Abstract
While gastric adenocarcinoma is the most serious consequence of Helicobacter pylori infection, not all infected persons develop this pathology. Individuals most at risk of this cancer are those in whom the bacteria colonize the acid-secreting region of the stomach and subsequently develop severe inflammation in the gastric corpus. It has been reported anecdotally that male mice become infected with greater numbers of H. pylori bacteria than female mice. While investigating this phenomenon, we found that increased H. pylori infection densities in male mice were not related to antibody production, and this phenomenon was not normalized by gonadectomy. However, the gastric pH in male 129/Sv mice was significantly elevated compared with that in female mice. Differences in colonization were evident within 1 day postinfection and significantly arose due to colonization of the gastric corpus region in male mice. This provided a potential model for comparing the effect of corpus colonization on the development of gastritis. This was explored using two models of H. pylori-induced inflammation, namely, 2-month infections of Muc1−/− mice and 6-month infections of wild-type 129/Sv mice. While H. pylori infection of female mice induced a severe, corpus-predominant atrophic gastritis, to our surprise, male mice developed minimal inflammation despite being colonized with significantly more H. pylori bacteria than female controls. Thus, colonization of the gastric corpus in male mice was associated with a loss of inflammation in that region. The suppression of inflammation concomitant with infection of the gastric corpus in male mice demonstrates a powerful localized suppression of inflammation induced at sites of H. pylori colonization.
INTRODUCTION
The pathological consequences of chronic infection by the gastric pathogen Helicobacter pylori include peptic ulcer disease and gastric adenocarcinoma, which globally is the second leading cause of death due to malignancy (20, 35). The key features believed to dictate whether an infected individual will develop these diseases are the severity and the localization of the inflammation that results from this infection. Individuals who develop antrum-predominant gastritis, while at risk of duodenal ulcers, appear protected from gastric cancer. In contrast, severe gastritis in the stomach corpus and low gastric acid production (hypochlorhydria) are major risk factors for gastric cancer. Any factor that facilitates H. pylori colonization of the gastric corpus and/or reduces gastric acid secretion may therefore contribute to host susceptibility to gastric cancer. For example, gastric pH can influence the gastric localization of H. pylori colonization within the stomach (13), and polymorphisms believed to increase production of the proinflammatory cytokine interleukin 1β (IL-1β), which is known to have potent acid-suppressive activity, are associated with an increased risk of gastric cancer in Caucasians (8).
Another host factor that appears to play an important role in regulating the inflammatory response to H. pylori infection is the mucin MUC1/Muc1 (human and mouse protein designations, respectively), which is expressed on the apical surface of gastric epithelial cells. In humans, polymorphisms in the MUC1 allele which result in short forms of this mucin are associated with an increased susceptibility to gastric cancer and H. pylori-induced gastritis (3, 32). Moreover, we have previously shown that a deficiency in Muc1 facilitates a significant increase in H. pylori colonization and the development of a much more severe, corpus-predominant gastritis in female mice compared to the case in wild-type controls (17).
The vast majority of mouse Helicobacter infection studies utilize female mice. However, several previous studies involving standard inbred-mouse strains (including C57BL/6 and BALB/c) have reported that male mice are colonized with significantly more H. pylori bacteria than are female mice (1, 23). While investigating the mechanism behind this phenomenon, we determined that H. pylori colonizes the corpus region in male mice, and this led us to evaluate the effects of the spatial distribution of H. pylori and resulting inflammation.
MATERIALS AND METHODS
Infection and quantification of H. pylori in mice.
H. pylori strain SS1 was cultured as described previously (17). Specific-pathogen (including Helicobacter)-free mice (C57BL/6, μMT, 129/Sv, and Muc1−/− mice) (12, 29) were bred within the Veterinary Science Animal Facility of the University of Melbourne. Age-matched mice (between 6 and 10 weeks old) were used for all experiments. Mice were infected with a single dose of 107 H. pylori SS1 bacteria, suspended in 100 μl brain heart infusion (BHI) via orogastric gavage. Animal experiments were approved by the University of Melbourne Animal Ethics Committee. Gastric colonization by H. pylori was quantified by colony formation assay as described previously (17).
Immunohistochemistry.
