Abstract
The stringent response is a regulatory system that allows bacteria to sense and adapt to nutrient-poor environments. The central mediator of the stringent response is the molecule guanosine 3′,5′-bispyrophosphate (ppGpp), which is synthesized by the enzymes RelA and SpoT and which is also degraded by SpoT. Our laboratory previously demonstrated that a relA mutant of Pseudomonas aeruginosa, the principal cause of lung infections in cystic fibrosis patients, was attenuated in virulence in a Drosophila melanogaster feeding model of infection. In this study, we examined the role of spoT in P. aeruginosa virulence. We generated an insertion mutation in spoT within the previously constructed relA mutant, thereby producing a ppGpp-devoid strain. The relA spoT double mutant was unable to establish a chronic infection in D. melanogaster and was also avirulent in the rat lung agar bead model of infection, a model in which the relA mutant is fully virulent. Synthesis of the virulence determinants pyocyanin, elastase, protease, and siderophores was impaired in the relA spoT double mutant. This mutant was also defective in swarming and twitching, but not in swimming motility. The relA spoT mutant and, to a lesser extent, the relA mutant were less able to withstand stresses such as heat shock and oxidative stress than the wild-type strain PAO1, which may partially account for the inability of the relA spoT mutant to successfully colonize the rat lung. Our results indicate that the stringent response, and SpoT in particular, is a crucial regulator of virulence processes in P. aeruginosa.
INTRODUCTION
Pseudomonas aeruginosa is an environmentally and metabolically diverse bacterium that is, perhaps, most notorious for its role in chronic lung infections in cystic fibrosis (CF) patients, with 55% of all CF patients and approximately 80% of adult CF patients being colonized by P. aeruginosa (15). This organism also causes a variety of other community-acquired and nosocomial infections, including microbial keratitis associated with contact lens usage (63), ventilator-associated pneumonia (30), and burn wound infections (14). The diverse infections caused by P. aeruginosa are of significant concern to the medical community and can be partly attributed to the pathogen's versatility and large virulence arsenal. The synthesis of virulence factors is precisely attuned to environmental conditions by several regulatory systems, of which the stringent response was of particular interest to our study.
The stringent response was originally described as an adaptation to amino acid starvation, whereby stable RNA and ribosomal protein synthesis is downregulated while proteolytic and amino acid biosynthetic enzymes are upregulated, as studied primarily in Escherichia coli (10). Currently, it is recognized that the stringent response can be induced by starvation for a variety of nutrients resulting in a broad-scale adaptation to stressful conditions. Such adaptations include regulatory changes to central metabolic pathways, induction of the RpoS-dependent general stress response, and repression of DNA replication (19, 57). The stringent response, therefore, functions as a switch between “feast or famine” conditions, which is used by bacteria to determine resource allocation to either reproductive or cell maintenance functions (39). Additionally, the stringent response is utilized by many bacterial pathogens, including members of the gamma-, alpha-, and epsilonproteobacteria, Actinobacteria, Firmicutes, and spirochetes, to control virulence functions (reviewed by Dalebroux et al. [16]). The central signaling molecules of the stringent response are two guanine nucleotides, guanosine 3′,5′-bispyrophosphate (ppGpp) and guanosine 3′-diphosphate 5′-triphosphate (pppGpp), collectively referred to as (p)ppGpp (10). These molecules, which exert their cellular effects by binding to and modifying the transcription pattern of RNA polymerase, are produced by enzymes termed Rel/Spo homologues. In all beta- and gammaproteobacteria studied thus far, there are two such enzymes, RelA and SpoT (27). RelA, the foremost producer of (p)ppGpp in E. coli (8), is activated by ribosomal stalling caused by amino acid starvation (23). SpoT is a secondary producer of (p)ppGpp and is solely responsible for degrading these signaling molecules (4, 24, 32). The environmental cues sensed by SpoT are not well-defined but include starvation for carbon (65), iron (60), phosphate (52), and fatty acids (49). Fatty acid starvation may be sensed via a direct interaction between SpoT and the acyl carrier protein in both E. coli and P. aeruginosa (2). Additionally, alkaline pH triggers ppGpp accumulation in P. aeruginosa in a SpoT-dependent manner (4). These inducing factors may mediate a switch between the two opposing enzymatic activities of SpoT (1), allowing a sensitive response to external conditions.
Several groups have begun to examine the stringent response in P. aeruginosa and its role in regulating expression of virulence factors. Two studies have demonstrated that RelA is a positive regulator of the quorum-sensing circuitry of P. aeruginosa (20, 58). Since quorum sensing is an important regulator of virulence functions in P. aeruginosa (33), Erickson et al. examined the virulence of a relA mutant (20), finding that this strain secreted reduced amounts of the extracellular protease elastase but increased amounts of the secondary metabolite pyocyanin. Preliminary experiments showed that the relA mutant was fully virulent in the rat lung agar bead model (20). Intriguingly, the relA mutant was attenuated in virulence in the Drosophila melanogaster feeding model of infection (20). To explain the different virulence potentials of the relA mutant in these two model systems, we postulated that (p)ppGpp may be required for full virulence of P. aeruginosa but that a functional copy of SpoT may be sufficient to fulfill this requirement in some situations, such as in the rat lung. In support of this idea, Boes et al. (4) recently showed that a P. aeruginosa relA mutant has some ability to produce ppGpp but that a relA spoT double mutant is ppGpp negative. They also showed that SpoT positively regulates a set of universal stress proteins with roles in anaerobic energy stress adaptation, which may contribute to survival within a host (4).
In this study, we assessed the role of spoT in P. aeruginosa virulence by creating a relA spoT double mutant devoid of (p)ppGpp production. We examined the virulence of the double mutant in two chronic infection model systems and found that this strain was incapable of establishing a successful infection in either organism. We also examined this mutant in a model of acute infection where it seemed the stringent response did not play as large a role in pathogenesis. Further experiments showed that the double mutant was defective in both secretion of virulence factors and survival under a variety of stresses, suggesting that the stringent response promotes P. aeruginosa virulence through several distinct mechanisms.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Escherichia coli strain DH5α (22) was utilized for all cloning. Unless otherwise indicated, both P. aeruginosa and E. coli were cultured using Luria-Bertani (LB) broth or agar at 37°C.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description and/or genotype or phenotypea | Reference or source |
|---|---|---|
| Strain or plasmid | Description and/or genotype or phenotypea | Reference or source |
| Bacterial strains | ||
| P. aeruginosa | ||
| PAO1 | Prototypical laboratory strain | 26 |
| relA mutant | PAO1 with a gentamicin resistance cassette inserted into relA; Gmr | 20 |
| relA spoT mutant | PAO1 relA mutant with an Ω streptomycin/spectinomycin resistance cassette inserted into spoT; Gmr Smr/Spr | This study |
| E. coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA–argF)U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 λ−thi-1 gyrA96 relA1 | 22 |
| Plasmids | ||
| pCR2.1 | TA cloning vector; Apr Kmr | Invitrogen |
| pHP45Ω | Cloning vector pHP45 containing the Smr/Spr Ω element; Apr Smr/Spr | 43 |
| pEX18Tc | Gene replacement vector containing the MCS from pUC18 and the sacB gene; Tcr | 25 |
| pSS11 | pEX18Tc containing a spoT::Ω insert; Tcr Smr/Spr | This study |
| pUCP28T | E. coli–P. aeruginosa shuttle vector derived from general cloning vector pUC18; Tpr | 47 |
| pSS6 | pUCP28T containing rpoZ–spoT from PAO1; Tpr | This study |
MCS, multiple cloning site.
Creation and complementation of the relA spoT double mutant.
