scholar]
- 36. Popoff M. R., Boquet P. 1988. Clostridium spiroforme toxin is a binary toxin which ADP-ribosylates cellular actin. Biochem. Biophys. Res. Commun. 152:1361–1368 [DOI] [PubMed] [Google Scholar]
- 37. Popoff M. R., Rubin E. J., Gill D. M., Boquet P. 1988. Actin-specific ADP-ribosyltransferase produced by a Clostridium difficile strain. Infect. Immun. 56:2299–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Popov S. G., et al. 2002. Lethal toxin of Bacillus anthracis causes apoptosis of macrophages. Biochem. Biophys. Res. Commun. 293:349–355 [DOI] [PubMed] [Google Scholar]
- 39. Pratt W. B., Toft D. O. 1997. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 18:306–360 [DOI] [PubMed] [Google Scholar]
- 40. Ratts R., et al. 2003. The cytosolic entry of diphtheria toxin catalytic domain requires a host cell cytosolic translocation factor complex. J. Cell Biol. 160:1139–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Richter K., et al. 2008. Conserved conformational changes in the ATPase cycle of human Hsp90. J. Biol. Chem. 283:17757–17765 [DOI] [PubMed] [Google Scholar]
- 42. Sandvig K., Olsnes S. 1980. Diphtheria toxin entry into cells is facilitated by low pH. J. Cell Biol. 87:828–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schering B., Bärmann M., Chhatwal G. S., Geipel U., Aktories K. 1988. ADP-ribosylation of skeletal muscle and non-muscle actin by Clostridium perfringens iota toxin. Eur. J. Biochem. 171:225–229 [DOI] [PubMed] [Google Scholar]
- 44. Schmid F. X. 1993. Prolyl isomerase: enzymatic catalysis of slow protein-folding reactions. Annu. Rev. Biophys. Biomol. Struct. 22:123–142 [DOI] [PubMed] [Google Scholar]
- 45. Schmid F. X., Mayr L. M., Mucke M., Schonbrunner E. R. 1993. Prolyl isomerases: role in protein folding. Adv. Protein Chem. 44:25–66 [DOI] [PubMed] [Google Scholar]
- 46. Schwan C., et al. 2009. Clostridium difficile toxin CDT induces formation of microtubule-based protrusions and increases adherence of bacteria. PLoS Pathog. 5:e1000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Songer J. G. 1996. Clostridial enteric diseases of domestic animals. Clin. Microbiol. Rev. 9:216–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Stiles B. G., Hale M. L., Marvaud J. C., Popoff M. R. 2002. Clostridium perfringens iota toxin: characterization of the cell-associated iota b complex. Biochem. J. 367:801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stiles B. G., Hale M. L., Marvaud J.-C., Popoff M. 2000. Clostridium perfringens iota toxin: binding studies and characterization of cell surface receptor by fluorescence-activated cytometry. Infect. Immun. 68:3475–3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Stiles B. G., Wilkens T. D. 1986. Purification and characterization of Clostridium perfringens iota toxin: dependence on two nonlinked proteins for biological activity. Infect. Immun. 54:683–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stiles B. G., Wilkins T. D. 1986. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon 24:767–773 [DOI] [PubMed] [Google Scholar]
- 52. Stubbs S., et al. 2000. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol. Lett. 186:307–312 [DOI] [PubMed] [Google Scholar]
- 53. Taylor M., et al. 2010. Hsp90 is required for transfer of the cholera toxin A1 subunit from the endoplasmic reticulum to the cytosol. J. Biol. Chem. 285:31261–31267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wegner A., Aktories K. 1988. ADP-ribosylated actin caps the barbed ends of actin filaments. J. Biol. Chem. 263:13739–13742 [PubMed] [Google Scholar]
- 55. Yang G., et al. 2008. Expression of recombinant Clostridium difficile toxin A and B in Bacillus megaterium. BMC Microbiol. 8:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Young J. A., Collier R. J. 2007. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu. Rev. Biochem. 76:243–265 [DOI] [PubMed] [Google Scholar]
- 57. Zornetta I., et al. 2010. Imaging the cell entry of the anthrax oedema and lethal toxins with fluorescent protein chimeras. Cell. Microbiol. 12:1435–1445 [DOI] [PubMed] [Google Scholar]