Longitudinally bisected half stomachs were embedded in optimum cutting temperature compound (OCT; Sakura Finetech, Tokyo, Japan), and then 7-μm cryosections were cut (with a CM1900 Cryostat; Leica Microsystems, Solms, Germany) onto glass slides. Immunohistochemistry was performed on acetone-fixed tissues as previously described (33). A polyclonal rabbit antiserum (1/200) raised against a glycine extract of H. pylori was used to localize H. pylori in the mucosa (11). Slides were examined with a Leica DMLB microscope, and images were captured with a digital Leica DFC350FX camera (Leica Microsystems, Wetzlar, Germany). Labeled H. pylori bacteria were quantified on blinded slides by counting fluorescent focal points in 3 independent fields of view at ×400 magnification.
Histological assessment of gastritis.
Longitudinally bisected half stomachs were fixed in 10% neutral buffered formalin and embedded in paraffin, and 4-μm-thick sections were cut. Sections were stained with hematoxylin-eosin (H&E) and scored blindly under light microscopy. Inflammation was assessed in two separate tissue sections for each animal by using the following three parameters: (i) cellular infiltration, graded from 0 to 6, where 0 represents none, 1 represents mild multifocal infiltration, 2 represents mild widespread or moderately multifocal infiltration, 3 represents mild widespread and moderate multifocal infiltration or severe multifocal infiltration, 4 represents moderate widespread infiltration, 5 represents moderate widespread and severe multifocal infiltration, and 6 represents severe widespread infiltration, (ii) “mucus metaplasia” (large, pale, globular cells in the corpus), and (iii) “functional atrophy” (loss of parietal and chief cells) (mucus metaplasia and functional atrophy were graded from 0 to 3, corresponding to absent, mild, moderate, and severe, respectively).
Gonadectomy.
Mice were anesthetized by intraperitoneal injection of 75 mg/kg of body weight ketamine hydrochloride and 15 mg/kg xylazine hydrochloride (Ilium, Sydney, New South Wales, Australia). Isoflurane (VCA, King's Park, Western Australia, Australia) was used to maintain anesthesia. Mice were bilaterally ovariectomized by creating a single dorsal paramedian incision in the skin and removing the ovaries and attached adipose tissue through small 0.25-cm incisions in the peritoneum. Orchiectomy was performed by removing the epididymis and testicles after ligating the attached vas deferens and blood vessels. Michel wound clips (Aesculap, Tuttlingen, Germany) were used to close incisions in the skin.
Gastric acid assays.
For measurement of acid production, the pylori of overnight-fasted mice were ligated under anesthesia via a single small ventral paramedian incision through the skin and peritoneum as previously described (28). Three hours later, mice were euthanized, stomach contents were collected by flushing with 2 ml of distilled water, and the acidity of the solution was measured with a pH meter (Cyberscan pH 500; Eutech Instruments).
Statistics.
Statistical analyses were performed using SPSS software, version 17.0. For comparisons of histological grading scores, data were compared by nonparametric Mann-Whitney analysis. For all other analyses, log-transformed data were compared by analysis of variance (ANOVA), with Dunnett's post hoc analysis where appropriate.
RESULTS
Increased colonization of male mice occurs in the absence of antibodies, within 1 day of infection, and independently of inflammation.
Infection of wild-type C57BL/6 and μMT mice (deficient in B cells) with H. pylori revealed that male mice from both groups were colonized by greater numbers of H. pylori bacteria than female controls (Fig. 1). Moreover, experimental infection of 129/Sv mice with H. pylori revealed that colonization was consistently and significantly higher in males than in females by at least 2-fold, out to at least 3 months postinfection (Fig. 2). This observation was highly reproducible, with the colonization difference at 2 weeks occurring in 7 independent experiments. Most notably, male mice were infected with approximately 100-fold more H. pylori bacteria than female mice were, as early as 1 day postinfection (Fig. 2). These observations indicate that the increased colonization in male mice occurs on different genetic backgrounds and is not related to antibody production or the development of inflammation, which does not develop within this time frame. Rather, these data suggested the impact of a fundamental physiological difference between the gastric mucosa of the male and female mice.
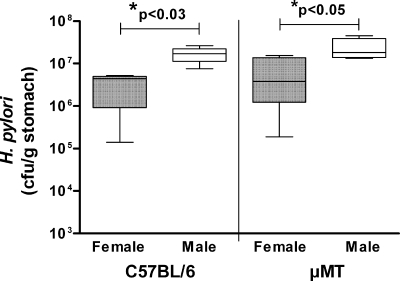
Fig. 1.
Elevated levels of Helicobacter pylori colonization in male mice is unrelated to antibody production. Male and female wild-type or B-cell deficient (μMT) C57BL/6 mice (n = 5) were infected with H. pylori for 2 months, and then gastric bacterial colonization was determined by colony formation assay. Box plots present the median (horizontal bar), interquartile range (box), and 10th and 90th percentile ranges (whiskers). For both groups of mice, males had significantly higher levels of colonization than females (*; ANOVA).