The region of the P. aeruginosa PAO1 (parental, wild-type strain) genome (54, 64) encompassing spoT was PCR amplified using primers SpoFor3 (For stands for forward) (5′-CTGGTCGATGAGAACGTCG-3′) and SpoRev1 (Rev stands for reverse) (5′-GATAACGGTCTTGGTCATGG-3′) and cloned into pCR2.1. An insertion mutation was generated by liberating the Ω cassette, encoding streptomycin and spectinomycin resistance, from pHP45Ω (43) by SmaI digestion and inserting it into the unique NruI site in spoT. This spoT::Ω construct was then transferred into the suicide plasmid pEX18Tc (25) by an XbaI/SstI double digestion, thereby producing pSS11. pSS11 was subsequently electroporated into the PAO1 relA mutant as previously described (11). The genotypes of putative double mutants were verified by PCR amplification and Southern hybridization (data not shown).
Plasmid pSS6, which contains the rpoZ and spoT open reading frames from P. aeruginosa strain PAO1 cloned into the vector pUCP28T, was used to complement the spoT mutation. Since pUCP28T does not contain a promoter suitable for driving expression of the cloned insert in P. aeruginosa, we needed to include the endogenous promoter of the spoT gene in order for the construct to be expressed. rpoZ was included in pSS6 because a similar plasmid containing only spoT and its upstream intergenic region failed to complement any mutant phenotype that we examined (data not shown) and because rpoZ and spoT are predicted to be cotranscribed (Pseudomonas Genome Database v2 [http://www.pseudomonas.com]). We therefore concluded that the promoter driving spoT expression is located within or upstream of rpoZ. The complementation region was amplified from strain PAO1 using primers SpoFor2 (5′-GACGCCCAGCAGCAACGC-3′) and SpoRev1.
(p)ppGpp assays.
Production of (p)ppGpp by strains of P. aeruginosa was detected using a radiolabeling assay (9). Strains were cultured overnight on LB agar and then resuspended in 1 ml of 1× minimal medium containing morpholinepropanesulfonic acid (MOPS) (38). After the strains were washed once in the same medium, the optical density at 600 nm (OD600) of the cell suspension was normalized to 0.5 in 100 μl of the appropriate starvation medium. For amino acid starvation, 1× MOPS was supplemented with 0.2% glucose, 1 mg/ml serine hydroxamate (Sigma), and 0.5 mg/ml l-valine (Sigma). For carbon starvation, no supplements were added to the medium containing 1× MOPS. For iron starvation, the iron(II) sulfate was omitted from the 1× MOPS mixture, and the medium was supplemented with 0.2% glucose, 100 μg/ml Casamino Acids, and 2.5 mM 2,2′-dipyridyl (Sigma). 32Pi (PerkinElmer) was added to each well to an activity of 20 μCi/ml; labeling was carried out at 37°C for 1 h. Nucleotides were extracted by adding 100 μl of cold 90% formic acid to each sample. Cells were then lysed by two freeze-thaw cycles on dry ice. For analysis of cellular nucleotide pools, 5 μl of each cell lysate was spotted onto a cellulose-polyethyleneimine thin-layer chromatography (TLC) plate (Selecto Scientific), which was developed in 1.5 M KH2PO4, pH 3.4. Radioactivity was detected by exposing the dried TLC plate to X-ray film.
Fly feeding model of infection.
The Drosophila melanogaster fly feeding assay was performed by the method of Chugani et al. (12). P. aeruginosa strains were cultured overnight with maximum aeration at 37°C in brain heart infusion (BHI) broth. Cultures were normalized to an OD600 of 3.0 in 2 ml of BHI broth, then pelleted, and resuspended in 175 μl of sterile 5% sucrose. These bacterial cultures were placed on sterile filters atop 5% sucrose agar medium in fly vials. Three- to 5-day-old male D. melanogaster strain Oregon R flies were starved for 5 h before being placed in infection assay vials, with 10 to 12 flies per vial. Flies were incubated at 28°C, and their mortality was monitored daily for 14 days. To determine the survival of P. aeruginosa strains on feeding filters, one filter from each strain was removed from its feeding vial every 2 days throughout the course of the experiment. The filter was vortexed vigorously in 5 ml of LB broth to resuspend bacteria, and the resulting cell suspension was plated for viable cell counting.
Fly nicking model of infection.
The fly nicking model was performed by the method of D'Argenio et al. (17). Bacteria were cultured overnight in BHI broth at 37°C. The following day 3- to 5-day-old female D. melanogaster flies were anesthetized, using a tile cooled on ice, and nicked in the abdomen with a 27.5-gauge needle dipped in a bacterial culture normalized to an OD600 of 1.0. Once inoculated, the flies were transferred to vials containing cornmeal agar medium, and survival was monitored for up to 36 h.
Rat lung agar bead model of infection.
The rat lung agar bead infections were performed by the method of Cash et al. (7). Two experiments were performed; the first experiment compared wild-type strain PAO1 with the relA mutant, and the second compared strain PAO1 with the relA spoT mutant. In both experiments, a minimum of seven male Sprague-Dawley rats were infected with each strain by placing agar beads enmeshed with approximately 104 CFU of bacteria in the lungs. After 7 days of incubation, the rats were sacrificed. Three rats per strain were examined for lung pathology, and at least four rats per strain were analyzed for quantitative bacteriology. For quantitative histopathology, lung involvement or consolidation was characterized by infiltration of inflammatory cells, neutrophils, and mononuclear cells in the airway lumen as well as visible epithelial desquamation.
Virulence factor assays.
Pyocyanin production was assessed using the chloroform-hydrochloric acid extraction method of Carty et al. (6). Cultures to be assayed were incubated in LB broth with maximum aeration at 37°C for 18 h. The reverse-elastin plate assay and elastin-Congo red assay for detection of elastase production were performed by the method of Rust et al. (45), with cultures grown in BHI broth at 37°C for 20 h. Overall protease production was tested on dialyzed BHI agar containing skim milk (BHI-skim milk agar) (50), with cultures grown in the same manner as for elastase assays. Siderophore production was assayed using chrome azurol S (CAS) agar by the method of Schwyn and Neilands (48). Lipase activity was measured using a turbidimetric assay based on the cleavage of a Tween 20 substrate (62). The radiometric ADP-ribosylation assay for exotoxin A production was performed by the method of Chung and Collier (13). Overnight cultures were normalized to an OD600 of 0.3 before they were placed on the agar in all plate-based assays. All virulence factor assays were performed three or four times in triplicate.
Motility assays.
Swarming assays were carried out by the method of Kohler et al. (29). The swarm medium used on the plates was M8 medium supplemented with 0.2% glucose, 0.05% sodium glutamate, and 0.5% agar. The plates were inoculated with 1 μl of a normalized culture (OD600 of 0.2) and incubated at 37°C for 48 h. Twitching assays were performed by the method of Darzins (18). A sterile toothpick was used to stab bacteria to the bottom of a LB plate with 1% agar. The plates were incubated at 37°C for 48 h. The agar was then carefully removed, and the petri plate was air dried and stained for 5 min with 1% Coomassie blue. Motility zones were measured after rinsing with distilled water. For the swimming motility assay, 1 μl of a bacterial culture standardized to an OD540 of 1.0 was inoculated into the center of an LB plate containing 0.3% agar and then incubated at 37°C for 24 h (21).
Stress tolerance assays.
Assays to examine the sensitivity of P. aeruginosa strains to heat shock, osmotic stress, and oxidative stress were performed essentially by the method of Suh et al. (55). For the osmotic stress assays, 4 M sodium chloride and 2 M sucrose were used as the ionic and nonionic solutes, respectively. The hydrogen peroxide disc diffusion assay was performed using Vogel-Bonner minimal medium (VBMM) (61) rather than LB, since we expected to see a larger difference between the wild-type and ppGpp-deficient strains in the nutrient-limiting environment of VBMM. All stress tolerance assays were performed in triplicate or quadruplicate.
Competition assay.