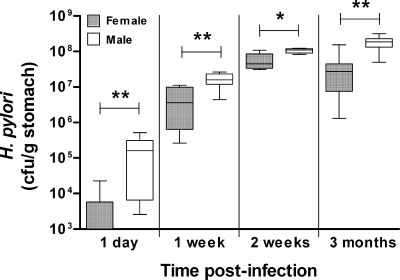
Fig. 2.
H. pylori colonization levels in male mice were elevated compared with those in females as early as 1 day postinfection. Male or female 129/Sv mice were infected with 107 H. pylori, and gastric CFU were assessed 1 day (n = 8), 1 week (n = 8), 2 weeks (n = 5), and 3 months (n = 8) later. Box plots present the median (horizontal bar), interquartile range (box), and 10th and 90th percentile ranges (whiskers). *, P < 0.03; **, P < 0.008 (ANOVA).
Gonadectomy does not normalize H. pylori colonization in male and female mice.
The most obvious physiological difference between males and females is the sex hormones. To examine the potential role of the major sex hormones, gonads were removed from male and female mice and the levels of H. pylori colonization were compared with those of gender controls that underwent sham surgery. Removal of the testes had no effect on the level of colonization of male mice (Fig. 3). In contrast, female mice from which the ovaries were removed had significantly lower levels of colonization than control females (Fig. 3). Ohtani et al., who previously reported a similar reduction in H. pylori colonization in ovariectomized INS-GAS mice, showed that this effect could be overcome by treatment with 17β-estradiol (19), which is known to modulate cytokine production in mice (2, 25). It is possible that the reduced colonization in ovariectomized mice indicates that estradiol inhibits an innate protective response that partially controls H. pylori infection, although this is highly speculative.
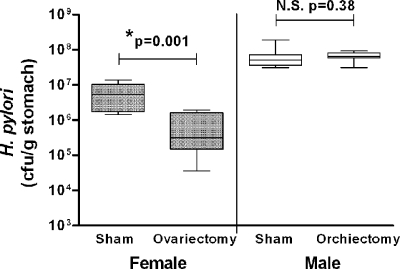
Fig. 3.
Differential colonization in male and female mice is not normalized by gonadectomy. Groups of male and female 129/Sv mice (n = 8) underwent gonadectomy, while matched control groups underwent sham surgery. Two weeks postoperation, all mice were infected with H. pylori, and gastric CFU were assessed a further 2 weeks later. Plots present the median (horizontal bar), interquartile range (boxed region), and 10th and 90th percentiles (error bars). While removal of testes (orchiectomy) had no effect on H. pylori colonization in males, removal of ovaries (ovariectomy) produced a significant reduction in bacterial numbers in female mice (*; ANOVA). N.S., not significant.
Hence, while in our study ovariectomy clearly induced a biological effect, it did not normalize the levels of H. pylori in male and female mice (it in fact increased the colonization difference), thereby indicating that gonadal sex hormones are not directly involved in this dichotomy.
H. pylori colonizes the gastric corpus of male mice but not that of female mice.
It has long been recognized that gastric H. pylori infection in female mice is predominantly confined to the body-antrum and body-cardia transitional zones, as well as the antrum (14). However, to our knowledge, the distribution of H. pylori colonization in male mice has not previously been characterized. We therefore compared the distributions of H. pylori bacteria in male and 129/Sv female mice histologically.
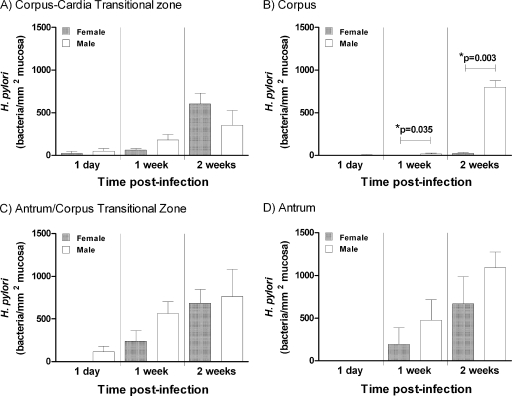
Consistent with previous studies, H. pylori colonized predominantly the antrum and transitional zones on either side of the corpus, but not the corpus itself, in female mice. However, at 2 weeks there was a highly significant 33-fold increase in H. pylori colonization of the corpus in male mice (Fig. 4). Colonization in the corpus was virtually absent in female mice. There was no significant difference between males and females in colonization of any other gastric region (Fig. 4). Hence, the different total levels of gastric CFU observed in male and female mice were entirely explained by the differential colonization of the corpus.