The ability of P. aeruginosa PAO1 and the relA spoT double mutant to survive in an environment depleted of nutrients was assessed in a competition assay. Strains were cultured individually overnight in BHI broth and then coinoculated into a diluted rich medium (0.1× BHI broth), with the starting cell density normalized to an OD600 of 0.002 for strain PAO1 and 0.02 for the relA spoT mutant. The mixed culture was incubated at 37°C with aeration, without supplementing with fresh medium. The culture was sampled daily for an overall viable cell count as well as to determine the proportion comprised by each strain. Viable cell counting was performed by serially diluting the culture and plating onto LB agar. For determination of strain ratios, the mixed culture was plated on LB agar for isolated colonies, 100 of which were randomly selected to be patched onto plates containing antibiotics selective for the relA spoT mutant. Control pure cultures of each strain were prepared and plated as described above. Three replicate experiments were performed.
Statistical analysis.
All data were analyzed and graphed using Prism version 5.0a (GraphPad Software, Inc.). All comparisons, with the exception of fly survival curves, were made using a one-way analysis of variance followed by Tukey's multiple-comparison test. Fly survival curves were compared using the Mantel-Cox log rank test.
RESULTS
Construction of a P. aeruginosa relA spoT double mutant.
In this study, our primary objective was to characterize the involvement of ppGpp and SpoT, in particular, in the virulence of P. aeruginosa. We created a strain lacking both known ppGpp synthetase-encoding genes by generating an insertion mutation in the spoT open reading frame within a previously constructed relA mutant (20). The same construct was introduced into the wild-type strain PAO1, but all attempts at creating a spoT mutation in a relA+ background failed (see Discussion). In accordance with the report by Boes et al. (4), no (p)ppGpp synthesis could be detected in the relA spoT double mutant under any growth condition tested, including starvation for nutrients such as amino acids, carbon, and iron (see Fig. S1 in the supplemental material). We were also unable to detect (p)ppGpp synthesis in the relA mutant under these conditions, although a small amount of ppGpp was produced by the relA spoT mutant complemented in trans with spoT (on pSS6) during carbon starvation (Fig. S1B). Since this strain lacks relA, we conclude that this ppGpp was synthesized in a SpoT-dependent manner.
A P. aeruginosa relA spoT mutant is severely attenuated in virulence in two chronic infection models.
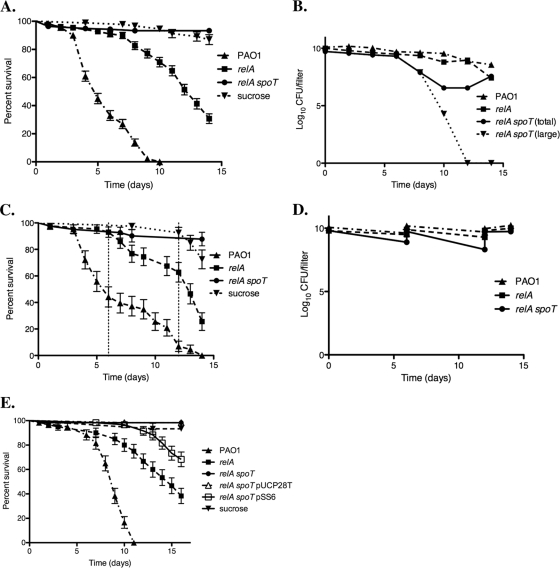
To determine whether P. aeruginosa stringent response mutants are defective in virulence, we orally inoculated D. melanogaster with wild-type or mutant bacteria and monitored fly mortality for 14 days. Figure 1A shows that the relA spoT double mutant was significantly less virulent than both the wild-type strain PAO1 and the relA mutant (P < 0.0001) and did not kill significantly more flies than sterile sucrose (P = 0.0904). Enumeration of viable bacteria on the fly feeding filters indicated that the relA spoT mutant maintained a high CFU count until only day 6; subsequently, the CFU counts plunged rapidly (Fig. 1B). Notably, at around day 10, the colony morphology of the relA spoT mutant also changed, with tiny punctiform colonies rapidly outnumbering the large, irregular colonies that are typical of this strain (data not shown). To remedy this difference in bacterial survival on the filters, the fly feeding experiments were repeated, with flies transferred to freshly prepared bacterial filters every 6 days. Viable cell counts of these filters revealed that the relA spoT mutant was able to maintain filter colonization at levels comparable to both PAO1 and the relA mutant throughout the experiment (Fig. 1D), and no colony morphology variants were observed. Increased filter colonization did not affect the survival of the infected flies, with the relA spoT double mutant still causing no additional fly lethality over the sucrose control (P = 0.1174) (Fig. 1C). Therefore, the reduced survival of the relA spoT mutant on the fly feeding filters is unlikely to account for the inability of this strain to produce a lethal infection in flies. In addition, the virulence of the relA spoT mutant could be partially restored by complementation with spoT in trans (Fig. 1E). Although still less virulent than the relA mutant, the complemented strain, the relA spoT mutant carrying the pSS6 plasmid, killed significantly more flies than either the relA spoT mutant or the vector control strain, the relA spoT mutant carrying pUCP28T (P < 0.0001).
Fig. 1.
A P. aeruginosa relA spoT double mutant is avirulent in the fly feeding model of infection. (A, C, and E) Mortality of flies infected with P. aeruginosa PAO1 (▴), relA mutant (▪), and relA spoT (•) mutant, as well as the negative control of sterile sucrose (▾), was monitored for 14 days following infection. In panel A, the data points making up the survival curve and standard errors of the means (SEM) (error bars) were generated from three replicate experiments with 30 to 60 flies per strain. In panel C, flies were transferred to fresh bacterial filters on days 6 and 12 (indicated by the vertical broken lines); the data making up the survival curve and SEM were generated from one experiment with 40 flies per strain. In panel E, the vector control strain, the relA spoT mutant carrying pUCP28T (relA spoT pUCP28T) (▵), and the complemented strain, the relA spoT mutant carrying pSS6 (relA spoT pSS6) (□) were also examined; the data points making up the survival curve and SEM were from one experiment with 60 flies per strain. (B and D) Enumeration of viable bacteria remaining on fly feeding filters containing strain PAO1 (▴), relA mutant (▪), and relA spoT mutant (•) throughout the experiment. CFUs were determined in triplicate for each filter. Graphed data represent the average CFU/filter, with error bars (mainly too small to be visible) denoting SEM. In panel B, one filter per strain was removed every 2 days for the duration of the experiment. Note that the colony morphology of the relA spoT mutant changed throughout the experiment from the original large colonies (▾) to a small-colony variant; thus, both total counts and large-colony counts are shown for this strain. In panel D, flies were transferred to fresh bacterial filters on days 6 and 12. CFU counting was performed on the inoculum on days 0, 6, and 12, and also on the removed filters on days 6, 12, and 14 (end of experiment). In this experiment, no small colonies were observed for the relA spoT mutant.
To assess the virulence of stringent response mutants in a mammalian model, two sets of rat lung agar bead infections were performed. Figure 2 shows that there was no significant difference between the mean bacterial load of rats (n = 4) infected with strain PAO1 and the relA mutant in experiment 1. Likewise, there was similar pathology and involvement of tissue in the lungs of rats infected with either strain, suggesting that the relA mutant was as virulent as the parental strain in the rat lung infection model. In contrast, in experiment 2, there was a significant difference between the mean total bacterial loads of rats (n = 4) infected with strain PAO1 (1.64 × 105 CFU/ml) and the relA spoT double mutant (no culturable cells) after 7 days of infection (Fig. 2). As expected, strain PAO1 caused an influx of neutrophils and monocytes, with accompanying epithelial desquamation involving on average 47% of the lung tissue (n = 3), whereas there was no lung pathology evident in the rats infected with the relA spoT mutant (n = 3). These results suggested that the relA spoT double mutant was unable to survive, was effectively cleared from the rat lung within 7 days, and was unable to provoke any tissue damage during the infection.
Fig. 2.