Fig. 4.
H. pylori is more abundant in the corpus of male mice than in that of female mice. (A to D) Stomach sections from male and female 129/Sv mice, infected for 1 day, 1 week, or 2 weeks (n = 4), were stained for H. pylori by immunofluorescence, and different gastric regions were graded for colonization levels. *, significantly different (ANOVA). Similar results were obtained when sections stained with Giemsa stain were evaluated (data not shown).
Elevated pH levels in male mice.
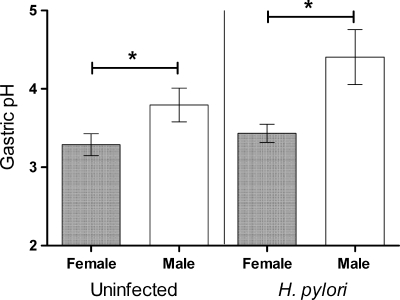
Differential colonization of the acid-secreting corpus in males and females raised the possibility that the dichotomy may be due to gender differences in acid production. We therefore compared pH levels in the gastric juices of male and female 129/Sv mice, either uninfected or infected with H. pylori for 2 weeks. Supporting our hypothesis, the pH of gastric juice from male mice was indeed significantly higher than that of gastric juice from female mice, irrespective of infection status (Fig. 5). The fact that a difference in gastric pH was observed without infection supports a role for this physiological difference underlying the elevated H. pylori colonization observed in male mice within 1 day.
Fig. 5.
Uninfected and H. pylori-infected male mice have elevated gastric pH. Acidity in gastric washes from male and female 129/Sv mice, either uninfected or infected with H. pylori for 2 weeks (n = 10), were measured by using a pH meter (mean ± standard error of the mean [SEM]). Gastric pH was significantly affected by gender (P = 0.002 [*]) but not by 2 weeks of H. pylori infection (P = 0.102) (two-way ANOVA).
To test this further, we attempted to manipulate gastric pH levels by orally treating 129/Sv mice daily with 1.5 mg of the proton pump inhibitor omeprazole during a 1-week H. pylori infection. This treatment resulted in colonization of the gastric mucosa by a range of other organisms (indicated by multiple contaminants on the colony formation assay plates of treated mice as opposed to untreated mice; data not shown). Such overgrowth made interpretation of any effects on H. pylori colonization impossible and indicates that the use of acid suppressants may be too potent to accurately reproduce subtle differences in pH (i.e., from pH 3 to pH 4) as observed in male and female 129/Sv mice.
Increased Helicobacter pylori colonization of male mice is associated with suppression of atrophic gastritis.
H. pylori colonization of the gastric corpus is a major risk factor in the etiology of gastric adenocarcinoma (30). Therefore, the determination that male mice had elevated colonization of the gastric corpus raised the potential to use this dichotomy to examine pathogenic processes associated with chronic H. pylori infection. As conventional mice develop only very mild gastritis when infected with H. pylori, we initially used Muc1−/− mice; we have previously shown that the females of this type develop both greater colonization and more severe gastritis than wild-type controls (17). Increased H. pylori colonization levels concurrent with severe gastritis in female Muc1−/− mice are perhaps unique, as inflammation is usually associated with decreased colonization. We have shown previously that Muc1 restricts H. pylori attachment to the epithelial surface (16), and the increased adherence in Muc1−/− mice likely protects the bacteria from the detrimental effects of severe inflammation.
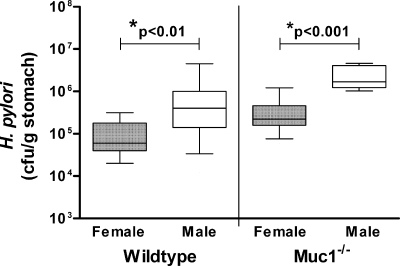
First, we confirmed that male Muc1−/− mice also have significantly elevated colonization levels when infected with H. pylori, relative to matching female controls (Fig. 6). Next, we evaluated the severity of inflammation in these animals. Following 2 months of infection, there was no difference in inflammation between male and female wild-type 129/Sv mice (Table 1); the inflammation remained mild and provided further confirmation that the difference in colonization between these animals was not caused by variability in their inflammatory response. However, while H. pylori infection of female Muc1−/− mice induced a moderate atrophic gastritis, infection of male Muc1−/− mice unexpectedly resulted in only an extremely mild gastritis with no atrophy (Table 1). This was despite the male Muc1−/− mice being infected with approximately 10-fold more H. pylori bacteria than female Muc1−/− mice (Fig. 6).
Fig. 6.
Male 129/Sv and Muc1-deficient (Muc1−/−) mice have elevated levels of Helicobacter pylori colonization compared with female controls. Male and female wild-type or Muc1−/− 129/Sv mice (n = 11) were infected with H. pylori for 2 months, and then gastric bacterial colonization was determined by colony formation assay. Box plots present the median (horizontal bar), interquartile range (box), and 10th and 90th percentile ranges (whiskers). Male and female Muc1−/− mice had significantly greater levels of H. pylori colonization than male and female wild-type mice, respectively (P < 0.05; ANOVA). For all groups of mice, males had significantly higher levels of colonization than females (*; ANOVA).
Table 1.
Female 129/Sv mice but not male 129/Sv mice develop Helicobacter pylori-induced atrophic gastritis
| Expt and micea | Length of infection (mo) | Median grade of pathology (interquartile range)b |
||
|---|---|---|---|---|
| Cellular infiltration (0–6) | Mucus metaplasia (0–3) | Atrophy (0–3) | ||
| Expt 1 | ||||
| Male 129/Sv | 2 | 1 (0.5–1.0) | 0 (0–0) | 0 (0–0) |
| Female 129/Sv | 2 | 1 (1–2.5) | 0 (0–0) | 0 (0–0.5) |
| Male Muc1−/− | 2 | 0.5 (0–2) | 0 (0–0) | 0 (0–1) |
| Female Muc1−/− | 2 | 3 (2.5–4.0)* | 0 (0–0) | 2 (1–2.5)* |
| Expt 2 | ||||
| Male 129/Sv | 0 (uninfected) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Female 129/Sv | 0 (uninfected) | 0 (0–0) | 0 (0–0) | 0 (0–0) |
| Male 129/Sv | 6 | 1 (1–2.5) | 0 (0–0) | 0.5 (0–1.5) |
| Female 129/Sv | 6 | 4 (4–4)* | 0 (0–0) | 3 (2.5–3.0)* |
In experiment 1, male and female wild-type or Muc1−/− 129/Sv mice (n = 11) were infected with H. pylori for 2 months, and then gastric pathology was assessed histologically. Gastritis in all mice was observed only in the corpus. Only the H. pylori-infected female Muc1−/− mice developed atrophic gastritis. In experiment 2, male and female wild-type 129/Sv mice (n = 8) were infected with H. pylori for 6 months or left uninfected. While the infected female mice developed a severe atrophic gastritis, infected male mice developed only a very mild gastritis. This demonstrates that the gender effect on gastritis was not related to Muc1 expression but was common for 129/Sv mice.
*, significantly greater cellular infiltrate and atrophy than in all other groups (P < 0.04; Mann-Whitney U test).
In a second experiment, we infected wild-type 129/Sv mice with H. pylori for 6 months, which provides sufficient time for these animals to develop gastritis. At this later time point, the male mice still maintained significantly higher H. pylori colonization levels than female mice (median CFU [interquartile ranges] were 185 × 106 [5 × 106 to 217 × 106] for males and 0.5 × 106 [0.2 × 106 to 1 × 106] for females; ANOVA P = 0.005). Importantly, while the female wild-type mice developed a severe atrophic gastritis, the male mice did not, demonstrating that the gender difference in Helicobacter-induced gastritis was not related to Muc1 but also occurred in wild-type mice (Table 1).
DISCUSSION
Our studies were initially intended to examine the mechanism behind gender differences in H. pylori colonization levels. While differences between immune responses to H. pylori infection, including IgG levels and cytokine responses, of male and female mice and gerbils have been reported (5, 10, 19), the rapidity of the differential colonization (i.e., within 1 day) and the different numbers of H. pylori observed in male and female antibody-deficient mice argue against a role for host immunity or inflammation in this phenomenon. It also appears that the major sex hormones are not involved, as the removal of testes and ovaries 2 weeks prior to infection failed to normalize the subsequent colonization levels in male and female mice. These studies have, however, revealed several novel observations with fundamental relevance for understanding the relationship between H. pylori infection and the development of disease.