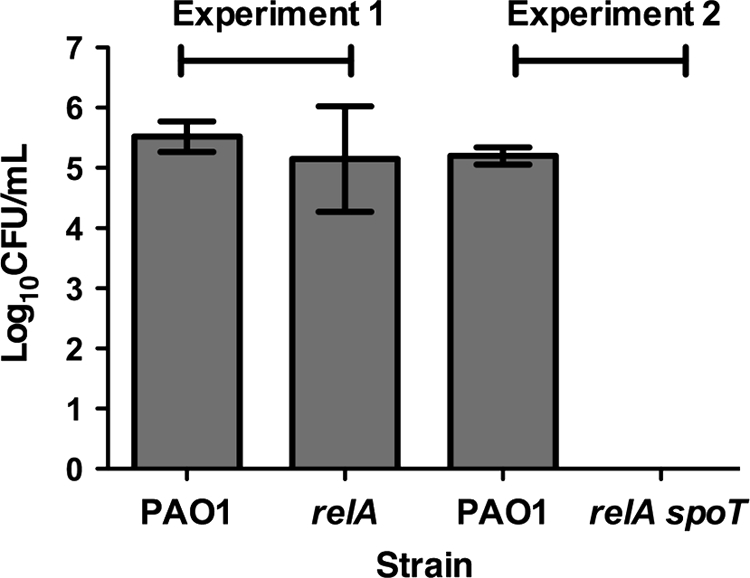
A P. aeruginosa relA spoT double mutant is avirulent in the rat lung infection model. Quantitative bacteriology of rat lung agar bead infections with P. aeruginosa PAO1 and the relA and relA spoT mutants on day 7 of the infection. Data represent the mean of total bacterial counts ± SEM from the lungs of four animals for each strain. No significant differences were observed between the means of strain PAO1 and the relA mutant. There was a significant difference between the mean values for the colony counts of strain PAO1 and the relA spoT mutant (P < 0.0001).
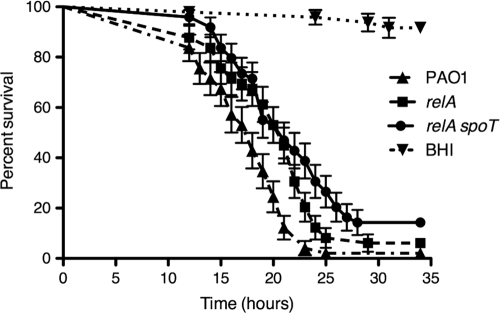
Both of these models are representative of chronic infections. Thus, we also performed fly nicking experiments as an acute infection model. Figure 3 shows that both the relA and relA spoT mutants were significantly less virulent than the parental strain PAO1 (P = 0.0034 and P < 0.0001, respectively). However, there was not a significant difference between the survival curves for the two mutant strains. This suggested that the stringent response, and SpoT in particular, might not be as important in this model of acute infection as in the chronic infection model above.
Fig. 3.
The virulence of the P. aeruginosa relA spoT double mutant is similar to that of the relA mutant, but both are less virulent than P. aeruginosa PAO1 in the Drosophila nicking infection model. Survival curves for D. melanogaster challenged with wild-type PAO1 or the stringent response mutants are shown. Flies were nicked in the abdomen with a needle dipped into a standardized culture of P. aeruginosa. Fly mortality was measured hourly on 3 sets of 30 flies. Significant differences were observed between the PAO1 survival curve and the survival curve of either the relA mutant (P = 0.0034) or the relA spoT mutant (P < 0.0001). There is not a significant difference between the survival curves for the relA and relA spoT mutants (P = 0.113).
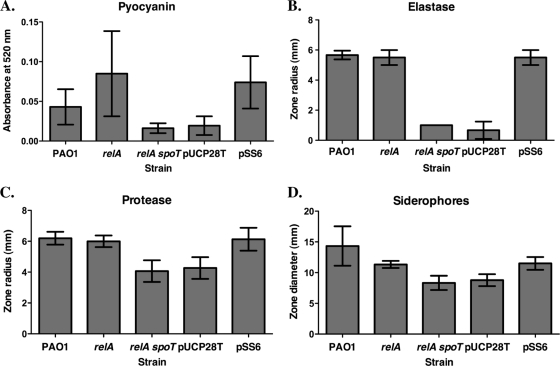
ppGpp-deficient strains of P. aeruginosa are impaired in virulence factor production.
In order to understand the mechanisms by which the stringent response affects pathogenesis, we characterized the production of virulence factors by our ppGpp-deficient strains. When grown in LB, the relA spoT mutant displayed a notable lack of pigmentation (data not shown). This observation prompted us to examine this strain's production of pyocyanin, a blue redox-active compound secreted by many strains of P. aeruginosa (34). As illustrated in Fig. 4A, pyocyanin production was virtually abolished in the double mutant. Like Erickson et al. (20), we found an elevated level of pyocyanin in supernatants obtained from the relA mutant compared to those obtained from strain PAO1 (P value of <0.05 when comparing the value for the relA mutant to the value of either strain PAO1 or the relA spoT mutant) (Fig. 4A). Complementation with pSS6 significantly enhanced pyocyanin production (P < 0.05 compared to vector control pUCP28T).
Fig. 4.
Virulence factor synthesis is diminished in a P. aeruginosa relA spoT double mutant. All virulence factor assay data represent the means of three or four replicate experiments, performed in triplicate; the error bars indicate the standard deviations. The relA spoT mutant carrying pUCP28T and the relA spoT mutant carrying pSS6 are shown as pUCP28T and pSS6, respectively, in the figure. (A) Pyocyanin production was determined by extraction of culture supernatants with chloroform and hydrochloric acid; the absorbance at 520 nm of the resulting aqueous phase corresponds to the concentration of pyocyanin. (B) Elastase production was quantified as the size of zones of clearing produced by bacterial colonies on reverse-elastin agar. (C) Protease production was measured as the size of zones of clearing produced by bacterial colonies on dialyzed BHI-skim milk agar. (D) Siderophore production was assessed as the size of the orange halos produced by bacterial colonies on low-iron CAS blue agar.
We examined elastase production by wild-type and mutant strains by measuring zones of clearing in reverse-elastin agar. The zones observed for the relA spoT mutant were extremely small to nonexistent (Fig. 4B) (reduced compared to either other strain [P < 0.05]), and elastase production was restored by complementation with pSS6 (P < 0.05 for the value for the relA spoT mutant carrying pSS6 compared to the value for the relA spoT mutant carrying pUCP28T). Comparable results were obtained using the more-quantitative elastin-Congo red assay (45) (A595 values of 0.280 ± 0.055 for strain PAO1, 0.235 ± 0.088 for the relA mutant, and 0.023 ± 0.008 for the relA spoT mutant; the value for the relA spoT mutant was lower than that of any other strain [P < 0.05]).
Since ppGpp production is stimulated by iron starvation (60), we assessed the ability of stringent response mutants to synthesize several iron-regulated virulence factors: protease, siderophores, lipase, and exotoxin A. Dialyzed BHI-skim milk agar plates were used to examine overall protease production by strains of P. aeruginosa. Although there was no significant difference in protease production by the relA mutant compared to P. aeruginosa PAO1 (P > 0.05), the relA spoT double mutant displayed a significant reduction (P < 0.05) in protease production, with zones of clearing being approximately 66% of PAO1 zone sizes (Fig. 4C). With this modest reduction in total protease activity and since such a strong reduction in elastase activity was observed in the relA spoT mutant (Fig. 4B), it is possible that production of other proteases, such as alkaline protease and protease IV, was unaffected in this mutant. Again, complementation with pSS6 significantly increased protease production by the relA spoT mutant compared to the vector control (P < 0.05).
To assess overall siderophore production by P. aeruginosa, we spotted cultures grown in low-iron medium onto chrome azurol S (CAS) agar. The relA spoT mutant produced orange halos indicative of siderophore production that were significantly smaller than those produced by strain PAO1 (P < 0.05), and this defect in siderophore synthesis could be complemented with pSS6 (P < 0.05 compared to the relA spoT mutant carrying pUCP28T) (Fig. 4D).