First, we found that, in 129/Sv mice, sex hormone-independent gender differences in acid secretion appear to alter the mucosal niche for H. pylori, which explains differences in both total gastric density of infection and distribution of bacteria in different regions of the stomach. Acid secretion in the corpus is believed to have a major impact on both the distribution of H. pylori within the human gastric mucosa and development of associated pathologies (13). In fact, acid production has been proposed as a key factor dictating whether H. pylori colonizes the antrum or corpus in humans, with patients with higher acid output having antrum-predominant gastritis (31). As this pH difference was evident even without H. pylori infection, elevated gastric pH in male mice could result in increased colonization within 1 day postinfection and therefore is consistent with this pH difference being the likely explanation for differential bacterial levels in male and female mice.
The regulation of gastric acid secretion is highly complex. In an attempt to identify the mechanism behind this difference in gastric pH, we used real-time PCR to measure gastric mRNA levels of a range of mediators known to influence acid secretion. However, we found no association between acid levels in male and female 129/Sv mice and the expression of gastrin, somatostatin, ghrelin, H+K+-ATPase, histidine decarboxylase, and the potassium pump Kcnq1 (data not shown). Hence, the reason for the pH difference in these mice remains unknown.
As widespread gender differences in gastric pH do not occur in humans (15, 27), this is most likely a mouse phenomenon. However, this finding does have important implications for the influence of interindividual variations in acid secretion, due to genetic and environmental factors, on the natural history of H. pylori infection and the development of disease in humans.
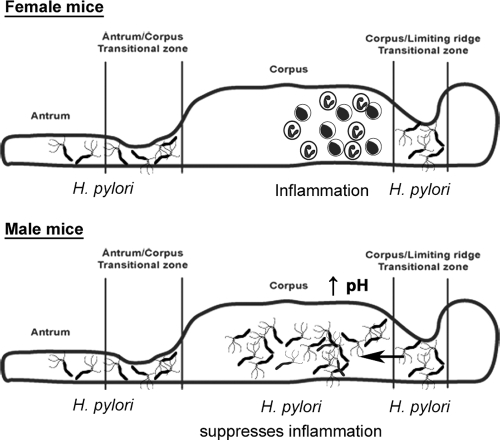
The second and most important observation is that H. pylori appears capable of actively suppressing the inflammatory response in its local gastric microenvironment, via a mechanism different from any effects on the generalized immune response mounted by the animal. In female mice, the highest density of H. pylori colonization occurs in the antrum and the transitional zones on either side of the corpus, but bacteria are not found in the female gastric corpus in large numbers. However, the most severe inflammatory response in females does not develop in the antrum and transitional zones but rather in the corpus (17, 34), where few bacteria were located. This demonstrates that, despite the ability of this host to mount a response to these bacteria, there is a somewhat paradoxical disconnect between the infected and inflamed regions of the mucosa. In male mice, however, H. pylori colonizes the corpus in large numbers (probably due to the elevated pH) as well as all other gastric regions, and little or no inflammation occurred in these mice. This strongly suggests that it is the colonization of the corpus in male mice that actively suppresses the local inflammatory response. A model summarizing this relationship is proposed in Fig. 7. This suppression of inflammation in the corpus occurs even when other inflammation-promoting factors (such as loss of Muc1) are present.
Fig. 7.
Schematic model for reduced inflammation in male Muc1−/− mice. In female mice, which have greater gastric acidity, H. pylori colonization is restricted to either side of the corpus. The resulting inflammation does not develop at the main sites of infection in female mice but rather develops in the corpus, where very few bacteria are located. We hypothesize that the lack of gastritis at these sites of infection is due to a localized anti-inflammatory action of H. pylori. In male mice, however, the lower gastric acidity allows H. pylori to colonize the corpus. As bacteria are now present in the corpus, they are also able to suppress the severe inflammatory response that normally develops at this site, for example, in Muc1−/− mice or long-term-infected wild-type mice.
We propose the following hypothetical mechanism by which H. pylori may mediate this localized suppression of inflammation. An effective T-cell response has been shown to be essential for the generation of the inflammatory response to H. pylori infection in mice. Recombination-activating gene (RAG) knockout and SCID (severe combined immunodeficient) mice do not develop Helicobacter-induced gastritis (7, 24), demonstrating the importance of T cells in this process. It is likely very important with regard to its ability to chronically colonize the gastric mucosa of its host that H. pylori has developed mechanisms to suppress these T-cell responses (6, 18, 21, 26). In particular, γ-glutamyl transpeptidase (GGT) from H. pylori has been shown to be very potent at inhibiting T-cell responses in vitro (26) and was recently shown to induce Foxp3 expression and therefore may be capable of inducing a localized regulatory T-cell response (9). Foxp3+ regulatory T cells are important suppressors of H. pylori-induced gastritis (22). Moreover, γ-glutamyl transpeptidase is essential for H. pylori colonization, as mutation of this enzyme in the mouse-colonizing strain SS1 removed the ability of the bacteria to infect mice (4). We suggest that this GGT mutant may have been unable to colonize mice because it was no longer able to suppress the localized T-cell proinflammatory response mounted by the host. Strategies that aim to neutralize the activity of GGT in vivo may therefore provide a potential novel therapeutic approach.