The production of lipase and exotoxin A by wild-type and ppGpp synthase-deficient strains of P. aeruginosa was also measured. However, no significant differences were detected between the relA spoT mutant and either strain PAO1 or the relA mutant for the production of either exotoxin A or lipase (data not shown).
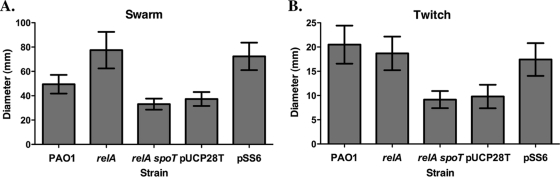
SpoT is important for swarming and twitching motilities.
Since motility is also crucial during the colonization stage of a P. aeruginosa infection (28), we tested P. aeruginosa PAO1 and our mutant strains for swimming, swarming, and twitching motility. Interestingly, there were no significant differences between strain PAO1 and either the relA or relA spoT mutant in flagellum-dependent swimming motility (data not shown). However, the relA spoT mutant was significantly impaired in both swarming (flagellum-dependent) (P < 0.001) and twitching (pilus-dependent) motility (P < 0.0001) compared to PAO1, the parental strain (Fig. 5). The relA mutant displayed increased swarming motility compared to PAO1, with extensive tendril formation, but had no significant change in twitching motility (Fig. 5). Complementation of the relA spoT mutant with pSS6 produced motility zones equivalent to those of the relA mutant (Fig. 5). These results demonstrate that ppGpp is required for optimal twitching and swarming motility but that swimming is unaffected, suggesting that the stringent response mutants are able to produce functional flagella but may be deficient in the production of pili or surfactants such as rhamnolipid.
Fig. 5.
Swarming and twitching motilities are reduced in the relA spoT mutant compared to the relA mutant and the parental PAO1 strain. The graphs show the means of motility from three replicate experiments performed in triplicate for strain PAO1, relA mutant, relA spoT mutant, the vector control strain, the relA spoT mutant carrying pUCP28T (“pUCP28T” in the figure), and the complemented strain, the relA spoT mutant carrying pSS6 (“pSS6” in the figure), overexpressing a plasmid-borne copy of spoT. Error bars denote the SEM. (A and B) The mean diameters of spread for swarming motility (A) and twitching motility (B) are shown.
ppGpp-deficient P. aeruginosa mutants are more susceptible to abiotic stresses.
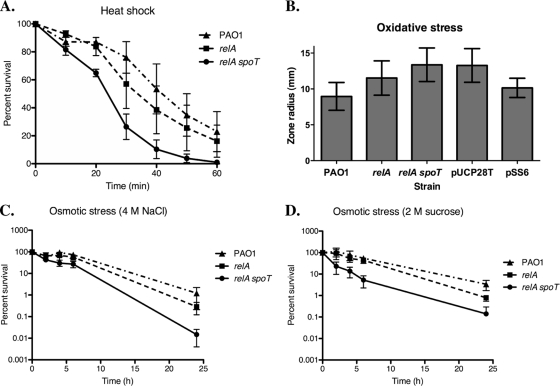
We hypothesized that the poor survival of the relA spoT mutant in the rat lung assay may reflect an increased sensitivity of this strain to various stresses, including heat shock, oxidative stress, high osmolarity, and nutrient starvation. Figure 6A shows that of the three P. aeruginosa strains, the relA spoT mutant was the strain most susceptible to heat shock. The difference in survival between strain PAO1 and the relA mutant was not as dramatic, but on average, PAO1 was able to withstand heat shock better than the relA mutant (Fig. 6A). These results suggest that ppGpp improved survival during heat stress and that even the basal level of ppGpp present in the relA mutant was sufficient to provide some heat protection to the bacterium compared to the relA spoT mutant.
Fig. 6.
Stringent response mutants of P. aeruginosa are more susceptible to abiotic stresses. The abilities of strain PAO1 (▴), relA mutant (▪), and relA spoT double mutant (•) to survive a variety of stresses were examined. All data are from three or four replicate experiments; error bars denote standard deviations. (A) Diluted cultures were heat shocked at 50°C for 60 min, with duplicate samples taken every 10 min to assess the number of viable cells remaining. Cell survival is expressed as a percentage of the viable cell count at time zero. (B) The sensitivity of strains to oxidative stress was assessed by measuring the size of the zones of clearing in the bacterial lawn surrounding discs soaked in 30% hydrogen peroxide. In this assay, the vector control strain, the relA spoT mutant carrying pUCP28T (“pUCP28T” in the figure), and the complemented strain, the relA spoT mutant carrying pSS6 (“pSS6” in the figure), were also tested. (C and D) Sensitivity to osmotic stress was examined by resuspending cultures in minimal medium containing a high concentration of solute (4 M sodium chloride in panel C and 2 M sucrose in panel D). Viable cell counting was performed after 2, 4, 6, and 24 h, with cell survival represented as the percentage of the viable cell count at time zero.
The stringent response may also promote survival under conditions of oxidative stress, which bacteria face during stationary phase (40) and during colonization of mucosal tissues. In this study, we used sensitivity to hydrogen peroxide as a measurement of oxidative stress resistance. The differences in hydrogen peroxide susceptibility between all three strains were statistically significant (P < 0.05), with the relA mutant slightly but significantly more sensitive than strain PAO1, and the relA spoT mutant, in turn, more sensitive than the relA mutant (Fig. 6B). In this assay, complementation of the relA spoT mutant with pSS6 effectively reduced the hydrogen peroxide sensitivity of the relA spoT mutant to less than that of the relA mutant (Fig. 6B). These results demonstrate that, at least for certain phenotypes, overexpression of spoT was able to partially compensate for the lack of a functional copy of relA.
The tolerance of wild-type and mutant strains of P. aeruginosa to high osmolarity was assessed by exposing cultures grown to stationary phase to high concentrations of one ionic solute, 4 M sodium chloride, and one nonionic solute, 2 M sucrose. Once again, the relA spoT double mutant was the most susceptible strain, while strain PAO1 was the most resistant strain, with the relA mutant intermediate in sensitivity (Fig. 6C and D). Interestingly, the relA spoT mutant displayed an increase in susceptibility to 2 M sucrose beginning at 2 h postexposure through to the end of the experiment (Fig. 6D), whereas this strain's increased sensitivity to sodium chloride took considerably longer to manifest (Fig. 6C). Therefore, ppGpp may be more important for survival with nonionic stressors, such as 2 M sucrose, than with ionic stressors, such as 4 M sodium chloride.
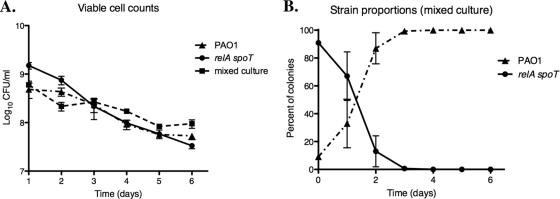
To verify the roles of RelA and SpoT in adaptation to nutrient-poor conditions, cultures of P. aeruginosa PAO1 and the relA spoT mutant were grown for 6 days in a nutrient-poor medium without supplementation with fresh medium. Using these conditions, we observed almost no difference in survival between strain PAO1 and the relA spoT mutant (Fig. 7A). In contrast, in a competition assay, where strain PAO1 and the relA spoT mutant were inoculated at a 1-to-10 ratio, PAO1 rapidly constituted the majority of the culture, in spite of its lower inoculum (Fig. 7B). In each replicate experiment, PAO1 represented 100% of the sampled colonies by day 3 or 4. These experiments demonstrated that, when faced with direct competition from an isogenic wild-type strain, the relA spoT mutant was unable to thrive under conditions of nutrient starvation.
Fig. 7.