In summary, our data provide new insights into the relationship between the preferred niche(s) that H. pylori colonizes with respect to both gastric pH and the development of gastritis. The most serious pathological outcome of H. pylori infection, gastric adenocarcinoma, develops as a consequence of a chronic inflammation in the acid-secreting corpus region of the stomach of infected individuals. Hence, our findings have particular relevance for understanding the relationships between the site of gastric infection and the development of pathology. Importantly, we believe these demonstrate for the first time that H. pylori suppresses the inflammatory response in its immediate microenvironment with great efficiency. As severe gastritis is associated with reduced H. pylori colonization (7), the ability to locally reduce the host inflammatory response would confer a considerable advantage to the pathogen and thereby facilitate chronic infection of the host. Individuals at risk for severe inflammation may have polymorphisms that affect the H. pylori-mediated local immunosuppressive mechanism(s).
ACKNOWLEDGMENTS
We thank biostatistician Garry Anderson for assistance with data analyses.
This work was funded by project grant 543704 from the National Health and Medical Research Council of Australia and an Early Career Research grant from the University of Melbourne.
Footnotes
Published ahead of print on 1 August 2011.
REFERENCES
- 1. Aebischer T., Laforsch S., Hurwitz R., Brombacher F., Meyer T. F. 2001. Immunity against Helicobacter pylori: significance of interleukin-4 receptor alpha chain status and gender of infected mice. Infect. Immun. 69:556–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Calippe B., et al. 2010. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J. Immunol. 185:1169–1176 [DOI] [PubMed] [Google Scholar]
- 3. Carvalho F., et al. 1997. MUC1 gene polymorphism and gastric cancer—an epidemiological study. Glycoconj. J. 14:107–111 [DOI] [PubMed] [Google Scholar]
- 4. Chevalier C., Thiberge J. M., Ferrero R. L., Labigne A. 1999. Essential role of Helicobacter pylori gamma-glutamyltranspeptidase for the colonization of the gastric mucosa of mice. Mol. Microbiol. 31:1359–1372 [DOI] [PubMed] [Google Scholar]
- 5. Crabtree J. E., et al. 2004. Gastric mucosal cytokine and epithelial cell responses to Helicobacter pylori infection in Mongolian gerbils. J. Pathol. 202:197–207 [DOI] [PubMed] [Google Scholar]
- 6. Das S., et al. 2006. Expression of B7-H1 on gastric epithelial cells: its potential role in regulating T cells during Helicobacter pylori infection. J. Immunol. 176:3000–3009 [DOI] [PubMed] [Google Scholar]
- 7. Eaton K. A., Mefford M., Thevenot T. 2001. The role of T cell subsets and cytokines in the pathogenesis of Helicobacter pylori gastritis in mice. J. Immunol. 166:7456–7461 [DOI] [PubMed] [Google Scholar]
- 8. El-Omar E. M., et al. 2000. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 404:398–402 [DOI] [PubMed] [Google Scholar]
- 9. Fassi Fehri L., et al. 2010. Helicobacter pylori induces miR-155 in T cells in a cAMP-Foxp3-dependent manner. PLoS One 5:e9500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fox J. G., et al. 2003. Helicobacter pylori-associated gastric cancer in INS-GAS mice is gender specific. Cancer Res. 63:942–950 [PubMed] [Google Scholar]
- 11. Kaparakis M., et al. 2008. Macrophages are mediators of gastritis in acute Helicobacter pylori infection in C57BL/6 mice. Infect. Immun. 76:2235–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kitamura D., Roes J., Kuhn R., Rajewsky K. 1991. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature 350:423–426 [DOI] [PubMed] [Google Scholar]
- 13. Lee A., et al. 1995. Local acid production and Helicobacter pylori: a unifying hypothesis of gastroduodenal disease. Eur. J. Gastroenterol. Hepatol. 7:461–465 [PubMed] [Google Scholar]
- 14. Lee A., et al. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386–1397 [DOI] [PubMed] [Google Scholar]