A P. aeruginosa relA spoT double mutant is outcompeted by the wild-type strain PAO1 during prolonged coculture in diluted rich medium. Pure cultures of strain PAO1 and the relA spoT double mutant were grown overnight in BHI broth and then inoculated into diluted (0.1 strength) BHI either individually or mixed in a ratio of 10:1 for the relA spoT mutant to strain PAO1. These cultures were incubated at 37°C with aeration for 5 to 6 days without supplementation with fresh medium. (A) Results of viable cell counting for pure and mixed cultures, which was performed daily in triplicate for each culture. Error bars denote standard errors of the means. (B) Results of plating onto selective media to determine the proportion of cells in the mixed culture comprised by each strain. The mixed culture was plated daily on LB agar for single colonies, and 100 randomly selected colonies were subsequently restreaked onto media containing antibiotics selective for the relA spoT mutant. Data represent the means of three independent experiments; error bars denote standard deviations.
DISCUSSION
The ability to adapt to changing environmental conditions is a hallmark of many bacterial species. One mechanism allowing bacteria to adapt to fluctuations in nutrient availability is the stringent response. Most bacteria, with the exception of some obligate parasites, possess at least one enzyme capable of synthesizing the stringent response signal ppGpp, with the gammaproteobacteria using both RelA and SpoT for this purpose (36). As the host environment is frequently limiting for one or more essential nutrients, which therefore triggers the stringent response, many Gram-positive and Gram-negative pathogens use ppGpp to regulate virulence functions (16). In this study, we have examined the role of SpoT in the pathogenesis of P. aeruginosa. We have determined that the stringent response is critical for the virulence of P. aeruginosa in both the fly feeding and rat lung agar bead models of infection.
Most previous work on the stringent response has been performed using the bacterium E. coli; relA and relA spoT mutants have been constructed and thoroughly characterized (10). Interestingly, our P. aeruginosa relA spoT double mutant displayed a number of phenotypic differences compared to the E. coli double mutant. The P. aeruginosa relA spoT double mutant grew at rates comparable to those of the wild-type strain in both rich and minimal media (data not shown), whereas the E. coli double mutant has multiple and complex amino acid auxotrophies (65). Furthermore, the survival of the P. aeruginosa relA spoT mutant during prolonged culture in diluted rich medium was comparable to that of the wild-type strain PAO1 (Fig. 7A); we could detect a difference in survival only when the strains were cocultured (Fig. 7B). These findings imply that the relA spoT double mutant was not severely handicapped in either nutrient acquisition or survival of starvation. It is therefore possible that ppGpp is not as important for the expression of metabolic genes in P. aeruginosa as it is in E. coli. Our examination of ppGpp levels in the wild-type and mutant strains indicates that the majority of (p)ppGpp synthesis in P. aeruginosa is RelA dependent, as we were able to detect ppGpp synthesis in a strain lacking relA only when spoT was present on a multicopy plasmid (see Fig. S1 in the supplemental material). Since Boes et al. (4) were able to detect ppGpp synthesis in a P. aeruginosa relA mutant during stationary phase, we believe that SpoT is a functional ppGpp synthetase but that our assay conditions were insufficiently sensitive to detect the small amount of ppGpp remaining in the relA mutant. This too is in contrast to E. coli, wherein ppGpp accumulation in a relA single mutant is easily detected upon carbon starvation (65).
Although the relA spoT double mutant is viable under most conditions, we were unable to produce a spoT single mutant, despite repeated attempts using two different wild-type strains (PAO1 and PA14). A spoT mutant was reported and characterized by Viducic et al. (59), but we believe that a spoT mutation is extremely deleterious in relA+ strains of P. aeruginosa. Despite the relatively large size of the spoT open reading frame (2,105 bp), there is a lack of spoT mutants available in two different PAO1 transposon mutant libraries: the phoA/lacZ transposon library from the University of Washington Genome Centre and the Tn5–lux library from the Hancock laboratory at the University of British Columbia (Pseudomonas Genome Database v2 [http://www.pseudomonas.com]). spoT was also identified as a candidate essential gene in P. aeruginosa by Sakharkar et al. (46), using in silico predictions. In agreement with these facts, spoT is considered an essential gene in E. coli relA+ cells, likely because even the basal level of (p)ppGpp synthesis by RelA would prevent cell growth and division in the absence of the hydrolyzing activity of SpoT (65). Therefore, it is possible that the spoT mutant reported by Viducic et al. (59) contains a suppressor mutation that allows it to grow in spite of a lack of SpoT-mediated ppGpp hydrolysis.
Despite the viability of the relA spoT double mutant in vitro, it is severely compromised in its ability to produce disease in two models of infection. We observed an almost complete lack of lethality in the fly feeding model (Fig. 1), and in the rat lung model, there was no tissue damage or evidence of a sustained infection after 7 days of incubation with the relA spoT mutant (Fig. 2). It is important to note that the rat lung infections were not monitored prior to 7 days postinoculation; therefore, at this time we cannot say whether the relA spoT mutant was defective in its ability to establish an infection or to maintain a chronic infection in this model system. The importance of SpoT for virulence is supported by the finding that complementation of the relA spoT mutant with a spoT-encoding plasmid can partially restore lethality in the fly feeding model (Fig. 1E). The reason for the lack of full complementation in the fly model is currently unknown, but given that full complementation was observed in all of the in vitro virulence factor assays (Fig. 4), we believe that polar effects of our spoT mutation are unlikely. In combination, these results suggest that there is a generalized virulence defect in the double mutant. However, the reasons for this reduced ability to cause disease could be numerous. On the basis of our fly filter CFU counts and our starvation assay (Fig. 1B and 7A, respectively), we do not believe that the relA spoT mutant has a general growth or survival defect. However, since the relA spoT mutant is more susceptible to a variety of different stresses that may be encountered within a host (Fig. 6), this strain likely has a reduced ability to survive attacks mediated by the host immune system. In particular, the relA spoT double mutant is more sensitive to hydrogen peroxide, which the bacteria encounter while colonizing airway epithelia. The decreased production of virulence determinants such as pyocyanin by the relA spoT mutant (Fig. 4A) may account for decreased lung damage in the rat model (35) and is also relevant to oxidative killing. Pyocyanin counteracts the production of hydrogen peroxide in airway epithelial spaces (44), leading to increased bacterial survival while also killing host cells. Pyocyanin also kills bacteria, and it is possible that the inability of the relA spoT double mutant to persist in cocultures with the wild-type strain (Fig. 7B) is due to increased sensitivity to pyocyanin or other redox-active antimicrobial compounds.
The relA spoT double mutant also secretes reduced amounts of virulence factors that are known to be important for nutrient acquisition and thus survival within a host (Fig. 4). Interestingly, we noted that pyocyanin production was increased relative to wild-type levels in the relA mutant, yet the relA spoT double mutant was virtually devoid of pyocyanin (Fig. 4A) (20). Erickson et al. (20) suggested that the increased pyocyanin production by P. aeruginosa relA could be the result of its decreased levels of RpoS, a negative regulator of pyocyanin synthesis (55). We believe it is unlikely that RpoS levels could account for the decreased pyocyanin production by the relA spoT mutant, since the stress response assays suggest that RpoS levels are likely even lower in this strain (Fig. 6). Instead, we speculate that the reduced production of pyocyanin, elastase, and the siderophore pyoverdin by the relA spoT mutant can be attributed to altered expression or activity of the Las and/or Rhl quorum-sensing systems, which promote synthesis of all three of these virulence factors (5, 33, 42, 53). This raises the possibility that the decreased virulence of the relA spoT mutant might be at least partially due to a disruption of quorum-sensing signaling, which is known to contribute to the ability of P. aeruginosa to cause infections (3).
In addition to its defects in virulence factor production, the relA spoT double mutant displayed defects in swarming and twitching motility (Fig. 5), the latter of which is known to play an important role in pulmonary infections (56). The decreased ability of the relA spoT double mutant to cause chronic infections is most likely multifactorial, reflecting the pleiotropic nature of stringent response mutations. The reason for the relatively minor virulence defects of the stringent response mutants in the acute fly nicking model (Fig. 3) is not currently known but could reflect a decreased requirement for the stringent response when the bacteria need to survive within the host for a period of only hours rather than days.
In contrast to the avirulence of the relA spoT double mutant in both chronic models, the relA mutant was attenuated in virulence only in the fly feeding model (Fig. 1) (18). The fact that the relA mutant was fully virulent in the rat lung model suggests that the specific host environment dictates, to some extent, which genetic regulators are required for successful pathogenesis. These findings could imply that amino acids are not limiting in the rat lung and therefore, that RelA is not normally stimulated to produce ppGpp in this environment. In this case, the absence of a functional RelA would have little impact upon the virulence of P. aeruginosa. Recent studies have indicated that sputum samples from CF patients contain high concentrations of amino acids (41) and that P. aeruginosa expresses genes required for the transport and catabolism of amino acids when colonizing the CF lung (51). Therefore, RelA may not be essential for P. aeruginosa virulence in the CF lung, much like in the rat lung, because prevailing conditions may not induce the production of ppGpp by RelA.
In combination with previous reports, our study demonstrates the importance of the stringent response for P. aeruginosa stress survival. We previously demonstrated that a P. aeruginosa relA mutant produces a decreased amount of σS protein, the stationary-phase and general stress response alternative sigma factor (20). In E. coli, ppGpp also enhances the ability of σS to bind to core RNA polymerase, thereby allowing σS to play a greater role in activating transcription (31). These findings imply that ppGpp enhances both the amount and activity of σS. Therefore, we anticipated that the ppGpp-lacking relA spoT double mutant would display many similar phenotypes to an rpoS mutant of P. aeruginosa (55). Indeed, both mutants displayed increased sensitivity to heat shock, oxidative stress, and osmotic stress (55) (Fig. 4). In addition, P. aeruginosa stringent response mutants have reduced expression of several universal stress proteins that play a role in anaerobic stress survival (4). Thus, the stringent response coordinates the expression of a variety of stress response pathways that would be expected to promote P. aeruginosa survival in both environmental and host-associated habitats.
In summary, we have generated a P. aeruginosa relA spoT double mutant and examined the link between the stringent response and virulence in this organism. The ppGpp-devoid relA spoT strain has pronounced virulence defects in both the fly feeding model and the rat lung agar bead model of infection. Numerous virulence-associated phenotypes, including the production of secreted compounds, such as pyocyanin and siderophores, and degradative enzymes, such as elastase, as well as swarming and twitching motilities are altered in the stringent response mutants. These strains are also less able than the wild-type strain to withstand environmental insults. Since no orthologue of RelA or SpoT has been detected in animals (36), these ppGpp-synthesizing enzymes may constitute excellent drug targets. Alternatively, the P. aeruginosa relA spoT double mutant may be suitable for use as a live attenuated vaccine in certain situations, as already demonstrated for a ΔrelA ΔspoT strain of Salmonella enterica serovar Typhimurium (37). Although a live vaccine may not be appropriate for patients with CF, other groups that are susceptible to P. aeruginosa infection, such as long-term care patients and firefighters, might benefit from such a vaccine. Since SpoT plays a critical role in P. aeruginosa virulence, the development of therapeutic approaches targeting the stringent response merits further research.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by Natural Sciences and Engineering Research Council (NSERC) Discovery Grant RGPIN203595-07 to D.G.S. and Canadian Institutes of Health research grant MOP-36343 to D.E.W. D.E.W. is a Canada Research Chair in Microbiology. S.L.V. was supported by an NSERC postgraduate scholarship.
We are grateful to Monica Faria for performing the exotoxin A assays and Darshi Cheruvu for technical assistance.
Footnotes
Supplemental material for this article may be found at http://iai.asm.org/.
Published ahead of print on 25 July 2011.
REFERENCES
- 1. Battesti A., Bouveret E. 2006. Acyl carrier protein/SpoT interaction, the switch linking SpoT-dependent stress response to fatty acid metabolism. Mol. Microbiol. 62:1048–1063 [DOI] [PubMed] [Google Scholar]
- 2. Battesti A., Bouveret E. 2009. Bacteria possessing two RelA/SpoT-like proteins have evolved a specific stringent response involving the acyl carrier protein-SpoT interaction. J. Bacteriol. 191:616–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjarnsholt T., Givskov M. 2007. The role of quorum sensing in the pathogenicity of the cunning aggressor Pseudomonas aeruginosa. Anal. Bioanal. Chem. 387:409–414 [DOI] [PubMed] [Google Scholar]
- 4. Boes N., Schreiber K., Schobert M. 2008. SpoT-triggered stringent response controls usp gene expression in Pseudomonas aeruginosa. J. Bacteriol. 190:7189–7199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brint J. M., Ohman D. E. 1995. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 177:7155–7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carty N. L., et al. 2006. PtxR modulates the expression of QS-controlled virulence factors in the Pseudomonas aeruginosa strain PAO1. Mol. Microbiol. 61:782–794 [DOI] [PubMed] [Google Scholar]
- 7. Cash H. A., Woods D. E., McCullough B., Johanson W. G., Jr., Bass J. A. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119:453–459 [DOI] [PubMed] [Google Scholar]
- 8. Cashel M. 1969. The control of RNA synthesis in Escherichia coli. IV. Relevance of unusual phosphorylated compounds from amino acid-starved stringent strains. J. Biol. Chem. 244:3133–3141 [PubMed] [Google Scholar]
- 9. Cashel M. 1993. Detection of (p)ppGpp accumulation patterns in Escherichia coli mutants, p. 341–356 In Adolph K. W. (ed.), Molecular microbiology techniques, vol. 3 Academic Press, San Diego, CA [Google Scholar]
- 10. Cashel M., Gentry D., Hernandez V. J., Vinella D. 1996. The stringent response, p. 1458–1496 In Neidhardt F. C., et al. (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, 2nd ed. ASM Press, Washington, DC [Google Scholar]
- 11. Choi K. H., Kumar A., Schweizer H. P. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J. Microbiol. Methods 64:391–397 [DOI] [PubMed] [Google Scholar]
- 12. Chugani S. A., et al. 2001. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. U. S. A. 98:2752–2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung D. W., Collier R. J. 1977. Enzymatically active peptide from the ADP-ribosylating toxin of Pseudomonas aeruginosa. Infect. Immun. 16:832–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Church D., Elsayed S., Reid O., Winston B., Lindsay R. 2006. Burn wound infections. Clin. Microbiol. Rev. 19:403–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cystic Fibrosis Foundation 2008. Patient registry 2006 annual report. Cystic Fibrosis Foundation, Bethesda, MD [Google Scholar]
- 16. Dalebroux Z. D., Svensson S. L., Gaynor E. C., Swanson M. S. 2010. ppGpp conjures bacterial virulence. Microbiol. Mol. Biol. Rev. 74:171–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. D'Argenio D. A., Gallagher L. A., Berg C. A., Manoil C. 2001. Drosophila as a model host for Pseudomonas aeruginosa infection. J. Bacteriol. 183:1466–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Darzins A. 1993. The pilG gene product, required for Pseudomonas aeruginosa pilus production and twitching motility, is homologous to the enteric, single-domain response regulator CheY. J. Bacteriol. 175:5934–5944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Durfee T., Hansen A.-M., Zhi H., Blattner F. R., Jin D. J. 2008. Transcription profiling of the stringent response in Escherichia coli. J. Bacteriol. 190:1084–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erickson D. L., Lines J. L., Pesci E. C., Venturi V., Storey D. G. 2004. Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect. Immun. 72:5638–5645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fukuoka H., Homma M., Ichihara S. 2003. Flagellum-independent trail formation of Escherichia coli on semi-solid agar. Biosci. Biotechnol. Biochem. 67:1802–1805 [DOI] [PubMed] [Google Scholar]