- 15. Lindahl A., Ungell A. L., Knutson L., Lennernas H. 1997. Characterization of fluids from the stomach and proximal jejunum in men and women. Pharm. Res. 14:497–502 [DOI] [PubMed] [Google Scholar]
- 16. Lindén S. K., et al. 2009. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 5:e1000617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McGuckin M. A., et al. 2007. Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133:1210–1218 [DOI] [PubMed] [Google Scholar]
- 18. Molinari M., et al. 1998. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J. Exp. Med. 187:135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ohtani M., et al. 2007. Protective role of 17 beta-estradiol against the development of Helicobacter pylori-induced gastric cancer in INS-GAS mice. Carcinogenesis 28:2597–2604 [DOI] [PubMed] [Google Scholar]
- 20. Parkin D. M., Bray F., Ferlay J., Pisani P. 2005. Global cancer statistics, 2002. CA Cancer J. Clin. 55:74–108 [DOI] [PubMed] [Google Scholar]
- 21. Paziak-Domańska B., Chmiela M., Jarosinska A., Rudnicka W. 2000. Potential role of CagA in the inhibition of T cell reactivity in Helicobacter pylori infections. Cell. Immunol. 202:136–139 [DOI] [PubMed] [Google Scholar]
- 22. Rad R., et al. 2006. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 131:525–537 [DOI] [PubMed] [Google Scholar]
- 23. Raghavan S., Hjulstrom M., Holmgren J., Svennerholm A. M. 2002. Protection against experimental Helicobacter pylori infection after immunization with inactivated H. pylori whole-cell vaccines. Infect. Immun. 70:6383–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roth K. A., Kapadia S. B., Martin S. M., Lorenz R. G. 1999. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J. Immunol. 163:1490–1497 [PubMed] [Google Scholar]
- 25. Sakazaki F., Ueno H., Nakamuro K. 2008. 17beta-estradiol enhances expression of inflammatory cytokines and inducible nitric oxide synthase in mouse contact hypersensitivity. Int. Immunopharmacol. 8:654–660 [DOI] [PubMed] [Google Scholar]
- 26. Schmees C., et al. 2007. Inhibition of T-cell proliferation by Helicobacter pylori gamma-glutamyl transpeptidase. Gastroenterology 132:1820–1833 [DOI] [PubMed] [Google Scholar]
- 27. Shih G. L., Brensinger C., Katzka D. A., Metz D. C. 2003. Influence of age and gender on gastric acid secretion as estimated by integrated acidity in patients referred for 24-hour ambulatory pH monitoring. Am. J. Gastroenterol. 98:1713–1718 [DOI] [PubMed] [Google Scholar]
- 28. Shurman D., Kamen M., Necheles H. 1957. The Shay mouse: production of gastric ulcers. J. Appl. Physiol. 11:329–330 [DOI] [PubMed] [Google Scholar]
- 29. Spicer A. P., Rowse G. J., Lidner T. K., Gendler S. J. 1995. Delayed mammary tumor progression in Muc-1 null mice. J. Biol. Chem. 270:30093–30101 [DOI] [PubMed] [Google Scholar]
- 30. Uemura N., et al. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784–789 [DOI] [PubMed] [Google Scholar]
- 31. Van Zanten S. J. O. V., Dixon M. F., Lee A. 1999. The gastric transitional zones: neglected links between gastroduodenal pathology and Helicobacter ecology. Gastroenterology 116:1217–1229 [DOI] [PubMed] [Google Scholar]
- 32. Vinall L. E., et al. 2002. Altered expression and allelic association of the hypervariable membrane mucin MUC1 in Helicobacter pylori gastritis. Gastroenterology 123:41–49 [DOI] [PubMed] [Google Scholar]
- 33. Walduck A., Schmitt A., Lucas B., Aebischer T., Meyer T. F. 2004. Transcription profiling analysis of the mechanisms of vaccine-induced protection against H. pylori. FASEB J. 18:1955–1957 [DOI] [PubMed] [Google Scholar]
- 34. Wee J. L., et al. 2010. Protease-activated receptor-1 down-regulates the murine inflammatory and humoral response to Helicobacter pylori. Gastroenterology 138:573–582 [DOI] [PubMed] [Google Scholar]
- 35. World Health Organization 2008. The global burden of disease: 2004 update. World Health Organization, Geneva, Switzerland [Google Scholar]