- 22. Hanahan D. 1985. Techniques for transformation of E. coli, p. 109 In Glover D. M. (ed.), DNA cloning: a practical approach, vol. 1 IRL Press, McLean, VA [Google Scholar]
- 23. Haseltine W. A., Block R. 1973. Synthesis of guanosine tetra- and pentaphosphate requires the presence of a codon-specific, uncharged tRNA in the acceptor site of ribosomes. Proc. Natl. Acad. Sci. U. S. A. 70:1564–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heinemeyer E. A., Richter D. 1978. Mechanism of the in vitro breakdown of GDP 3′-diphosphate in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 75:4180–4183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hoang T. T., Karkhoff-Schweizer R. R., Kutchma A. J., Schweizer H. P. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86 [DOI] [PubMed] [Google Scholar]
- 26. Holloway B. W., Krishnapillai V., Morgan A. F. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 43:73–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jain V., Kumar M., Chatterji D. 2006. ppGpp: stringent response and survival. J. Microbiol. 44:1–10 [PubMed] [Google Scholar]
- 28. Kipnis E., Sawa T., Wiener-Kronish J. 2006. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med. Mal. Infect. 36:78–91 [DOI] [PubMed] [Google Scholar]
- 29. Kohler T., Curty L. K., Barja F., van Delden C., Pechere J. C. 2000. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J. Bacteriol. 182:5990–5996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koulenti D., Rello J. 2006. Gram-negative bacterial pneumonia: aetiology and management. Curr. Opin. Pulmon. Med. 12:198–204 [DOI] [PubMed] [Google Scholar]
- 31. Kvint K., Farewell A., Nystrom T. 2000. RpoS-dependent promoters require guanosine tetraphosphate for induction even in the presence of high levels of sigma(s). J. Biol. Chem. 275:14795–14798 [DOI] [PubMed] [Google Scholar]
- 32. Laffler T., Gallant J. A. 1974. Stringent control of protein synthesis in E. coli. Cell 3:47–49 [DOI] [PubMed] [Google Scholar]
- 33. Latifi A., et al. 1995. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol. Microbiol. 17:333–343 [DOI] [PubMed] [Google Scholar]
- 34. Lau G. W., Hassett D. J., Ran H., Kong F. 2004. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 10:599–606 [DOI] [PubMed] [Google Scholar]
- 35. Lau G. W., Ran H., Kong F., Hassett D. J., Mavrodi D. 2004. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 72:4275–4278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mittenhuber G. 2001. Comparative genomics and evolution of genes encoding bacterial (p)ppGpp synthetases/hydrolases (the Rel, RelA and SpoT proteins). J. Mol. Microbiol. Biotechnol. 3:585–600 [PubMed] [Google Scholar]
- 37. Na H. S., et al. 2006. Immune response induced by Salmonella typhimurium defective in ppGpp synthesis. Vaccine 24:2027–2034 [DOI] [PubMed] [Google Scholar]
- 38. Neidhardt F. C., Bloch P. L., Smith D. F. 1974. Culture medium for enterobacteria. J. Bacteriol. 119:736–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nystrom T. 2004. Growth versus maintenance: a trade-off dictated by RNA polymerase availability and sigma factor competition? Mol. Microbiol. 54:855–862 [DOI] [PubMed] [Google Scholar]
- 40. Nystrom T. 2004. Stationary-phase physiology. Annu. Rev. Microbiol. 58:161–181 [DOI] [PubMed] [Google Scholar]
- 41. Palmer K. L., Aye L. M., Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J. Bacteriol. 189:8079–8087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Passador L., Cook J. M., Gambello M. J., Rust L., Iglewski B. H. 1993. Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260:1127–1130 [DOI] [PubMed] [Google Scholar]
- 43. Prentki P., Krisch H. M. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303–313 [DOI] [PubMed] [Google Scholar]
- 44. Rada B., Lekstrom K., Damian S., Dupuy C., Leto T. L. 2008. The Pseudomonas toxin pyocyanin inhibits the dual oxidase-based antimicrobial system as it imposes oxidative stress on airway epithelial cells. J. Immunol. 181:4883–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rust L., Messing C. R., Iglewski B. H. 1994. Elastase assays. Methods Enzymol. 235:554–562 [DOI] [PubMed] [Google Scholar]
- 46. Sakharkar K. R., Sakharkar M. K., Chow V. T. 2004. A novel genomics approach for the identification of drug targets in pathogens, with special reference to Pseudomonas aeruginosa. In Silico Biol. 4:355–360 [PubMed] [Google Scholar]
- 47. Schweizer H. P. 1996. Improved methods for gene analysis and expression in Pseudomonas spp., p. 229–236 In Nakazawa T., Furukawa K., Haas D., Silver S. (ed.), Molecular biology of pseudomonads. ASM Press, Washington, DC [Google Scholar]
- 48. Schwyn B., Neilands J. B. 1987. Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160:47–56 [DOI] [PubMed] [Google Scholar]
- 49. Seyfzadeh M., Keener J., Nomura M. 1993. spoT-dependent accumulation of guanosine tetraphosphate in response to fatty acid starvation in Escherichia coli. Proc. Natl. Acad. Sci. U. S. A. 90:11004–11008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sokol P. A., Ohman D. E., Iglewski B. H. 1979. A more sensitive plate assay for detection of protease production by Pseudomonas aeruginosa. J. Clin. Microbiol. 9:538–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Son M. S., Matthews W. J., Jr., Kang Y., Nguyen D. T., Hoang T. T. 2007. In vivo evidence of Pseudomonas aeruginosa nutrient acquisition and pathogenesis in the lungs of cystic fibrosis patients. Infect. Immun. 75:5313–5324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Spira B., Silberstein N., Yagil E. 1995. Guanosine 3′,5′-bispyrophosphate (ppGpp) synthesis in cells of Escherichia coli starved for Pi. J. Bacteriol. 177:4053–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stintzi A., Evans K., Meyer J. M., Poole K. 1998. Quorum-sensing and siderophore biosynthesis in Pseudomonas aeruginosa: lasR/lasI mutants exhibit reduced pyoverdine biosynthesis. FEMS Microbiol. Lett. 166:341–345 [DOI] [PubMed] [Google Scholar]
- 54. Stover C. K., et al. 2000. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature 406:959–964 [DOI] [PubMed] [Google Scholar]
- 55. Suh S. J., et al. 1999. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J. Bacteriol. 181:3890–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang H., Kays M., Prince A. 1995. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect. Immun. 63:1278–1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Traxler M. F., et al. 2008. The global, ppGpp-mediated stringent response to amino acid starvation in Escherichia coli. Mol. Microbiol. 68:1128–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. van Delden C., Comte R., Bally A. M. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183:5376–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Viducic D., et al. 2006. Functional analysis of spoT, relA and dksA genes on quinolone tolerance in Pseudomonas aeruginosa under nongrowing condition. Microbiol. Immunol. 50:349–357 [DOI] [PubMed] [Google Scholar]
- 60. Vinella D., Albrecht C., Cashel M., D'Ari R. 2005. Iron limitation induces SpoT-dependent accumulation of ppGpp in Escherichia coli. Mol. Microbiol. 56:958–970 [DOI] [PubMed] [Google Scholar]
- 61. Vogel H. J., Bonner D. M. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97–106 [PubMed] [Google Scholar]
- 62. von Tigerstrom R. G., Stelmaschuk S. 1989. The use of Tween 20 in a sensitive turbidimetric assay of lipolytic enzymes. Can. J. Microbiol. 35:511–514 [DOI] [PubMed] [Google Scholar]
- 63. Willcox M. D. 2007. Pseudomonas aeruginosa infection and inflammation during contact lens wear: a review. Optom. Vis. Sci. 84:273–278 [DOI] [PubMed] [Google Scholar]
- 64. Winsor G. L., et al. 2005. Pseudomonas aeruginosa Genome Database and PseudoCAP: facilitating community-based, continually updated, genome annotation. Nucleic Acids Res. 33:D338–D343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xiao H., et al. 1991. Residual guanosine 3′,5′-bispyrophosphate synthetic activity of relA null mutants can be eliminated by spoT null mutations. J. Biol. Chem. 266:5980–5990 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.